Systemic Sclerosis: A Key Model of Endothelial Dysfunction
Abstract
1. Systemic Sclerosis and Endothelial Dysfunction: Background and Rationale
2. Materials and Methods
3. Endothelial Cell Damage
4. Impaired Vasculogenesis
5. Impaired Angiogenesis
6. Endothelial to Mesenchymal Transition
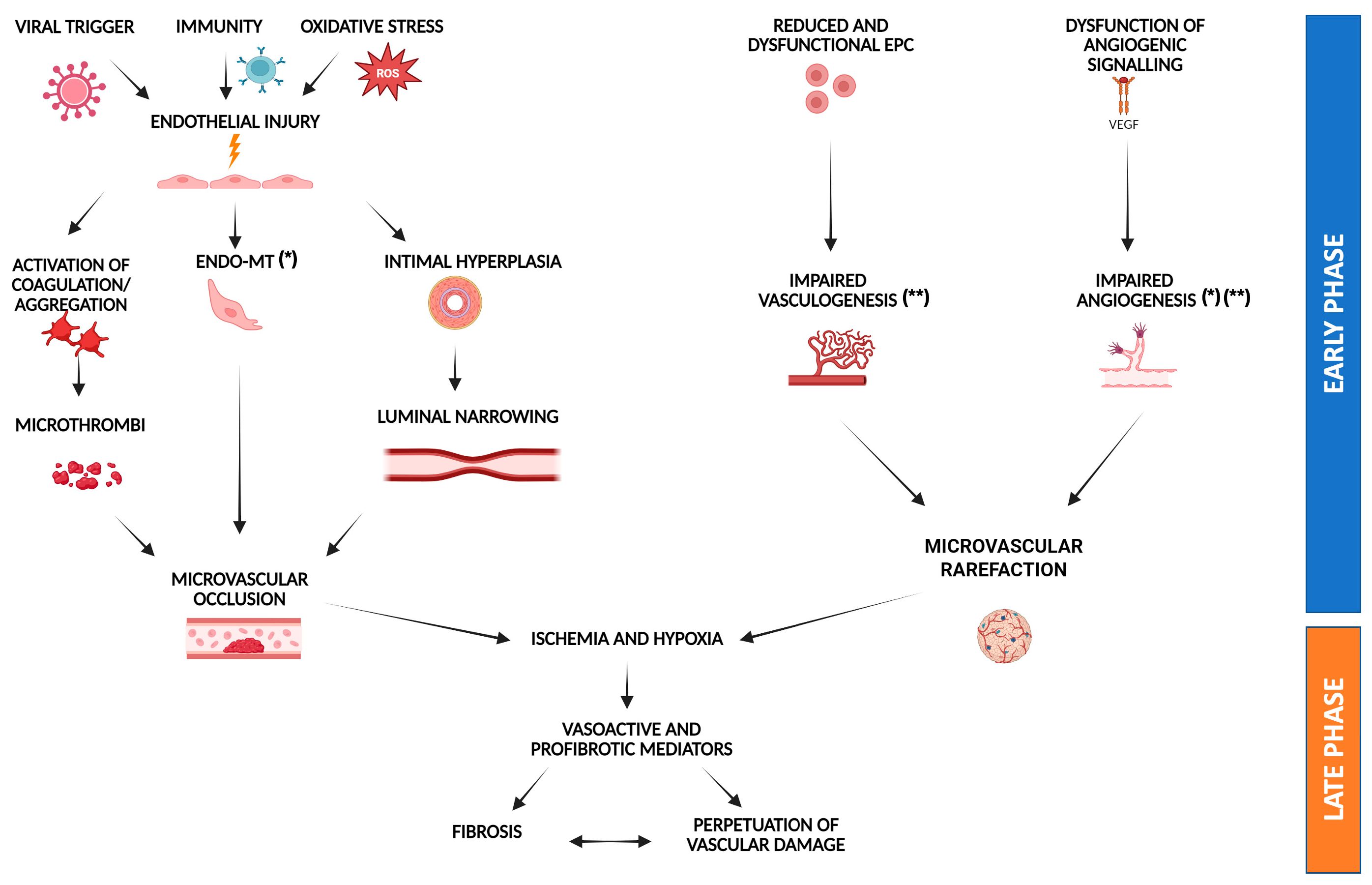
7. Endothelial Dysfunction in Systemic Sclerosis: A Mechanistic Perspective
8. Clinical Manifestations and Therapeutic Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SMA | α-smooth muscle actin |
| AECAs | Autoantibodies directed against endothelial cell antigens |
| Ang-1 and 2 | Angiopoietin-1 and 2 |
| CCL2 | C-C motif chemokine ligand 2 |
| CTGF | Connective tissue growth factor |
| EBV | Epstein–Barr virus |
| eNOS | Endothelial nitric oxide synthase |
| EndoMT | Endothelial to mesenchymal transition |
| EPCs | Endothelial progenitor cells |
| ET-1 | Endothelin-1 |
| FGF-2 | Fibroblast growth factor-2 |
| FLI1 | Friend leukemia virus integration 1 |
| FMD | Flow-mediated dilation |
| HCMV | Human cytomegalovirus |
| HLA | Human Leukocyte Antigen |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-6 | Interleukin-6 |
| ILD | Interstitial lung disease |
| IMT | Intima-media thickness |
| LTB4 | Leukotriene B4 |
| NO | Nitric oxide |
| PAH | Pulmonary arterial hypertension |
| PDE4 | Phosphodiesterase 4 |
| PDGF | Platelet-derived growth factor |
| ROS | Reactive oxygen species |
| SDF-1 | Stromal cell-derived factor-1 |
| SRC | Scleroderma renal crisis |
| TGF-β | Transforming growth factor-beta |
| TLR9 | Toll-like receptor 9 |
| TNF-α | Tumor necrosis factor-alpha |
| sJAM-1 | Soluble Junctional Adhesion Molecule-1 |
| SSc | Systemic sclerosis |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VEGF165b | Vascular Endothelial Growth Factor 165b |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| vWF | Von Willebrand factor |
References
- Denton, C.P.; Khanna, D. Systemic Sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, E.; Lyons, M.; Tran, K.; Pattanaik, D. Endothelial Dysfunction in Systemic Sclerosis. Int. J. Mol. Sci. 2023, 24, 14385. [Google Scholar] [CrossRef] [PubMed]
- Maehara, T.; Kaneko, N.; Perugino, C.A.; Mattoo, H.; Kers, J.; Allard-Chamard, H.; Mahajan, V.S.; Liu, H.; Murphy, S.J.H.; Ghebremichael, M.; et al. Cytotoxic CD4+ T Lymphocytes May Induce Endothelial Cell Apoptosis in Systemic Sclerosis. J. Clin. Investig. 2020, 130, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Sgonc, R.; Gruschwitz, M.S.; Boeck, G.; Sepp, N.; Gruber, J.; Wick, G. Endothelial Cell Apoptosis in Systemic Sclerosis Is Induced by Antibody-Dependent Cell-Mediated Cytotoxicity via CD95. Arthritis Rheum. 2000, 43, 2550–2562. [Google Scholar] [CrossRef] [PubMed]
- Corallo, C.; Franci, B.; Lucani, B.; Montella, A.; Chirico, C.; Gonnelli, S.; Nuti, R.; Giordano, N. From Microvasculature to Fibroblasts: Contribution of Anti-Endothelial Cell Antibodies in Systemic Sclerosis. Int. J. Immunopathol. Pharmacol. 2015, 28, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Moroncini, G.; Mori, S.; Tonnini, C.; Gabrielli, A. Role of Viral Infections in the Etiopathogenesis of Systemic Sclerosis. Clin. Exp. Rheumatol. 2013, 31, 3–7. [Google Scholar] [PubMed]
- Pandey, J.P.; LeRoy, E.C. Human Cytomegalovirus and the Vasculopathies of Autoimmune Diseases (Especially Scleroderma), Allograft Rejection, and Coronary Restenosis. Arthritis Rheum. 1998, 41, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Muryoi, T.; Kasturi, K.N.; Kafina, M.J.; Cram, D.S.; Harrison, L.C.; Sasaki, T.; Bona, C.A. Antitopoisomerase I Monoclonal Autoantibodies from Scleroderma Patients and Tight Skin Mouse Interact with Similar Epitopes. J. Exp. Med. 1992, 175, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.; Bason, C.; Navone, R.; Millo, E.; Damonte, G.; Corrocher, R.; Puccetti, A. Systemic Sclerosis Immunoglobulin G Autoantibodies Bind the Human Cytomegalovirus Late Protein UL94 and Induce Apoptosis in Human Endothelial Cells. Nat. Med. 2000, 6, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Rosato, E.; York, M.; Gewurz, B.E.; Trojanowska, M.; Farina, G.A. Innate Immune Modulation Induced by EBV Lytic Infection Promotes Endothelial Cell Inflammation and Vascular Injury in Scleroderma. Front. Immunol. 2021, 12, 651013. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Nuovo, G.; Ferri, C.; Crowson, A.N.; Giuggioli, D.; Sebastiani, M. Parvoviral Infection of Endothelial Cells and Stromal Fibroblasts: A Possible Pathogenetic Role in Scleroderma. J. Cutan. Pathol. 2004, 31, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Giovannetti, A.; Gambardella, L.; Malorni, W.; Pietraforte, D.; Straface, E. Oxidative Stress in the Pathogenesis of Systemic Scleroderma: An Overview. J. Cell Mol. Med. 2018, 22, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Abraham, D.; Ong, V. The Yin and Yang of IL-17 in Systemic Sclerosis. Front. Immunol. 2022, 13, 885609. [Google Scholar] [CrossRef] [PubMed]
- Noack, M.; Beringer, A.; Miossec, P. Additive or Synergistic Interactions Between IL-17A or IL-17F and TNF or IL-1β Depend on the Cell Type. Front. Immunol. 2019, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Mogami, A.; Saito, R.; Seki, N.; Ishigaki, S.; Takei, H.; Yoshimoto, K.; Chiba, K.; Takeuchi, T.; Kaneko, Y. Interleukin-17A Is a Potential Therapeutic Target Predicted by Proteomics for Systemic Sclerosis Patients at High Risk of Pulmonary Arterial Hypertension. Sci. Rep. 2024, 14, 29484. [Google Scholar] [CrossRef] [PubMed]
- Schachna, L.; Wigley, F.M. Targeting Mediators of Vascular Injury in Scleroderma. Curr. Opin. Rheumatol. 2002, 14, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Privitera, D.; Bodio, C.; Lonati, P.A.; Borghi, M.O.; Ingegnoli, F.; Meroni, P.L.; Chighizola, C.B. Scleroderma-Specific Autoantibodies Embedded in Immune Complexes Mediate Endothelial Damage: An Early Event in the Pathogenesis of Systemic Sclerosis. Arthritis Res. Ther. 2020, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Tabib, T.; Khanna, D.; Assassi, S.; Domsic, R.; Lafyatis, R. Single-Cell Transcriptomes and Chromatin Accessibility of Endothelial Cells Unravel Transcription Factors Associated with Dysregulated Angiogenesis in Systemic Sclerosis. Ann. Rheum. Dis. 2024, 83, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Renaud, L.; Wilson, C.L.; Lafyatis, R.; Schnapp, L.M.; Feghali-Bostwick, C.A. Transcriptomic Characterization of Lung Pericytes in Systemic Sclerosis-Associated Pulmonary Fibrosis. iScience 2024, 27, 110010. [Google Scholar] [CrossRef] [PubMed]
- Chepy, A.; Vivier, S.; Bray, F.; Chauvet, C.; Lescoat, A.; Elhannani, A.; Figeac, M.; Guilbert, L.; Leprêtre, F.; Bourel, L.; et al. Immunoglobulins G from Patients with Systemic Sclerosis Modify the Molecular Signatures of Endothelial Cells. RMD Open 2025, 11, e004290. [Google Scholar] [CrossRef] [PubMed]
- Rius Rigau, A.; Li, Y.-N.; Matei, A.-E.; Györfi, A.-H.; Bruch, P.-M.; Koziel, S.; Devakumar, V.; Gabrielli, A.; Kreuter, A.; Wang, J.; et al. Characterization of Vascular Niche in Systemic Sclerosis by Spatial Proteomics. Circ. Res. 2024, 134, 875–891. [Google Scholar] [CrossRef] [PubMed]
- BELLANDO-RANDONE, S.; MATUCCI-CERINIC, M. The Measurement of the Endothelial Glycocalyx as a New Biomarker of Endothelial Derangement in Systemic Sclerosis: A Challenge for the Future. J. Rheumatol. 2017, 44, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Alphonsus, C.S.; Rodseth, R.N. The Endothelial Glycocalyx: A Review of the Vascular Barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Nijst, P.; Kiefer, K.; Tang, W.H.W. Endothelial Glycocalyx as Biomarker for Cardiovascular Diseases: Mechanistic and Clinical Implications. Curr. Heart Fail. Rep. 2017, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Armengol, G.; Le Besnerais, M.; Lévesque, H.; Benhamou, Y. New Insights into Systemic Sclerosis Related Microcirculatory Dysfunction by Assessment of Sublingual Micr\ocirculation and Vascular Glycocalyx Layer. Results A Prelim. Study Microvasc. Res. 2015, 99, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Gates, P.E.; Vink, H.; Frech, T.M.; Donato, A.J. Automated Measurement of Microvascular Function Reveals Dysfunction in Systemic Sclerosis: A Cross-Sectional Study. J. Rheumatol. 2017, 44, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Tas, S.W.; Maracle, C.X.; Balogh, E.; Szekanecz, Z. Targeting of Proangiogenic Signalling Pathways in Chronic Inflammation. Nat. Rev. Rheumatol. 2016, 12, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Juin, F.; Wipff, J.; Couraud, P.O.; Chiocchia, G.; Kahan, A.; Boileau, C.; Uzan, G.; Allanore, Y. Circulating Endothelial Progenitor Cells in Systemic Sclerosis: Association with Disease Severity. Ann. Rheum. Dis. 2008, 67, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Meune, C.; Ruiz, B.; Couraud, P.O.; Uzan, G.; Boileau, C.; Kahan, A.; Chiocchia, G.; Allanore, Y. Angiogenic Biomarkers Predict the Occurrence of Digital Ulcers in Systemic Sclerosis. Ann. Rheum. Dis. 2012, 71, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, M.; Okazaki, Y.; Yasuoka, H.; Kawakami, Y.; Ikeda, Y. Defective Vasculogenesis in Systemic Sclerosis. Lancet 2004, 364, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, M.; Okazaki, Y. Brief Report: Impaired In Vivo Neovascularization Capacity of Endothelial Progenitor Cells in Patients With Systemic Sclerosis. Arthritis Rheumatol. 2014, 66, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Carrai, V.; Miniati, I.; Guiducci, S.; Capaccioli, G.; Alterini, R.; Saccardi, R.; Conforti, M.L.; Rigacci, L.; Rotunno, G.; Bosi, A.; et al. Evidence for Reduced Angiogenesis in Bone Marrow in SSc: Immunohistochemistry and Multiparametric Computerized Imaging Analysis. Rheumatology 2012, 51, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Okazaki, Y.; Inoue, Y.; Tamura, Y.; Yasuoka, H.; Takeuchi, T.; Kuwana, M. Elevated Levels of Pentraxin 3 in Systemic Sclerosis: Associations With Vascular Manifestations and Defective Vasculogenesis. Arthritis Rheumatol. 2015, 67, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Evans, S.; Yan, B.; Povsic, T.J.; Tapson, V.; Goldschmidt-Clermont, P.J.; Dong, C. Transcriptional Regulation of Bim by FOXO3a and Akt Mediates Scleroderma Serum–Induced Apoptosis in Endothelial Progenitor Cells. Circulation 2008, 118, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Al-Soudi, A.; Kaaij, M.H.; Tas, S.W. Endothelial Cells: From Innocent Bystanders to Active Participants in Immune Responses. Autoimmun. Rev. 2017, 16, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Pratesi, S.; Romano, E.; Rosa, I.; Bruni, C.; Bellando-Randone, S.; Guiducci, S.; Maggi, E.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Decreased Circulating Lymphatic Endothelial Progenitor Cells in Digital Ulcer-Complicated Systemic Sclerosis. Ann. Rheum. Dis. 2019, 78, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Okazaki, Y.; Seta, N.; Satoh, T.; Takahashi, K.; Ikezawa, Z.; Kuwana, M. Enhanced Angiogenic Potency of Monocytic Endothelial Progenitor Cells in Patients with Systemic Sclerosis. Arthritis Res. Ther. 2010, 12, R205. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Yasuoka, H.; Satoh, T.; Okazaki, Y.; Yamaguchi, Y.; Kuwana, M. Versican Is Upregulated in Circulating Monocytes in Patients with Systemic Sclerosis and Amplifies a CCL2-Mediated Pathogenic Loop. Arthritis Res. Ther. 2013, 15, R74. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.I.; Zhang, D.-N.; Cooke, J.P.; Ho, H.-K.V.; Avalos, E.; Herrera, R.; Herron, G.S. Differential Expression of Nitric Oxide by Dermal Microvascular Endothelial Cells from Patients with Scleroderma. Vasc. Med. 2000, 5, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-J.; Min, D.-J.; Cho, M.-L.; Min, S.-Y.; Kim, S.-J.; Lee, S.-S.; Park, K.-S.; Seo, Y.-I.; Kim, W.-U.; Park, S.-H.; et al. Elevated Vascular Endothelial Growth Factor in Systemic Sclerosis. J. Rheumatol. 2003, 30, 1529–1533. [Google Scholar] [PubMed]
- Manetti, M.; Guiducci, S.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Impaired Angiogenesis in Systemic Sclerosis: The Emerging Role of the Antiangiogenic VEGF165b Splice Variant. Trends Cardiovasc. Med. 2011, 21, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Jakubus, M.; Kowal-Bielecka, O.; Chodorowska, G.; Bielecki, M.; Krasowska, D. Angiopoietins-1 and -2 Are Differentially Expressed in the Sera of Patients with Systemic Sclerosis: High Angiopoietin-2 Levels Are Associated with Greater Severity and Higher Activity of the Disease. Rheumatology 2011, 50, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, P.; Kahaleh, B. Association between Enhanced Type I Collagen Expression and Epigenetic Repression of the FLI1 Gene in Scleroderma Fibroblasts. Arthritis Rheum. 2006, 54, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Capece, D.; Zazzeroni, F.; Liakouli, V.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Pecetti, G.; et al. The Endothelial-Mesenchymal Transition in Systemic Sclerosis Is Induced by Endothelin-1 and Transforming Growth Factor-β and May Be Blocked by Macitentan, a Dual Endothelin-1 Receptor Antagonist. J. Rheumatol. 2015, 42, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A. Role of Endothelial to Mesenchymal Transition in the Pathogenesis of the Vascular Alterations in Systemic Sclerosis. Int. Sch. Res. Not. Rheumatol. 2013, 2013, 835948. [Google Scholar] [CrossRef] [PubMed]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.A.; Piera-Velazquez, S.; Farber, J.L.; Feghali-Bostwick, C.; Jiménez, S.A. Endothelial Cells Expressing Endothelial and Mesenchymal Cell Gene Products in Lung Tissue From Patients With Systemic Sclerosis–Associated Interstitial Lung Disease. Arthritis Rheumatol. 2016, 68, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Romano, E.; Rosa, I.; Guiducci, S.; Bellando-Randone, S.; De Paulis, A.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Endothelial-to-Mesenchymal Transition Contributes to Endothelial Dysfunction and Dermal Fibrosis in Systemic Sclerosis. Ann. Rheum. Dis. 2017, 76, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lv, J.; Jiang, Z.; He, H.; Chen, C.; Xiong, Y.; Zhu, X.; Xue, Y.; Yu, Y.; Yang, S.; et al. Promotion of Myofibroblast Differentiation and Tissue Fibrosis by the Leukotriene B4–Leukotriene B4 Receptor Axis in Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence That Systemic Sclerosis Is a Vascular Disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Bohn, E.; Kröber, S.M.; Xiao, Y.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic Cells Induce Migration of Phagocytes via Caspase-3-Mediated Release of a Lipid Attraction Signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, G.P.; Brown, K.K.; Schiemann, W.P.; Serls, A.E.; Parr, J.E.; Geraci, M.W.; Schwarz, M.I.; Cool, C.D.; Worthen, G.S. Pigment Epithelium–Derived Factor in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2004, 170, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.; Denton, C.P.; Pope, J.E.; Matucci-Cerinic, M. Digital Ulcers: Overt Vascular Disease in Systemic Sclerosis. Rheumatology 2006, 48, iii19–iii24. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Lücht, C.; Hosp, I.; Zhao, H.; Wu, D.; Heidecke, H.; Witowski, J.; Budde, K.; Riemekasten, G.; Catar, R. Autoantibodies from Patients with Scleroderma Renal Crisis Promote PAR-1 Receptor Activation and IL-6 Production in Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11793. [Google Scholar] [CrossRef] [PubMed]
- Penn, H.; Quillinan, N.; Khan, K.; Chakravarty, K.; Ong, V.H.; Burns, A.; Denton, C.P. Targeting the Endothelin Axis in Scleroderma Renal Crisis: Rationale and Feasibility. QJM Int. J. Med. 2013, 106, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.; Corallo, C.; Chirico, C.; Brazzi, A.; Marinetti, A.; Fioravanti, A.; Valenti, R.; Nuti, R.; Pecetti, G. Pulmonary Arterial Hypertension in Systemic Sclerosis: Diagnosis and Treatment According to the European Society of Cardiology and European Respiratory Society 2015 Guidelines. J. Scleroderma Relat. Disord. 2019, 4, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, P.; Guggino, G.; Manzi, G.; Ruscitti, P.; Berardicurti, O.; Panzera, N.; Grazia, N.; Badagliacca, R.; Riccieri, V.; Vizza, C.D.; et al. Interleukin-32 in Systemic Sclerosis, a Potential New Biomarker for Pulmonary Arterial Hypertension. Arthritis Res. Ther. 2020, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and Risk Factors for Death in Systemic Sclerosis: A Study from the EULAR Scleroderma Trials and Research (EUSTAR) Database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Li, X.; Li, W.; Yang, Z.; Jiao, R.; Wang, Q.; Meng, L.; Zhang, T.; Liu, J.; et al. Nerandomilast Improves Bleomycin-Induced Systemic Sclerosis-Associated Interstitial Lung Disease in Mice by Regulating the TGF-Β1 Pathway. Inflammation 2024. [Google Scholar] [CrossRef] [PubMed]
- Reininger, D.; Wolf, F.; Mayr, C.H.; Wespel, S.L.; Laufhaeger, N.; Geillinger-Kästle, K.; Dick, A.; Gantner, F.; Nickolaus, P.; Herrmann, F.E. Insights Into the Cellular and Molecular Mechanisms Behind the Antifibrotic Effects of Nerandomilast. Am. J. Respir. Cell Mol. Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Reininger, D.; Wolf, F.; Mayr, C.; Nickolaus, P.; Hermann, F.E. P146 Staying in the Flow: PDE4B Inhibitor Nerandomilast (BI 1015550) Improves Features of Vascular Dysfunction in Lung Fibrosis in Vitro and in Vivo. Rheumatology 2025, 64, keaf142. [Google Scholar] [CrossRef]
- Hamada, N.; Fukushima, K.; Motooka, D.; Hayashi, Y.; Fukui, E.; Okuzaki, D.; Shintani, Y.; Akira, S. Exploration of Mechanism of Action of Nerandomilast in Pulmonary Fibrosis Through Single Cell Rna Sequence and Spatial Transcriptomic Analysis [abstract]. Am. J. Respir. Crit. Care Med. 2025, 211, A7626. [Google Scholar] [CrossRef]
| Mechanism | Molecular/Cellular Factors Involved | Pathological Consequences |
|---|---|---|
| Immune-mediated mechanism | CD8+ cytotoxic T cells, anti-endothelial cell antibodies (AECAs), HLA class I/II expression | Endothelial activation and apoptosis, increased IL-6, ICAM-1, TGF-β1, fibrosis, vasoconstriction |
| Oxidative stress | Reactive oxygen species (ROS), decreased nitric oxide (NO), ischemia–reperfusion injury | Vasoconstriction, platelet aggregation, endothelial apoptosis |
| Viral infections | HCMV (UL94), EBV (TLR9, IFN pathways), parvovirus B19 | Endothelial cytotoxicity, apoptosis, inflammation |
| Impaired vasculogenesis | Decreased EPCs (CD34+ CD133+ VEGFR2+), bone marrow fibrosis, pentraxin-3, Akt-FOXO3a-Bim pathway | Reduced vascular repair, digital ulcers, pulmonary arterial hypertension |
| Impaired angiogenesis | eNOS deficiency, VEGF165b (antiangiogenic isoform), reduced Ang-1, increased Ang-2, FLI1 deficiency | Capillary rarefaction, chronic hypoxia, endothelial dysfunction |
| Endothelial-to-mesenchymal transition (EndoMT) | TGF-β1, endothelin-1, Wnt3a, hypoxia, microRNAs, IL-1β, TNF-α, oxidative stress, PI3K/Akt/mTOR, LTB4/BLT1 | Myofibroblast differentiation, vascular remodeling, fibrosis |
| Pro-thrombotic state and vascular remodeling | Coagulation cascade activation, platelet aggregation, CTGF, chronic hypoxia | Intimal hyperplasia, luminal narrowing, ischemia, fibrosis |
| Biomarker | Biological Role | Alteration in SSc | Clinical Relevance |
|---|---|---|---|
| ET-1 | Potent vasoconstrictor; promotes fibrosis and vascular remodeling | Elevated in serum and tissues | Correlates with severity of vasculopathy, digital ulcers, PAH, and renal crisis |
| VCAM-1 | Promotes leukocyte adhesion to activated endothelium | Increased expression on endothelial cells and in circulation | Marker of endothelial activation; associated with inflammation and early microvascular damage |
| vWF | Mediates platelet adhesion; marker of endothelial injury | Elevated plasma levels | Indicator of endothelial damage and thrombotic risk; associated with PAH and digital ulcers |
| IL-6 | Pro-inflammatory cytokine; promotes acute-phase response | Elevated serum levels | Predicts disease progression, vascular complications (e.g., SRC, PAH), and fibrosis |
| sJAM-1 | Maintains endothelial integrity; regulates leukocyte transmigration | Increased in circulation | Associated with endothelial disruption; elevated in early SSc and vasculopathic complications |
| E-selectin | Facilitates leukocyte rolling on activated endothelium | Elevated serum levels | Reflects endothelial activation; linked to inflammation and Raynaud’s phenomenon |
| Ang-2 | Antagonist of Ang-1; disrupts endothelial stability | Elevated in serum | Associated with vascular regression and disease severity; predictor of PAH |
| VEGF | Stimulates angiogenesis | Elevated, especially antiangiogenic VEGF165b isoform | Paradoxical role: increased levels yet impaired angiogenesis; related to capillary rarefaction |
| NO | Vasodilator; maintains vascular tone and inhibits platelet aggregation | Reduced bioavailability | Contributes to vasoconstriction, endothelial dysfunction, and impaired tissue perfusion |
| TGF-β | Profibrotic cytokine; regulates immune responses and extracellular matrix production | Overexpressed in affected tissues | Central mediator of fibrosis and vasculopathy; therapeutic target in SSc |
| TNF-α | Tumor necrosis factor-alpha | Pro-inflammatory cytokine; enhances endothelial activation, leukocyte adhesion, and perpetuates vascular inflammation | Promotes chronic inflammation and endothelial activation; associated with digital ulcers and fibrotic progression in SSc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccone, V.; Falsetti, L.; Contegiacomo, S.; Cataldi, S.; Benfaremo, D.; Moroncini, G. Systemic Sclerosis: A Key Model of Endothelial Dysfunction. Biomedicines 2025, 13, 1771. https://doi.org/10.3390/biomedicines13071771
Zaccone V, Falsetti L, Contegiacomo S, Cataldi S, Benfaremo D, Moroncini G. Systemic Sclerosis: A Key Model of Endothelial Dysfunction. Biomedicines. 2025; 13(7):1771. https://doi.org/10.3390/biomedicines13071771
Chicago/Turabian StyleZaccone, Vincenzo, Lorenzo Falsetti, Silvia Contegiacomo, Serena Cataldi, Devis Benfaremo, and Gianluca Moroncini. 2025. "Systemic Sclerosis: A Key Model of Endothelial Dysfunction" Biomedicines 13, no. 7: 1771. https://doi.org/10.3390/biomedicines13071771
APA StyleZaccone, V., Falsetti, L., Contegiacomo, S., Cataldi, S., Benfaremo, D., & Moroncini, G. (2025). Systemic Sclerosis: A Key Model of Endothelial Dysfunction. Biomedicines, 13(7), 1771. https://doi.org/10.3390/biomedicines13071771






