Targeted and Biomimetic Nanoparticles for Atherosclerosis Therapy: A Review of Emerging Strategies
Abstract
1. Introduction
- Qualifying articles relating to the use of nanoparticles in the treatment of atherosclerosis;
- Qualifying both in vivo and in vitro studies;
- Qualifying both abstracts and full-text articles.
- Articles in a language other than English or Polish;
- Articles from before 2020;
- Articles with content that does not correspond to the subject of the article;
- Articles that do not clearly define the effect of nanoparticles on atherosclerosis.
2. Macrophage-Targeted Nanoparticles
3. Endothelial Cell-Targeted Nanoparticles
4. Vascular Smooth Muscle Cells (VSMCs)-Targeted Nanoparticles
5. Lowering LDL Levels
6. Anti-Inflammatory and Anti-Oxidative Acting
7. Platelet Membrane-Coated Nanoparticles
8. Neutrophil Membrane-Coated Nanoparticles
9. Erythrocyte-Membrane Coated Nanoparticles
10. Challenges and Future Directions in Nanoparticle-Based Atherosclerosis Therapy
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, P.; Ren, J.; Yang, L. Nanoparticles in the New Era of Cardiovascular Therapeutics: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 5205. [Google Scholar] [CrossRef] [PubMed]
- WHO Reports. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 15 March 2025).
- Tu, S.; He, W.; Han, J.; Wu, A.; Ren, W. Advances in imaging and treatment of atherosclerosis based on organic nanoparticles. APL Bioeng. 2022, 6, 041501. [Google Scholar] [CrossRef] [PubMed]
- Aili, T.; Zong, J.B.; Zhou, Y.F.; Liu, Y.X.; Yang, X.L.; Hu, B.; Wu, J.H. Recent advances of self-assembled nanoparticles in the diagnosis and treatment of atherosclerosis. Theranostics 2024, 14, 7505–7533. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, H.; Chen, Y.; Wu, R. Nanomedicine for Diagnosis and Treatment of Atherosclerosis. Adv. Sci. 2023, 10, e2304294. [Google Scholar] [CrossRef]
- Evgenii, G.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar]
- Meng, H.; Ruan, J.; Yan, Z.; Chen, Y.; Liu, J.; Li, X.; Meng, F. New Progress in Early Diagnosis of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 8939. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; He, W.; Yao, C.; Ma, X.; Ren, W.; Mai, Y.; Wu, A. Applications of inorganic nanoparticles in the diagnosis and therapy of atherosclerosis. Biomater. Sci. 2020, 8, 3784–3799. [Google Scholar] [CrossRef]
- Zhang, X.; Centurion, F.; Misra, A.; Patel, S.; Gu, Z. Molecularly targeted nanomedicine enabled by inorganic nanoparticles for atherosclerosis diagnosis and treatment. Adv. Drug Deliv. Rev. 2023, 194, 11470. [Google Scholar] [CrossRef]
- Yang, L.; Zang, G.; Li, J.; Li, X.; Li, Y.; Zhao, Y. Cell-derived biomimetic nanoparticles as a novel drug delivery system for atherosclerosis: Predecessors and perspectives. Regen. Biomater. 2020, 7, 349–358. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, J.; Chong, S.Y.; Ting, H.J.; Tang, X.; Yang, L.; Zhang, S.; Qi, X.; Pei, P.; Yi, Z.; et al. Dual-Function Nanoscale Coordination Polymer Nanoparticles for Targeted Diagnosis and Therapeutic Delivery in Atherosclerosis. Small 2024, 20, e2401659. [Google Scholar] [CrossRef]
- Ouyang, J.; Xie, A.; Zhou, J.; Liu, R.; Wang, L.; Liu, H.; Kong, N.; Tao, W. Minimally invasive nanomedicine: Nanotechnology in photo-/ultrasound-/radiation-/magnetism-mediated therapy and imaging. Chem. Soc. Rev. 2022, 51, 4996–5041. [Google Scholar] [CrossRef]
- Dai, T.; He, W.; Tu, S.; Han, J.; Yuan, B.; Yao, C.; Ren, W.; Wu, A. Black TiO2 nanoprobe-mediated mild phototherapy reduces intracellular lipid levels in atherosclerotic foam cells via cholesterol regulation pathways instead of apoptosis. Bioact. Mater. 2022, 17, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Perera, B.; Wu, Y.; Nguyen, N.T.; Ta, H.T. Advances in drug delivery to atherosclerosis: Investigating the efficiency of different nanomaterials employed for different type of drugs. Mater. Today Bio 2023, 22, 100767. [Google Scholar] [CrossRef]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.P.; Luo, S.X.; Fan, X.Q.; Li, D.; Tong, X.Y. Macrophage-targeted nanomedicine for the diagnosis and management of atherosclerosis. Front. Pharmacol. 2022, 13, 1000316. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yurdagul, A., Jr.; Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 2020, 12, eaay1063. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, J.; Chen, J.; Li, Y.; Bu, T.; Li, Z.; Zhang, L.; Sun, W.; Zhou, T.; Hu, W.; et al. Targeted delivery of MerTK protein via cell membrane engineered nanoparticle enhances efferocytosis and attenuates atherosclerosis in diabetic ApoE-/-Mice. J. Nanobiotechnology 2024, 22, 178. [Google Scholar] [CrossRef]
- Patel, Y.; Manturthi, S.; Tiwari, S.; Gahunia, E.; Courtemanche, A.; Gandelman, M.; Côté, M.; Gadde, S. Development of Pro-resolving and Pro-efferocytic Nanoparticles for Atherosclerosis Therapy. ACS Pharmacol. Transl. Sci. 2024, 7, 3086–3095. [Google Scholar] [CrossRef]
- Nankivell, V.; Vidanapathirana, A.K.; Hoogendoorn, A.; Tan, J.T.M.; Verjans, J.; Psaltis, P.J.; Hutchinson, M.R.; Gibson, B.C.; Lu, Y.; Goldys, E.; et al. Targeting macrophages with multifunctional nanoparticles to detect and prevent atherosclerotic cardiovascular disease. Cardiovasc. Res. 2024, 120, 819–838. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.; Kong, N.; Xiao, Y.; Yurdagul, A., Jr.; Tabas, I.; Tao, W. Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. Nat. Protoc. 2022, 17, 748–780. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Cheng, M.; Zhang, X.; Chen, X. Targeting macrophages using nanoparticles: A potential therapeutic strategy for atherosclerosis. J. Mater. Chem. B 2021, 9, 3284–3294. [Google Scholar] [CrossRef]
- Mao, Y.; Ren, J.; Yang, L. Advances of nanomedicine in treatment of atherosclerosis and thrombosis. Environ. Res. 2023, 238 Pt 2, 116637. [Google Scholar] [CrossRef]
- Rao, L.; Zhao, S.K.; Wen, C.; Tian, R.; Lin, L.; Cai, B.; Sun, Y.; Kang, F.; Yang, Z.; He, L.; et al. Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Adv. Mater. 2020, 32, e2004853. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, Y.; Cheraga, N.; Abodunrin, O.D.; Qu, K.Y.; Qiao, L.; Ma, Y.Q.; Hang, Y.; Huang, N.P.; Chen, L.J. M2 Macrophage Membrane-Camouflaged Fe3 O4 -Cy7 Nanoparticles with Reduced Immunogenicity for Targeted NIR/MR Imaging of Atherosclerosis. Small 2024, 20, e2304110. [Google Scholar] [CrossRef]
- Castro, R.; Adair, J.H.; Mastro, A.M.; Neuberger, T.; Matters, G.L. VCAM-1-targeted nanoparticles to diagnose, monitor and treat atherosclerosis. Nanomedicine 2024, 19, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef]
- Xu, H.; She, P.; Ma, B.; Zhao, Z.; Li, G.; Wang, Y. ROS responsive nanoparticles loaded with lipid-specific AIEgen for atherosclerosis-targeted diagnosis and bifunctional therapy. Biomaterials 2022, 288, 121734. [Google Scholar] [CrossRef]
- Vi, G.; Hao, L.; Li, X.; Xu, W.; Liu, F.; Peng, Q.; Lv, S. VCAM-1-targeted and PPARδ-agonist-loaded nanomicelles enhanced suppressing effects on apoptosis and migration of oxidized low-density lipoprotein-induced vascular smooth muscle cells. Biosci. Rep. 2020, 40, BSR20200559. [Google Scholar]
- Liu, Y.; He, M.; Yuan, Y.; Nie, C.; Wei, K.; Zhang, T.; Chen, T.; Chu, X. Neutrophil-Membrane-Coated Biomineralized Metal-Organic Framework Nanoparticles for Atherosclerosis Treatment by Targeting Gene Silencing. ACS Nano 2023, 17, 7721–7732. [Google Scholar] [CrossRef]
- Fang, F.; Ni, Y.; Yu, H.; Yin, H.; Yang, F.; Li, C.; Sun, D.; Pei, T.; Ma, J.; Deng, L.; et al. Inflammatory endothelium-targeted and cathepsin responsive nanoparticles are effective against atherosclerosis. Theranostics 2022, 12, 4200–4220. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Feng, T.; Li, J.; Zhang, H.; Wang, Q.; Chen, Y.; Wang, G.; Shen, Y.; Liu, X. Cathepsin K contributed to disturbed flow-induced atherosclerosis is dependent on integrin-actin cytoskeleton-NF-κB pathway. Genes Dis. 2022, 10, 583–595. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.S.; Brott, B.C.; Jun, H.W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS Responsive Nanoplatform with Two-Photon AIE Imaging for Atherosclerosis Diagnosis and Two-Pronged Therapy. Small 2020, 16, e2003253. [Google Scholar] [CrossRef]
- He, J.; Gao, Y.; Yang, C.; Guo, Y.; Liu, L.; Lu, S.; He, H. Navigating the landscape: Prospects and hurdles in targeting vascular smooth muscle cells for atherosclerosis diagnosis and therapy. J. Control. Release 2024, 366, 261–281. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in atherosclerosis regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Deroissart, J.; Porsch, F.; Koller, T.; Binder, C.J. Anti-inflammatory and Immunomodulatory Therapies in Atherosclerosis. Handb. Exp. Pharmacol. 2022, 270, 359–404. [Google Scholar] [PubMed]
- Pedro-Botet, J.; Elisenda, C.; David, B. Atherosclerosis and inflammation. New therapeutic approaches. Med. Clínica 2020, 155, 256–262. [Google Scholar]
- Miano, J.M.; Edward, A.F.; Mark, W.M. Fate and state of vascular smooth muscle cells in atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar] [CrossRef]
- Liu, Y.X.; Yuan, P.Z.; Wu, J.H.; Hu, B. Lipid accumulation and novel insight into vascular smooth muscle cells in atherosclerosis. J. Mol. Med. 2021, 99, 1511–1526. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Martin, R.B. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.D.; Poon, C.; Wang, J.; Joo, J.; Ong, V.; Jiang, Z.; Cheng, K.; Plotkin, A.; Magee, G.A.; Chung, E.J. miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype. Biomaterials 2021, 273, 120810. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Zhang, W.; Qin, C.; Zhu, Y.; Fang, Y.; Wang, Y.; Tang, C.; Cao, F. Osteopontin-Targeted and PPARδ-Agonist-Loaded Nanoparticles Efficiently Reduce Atherosclerosis in Apolipoprotein E-/-Mice. ACS Omega 2022, 7, 28767–28778. [Google Scholar] [CrossRef]
- Jang, B.; Zhang, D.; Ma, Z.; Yang, X.; Liu, L.; Xing, H.; Feng, L.; Song, J.; Zhao, X.; Song, X.; et al. MicroRNAs in vascular smooth muscle cells: Mechanisms, therapeutic potential, and advances in delivery systems. Life Sci. 2025, 364, 123424. [Google Scholar] [CrossRef]
- Patel, N.; Avery, E.; Huang, Y.; Chung, E.J. Developing Therapeutically Enhanced Extracellular Vesicles for Atherosclerosis Therapy. Adv. Healthc. Mater. 2025, 14, e2404398. [Google Scholar] [CrossRef] [PubMed]
- Maiseyeu, A.; Di, L.; Ravodina, A.; Barajas-Espinosa, A.; Sakamoto, A.; Chaplin, A.; Zhong, J.; Gao, H.; Mignery, M.; Narula, N.; et al. Plaque-targeted, proteolysis-resistant, activatable and MRI-visible nano-GLP-1 receptor agonist targets smooth muscle cell differentiation in atherosclerosis. Theranostics 2022, 12, 2741–2757. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Y.; Zhu, X.; Miao, L.; Liang, X.; Duan, J.; Li, H.; Tian, X.; Pang, L.; Wei, Y.; et al. Significant difference between sirolimus and paclitaxel nanoparticles in anti-proliferation effect in normoxia and hypoxia: The basis of better selection of atherosclerosis treatment. Bioact. Mater. 2020, 6, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Dayar, E.; Olga, P. Targeted Strategy in Lipid-Lowering Therapy. Biomedicines 2022, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and Nanoparticles for Statin Delivery: Current Use and Future Perspectives in Cardiovascular Disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef]
- Gao, M.; Tang, M.; Ho, W.; Teng, Y.; Chen, Q.; Bu, L.; Xu, X.; Zhang, X.Q. Modulating Plaque Inflammation via Targeted mRNA Nanoparticles for the Treatment of Atherosclerosis. ACS Nano 2023, 17, 17721–17739. [Google Scholar] [CrossRef]
- Fang, Q.; Lu, X.; Zhu, Y.; Lv, X.; Yu, F.; Ma, X.; Liu, B.; Zhang, H. Development of a PCSK9-targeted nanoparticle vaccine to effectively decrease the hypercholesterolemia. Cell Rep. Med. 2024, 5, 101614. [Google Scholar] [CrossRef]
- Valenti, V.; Noto, D.; Giammanco, A.; Fayer, F.; Spina, R.; Altieri, G.I.; Ingrassia, V.; Scrimali, C.; Barbagallo, C.M.; Brucato, F.; et al. PCSK9-D374Y mediated LDL-R degradation can be functionally inhibited by EGF-A and truncated EGF-A peptides: An in vitro study. Atherosclerosis 2020, 292, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Jaafari, M.R.; Afshar, M.; Banach, M.; Sahebkar, A. PCSK9 immunization using nanoliposomes: Preventive efficacy against hypercholesterolemia and atherosclerosis. Arch. Med. Sci. AMS 2021, 17, 1365–1377. [Google Scholar] [CrossRef]
- Dawoud, M.H.S.H.S.; Fayez, A.M.M.; Mohamed, R.A.A.; Sweed, N.M.M. Enhancement of the Solubility of Rosuvastatin Calcium by Nanovesicular Formulation: A Systematic Study Based on a Quality by Design Approach. Proceedings 2021, 78, 34. [Google Scholar]
- Ou, L.C.; Zhong, S.; Ou, J.S.; Tian, J.W. Application of targeted therapy strategies with nanomedicine delivery for atherosclerosis. Acta Pharmacol. Sin. 2021, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Dai, Y.; Chong, L.; Wei, M.; Xing, M.; Zhang, C.; Li, J. Pro-efferocytic macrophage membrane biomimetic nanoparticles for the synergistic treatment of atherosclerosis via competition effect. J. Nanobiotechnology 2022, 20, 506. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xiao, J.; Liu, H.; Liu, H. Selenium nanoparticles alleviate hyperlipidemia and vascular injury in ApoE-deficient mice by regulating cholesterol metabolism and reducing oxidative stress. Met. Integr. Biometal Sci. 2020, 12, 204–217. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Q.B.; Xu, H.; Adi, D.; Ding, Y.W.; Luo, W.J.; Zhu, W.Z.; Xu, J.C.; Zhao, X.; Shi, X.J.; et al. siRNA nanoparticle targeting Usp20 lowers lipid levels and ameliorates metabolic syndrome in mice. J. Lipid Res. 2024, 65, 100626. [Google Scholar] [CrossRef]
- du Toit, L.C.; Hulisani Demana, P.; Essop Choonara, Y. A nano-enabled biotinylated anti-LDL theranostic system to modulate systemic LDL cholesterol. Int. J. Pharm. 2022, 628, 122258. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Deng, Y.; Li, T.; Tian, Q.; Yuan, Z.; Ma, L.; Cheng, C.; Guo, Q.; Qiu, L. Biocatalytic and antioxidant nanostructures for ROS scavenging and biotherapeutics. Adv. Funct. Mater. 2021, 31, 2101804. [Google Scholar] [CrossRef]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Khotina, V.; Ivanova, E.A.; Orekhov, A.N. NADPH Oxidases and Their Role in Atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Mao, J.; Wang, X.; Yang, R.; Wang, C.; Li, C.; Zhou, X. Advances in treatment strategies based on scavenging reactive oxygen species of nanoparticles for atherosclerosis. J. Nanobiotechnology 2023, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Wang, T.Y.; Rousseau, J.; Orlando, M.; Mungaray, M.; Michaud, C.; Plaisier, C.; Chen, Z.B.; Wang, K.C. Biomimetic nanodrug targets inflammation and suppresses YAP/TAZ to ameliorate atherosclerosis. Biomaterials 2024, 306, 122505. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, Y.; Wang, H.; Yu, M.; Hong, K.; Li, D.; Li, R.; Wen, B.; Hu, D.; Chang, L.; et al. Phospholipid nanoparticles: Therapeutic potentials against atherosclerosis via reducing cholesterol crystals and inhibiting inflammation. EBioMedicine 2021, 74, 103725. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, M.; Dai, W.; Xiao, X.; Li, X.; Zhu, Y.; Shi, X.; Li, Z. Targeted nanoparticles triggered by plaque microenvironment for atherosclerosis treatment through cascade effects of reactive oxygen species scavenging and anti-inflammation. J. Nanobiotechnology 2024, 22, 440. [Google Scholar] [CrossRef]
- Xiao, J.; Li, N.; Xiao, S.; Wu, Y.; Liu, H. Comparison of Selenium Nanoparticles and Sodium Selenite on the Alleviation of Early Atherosclerosis by Inhibiting Endothelial Dysfunction and Inflammation in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11612. [Google Scholar] [CrossRef]
- Sheng, J.; Zu, Z.; Zhang, Y.; Zhu, H.; Qi, J.; Zheng, T.; Tian, Y.; Zhang, L. Targeted therapy of atherosclerosis by zeolitic imidazolate framework-8 nanoparticles loaded with losartan potassium via simultaneous lipid-scavenging and anti-inflammation. J. Mater. Chem. B 2022, 10, 5925–5937. [Google Scholar] [CrossRef]
- Dai, Y.; Sha, X.; Song, X.; Zhang, X.; Xing, M.; Liu, S.; Xu, K.; Li, J. Targeted Therapy of Atherosclerosis Vulnerable Plaque by ROS-Scavenging Nanoparticles and MR/Fluorescence Dual-Modality Imaging Tracing. Int. J. Nanomed. 2022, 17, 5413–5429. [Google Scholar] [CrossRef]
- Shen, M.; Li, H.; Yao, S.; Wu, X.; Liu, S.; Yang, Q.; Zhang, Y.; Du, J.; Qi, S.; Li, Y. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112164. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Ma, X.; Zhang, B.; Huang, Y.; Zhao, J.; Wang, S.; Li, Y.; Zhu, Y.; Xiong, J.; et al. Synthesis and Characterization of Fucoidan-Chitosan Nanoparticles Targeting P-Selectin for Effective Atherosclerosis Therapy. Oxidative Med. Cell. Longev. 2022, 2022, 8006642. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, F.; Qian, X.; Sun, J.; Ge, Z.; Yang, L.; Cheng, Z. Anti-inflammatory cytokine IL10 loaded cRGD liposomes for the targeted treatment of atherosclerosis. J. Microencapsul. 2021, 38, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yang, J.; Lei, W.; Xiao, Z.; Zhou, P.; Zheng, S.; Zhu, P. Anti-Oxidative, Anti-Apoptotic, and M2 Polarized DSPC Liposome Nanoparticles for Selective Treatment of Atherosclerosis. Int. J. Nanomed. 2023, 18, 579–594. [Google Scholar] [CrossRef]
- Safdar, A.; Wang, P.; Muhaymin, A.; Nie, G.; Li, S. From bench to bedside: Platelet biomimetic nanoparticles as a promising carriers for personalized drug delivery. J. Control. Release 2024, 373, 128–144. [Google Scholar] [CrossRef]
- Kunde, S.S.; Sarika, W. Platelet membrane camouflaged nanoparticles: Biomimetic architecture for targeted therapy. Int. J. Pharm. 2021, 598, 120395. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yue, K.; Yan, X.; Zhong, W.; Zhang, G.; Wang, L.; Zhang, H.; Zhang, X. Inhibition of platelet adhesion to exposed subendothelial collagen by steric hindrance with blocking peptide nanoparticles. Colloids Surf. B Biointerfaces 2024, 237, 113866. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C 2020, 106, 110298. [Google Scholar] [CrossRef]
- Choi, B.; Park, W.; Park, S.B.; Rhim, W.K.; Han, D.K. Recent trends in cell membrane-cloaked nanoparticles for therapeutic applications. Methods 2020, 177, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhang, Y.; Lu, M.; Wang, Z.; Nong, X.; Wen, G.; Zhang, W. cRGD-platelet@MnO/MSN@PPARα/LXRα Nanoparticles Improve Atherosclerosis in Rats by Inhibiting Inflammation and Reducing Blood Lipid. Curr. Pharm. Biotechnol. 2025, 26, 740–753. [Google Scholar] [CrossRef]
- Zhou, J.; Niu, C.; Huang, B.; Chen, S.; Yu, C.; Cao, S.; Pei, W.; Guo, R. Platelet Membrane Biomimetic Nanoparticles Combined With UTMD to Improve the Stability of Atherosclerotic Plaques. Front. Chem. 2022, 10, 868063. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, Z.Y.; Song, Z.F.; Yu, M.; Huang, J.; Qian, H.Y. Platelet membrane-modified exosomes targeting plaques to activate autophagy in vascular smooth muscle cells for atherosclerotic therapy. Drug. Deliv. Transl. Res. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Lin, J.; Yang, M.; Li, C.; Wu, P.; Zou, J.; Jiang, Y.; Shao, J. Platelet membrane-cloaked selenium/ginsenoside Rb1 nanosystem as biomimetic reactor for atherosclerosis therapy. Colloids Surf. B Biointerfaces 2022, 214, 112464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Nie, G. Biomembrane-based nanostructures for cancer targeting and therapy: From synthetic liposomes to natural biomembranes and membrane-vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113974. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, N.; Li, Q.; Chen, J.; Wang, Q.; Yang, H.; Tan, H.; Gao, J.; Dong, Z.; Pang, Z.; et al. Biomimetic liposomes hybrid with platelet membranes for targeted therapy of atherosclerosis. Chem. Eng. J. 2021, 408, 127296. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, T.; Feng, J.; Shang, J.; Zhang, B.; Dong, Q.; Zhang, Z.; Sun, C. Application of therapeutical nanoparticles with neutrophil membrane camouflaging for inflammatory plaques targeting against atherosclerosis. Mater. Today Bio 2024, 30, 101397. [Google Scholar] [CrossRef]
- Yamashita, T.; Sasaki, N.; Kasahara, K.; Hirata, K. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J. Cardiol. 2015, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, C.; Feng, J.; Long, H.; Wang, Y.; Wang, P.; Hu, C.; Yue, Y.; Zhang, C.; Liu, Z.; et al. Anti-inflammatory mechanisms of neutrophil membrane-coated nanoparticles without drug loading. J. Control. Release 2024, 369, 12–24. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Wang, S.; Liu, N. Neutrophil membrane biomimetic delivery system (Ptdser‐NM‐Lipo/Fer‐1) designed for targeting atherosclerosis therapy. IET Nanobiotechnology 2023, 17, 387–395. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Q.; Zhang, S.; Lan, Q.; Wang, R.; Tan, E.; Zhou, L.; Wang, C.; Wang, H.; Cheng, Y. Polypyridiniums with Inherent Autophagy-Inducing Activity for Atherosclerosis Treatment by Intracellularly Co-Delivering Two Antioxidant Enzymes. Adv. Mater. 2024, 36, e2409015. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Wang, Y.; Yang, L.; Zhuang, W.; Li, G.; Wang, Y. Biomimetic-Coated Nanoplatform with Lipid-Specific Imaging and ROS Responsiveness for Atherosclerosis-Targeted Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 35410–35421. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Han, Z.; Zhu, L.; He, Z.; Mou, N.; Duan, X.; Chen, Q.; Qin, X.; Zhang, K.; Qu, K.; et al. Red Blood Cell Membrane Spontaneously Coated Nanoprodrug Based on Phosphatidylserine for Antiatherosclerosis Applications. ACS Appl. Mater. Interfaces 2024, 16, 46578–46589. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Li, X.; Liu, F.; Cheng, X.; Ran, H.; Wang, Z.; Li, Y.; Feng, Y.; Liang, L.; et al. Anti-CXCR2 antibody-coated nanoparticles with an erythrocyte-platelet hybrid membrane layer for atherosclerosis therapy. J. Control. Release 2023, 356, 610–622. [Google Scholar] [CrossRef]
- Prilepskii, A.Y.; Serov, N.S.; Kladko, D.V.; Vinogradov, V.V. Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis. Pharmaceutics 2020, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Reiner, Ž.; Carbone, F.; Montecucco, F.; Sahebkar, A. The Therapeutic Potential of Nanoparticles to Reduce Inflammation in Atherosclerosis. Biomolecules 2019, 9, 416. [Google Scholar] [CrossRef]
- De Negri Atanasio, G.; Ferrari, P.F.; Campardelli, R.; Perego, P.; Palombo, D. Innovative nanotools for vascular drug delivery: The atherosclerosis case study. J. Mater. Chem. B 2021, 9, 8558–8568. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Du, L.; Lin, R.; Ding, Z.; Guo, Z.; Wei, J.; Li, Y. How Advanced Is Nanomedicine for Atherosclerosis? Int. J. Nanomed. 2025, 20, 3445–3470. [Google Scholar] [CrossRef]
- Pang, A.S.; Dinesh, T.; Pang, N.Y.; Dinesh, V.; Pang, K.Y.; Yong, C.L.; Lee, S.J.J.; Yip, G.W.; Bay, B.H.; Srinivasan, D.K. Nanoparticles as Drug Delivery Systems for the Targeted Treatment of Atherosclerosis. Molecules 2024, 29, 2873. [Google Scholar] [CrossRef]
- Lin, L.; Chen, L.; Yan, J.; Chen, P.; Du, J.; Zhu, J.; Yang, X.; Geng, B.; Li, L.; Zeng, W. Advances of nanoparticle-mediated diagnostic and theranostic strategies for atherosclerosis. Front. Bioeng. Biotechnol. 2023, 11, 1268428. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
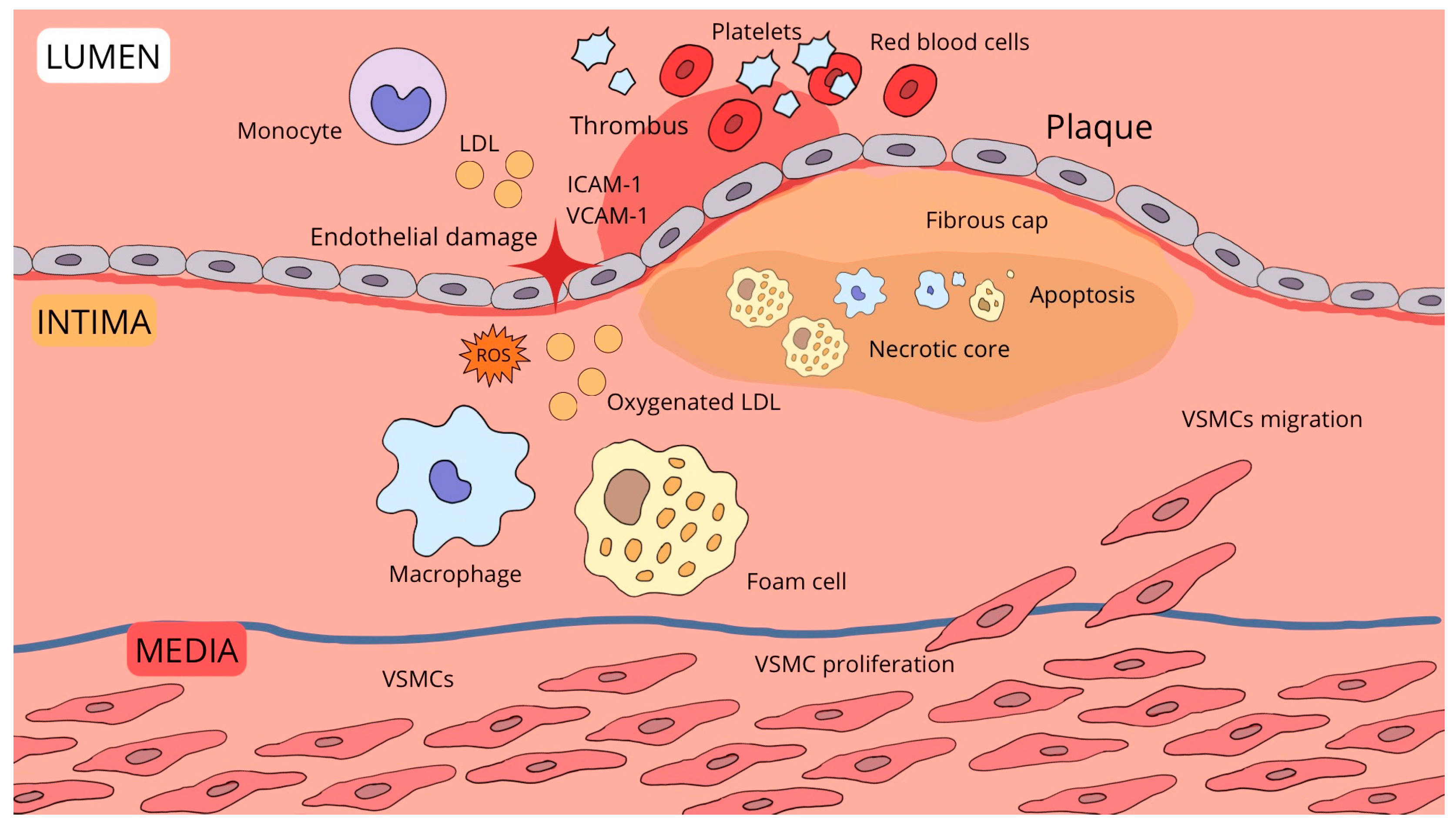
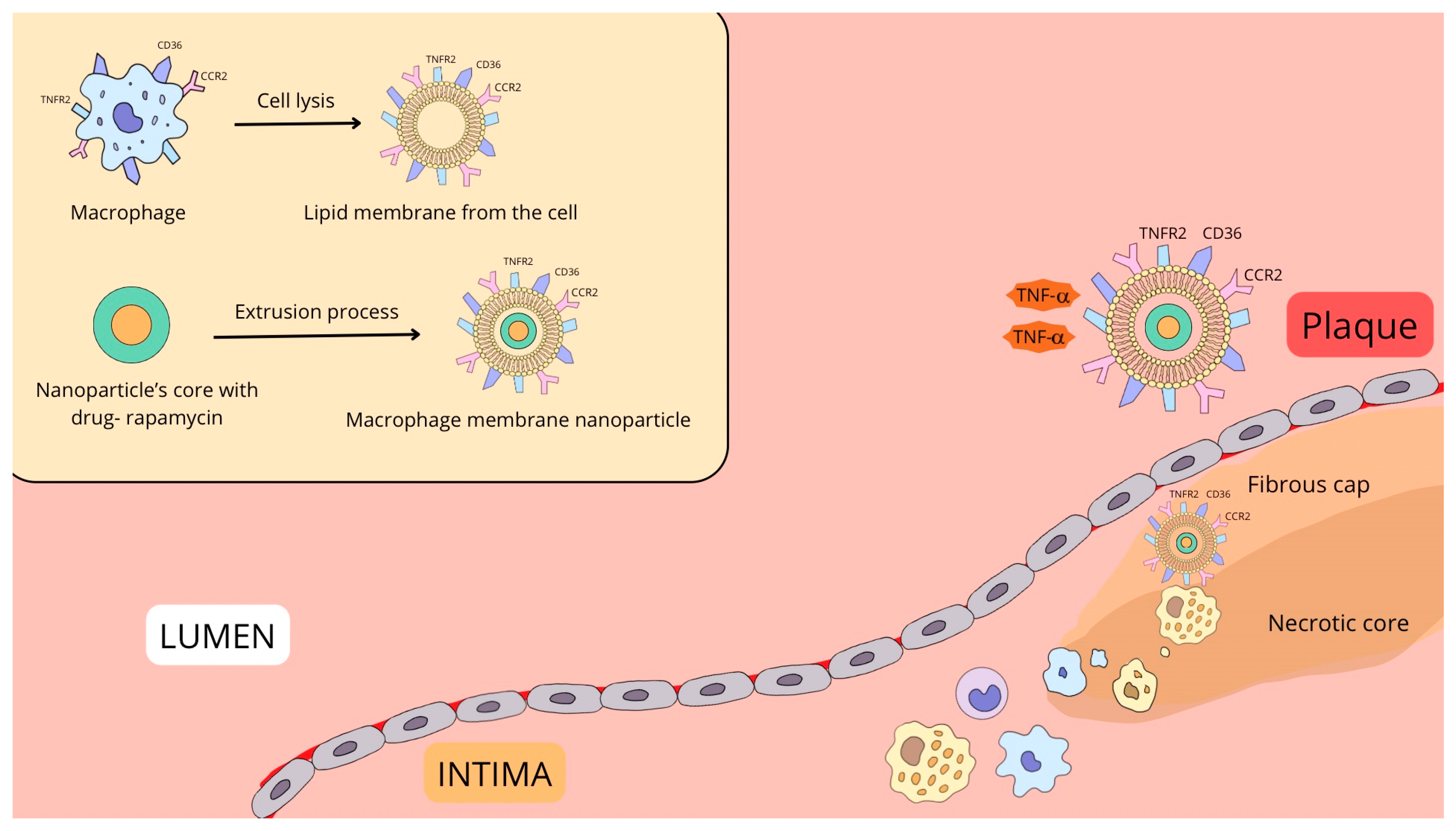
| Feature | Macrophage Membrane (MM-NP) | Platelet Membrane (PLT-NP) | Neutrophil Membrane (NM-NP) | Erythrocyte Membrane (RBC-NP) |
|---|---|---|---|---|
| Key Surface Molecules | Proinflammatory and adhesion receptors, e.g., CCR2, CD36, TNFR2. | Adhesion and pro-thrombotic molecules, e.g., P-selectin, integrins, and CD40L. | Chemotactic and adhesion receptors, e.g., chemokine receptors, CD18 (for ICAM-1 interaction). | Proteins providing immune “camouflage” (e.g., CD47) that prevent phagocytosis. |
| Main Targeting Mechanism | Homotypic targeting: natural attraction to inflammatory sites where other macrophages accumulate. | Targeting of damaged sites: adhesion to damaged vascular endothelium and the exposed extracellular matrix (ECM). | Active inflammation-seeking: natural ability to migrate towards inflammatory signals (chemotaxis) and perform trans-endothelial migration. | Passive accumulation: no active targeting; utilizing extended circulation to accumulate in sites of enhanced vascular permeability. |
| Main Therapeutic Advantages | -Active targeting of inflammatory foci. -Simultaneous neutralization of proinflammatory cytokines. -Evasion of rapid clearance by the reticuloendothelial system (RES) | -Excellent biocompatibility and low immunogenicity. -Targeting of damaged vessels and clots -Protection of the therapeutic cargo from degradation. | -Highest ability to locate acute inflammation. -Effective immune masking and prolonged circulation time -Precise drug delivery to inflammatory sites. | -Longest circulation time in the bloodstream. -Excellent biocompatibility and evasion of RES uptake. -Ability to be surface-modified for added targeting functions. |
| Example | Fe3O4@M2 NPs for imaging atherosclerotic lesions. | RAP@PLT NPs delivering rapamycin; PM@Se/Rb1 NPs with selenium and ginsenoside. | NNP-ST with simvastatin and SPIO; liposomes with Fer-1 coated with neutrophil membranes. | RBC/LFP@PMMP for simultaneous diagnosis and therapy; hybrid [RBC-P]NPs targeting CXCR2. |
| Nanoparticle Type and Name | Target Site/Molecule | Mechanism of Action | Therapeutic Outcome | Reference |
|---|---|---|---|---|
| Metallic and Metal-Organic Framework (MOF) NPs | ||||
| Metallic NP (Fe3O4@M2 NPs) | Active macrophages (CCR2 receptor) | TM2-macrophage membrane coating facilitates recognition and uptake for imaging. | Effective imaging of atherosclerotic plaques via MRI and near-infrared fluorescence; high safety. | [26] |
| MOF NP (LP@ZIF-8) | Atherosclerotic plaque | Delivery of losartan potassium. | Combined anti-inflammatory action and autophagy induction. | [69] |
| Metallic NP (GPRD) | Atherosclerotic plaque | Dual-modal imaging (MR/fluorescence) and enzymatic neutralization of ROS. | Significant reduction in inflammation and foam cell formation. | [70] |
| Polymeric NPs | ||||
| Polymeric NP (MM/RAPNPs) | Activated endothelial cells | Macrophage membrane-coated PLGA NPs deliver rapamycin. | Accumulated in lesions, inhibited disease progression, and showed good tolerance. | [28] |
| Polymeric NP (LFP/PCDPD) | Damaged endothelium (VCAM-1, CD44) | Dextran-based NPs for ROS-responsive release of prednisolone and lipid removal. | Targeted accumulation, drug release, lipid removal, and effective therapy. | [29] |
| Polymeric NP (RAP@T/R NPs) | Inflammatory endothelial cells (αvβ3 integrin) | c(RGDfC) peptide-targeted PLGA-PEG NPs for cathepsin K-sensitive release of rapamycin. | Selective accumulation, controlled drug release, reduced inflammation, and inhibited plaque progression. | [32] |
| Functionalized Polymeric NP (GW1516@NP-OPN) | VSMCs (Osteopontin) | Anti-OPN antibody-targeted delivery of GW1516 to activate the TGF-β/FAK pathway. | Inhibited VSMC migration and apoptosis; reduced atherosclerotic lesion area. | [44] |
| Polymeric NP (SRM-NPs) | Endothelial and smooth muscle cells | Delivery of sirolimus. | Inhibited cell proliferation and glycolysis under hypoxic conditions typical of plaques. | [48] |
| Polymeric NP (PLGA-statins) | Interleukin-10 (IL-10) Atherosclerotic plaques | Encapsulation of statins in biodegradable PLGA for controlled release. | Better stability, controlled release, and higher therapeutic efficacy at lower doses. | [49,50] |
| Polymeric NP (LLC NPs) | Endothelial cells (P-selectin) | LMWH and lipoic acid-based NPs for ROS-responsive release of curcumin. | Significant inhibition of atherosclerotic lesion development. | [67] |
| Polymeric NP (CFNs) | Atherosclerotic plaques (P-selectin) | Fucoidan and chitosan NPs neutralize ROS and inflammatory cytokines. | Limited disease progression. | [72] |
| Lipid-Based and Biomimetic NPs | ||||
| Nanomicelles (TM-GW) | VCAM-1 on HAVSMCs | Targeted delivery of a PPARδ receptor agonist. | Increased uptake and more effective inhibition of cell apoptosis and migration. | [30] |
| Nanomicelles (miR-145 micelles) | VSMCs (CCR2 receptor) | Targeted delivery of miR-145. | Restored protective phenotype of VSMCs and prevented lesion development. | [43] |
| Lipid NP (mRNA-NP) | M2 macrophages in plaques | Delivery of mRNA encoding for the anti-inflammatory cytokine IL-10. | Increased IL-10 expression, reduced oxidative stress, and stabilized plaques. | [51] |
| Liposomal Vaccine (L-IFPTA+) | Immune System (induces antibody production) | Liposomes presenting a PCSK9-mimicking peptide to induce an immune response. | Induced a durable immune response and lowered LDL levels. | [54] |
| Macrophage-Membrane Coated Liposome (MM@Lips-SHP1i) | Oxidized LDL in plaques | Competes with endogenous macrophages for oxLDL uptake; delivers SHP-1 inhibitor. | Reduced foam cell formation and enhanced efferocytosis, limiting plaque progression. | [57] |
| Lipid NP (siRNA-NP) | USP20 (regulator of cholesterol synthesis) | Delivery of siRNA targeting USP20. | Lowered lipids, improved glucose metabolism, and prevented atherosclerosis development. | [59] |
| HDL-like Particle (miNano) | Cholesterol crystals (CC) in plaques | Binds and dissolves cholesterol crystals, inhibiting the TLR4-NF-κB pathway. | Reduced CC and macrophage content; promoted efferocytosis. | [66] |
| Liposome (cRGD-Lipo) | Inflammatory sites | cRGD peptide-targeted delivery of IL-10. | Reduced expression of IL-1β and TNF-α; reduced oxidative stress. | [73] |
| Liposome (SE-LNPs) | Atherosclerotic plaques | Co-delivery of simvastatin and EGCG. | Reduced oxidative stress and apoptosis; promoted M2 macrophage polarization. | [74] |
| Biologically-Derived and Cell-Membrane Coated NPs | ||||
| Extracellular Vesicles (Modified EVs) | VSMCs (CCR2 receptor) | MCP-1 peptide-modified EVs deliver miR-145. | High therapeutic efficacy with a significantly lower miRNA payload, reducing side effects. | [46] |
| Ferritin Nanovaccine | Immune System (induces antibody production | Self-assembling ferritin nanoparticles presenting the catalytic domain of PCSK9. | Induced anti-PCSK9 antibodies, leading to lipid reduction and atherosclerosis inhibition. | [52,53] |
| Monocyte-Membrane Coated NP (MoNP) | Inflamed endothelium | Delivery of verteporfin to block the YAP/TAZ pathway. | Reduced inflammatory infiltrates and inhibited lesion progression. | [65] |
| Platelet-Membrane Coated NP (cRGD-platelet-NPs) | Atherosclerotic plaque | Delivery of LXRα and PPARα agonists. | Lowered LDL/triglycerides, raised HDL, promoted M2 polarization, and inhibited NF-κB. | [80] |
| Platelet-Membrane Coated NP (RAP@PLT NPs) | Atherosclerotic plaque (UTMD-assisted) | Targeted delivery of rapamycin. | Inhibited plaque progression and improved plaque stability. | [81] |
| Platelet-Exosome Hybrid NP (MSC-ExoP) | Atherosclerotic plaque (VSMCs) | Delivery of MSC-derived exosome cargo to activate autophagy in VSMCs. | Inhibited atherosclerosis progression by reducing lipid deposits and necrosis. | [82] |
| Platelet-Membrane Coated NP (PM@Se/Rb1 NPs) | Atherosclerotic plaque | Core of selenium and ginsenoside Rb1 provides antioxidant and anti-inflammatory effects. | Effective accumulation in plaques; anti-inflammatory and anti-angiogenic effects. | [83] |
| Neutrophil-Membrane Coated NP (ZIF-8 NP) | Endothelial cells (ICAM-1) | Delivery of anti-miR-155 ASOs via CD18-ICAM-1 interaction. | Reduced miR-155 expression, inhibited inflammation, and alleviated lesions. | [31] |
| Neutrophil-Membrane Coated NP (NNP-ST) | Inflammatory sites in plaques | PLGA core with simvastatin and SPIO for therapy and dual-modal imaging. | Effective immune masking, prolonged circulation, and clear therapeutic effect with minimal toxicity. | [88] |
| Neutrophil-Membrane Coated Liposome (PtdSer-NM-Lipo/Fer-1) | Atherosclerotic lesion sites | Delivery of the ferroptosis inhibitor ferrostatin-1. | Successfully inhibited the progression of atherosclerosis and ferroptosis. | [91] |
| Neutrophil-Membrane Coated NP (P5c Polymer NP) | Macrophages in plaques | Delivery of antioxidant enzymes (SOD, CAT) and induction of autophagy. | Reduced ROS, decreased senescent cells, and promoted M2 macrophage phenotype. | [92] |
| Erythrocyte-Membrane Coated Micelle (RBC/LFP@PMMP) | Atherosclerotic lesion sites | ROS-responsive release of prednisolone and fluorescence imaging of lipids. | Simultaneous diagnosis and therapy based on local biochemical changes. | [93] |
| Erythrocyte-Membrane Coated NP (RBC@P-LVTNPs) | Atherosclerotic plaque | ROS-responsive release of drug from a polypeptide-based core. | Demonstrated therapeutic efficacy and favorable biocompatibility. | [94] |
| Hybrid Erythrocyte-Platelet Membrane NP ([RBC-P]NPs) | Macrophages (CXCR2 receptor) | Targeted delivery of an anti-CXCR2 agent to block CXCL8-CXCR2 signaling | Reduced macrophage accumulation, plaque size, and intraplaque necrosis without side effects. | [95] |
| Other Systems | ||||
| Selenium NPs (SeNPs) | Systemic | Lowers cholesterol, increases HDL, and improves antioxidant enzyme profiles. | Reduced vascular damage. | [58] |
| Implantable System (IVISDDD) | Systemic (implantable) | LDL-level modulated release of fenofibrate. | Reduced total cholesterol and LDL in pigs, demonstrating precise treatment potential. | [60] |
| Activatable NPs | Atherosclerotic lesions | Local activation of a microdosed GLP-1R agonist within the plaque. | Proof-of-concept for local drug activation, bypassing systemic effects. | [47] |
| NP’s Type Under the Clinical Trial | Trial Phase | Aim of the Study | Outcome of the Trial | ClinicalTrials.Gov ID |
|---|---|---|---|---|
| Methotrexate Associated With LDL LDL-Like Nanoparticles | Unknown- Last known status was: Recruiting | The purpose of the study is to evaluate the safety and efficacy of an anti-inflammatory agent, methotrexate (MTX), in a cholesterol-rich non-protein nanoparticle (MTX-LDE) in patients with stable coronary disease. | No outcome available yet | NCT04616872 |
| Paclitaxel Associated With LDL-Like Nanoparticles (PAC-MAN) | Unknown- Last known status was: Active, not recruiting | The purpose of the study is to evaluate the safety and efficacy of an anti-proliferative agent, paclitaxel, in a cholesterol-rich non-protein nanoparticle (Paclitaxel -LDE) in patients with stable coronary disease. | No outcome available yet | NCT04148833 |
| Plasmonic resonance-mediated therapy using noble-metal NP-Gold Nanoparticles With Iron Oxide-Silica Shells | Terminated (The study was terminated under the political pressure of the Federal Security Service of the Russian Federation (FSB) and the Russian Society of Cardiology) | The aim of the study was to compare the safety and efficacy of a new therapy for atherosclerosis, involving plasmonic photothermal therapy with gold nanoparticles, with standard treatment using stenting. | No outcome available yet | NCT01436123 |
| Plasmonic Photothermal Therapy of Flow-Limiting Atherosclerotic Lesions With Silica-Gold Nanoparticles | Completed | The purpose of this first-in-man study was to compare the safety and feasibility of two novel nanoparticle delivery methods for plasmonic photothermal therapy of atherosclerosis with standard treatment by stenting. | The NANOM-PCI trial showed that plasmonic photothermal therapy with the use of silica-gold nanoparticles resulted in an unprecedented reduction in plaque volume (by an average of 84.1 mm3), in contrast to standard stenting, where plaque volume increased. This translated into significantly better clinical outcomes, including fewer thrombotic complications and higher patient survival in the group treated with the new method. | NCT01270139 |
| Nanoparticle Paclitaxel | Terminated (due to changing sponsor priorities, and was not based on safety or outcomes data) | The purpose of this study is to investigate the prevention of Restenosis following Revascularization of the superficial Femoral Artery (SFA) with the use of Paclitaxel NPs | Incomplete data related to the termination of the study | NCT00518284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Podgórski, R.; Serafin, I.; Aebisher, D. Targeted and Biomimetic Nanoparticles for Atherosclerosis Therapy: A Review of Emerging Strategies. Biomedicines 2025, 13, 1720. https://doi.org/10.3390/biomedicines13071720
Bartusik-Aebisher D, Podgórski R, Serafin I, Aebisher D. Targeted and Biomimetic Nanoparticles for Atherosclerosis Therapy: A Review of Emerging Strategies. Biomedicines. 2025; 13(7):1720. https://doi.org/10.3390/biomedicines13071720
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Rafał Podgórski, Iga Serafin, and David Aebisher. 2025. "Targeted and Biomimetic Nanoparticles for Atherosclerosis Therapy: A Review of Emerging Strategies" Biomedicines 13, no. 7: 1720. https://doi.org/10.3390/biomedicines13071720
APA StyleBartusik-Aebisher, D., Podgórski, R., Serafin, I., & Aebisher, D. (2025). Targeted and Biomimetic Nanoparticles for Atherosclerosis Therapy: A Review of Emerging Strategies. Biomedicines, 13(7), 1720. https://doi.org/10.3390/biomedicines13071720









