Impact of Biologics and Proton Pump Inhibitors on Gastrointestinal Infection Risk in Inflammatory Bowel Disease Patients: A Retrospective Analysis of Pathogen-Specific Outcomes and Treatment Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Variables of Interests
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Demographic Distribution of IBD Patients
3.2. Association Between Different GI Infection Categories and Treatment Groups
3.3. GI Infections Outcomes in IBD Patients Based on Different Biologic Agents
3.4. Infection Types Categorized by Treatment Groups
3.5. Infectious Gent in Non-Clostridial Infections Categorized by Treatment Groups
4. Discussion
4.1. Various Treatment Groups and Risk of GI Infections
4.1.1. Biologic Therapy and Risk of GI Infections
4.1.2. PPI Therapy and Risk of GI Infections
4.1.3. Combination of Biologics and PPI Therapy and Risk of GI Infections
4.2. GI Infection Risk with Various Biologic Agents
4.3. Infectious Type and Agents Across Treatment Groups
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BMI | Body mass index |
| CD | Crohn’s disease |
| CDI | Clostridioides difficile infections |
| C.diff | Clostridioides difficile |
| CI | Confidence interval |
| GI | Gastrointestinal |
| IBD | Inflammatory bowel disease |
| ICD-10 | International Classification of Disease, 10th Edition |
| IQR | Interquartile ranges |
| IRB | Institutional Review Board |
| MRN | medical record number |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| PPI | Proton pump inhibitor |
| TNFα | Tumor necrosis factor-alpha |
| UC | Ulcerative colitis |
Appendix A
| Infection Type | ||||
|---|---|---|---|---|
| Treatment | Bacterial | Parasite | Viral | Total |
| Biologics | 16 (69.57%) | 0 (0.00%) | 7 (30.43%) | 23 |
| PPIs | 49 (71.01%) | 0 (0.00%) | 20 (28.99%) | 69 |
| Biologics + PPIs | 29 (72.50%) | 0 (0.00%) | 11 (27.50%) | 40 |
| None | 30 (75.00%) | 1 (2.50%) | 9 (22.50%) | 40 |
| Total | 124 | 1 | 47 | 172 |
References
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Eldridge, F.; Raine, T. Understanding and Addressing the Psychological Burden of IBD. J. Crohns Colitis 2022, 16, 177–178. [Google Scholar] [CrossRef]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar]
- Lewin, S.; Velayos, F.S. Day-by-Day Management of the Inpatient With Moderate to Severe Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2020, 16, 449–457. [Google Scholar]
- Axelrad, J.E.; Cadwell, K.H.; Colombel, J.-F.; Shah, S.C. The role of gastrointestinal pathogens in inflammatory bowel disease: A systematic review. Ther. Adv. Gastroenterol. 2021, 14, 17562848211004493. [Google Scholar] [CrossRef]
- Dehghani, T.; Gholizadeh, O.; Daneshvar, M.; Nemati, M.M.; Akbarzadeh, S.; Amini, P.; Afkhami, H.; Kohansal, M.; Javanmard, Z.; Poortahmasebi, V. Association Between Inflammatory Bowel Disease and Viral Infections. Curr. Microbiol. 2023, 80, 195. [Google Scholar] [CrossRef]
- Irving, P.M.; de Lusignan, S.; Tang, D.; Nijher, M.; Barrett, K. Risk of common infections in people with inflammatory bowel disease in primary care: A population-based cohort study. BMJ Open Gastroenterol. 2021, 8, e000573. [Google Scholar] [CrossRef]
- Dalal, R.S.; Allegretti, J.R. Diagnosis and management of Clostridioides difficile infection in patients with inflammatory bowel disease. Curr. Opin. Gastroenterol. 2021, 37, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef] [PubMed]
- Vilcu, A.-M.; Sabatte, L.; Blanchon, T.; Souty, C.; Maravic, M.; Lemaitre, M.; Steichen, O.; Hanslik, T. Association Between Acute Gastroenteritis and Continuous Use of Proton Pump Inhibitors During Winter Periods of Highest Circulation of Enteric Viruses. JAMA Netw. Open 2019, 2, e1916205. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Dupont, H.L. Systematic review: The use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment. Pharmacol. Ther. 2011, 34, 1269–1281. [Google Scholar] [CrossRef]
- Kuo, C.J.; Lin, C.-Y.; Chen, C.-W.; Hsu, C.-Y.; Hsieh, S.-Y.; Chiu, C.-T.; Lin, W.-R. Risk of Enteric Infection in Patients with Gastric Acid Supressive Drugs: A Population-Based Case-Control Study. J. Pers. Med. 2021, 11, 1063. [Google Scholar] [CrossRef]
- Stoica, O.; Trifan, A.; Cojocariu, C.; Gîrleanu, I.; Maxim, R.; Stanciu, M.C. Incidence and risk factors of Clostridium difficile infection in patients with inflammatory bowel disease. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2015, 119, 81–86. [Google Scholar]
- Razik, R.; Rumman, A.; Bahreini, Z.; McGeer, A.; Nguyen, G.C. Recurrence of Clostridium difficile Infection in Patients with Inflammatory Bowel Disease: The RECIDIVISM Study. Am. J. Gastroenterol. 2016, 111, 1141–1146. [Google Scholar] [CrossRef]
- Balram, B.; Battat, R.; Al-Khoury, A.; D’aOust, J.; Afif, W.; Bitton, A.; Lakatos, P.L.; Bessissow, T. Risk Factors Associated with Clostridium difficile Infection in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohns Colitis 2018, 13, 27–38. [Google Scholar] [CrossRef]
- Maharshak, N.; Barzilay, I.; Zinger, H.; Hod, K.; Dotan, I. Clostridium difficile infection in hospitalized patients with inflammatory bowel disease: Prevalence, risk factors, and prognosis. Medicine 2018, 97, e9772. [Google Scholar] [CrossRef]
- Varma, S.; Trudeau, S.J.; Li, J.; E Freedberg, D. Proton pump Inhibitors and Risk of Enteric Infection in Inflammatory Bowel Disease: A Self-controlled Case Series. Inflamm. Bowel Dis. 2024, 30, 38–44. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Chen, D.M.; Pritchard, M.L.; Sandborn, W.J. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin. Gastroenterol. Hepatol. 2006, 4, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Joelson, A.; Green, P.H.R.; Lawlor, G.; Lichtiger, S.; Cadwell, K.; Lebwohl, B. Enteric Infections Are Common in Patients with Flares of Inflammatory Bowel Disease. Am. J. Gastroenterol. 2018, 113, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Bryan, L. Enteric Infections Among IBD Patients: Results From the Nationwide Emergency Department Sample, 2010–2012: P-033. Off. J. Am. Coll. Gastroenterol. ACG 2018, 113, S9. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, Q.-Y.; Fei, J.-X.; Zhang, Y.; Lin, M.-Y.; Jiang, S.-H.; Wang, P.; Chen, Y. Clostridium Difficile Infection Worsen Outcome of Hospitalized Patients with Inflammatory Bowel Disease. Sci. Rep. 2016, 6, 29791. [Google Scholar] [CrossRef]
- Martínez-Lozano, H.; Saralegui-Gonzalez, P.; Reigadas, E.; Fueyo-Peláez, P.R.; García-García, A.; Miranda-Bautista, J.; Alcalá, L.; Nieto, J.C.; Lobato-Matilla, M.E.; Marín-Jiménez, I.; et al. Risk factors for Clostridioides difficile infection among patients diagnosed with inflammatory intestinal and rheumatological diseases in the biologic era. BMC Gastroenterol. 2025, 25, 70. [Google Scholar] [CrossRef]
- Saad Alshahrani, A.; Mohammad, D.; Alzahrani, M.A.; Narula, N. Vedolizumab does not increase risk of clostridium difficile infection in patients with inflammatory bowel disease using vedolizumab: A retrospective cohort study. Saudi Pharm. J. 2023, 31, 101736. [Google Scholar] [CrossRef]
- D’Aoust, J.; Battat, R.; Bessissow, T. Management of inflammatory bowel disease with Clostridium difficile infection. World J. Gastroenterol. 2017, 23, 4986–5003. [Google Scholar] [CrossRef]
- Tosca, J.; Garcia, N.; Pascual, I.; Bosca-Watts, M.M.; Anton, R.; Sanahuja, A.; Mas, P.; Mora, F.; Minguez, M. Clinical assessment of risk factors for infection in inflammatory bowel disease patients. Int. J. Color. Dis. 2020, 35, 491–500. [Google Scholar] [CrossRef]
- Gong, S.S.; Fan, Y.H.; Han, Q.Q.; Lv, B.; Xu, Y. Nested case-control study on risk factors for opportunistic infections in patients with inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 2240–2250. [Google Scholar] [CrossRef]
- Mårild, K.; Söderling, J.; Axelrad, J.; Halfvarson, J.; Forss, A.; SWIBREG Study Group; Olén, O.; Ludvigsson, J.F. Histologic Activity in Inflammatory Bowel Disease and Risk of Serious Infections: A Nationwide Study. Clin. Gastroenterol. Hepatol. 2024, 22, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Vitikainen, K.; Kase, M.; Meriranta, L.; Molander, P.; Af Björkesten, C.G.; Anttila, V.J.; Arkkila, P. Higher disease activity of inflammatory bowel disease predisposes to Clostridioides difficile infection. Ther. Adv. Gastroenterol. 2025, 18, 17562848251318292. [Google Scholar] [CrossRef] [PubMed]
- Cholapranee, A.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 2017, 45, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sandborn, W.J.; Khan, K.J.; Hanauer, S.B.; Talley, N.J.; Moayyedi, P. Efficacy of Biological Therapies in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2011, 106, 644–659. [Google Scholar] [CrossRef]
- Sun, J.; Brooks, E.C.; Houshyar, Y.; Connor, S.J.; Paven, G.; Grimm, M.C.; Hold, G.L. Unravelling the Relationship Between Obesity and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2025, izaf098. [Google Scholar] [CrossRef]
- Greuter, T.; Porchet, F.; Braga-Neto, M.B.; Rossel, J.-B.; Biedermann, L.; Schreiner, P.; Scharl, M.; Schoepfer, A.M.; Safroneeva, E.; Straumann, A.; et al. Impact of obesity on disease activity and disease outcome in inflammatory bowel disease: Results from the Swiss inflammatory bowel disease cohort. United Eur. Gastroenterol. J. 2020, 8, 1196–1207. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Glass, K.; Du, W.; Banks, E.; Kirk, M.; Green, J. Use of Proton Pump Inhibitors and the Risk of Hospitalization for Infectious Gastroenteritis. PLoS ONE 2016, 11, e0168618. [Google Scholar] [CrossRef]
- Brophy, S.; Jones, K.H.; A Rahman, M.; Zhou, S.-M.; John, A.; Atkinson, M.D.; Francis, N.; A Lyons, R.; Dunstan, F. Incidence of Campylobacter and Salmonella infections following first prescription for PPI: A cohort study using routine data. Am. J. Gastroenterol. 2013, 108, 1094–1100. [Google Scholar] [CrossRef]
- Park, Y.H.; Seong, J.M.; Cho, S.; Han, H.W.; Kim, J.Y.; An, S.H.; Gwak, H.S. Effects of proton pump inhibitor use on risk of Clostridium difficile infection: A hospital cohort study. J. Gastroenterol. 2019, 54, 1052–1060. [Google Scholar] [CrossRef]
- Inghammar, M.; Svanström, H.; Voldstedlund, M.; Melbye, M.; Hviid, A.; Mølbak, K.; Pasternak, B. Proton-Pump Inhibitor Use and the Risk of Community-Associated Clostridium difficile Infection. Clin. Infect. Dis. 2021, 72, e1084–e1089. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Chen, C.; Liao, H.; Wang, M.; Hua, S.; Huang, B.; Xiong, Y.; Zhang, J.; Xu, Y. Updated meta-analysis of controlled observational studies: Proton-pump inhibitors and risk of Clostridium difficile infection. J. Hosp. Infect. 2018, 98, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Tawam, D.; Baladi, M.; Jungsuwadee, P.; Earl, G.; Han, J. The Positive Association between Proton Pump Inhibitors and Clostridium Difficile Infection. Innov. Pharm. 2021, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Liao, J.; Jian, Z.; Li, H.; Chen, X.; Liu, Q.; Liu, P.; Wang, Z.; Liu, X.; Yan, Q.; et al. Molecular epidemiology and clinical characteristics of Clostridioides difficile infection in patients with inflammatory bowel disease from a teaching hospital. J. Clin. Lab. Anal. 2022, 36, e24773. [Google Scholar] [CrossRef]
- Holmer, A.; Singh, S. Overall and comparative safety of biologic and immunosuppressive therapy in inflammatory bowel diseases. Expert Rev. Clin. Immunol. 2019, 15, 969–979. [Google Scholar] [CrossRef]
- Liang, Y.; Meng, Z.; Ding, X.-L.; Jiang, M. Effects of proton pump inhibitors on inflammatory bowel disease: An updated review. World J. Gastroenterol. 2024, 30, 2751–2762. [Google Scholar] [CrossRef]
- Lu, T.X.; Dapas, M.; Lin, E.; Peters, T.; Sakuraba, A. The influence of proton pump inhibitor therapy on the outcome of infliximab therapy in inflammatory bowel disease: A patient-level meta-analysis of randomised controlled studies. Gut 2021, 70, 2076–2084. [Google Scholar] [CrossRef]
- Szemes, K.; Farkas, N.; Sipos, Z.; Bor, R.; Fabian, A.; Szepes, Z.; Farkas, K.; Molnar, T.; Schafer, E.; Szamosi, T.; et al. Co-Administration of Proton Pump Inhibitors May Negatively Affect the Outcome in Inflammatory Bowel Disease Treated with Vedolizumab. Biomedicines 2024, 12, 158. [Google Scholar] [CrossRef]
- Choden, T.; Zhang, H.; Sakuraba, A. Influence of proton pump inhibitor use on clinical outcomes of patients with inflammatory bowel disease. Ann. Med. 2023, 55, 2198775. [Google Scholar] [CrossRef]
- Dalal, R.S.; Zhang, H.; Sakuraba, A. Risk of Gastrointestinal Infections After Initiating Vedolizumab and Anti-TNFα Agents for Ulcerative Colitis. J. Clin. Gastroenterol. 2023, 57, 714–720. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhang, Y.; Zhang, H.; Chen, C.; Zhu, S.; Zhou, Y.; Zhao, H.; Zong, Y. Risk of Clostridioides difficile infection in inflammatory bowel disease patients undergoing vedolizumab treatment: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 377. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V., Jr.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Gordon, K.B.; Rosenbaum, J.T.; Arikan, D.; Lau, W.L.; Li, P.; Faccin, F.; Panaccione, R. Long-Term Safety of Adalimumab in 29,967 Adult Patients From Global Clinical Trials Across Multiple Indications: An Updated Analysis. Adv. Ther. 2020, 37, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Peyrin-Biroulet, L.; Panaccione, R.; Rutgeerts, P.; Hanauer, S.B.; Ghosh, S.; Van Assche, G.; Robinson, A.M.; et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn’s disease or ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 219–228. [Google Scholar] [CrossRef]
- Karlqvist, S.; Sachs, M.C.; Eriksson, C.; Cao, Y.; Montgomery, S.; Ludvigsson, J.F.; Olén, O.; Halfvarson, J.; SWIBREG Study Group. Comparative Risk of Serious Infection With Vedolizumab vs Anti-Tumor Necrosis Factor in Inflammatory Bowel Disease: Results From Nationwide Swedish Registers. Am. J. Gastroenterol. 2024, 119, 2480–2492. [Google Scholar] [CrossRef]
- Dimopoulos-Verma, A.; Hong, S.; Axelrad, J.E. Enteric Infection at Flare of Inflammatory Bowel Disease Impacts Outcomes at 2 Years. Inflamm. Bowel Dis. 2024, 30, 1759–1766. [Google Scholar] [CrossRef]
- Arora, Z.; Mukewar, S.; Wu, X.; Shen, B. Risk factors and clinical implication of superimposed Campylobacter jejuni infection in patients with underlying ulcerative colitis. Gastroenterol. Rep. 2016, 4, 287–292. [Google Scholar]
- Jagirdhar, G.S.K.; Pulakurthi, Y.S.; Chigurupati, H.D.; Surani, S. Gastrointestinal tract and viral pathogens. World J. Virol. 2023, 12, 136–150. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Greenlund, K.; Carlson, S. Trends and demographic patterns in biologic and corticosteroid prescriptions for inflammatory bowel disease: Findings from electronic medical records, 2011–2020. J. Investig. Med. 2022, 70, 1771–1776. [Google Scholar] [CrossRef]
- Anderson, A.; Click, B.; Ramos-Rivers, C.; Cheng, D.; Babichenko, D.; Koutroubakis, I.E.; Hashash, J.G.; Schwartz, M.; Swoger, J.; Barrie, A.M., 3rd; et al. Lasting Impact of Clostridium difficile Infection in Inflammatory Bowel Disease: A Propensity Score Matched Analysis. Inflamm. Bowel Dis. 2017, 23, 2180–2188. [Google Scholar] [CrossRef]
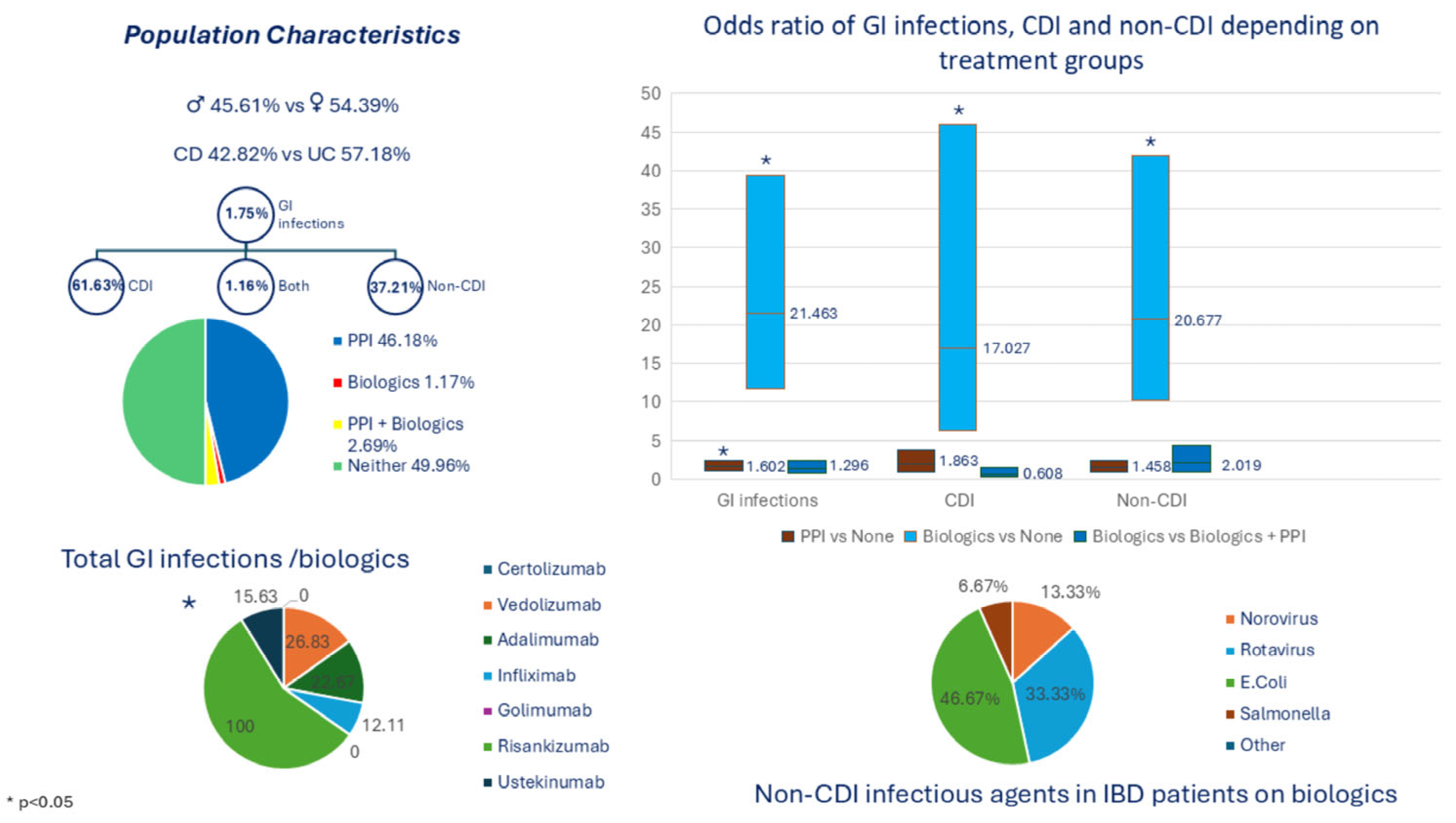
| Characteristic | ||
|---|---|---|
| Study Population (n) | 9849 | |
| Sex (%) | ||
| Male | 45.61% | |
| Female | 54.39% | |
| Race/Ethnicity (%) | ||
| White | 67.26% | |
| African American | 10.94% | |
| Hispanic/Latino | 8.19% | |
| Asian | 4.45% | |
| Multiracial or other | 6.80% | |
| Unknown | 2.37% | |
| Body Mass Index (Kg/m2) | ||
| Median (IQR) | 25.60 (22.3–29.8) | |
| Type of IBD (%) | ||
| Crohn’s Disease | 42.82% | |
| Ulcerative Colitis | 57.18% | |
| Treatment Groups (%/n) | ||
| PPIs Only | 46.18% (4548) | |
| Biologics Only | 1.17% (115) | |
| Both PPIs and Biologics | 2.69% (265) | |
| Neither PPIs nor Biologics | 49.96% (4921) | |
| GI Infection (%/n) | ||
| No | 98.25% (9677) | |
| Yes | 1.75% (172) | |
| a—Detected by GI PCR Only | 61.63% (106) | |
| b—Detected C. diff PCR Only | 37.21% (64) | |
| c—Detected by both | 1.16% (2) |
| Characteristic | Biologics | Biologics +PPIs | None | PPIs | p-Value | |
|---|---|---|---|---|---|---|
| Sex (%) | p = 0.5945 | |||||
| Male | 1.19% | 2.69% | 50.72% | 45.40% | ||
| Female | 1.14% | 2.69% | 49.07% | 47.11% | ||
| Race/Ethnicity (%) | p < 0.0001 * | |||||
| African American | 1.49% | 3.99% | 45.68% | 48.84% | ||
| Asian | 2.51% | 2.05% | 46.35% | 49.09% | ||
| Caucasian | 1.06% | 2.45% | 50.53% | 45.97% | ||
| Hispanic/Latino | 0.25% | 0.74% | 48.70% | 50.31% | ||
| Other/Multiracial | 2.24% | 6.12% | 54.18% | 37.46% | ||
| Body Mass Index (Kg/m2) | p < 0.0001 * | |||||
| Median (IQR) | 23.35 (20.30–26.70) | 26.10 (21.40–29.30) | 25.40 (22.10–29.60) | 25.80 (22.50–30.00) | ||
| Type of IBD (%) | p < 0.0001 * | |||||
| Crohn’s Disease | 1.38% | 3.53% | 49.28% | 45.81% | ||
| Ulcerative Colitis | 1.01% | 2.06% | 50.48% | 46.45% | ||
| GI infection (%/n) | p < 0.0001 * | |||||
| No | 0.95% | 2.33% | 50.44% | 46.29% | ||
| Yes | 13.37% | 23.26% | 23.26% | 40.12% |
| Odds Ratio Estimates | ||||
|---|---|---|---|---|
| Effect | Point Estimate | 95% Wald Confidence Limits | p-Value | |
| BMI | 0.995 | 0.970 | 1.021 | 0.6939 |
| Female vs. Male | 1.064 | 0.766 | 1.479 | 0.7115 |
| African American vs. Caucasian | 2.814 | 1.872 | 4.231 | <0.0001 * |
| Asian vs. Caucasian | 1.679 | 0.815 | 3.457 | 0.1599 |
| Hispanic/Latino vs. Caucasian | 1.259 | 0.621 | 2.550 | 0.5231 |
| Other/Multiracial vs. Caucasian | 2.304 | 1.396 | 3.805 | 0.0011 * |
| CD vs. UC | 0.901 | 0.646 | 1.256 | 0.5381 |
| Biologics vs. Biologics + PPIs | 1.296 | 0.699 | 2.401 | 0.4114 |
| Biologics vs. None | 21.463 | 11.693 | 39.396 | <0.0001 * |
| Biologics vs. PPIs | 13.396 | 7.551 | 23.764 | <0.0001 * |
| Biologics + PPIs vs. None | 16.561 | 10.239 | 26.786 | <0.0001 * |
| Biologics + PPIs vs. PPIs | 10.336 | 6.682 | 15.989 | <0.0001 * |
| PPIs vs. None | 1.602 | 1.064 | 2.413 | 0.0241 * |
| Hosmer-Lemeshow Test | p = 0.8395 | |||
| c-statistic | 0.740 | |||
| Odds Ratio Estimates and Wald Confidence Intervals | ||||
|---|---|---|---|---|
| Effect | Point Estimate | 95% Confidence Limits | p-Value | |
| African American vs. Caucasian | 6.104 | 3.290 | 11.325 | <0.0001 * |
| Asian vs. Caucasian | 3.198 | 1.127 | 9.081 | 0.029 * |
| Hispanic/Latino vs. Caucasian | 2.684 | 0.953 | 7.558 | 0.062 |
| Other/Multiracial vs. Caucasian | 4.873 | 2.370 | 10.016 | <0.0001 * |
| BMI | 0.957 | 0.915 | 1.000 | 0.049 * |
| Biologics vs. Biologics + PPIs | 0.608 | 0.241 | 1.533 | 0.2924 |
| Biologics vs. None | 17.027 | 6.299 | 46.022 | <0.0001 * |
| Biologics vs. PPIs | 9.138 | 3.655 | 22.850 | <0.0001 * |
| Biologics + PPIs vs. None | 28.027 | 13.656 | 57.518 | <0.0001 * |
| Biologics + PPIs vs. PPIs | 15.042 | 8.200 | 27.593 | <0.0001 * |
| PPIs vs. None | 1.863 | 0.929 | 3.737 | 0.080 |
| Hosmer-Lemeshow Test | p = 0.4546 | |||
| c-statistic | 0.821 | |||
| Odds Ratio Estimates | ||||
|---|---|---|---|---|
| Effect | Point Estimate | 95% Wald Confidence Limits | p Value | |
| African American vs. Caucasian | 1.513 | 0.870 | 2.632 | 0.143 |
| Asian vs. Caucasian | 1.211 | 0.474 | 3.093 | 0.689 |
| Hispanic/Latino vs. Caucasian | 0.865 | 0.343 | 2.177 | 0.757 |
| Other/Multiracial vs. Caucasian | 1.255 | 0.624 | 2.522 | 0.524 |
| BMI | 1.014 | 0.984 | 1.045 | 0.357 |
| Biologics vs. Biologics + PPIs | 2.019 | 0.944 | 4.315 | 0.071 |
| Biologics vs. None | 20.677 | 10.204 | 41.900 | <0.0001 * |
| Biologics vs. PPIs | 14.184 | 7.259 | 27.718 | <0.0001 * |
| Biologics + PPIs vs. None | 10.243 | 5.503 | 19.063 | <0.0001 * |
| Biologics + PPIs vs. PPIs | 7.026 | 3.941 | 12.527 | <0.0001 * |
| PPIs vs. None | 1.458 | 0.889 | 2.389 | 0.135 |
| Hosmer-Lemeshow Test | p = 0.4131 | |||
| c-statistic | 0.689 | |||
| Biologic Therapy | Number of IBD Patients on Treatment | Any GI Infection (%) | Non-Clostridial Infection (%) | CDI (%) |
|---|---|---|---|---|
| Certolizumab | 4 | 0.00 | 0.00 | 0.00 |
| Vedolizumab | 41 | 26.83 | 19.51 | 9.76 |
| Adalimumab | 75 | 22.67 | 10.67 | 12.00 |
| Infliximab | 223 | 12.11 | 4.93 | 7.62 |
| Golimumab | 2 | 0.00 | 0.00 | 0.00 |
| Risankizumab | 2 | 100.00 | 100.00 | 0.00 |
| Ustekinumab | 32 | 15.63 | 12.50 | 3.13 |
| p value | 0.0102 * | 0.001 * | 0.715 |
| Treatment | Biologics (n) Perc. (%) | PPIs (n) Perc. (%) | Biologics+PPIs (n) Perc. (%) | None (n) Perc. (%) |
|---|---|---|---|---|
| Adenovirus | 0 | 0 | 0 | 1 |
| 0.00% | 0.00% | 0.00% | 3.57% | |
| Astrovirus | 0 | 1 | 1 | 1 |
| 0.00% | 2.13% | 5.56% | 3.57% | |
| Norovirus | 2 | 15 | 7 | 7 |
| 13.33% | 31.91% | 38.89% | 25.00% | |
| Rotavirus | 5 | 4 | 2 | 0 |
| 33.33% | 8.51% | 11.11% | 0.00% | |
| Sapovirus | 0 | 0 | 1 | 0 |
| 0.00% | 0.00% | 5.56% | 0.00% | |
| E. coli | 7 | 16 | 6 | 12 |
| 46.67% | 34.04% | 33.33% | 42.86% | |
| Campylobacter | 0 | 6 | 1 | 4 |
| 0.00% | 12.77% | 5.56% | 14.29% | |
| Salmonella | 1 | 4 | 0 | 1 |
| 6.67% | 8.51% | 0.00% | 3.57% | |
| Yersinia | 0 | 1 | 0 | 1 |
| 0.00% | 2.13% | 0.00% | 3.57% | |
| Giardia | 0 | 0 | 0 | 1 |
| 0.00% | 0.00% | 0.00% | 3.57% | |
| Total | 15 | 47 | 18 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njeim, R.; Moussa, E.; Wei, C.; Sleiman, J.; Dimachkie, R.; Deeb, L. Impact of Biologics and Proton Pump Inhibitors on Gastrointestinal Infection Risk in Inflammatory Bowel Disease Patients: A Retrospective Analysis of Pathogen-Specific Outcomes and Treatment Interactions. Biomedicines 2025, 13, 1676. https://doi.org/10.3390/biomedicines13071676
Njeim R, Moussa E, Wei C, Sleiman J, Dimachkie R, Deeb L. Impact of Biologics and Proton Pump Inhibitors on Gastrointestinal Infection Risk in Inflammatory Bowel Disease Patients: A Retrospective Analysis of Pathogen-Specific Outcomes and Treatment Interactions. Biomedicines. 2025; 13(7):1676. https://doi.org/10.3390/biomedicines13071676
Chicago/Turabian StyleNjeim, Ryan, Elie Moussa, Chapman Wei, Joelle Sleiman, Reem Dimachkie, and Liliane Deeb. 2025. "Impact of Biologics and Proton Pump Inhibitors on Gastrointestinal Infection Risk in Inflammatory Bowel Disease Patients: A Retrospective Analysis of Pathogen-Specific Outcomes and Treatment Interactions" Biomedicines 13, no. 7: 1676. https://doi.org/10.3390/biomedicines13071676
APA StyleNjeim, R., Moussa, E., Wei, C., Sleiman, J., Dimachkie, R., & Deeb, L. (2025). Impact of Biologics and Proton Pump Inhibitors on Gastrointestinal Infection Risk in Inflammatory Bowel Disease Patients: A Retrospective Analysis of Pathogen-Specific Outcomes and Treatment Interactions. Biomedicines, 13(7), 1676. https://doi.org/10.3390/biomedicines13071676






