Heart Rate Variability and Autonomic Dysfunction After Stroke: Prognostic Markers for Recovery
Abstract
1. Introduction
2. Pathophysiology of Stroke and Autonomic Dysfunction
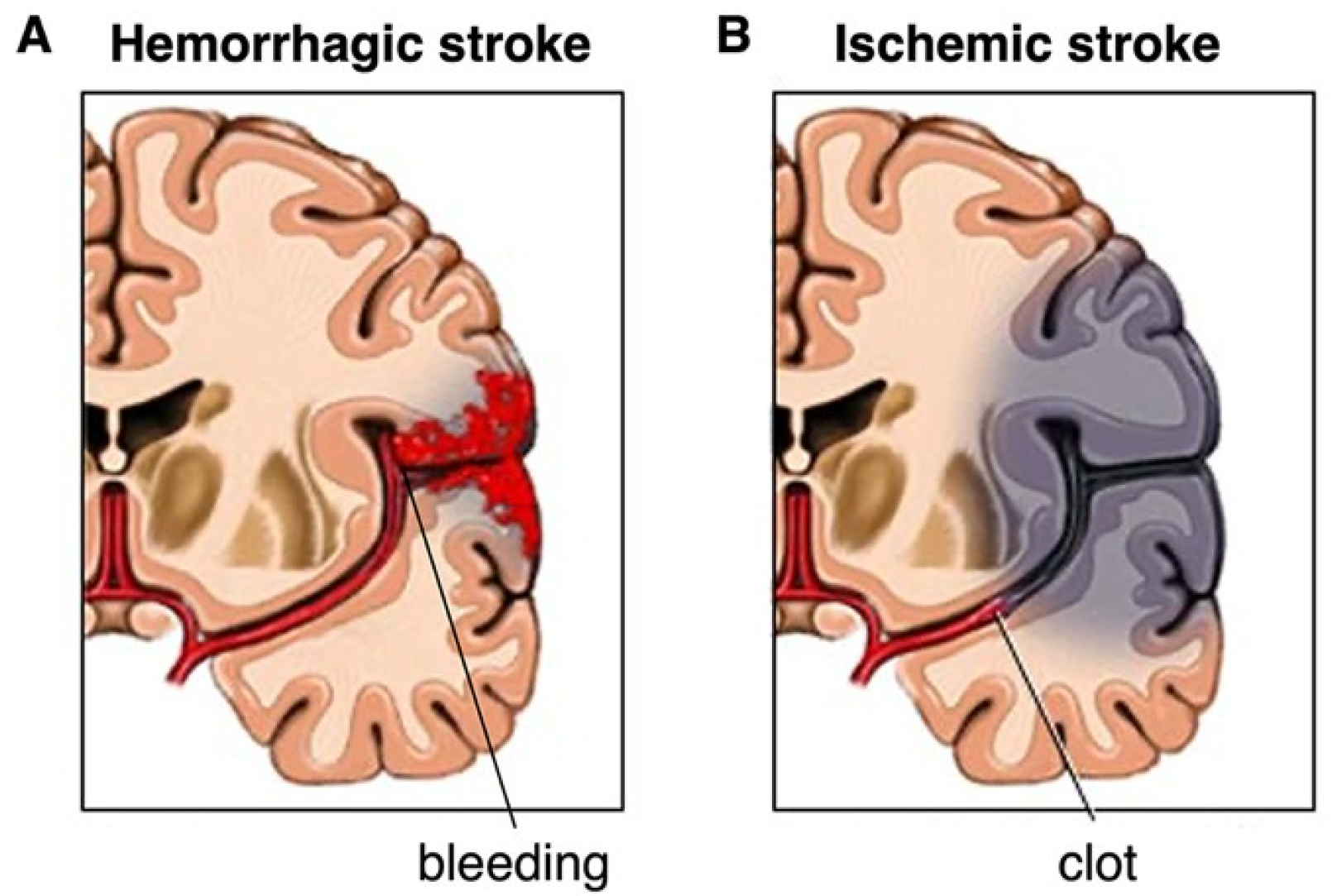
2.1. Types of Stroke: Ischemic and Hemorrhagic
2.2. Impact of Autonomic Dysfunction in Stroke
3. Heart Rate Variability: Measurement and Analysis
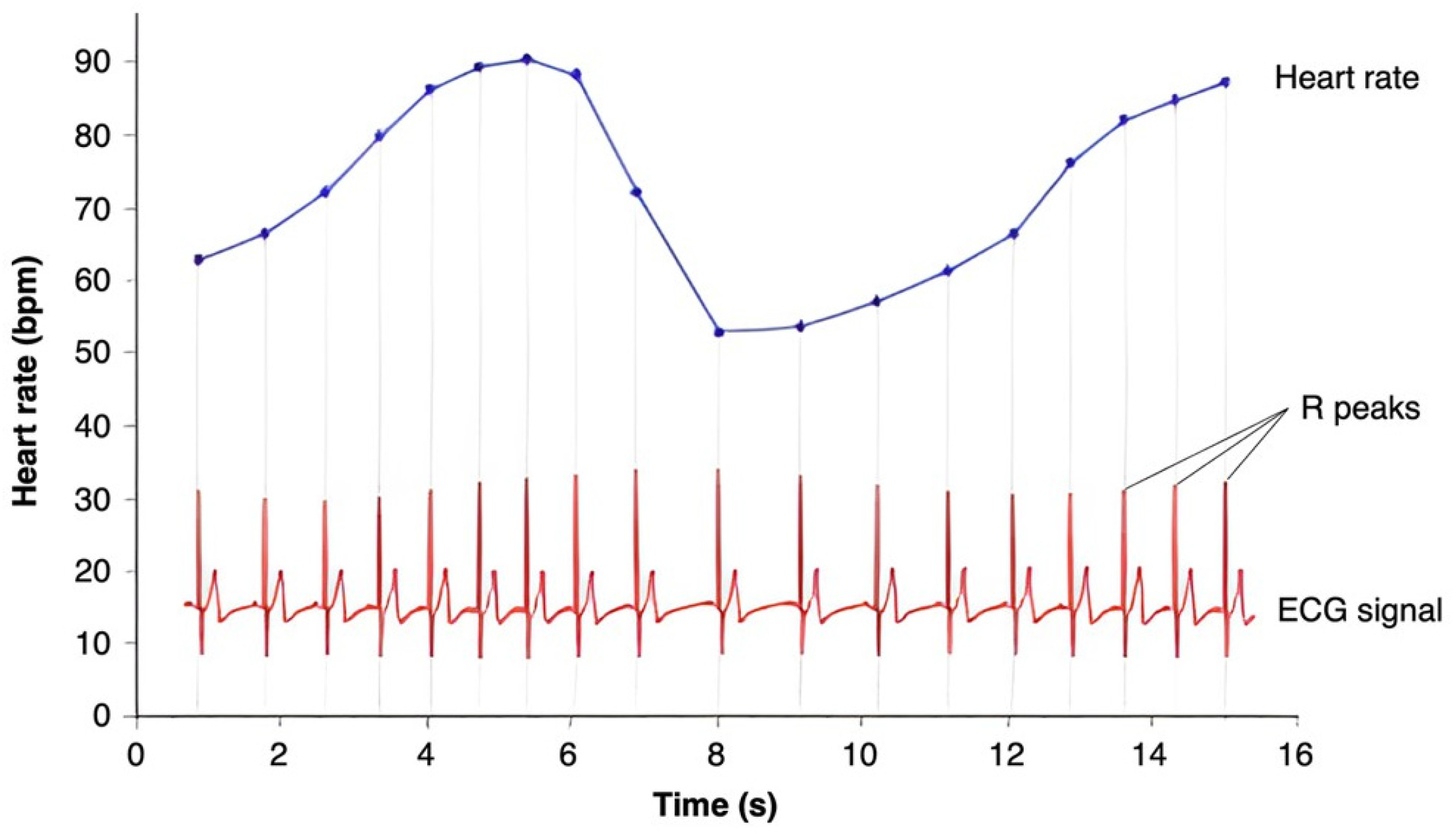
3.1. Basics of HRV
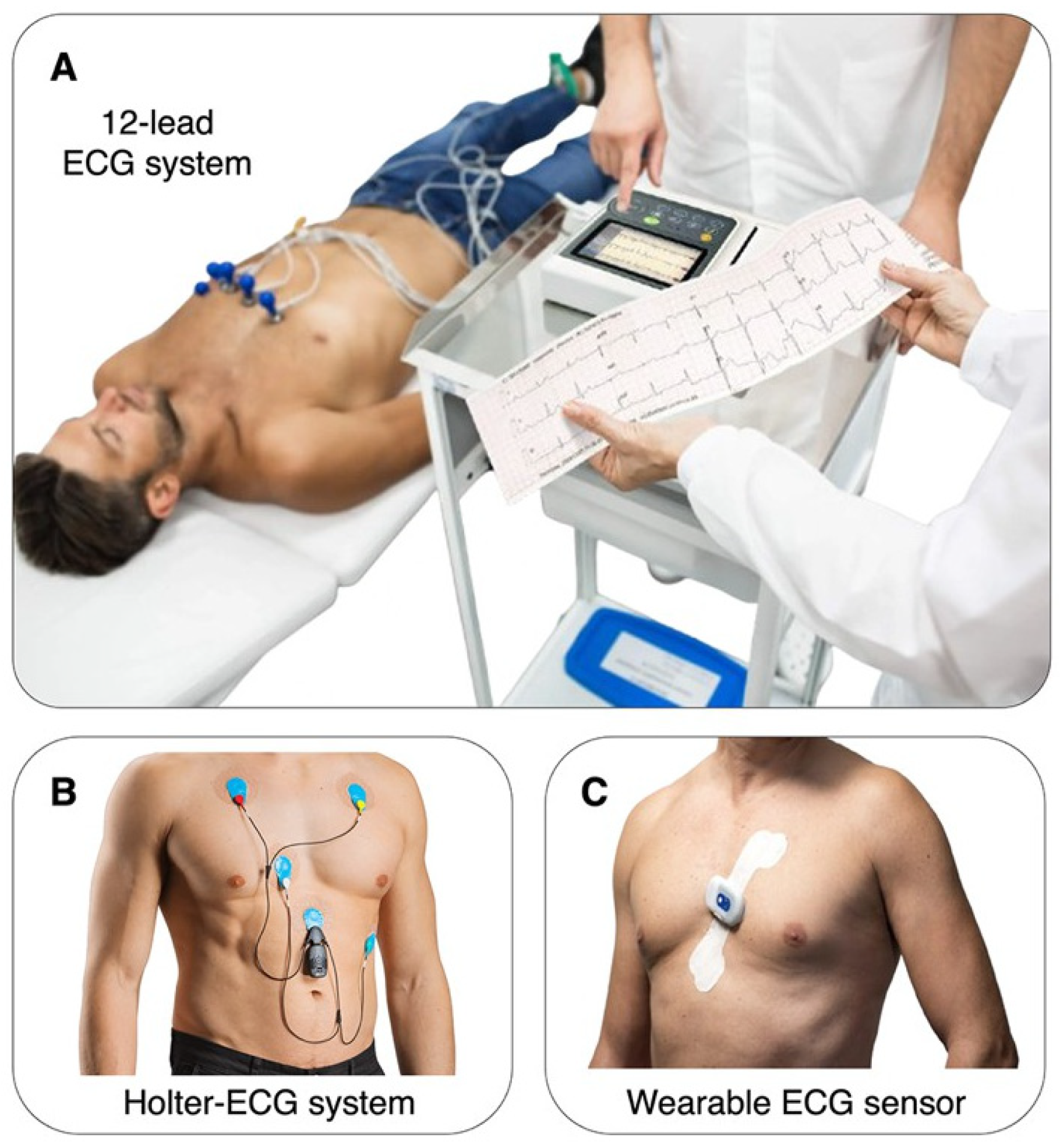
3.2. Methods of HRV Measurement
3.3. HRV Preprocessing
3.4. Extraction of Traditional Linear HRV Parameters
3.5. Extraction of Non-Linear HRV Parameters
4. HRV as a Biomarker in Stroke Recovery
4.1. Mechanisms Underlying HRV Changes Post-Stroke
4.2. Utility of HRV as a Prognostic Tool in Stroke
5. Clinical Applications
5.1. Risk Stratification Based on HRV
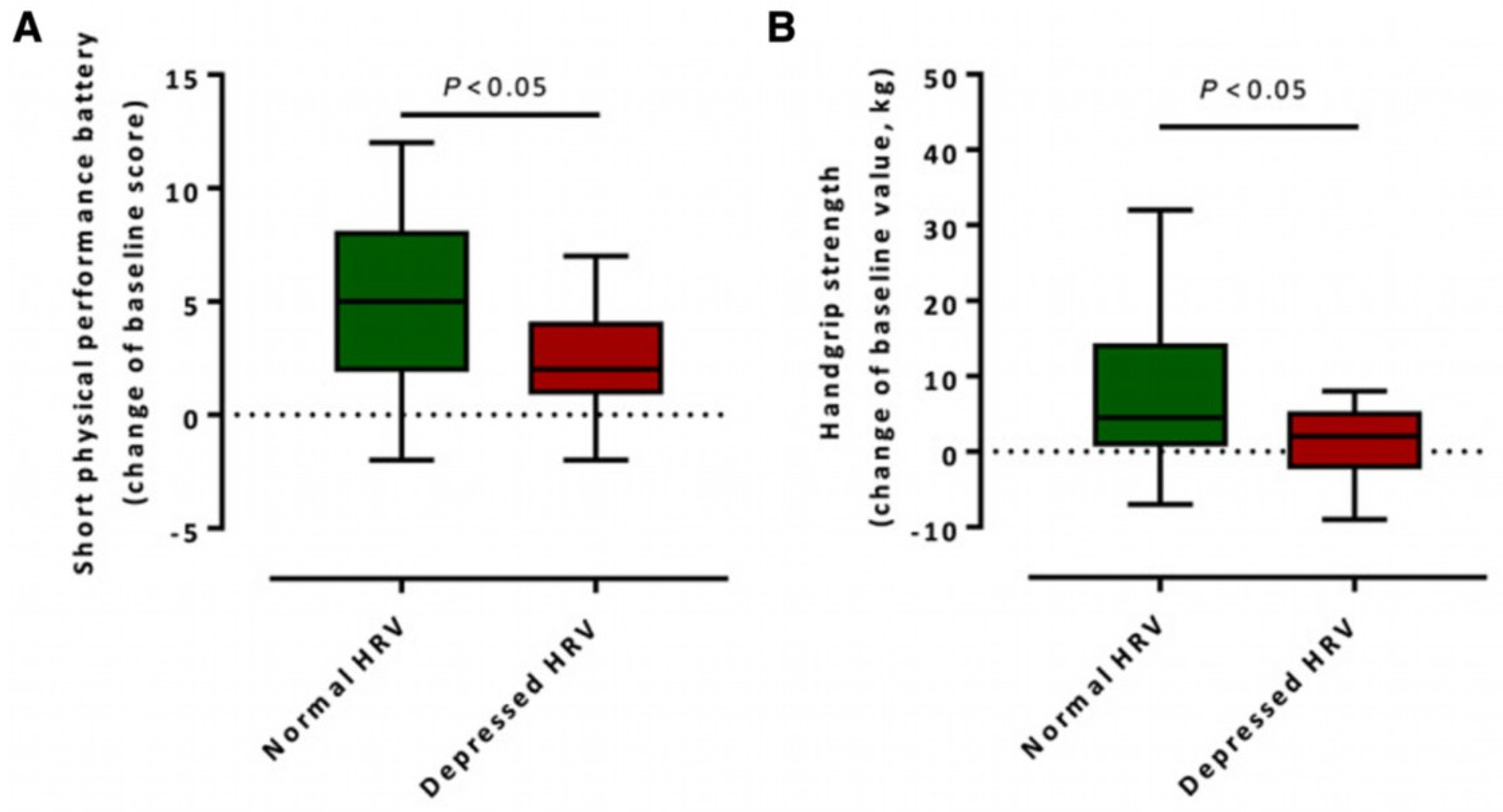
5.2. Monitoring Recovery Using HRV
5.3. Interventional Strategies to Improve HRV
6. Future Directions
6.1. Longitudinal Studies
6.2. Mechanistic Studies
6.3. Integration with Complementary Biomarkers
6.4. Methodological Challenges
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANS | Autonomic Nervous System |
| ApEn | Approximate Entropy |
| BNP | Brain Natriuretic Peptide |
| CRP | C-Reactive Protein |
| DFA | Detrended Fluctuation Analysis |
| ECG | Electrocardiogram |
| ENS | Enteric Nervous System |
| HF | High Frequency |
| HPA Axis | Hypothalamic–Pituitary–Adrenal Axis |
| HRV | Heart Rate Variability |
| IL-6 | Interleukin 6 |
| LE | Lyapunov Exponent |
| LF | Low Frequency |
| LF/HF | Low Frequency/High Frequency Ratio |
| MRI | Magnetic Resonance Imaging |
| pNN50 | Proportion of NN Intervals that Differ by More Than 50 ms |
| PNS | Parasympathetic Nervous System |
| PPG | Photoplethysmography |
| RMSSD | Root Mean Square of Successive Differences |
| SampEn | Sample Entropy |
| SDNN | Standard Deviation of Normal-to-Normal Intervals |
| SIE | Stroke-in-Evolution |
| SNS | Sympathetic Nervous System |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Pucciarelli, G.; Vellone, E.; Savini, S.; Simeone, S.; Ausili, D.; Alvaro, R.; Lee, C.S.; Lyons, K.S. Roles of Changing Physical Function and Caregiver Burden on Quality of Life in Stroke: A Longitudinal Dyadic Analysis. Stroke 2017, 48, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Tziaka, E.; Tsiakiri, A.; Vlotinou, P.; Christidi, F.; Tsiptsios, D.; Aggelousis, N.; Vadikolias, K.; Serdari, A. A Holistic Approach to Expressing the Burden of Caregivers for Stroke Survivors: A Systematic Review. Healthcare 2024, 12, 565. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.W.; Foley, N.C.; Bhogal, S.K.; Speechley, M.R. An Evidence-Based Review of Stroke Rehabilitation. Top. Stroke Rehabil. 2003, 10, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Damkjær, M.; Simonsen, S.A.; Heiberg, A.V.; Mehlsen, J.; West, A.S.; Jennum, P.; Iversen, H.K. Autonomic Dysfunction after Mild Acute Ischemic Stroke and Six Months after: A Prospective Observational Cohort Study. BMC Neurol. 2023, 23, 26. [Google Scholar] [CrossRef]
- Tenberg, A.; Tahara, N.; Grewal, A.; Herrera, A.; Klein, L.M.; Lebo, R.; Zink, E.K.; Bahouth, M.N. Dysautonomia and Activity in the Early Stroke Recovery Period. Neurol. Sci. 2024, 45, 2505–2521. [Google Scholar] [CrossRef]
- Armstrong, R.; Wheen, P.; Brandon, L.; Maree, A.; Kenny, R.-A. Heart Rate: Control Mechanisms, Pathophysiology and Assessment of the Neurocardiac System in Health and Disease. QJM Int. J. Med. 2022, 115, 806–812. [Google Scholar] [CrossRef]
- Jimenez-Ruiz, A.; Racosta, J.M.; Kimpinski, K.; Hilz, M.J.; Sposato, L.A. Cardiovascular Autonomic Dysfunction after Stroke. Neurol. Sci. 2021, 42, 1751–1758. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef]
- Korpelainen, J.T.; Sotaniemi, K.A.; Myllylä, V.V. Autonomic Nervous System Disorders in Stroke. Clin. Auton. Res. 1999, 9, 325–333. [Google Scholar] [CrossRef]
- Nayak, S.K.; Pradhan, B.; Mohanty, B.; Sivaraman, J.; Ray, S.S.; Wawrzyniak, J.; Jarzębski, M.; Pal, K. A Review of Methods and Applications for a Heart Rate Variability Analysis. Algorithms 2023, 16, 433. [Google Scholar] [CrossRef]
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart Rate Variability Biofeedback Improves Emotional and Physical Health and Performance: A Systematic Review and Meta Analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public. Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Zeid, S.; Buch, G.; Velmeden, D.; Söhne, J.; Schulz, A.; Schuch, A.; Tröbs, S.-O.; Heidorn, M.W.; Müller, F.; Strauch, K.; et al. Heart Rate Variability: Reference Values and Role for Clinical Profile and Mortality in Individuals with Heart Failure. Clin. Res. Cardiol. 2023, 113, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Muslumanoglu, L.; Akyuz, G.; Aki, S.; Karsidag, S.; Us, O. Evaluation of Autonomic Nervous System Functions in Post-Stroke Patients. Am. J. Phys. Med. Rehabil. 2002, 81, 721–725. [Google Scholar] [CrossRef]
- Bai, X.; Wang, N.; Si, Y.; Liu, Y.; Yin, P.; Xu, C. The Clinical Characteristics of Heart Rate Variability After Stroke: A Systematic Review. Neurologist 2023, 29, 133–141. [Google Scholar] [CrossRef]
- Dütsch, M.; Burger, M.; Dörfler, C.; Schwab, S.; Hilz, M.J. Cardiovascular Autonomic Function in Poststroke Patients. Neurology 2007, 69, 2249–2255. [Google Scholar] [CrossRef]
- Cha, K.H.; Kang, N.Y.; Huh, S.; Ko, S.-H.; Shin, Y.-I.; Min, J.H. The Effects of Autonomic Dysfunction on Functional Outcomes in Patients with Acute Stroke. Brain Sci. 2023, 13, 1694. [Google Scholar] [CrossRef]
- Baldo, J.V.; Schendel, K.; Lwi, S.J.; Herron, T.J.; Dempsey, D.G.; Muir, J.; Curran, B.C.; Paulraj, S.; Chok, J.; Cole, M.A. Mindfulness-Based Stress Reduction Intervention in Chronic Stroke: A Randomized, Controlled Pilot Study. Mindfulness 2021, 12, 2908–2919. [Google Scholar] [CrossRef]
- Lee, K.E.; Choi, M.; Jeoung, B. Effectiveness of Rehabilitation Exercise in Improving Physical Function of Stroke Patients: A Systematic Review. Int. J. Env. Res. Public. Health 2022, 19, 12739. [Google Scholar] [CrossRef]
- Buitrago-Ricaurte, N.; Cintra, F.; Silva, G.S. Heart Rate Variability as an Autonomic Biomarker in Ischemic Stroke. Arq. Neuro-Psiquiatr. 2020, 78, 724–732. [Google Scholar] [CrossRef]
- Schäfer, A.; Vagedes, J. How Accurate Is Pulse Rate Variability as an Estimate of Heart Rate Variability?: A Review on Studies Comparing Photoplethysmographic Technology with an Electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Elishkevitz, K.P.; Katz, K.; Nussinovitch, M.; Segev, S.; Volovitz, B.; Nussinovitch, N. Reliability of Ultra-Short ECG Indices for Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2011, 16, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sibrecht, G.; Piskorski, J.; Krauze, T.; Guzik, P. Heart Rate Asymmetry, Its Compensation, and Heart Rate Variability in Healthy Adults during 48-h Holter ECG Recordings. J. Clin. Med. 2023, 12, 1219. [Google Scholar] [CrossRef]
- Li, K.; Cardoso, C.; Moctezuma-Ramirez, A.; Elgalad, A.; Perin, E. Heart Rate Variability Measurement through a Smart Wearable Device: Another Breakthrough for Personal Health Monitoring? Int. J. Environ. Res. Public. Health 2023, 20, 7146. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, M.; Vanello, N.; Galperti, G.; Greco, A.; Scilingo, E.P. Assessing the Quality of Heart Rate Variability Estimated from Wrist and Finger PPG: A Novel Approach Based on Cross-Mapping Method. Sensors 2020, 20, 3156. [Google Scholar] [CrossRef]
- Beh, W.-K.; Yang, Y.-C.; Wu, A.-Y. Quality-Aware Signal Processing Mechanism of PPG Signal for Long-Term Heart Rate Monitoring. Sensors 2024, 24, 3901. [Google Scholar] [CrossRef]
- de Beukelaar, T.T.; Mantini, D. Monitoring Resistance Training in Real Time with Wearable Technology: Current Applications and Future Directions. Bioengineering 2023, 10, 1085. [Google Scholar] [CrossRef]
- Aziz, S.; Ahmed, S.; Alouini, M.-S. ECG-Based Machine-Learning Algorithms for Heartbeat Classification. Sci. Rep. 2021, 11, 18738. [Google Scholar] [CrossRef]
- Yadav, O.P.; Ray, S. A Novel Method of Preprocessing and Modeling ECG Signals with Lagrange–Chebyshev Interpolating Polynomials. Int. J. Syst. Assur. Eng. Manag. 2021, 12, 377–390. [Google Scholar] [CrossRef]
- Benchekroun, M.; Chevallier, B.; Istrate, D.; Zalc, V.; Lenne, D. Preprocessing Methods for Ambulatory HRV Analysis Based on HRV Distribution, Variability and Characteristics (DVC). Sensors 2022, 22, 1984. [Google Scholar] [CrossRef]
- Li, K.; Rüdiger, H.; Ziemssen, T. Spectral Analysis of Heart Rate Variability: Time Window Matters. Front. Neurol. 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Catai, A.M.; Pastre, C.M.; de Godoy, M.F.; da Silva, E.; de Medeiros Takahashi, A.C.; Vanderlei, L.C.M. Heart Rate Variability: Are You Using It Properly? Standardisation Checklist of Procedures. Braz. J. Phys. Ther. 2020, 24, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Mietus, J.E.; Peng, C.-K.; Henry, I.; Goldsmith, R.L.; Goldberger, A.L. The pNNx Files: Re-Examining a Widely Used Heart Rate Variability Measure. Heart 2002, 88, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Yuda, E. Assessment of Autonomic Function by Long-Term Heart Rate Variability: Beyond the Classical Framework of LF and HF Measurements. J. Physiol. Anthropol. 2021, 40, 21. [Google Scholar] [CrossRef]
- Hu, J.; Gao, J.; Tung, W. Characterizing Heart Rate Variability by Scale-Dependent Lyapunov Exponent. Chaos 2009, 19, 028506. [Google Scholar] [CrossRef]
- Beckers, F.; Ramaekers, D.; Aubert, A.E. Approximate Entropy of Heart Rate Variability: Validation of Methods and Application in Heart Failure. Cardiovasc. Eng. 2001, 1, 177–182. [Google Scholar] [CrossRef]
- Udhayakumar, R.K.; Karmakar, C.; Palaniswami, M. Approximate Entropy Profile: A Novel Approach to Comprehend Irregularity of Short-Term HRV Signal. Nonlinear Dyn. 2017, 88, 823–837. [Google Scholar] [CrossRef]
- Udhayakumar, R.K.; Karmakar, C.; Palaniswami, M. Understanding Irregularity Characteristics of Short-Term HRV Signals Using Sample Entropy Profile. IEEE Trans. Biomed. Eng. 2018, 65, 2569–2579. [Google Scholar] [CrossRef]
- Bojorges-Valdez, E.R.; Echeverría, J.C.; Valdés-Cristerna, R.; Peña, M.A. Scaling Patterns of Heart Rate Variability Data. Physiol. Meas. 2007, 28, 721–730. [Google Scholar] [CrossRef]
- Peña, M.A.; Echeverría, J.C.; García, M.T.; González-Camarena, R. Applying Fractal Analysis to Short Sets of Heart Rate Variability Data. Med. Biol. Eng. Comput. 2009, 47, 709–717. [Google Scholar] [CrossRef]
- von Rennenberg, R.; Krause, T.; Herm, J.; Hellwig, S.; Scheitz, J.F.; Endres, M.; Haeusler, K.G.; Nolte, C.H. Heart Rate Variability and Recurrent Stroke and Myocardial Infarction in Patients With Acute Mild to Moderate Stroke. Front. Neurol. 2021, 12, 772674. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-J.; Dewi, S.R.K.; Hsu, W.-T.; Hsu, T.-Y.; Liao, S.-F.; Chan, L.; Lin, M.-C. Exploring Relationships of Heart Rate Variability, Neurological Function, and Clinical Factors with Mortality and Behavioral Functional Outcome in Patients with Ischemic Stroke. Diagnostics 2024, 14, 1304. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Zhang, Y.; Feng, C.; An, Y.-H.; Li, Z.-P.; Yin, X.-S. Systemic Inflammation Response Index as a Clinical Outcome Evaluating Tool and Prognostic Indicator for Hospitalized Stroke Patients: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2023, 28, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guan, L.; Wang, Y. The Association of Autonomic Nervous System Function With Ischemic Stroke, and Treatment Strategies. Front. Neurol. 2020, 10, 1411. [Google Scholar] [CrossRef]
- Datta, A.; Saha, C.; Godse, P.; Sharma, M.; Sarmah, D.; Bhattacharya, P. Neuroendocrine Regulation in Stroke. Trends Endocrinol. Metab. 2023, 34, 260–277. [Google Scholar] [CrossRef]
- Wexler, T.L. Neuroendocrine Disruptions Following Head Injury. Curr. Neurol. Neurosci. Rep. 2023, 23, 213–224. [Google Scholar] [CrossRef]
- Manuel, J.; Färber, N.; Gerlach, D.A.; Heusser, K.; Jordan, J.; Tank, J.; Beissner, F. Deciphering the Neural Signature of Human Cardiovascular Regulation. Elife 2020, 9, e55316. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Lopes da Silva, A.; dos Santos Samary, C.; Leme Silva, P.; Dal Pizzol, F.; Pelosi, P.; Rocco, P.R.M. Brain–Heart Interaction after Acute Ischemic Stroke. Crit. Care 2020, 24, 163. [Google Scholar] [CrossRef]
- Aref, H.M.A.; Fahmy, N.A.; Khalil, S.H.; Ahmed, M.F.; ElSadek, A.; Abdulghani, M.O. Role of Interleukin-6 in Ischemic Stroke Outcome. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 12. [Google Scholar] [CrossRef]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Li, L.; Miyamoto, N.; Montaner, J.; et al. C-Reactive Protein, Interleukin-6, and Vascular Recurrence After Stroke: An Individual Participant Data Meta-Analysis. Stroke 2023, 54, 1289–1299. [Google Scholar] [CrossRef]
- Wang, F.; Luo, M.; Zhou, L.; Yang, L.; Lanzino, G.; Chang, H.-J.; Wellman, G.C. Endocrine Dysfunction Following Stroke. J. Neuroimmune Pharmacol. 2021, 16, 425–436. [Google Scholar] [CrossRef]
- Holman, E.A.; Cramer, S.C. Lifetime and Acute Stress Predict Functional Outcomes Following Stroke: Findings From the Longitudinal STRONG Study. Stroke 2023, 54, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Aftyka, J.; Staszewski, J.; Dębiec, A.; Pogoda-Wesołowska, A.; Żebrowski, J. Heart Rate Variability as a Predictor of Stroke Course, Functional Outcome, and Medical Complications: A Systematic Review. Front. Physiol. 2023, 14, 1115164. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Sun, Y.; Abuduxukuer, R.; Si, X.; Zhang, P.; Ren, J.; Fu, Y.; Zhang, K.; Liu, J.; Zhang, P.; et al. Heart Rate Variability Parameter Changes in Patients With Acute Ischemic Stroke Undergoing Intravenous Thrombolysis. J. Am. Heart Assoc. 2023, 12, e028778. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Huang, P.-W.; Tang, S.-C.; Shieh, J.-S.; Lai, D.-M.; Wu, A.-Y.; Jeng, J.-S. Complexity of Heart Rate Variability Can Predict Stroke-In-Evolution in Acute Ischemic Stroke Patients. Sci. Rep. 2015, 5, 17552. [Google Scholar] [CrossRef]
- Scherbakov, N.; Barkhudaryan, A.; Ebner, N.; von Haehling, S.; Anker, S.D.; Joebges, M.; Doehner, W. Early Rehabilitation after Stroke: Relationship between the Heart Rate Variability and Functional Outcome. ESC Heart Fail. 2020, 7, 2983–2991. [Google Scholar] [CrossRef]
- Chen, L.; Gao, H.; Wang, Z.; Gu, B.; Zhou, W.; Pang, M.; Zhang, K.; Liu, X.; Ming, D. Vagus Nerve Electrical Stimulation in the Recovery of Upper Limb Motor Functional Impairment after Ischemic Stroke. Cogn. Neurodyn 2024, 18, 3107–3124. [Google Scholar] [CrossRef]
- Mainali, S.; Darsie, M.E.; Smetana, K.S. Machine Learning in Action: Stroke Diagnosis and Outcome Prediction. Front. Neurol. 2021, 12, 734345. [Google Scholar] [CrossRef]
- Tang, S.; Xiong, L.; Fan, Y.; Mok, V.C.T.; Wong, K.S.; Leung, T.W. Stroke Outcome Prediction by Blood Pressure Variability, Heart Rate Variability, and Baroreflex Sensitivity. Stroke 2020, 51, 1317–1320. [Google Scholar] [CrossRef]
- Lischke, A.; Pahnke, R.; Mau-Moeller, A.; Behrens, M.; Grabe, H.J.; Freyberger, H.J.; Hamm, A.O.; Weippert, M. Inter-Individual Differences in Heart Rate Variability Are Associated with Inter-Individual Differences in Empathy and Alexithymia. Front. Psychol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Lischke, A.; Lemke, D.; Neubert, J.; Hamm, A.O.; Lotze, M. Inter-Individual Differences in Heart Rate Variability Are Associated with Inter-Individual Differences in Mind-Reading. Sci. Rep. 2017, 7, 11557. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, P.; Dumont, G.; Boivin, D. Circadian Variation of Heart Rate Variability during Different Sleep Stages. Sleep. Med. 2013, 14, e76. [Google Scholar] [CrossRef]
- Gander, P.H.; Connell, L.J.; Graeber, R.C. Masking of the Circadian Rhythms of Heart Rate and Core Temperature by the Rest-Activity Cycle in Man. J. Biol. Rhythm. 1986, 1, 119–135. [Google Scholar] [CrossRef]
- Saleem, S.; Khandoker, A.H.; Alkhodari, M.; Hadjileontiadis, L.J.; Jelinek, H.F. Investigating the Effects of Beta-Blockers on Circadian Heart Rhythm Using Heart Rate Variability in Ischemic Heart Disease with Preserved Ejection Fraction. Sci. Rep. 2023, 13, 5828. [Google Scholar] [CrossRef]
- Roy, A.; Guatimosim, S.; Prado, V.F.; Gros, R.; Prado, M.A.M. Cholinergic Activity as a New Target in Diseases of the Heart. Mol. Med. 2014, 20, 527–537. [Google Scholar] [CrossRef]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of Different Exercise Interventions on Heart Rate Variability and Cardiovascular Health Factors in Older Adults: A Systematic Review. Eur. Rev. Aging Phys. Act. 2021, 18, 24. [Google Scholar] [CrossRef]
- Guo, X.H.; Yi, G.; Batchvarov, V.; Gallagher, M.M.; Malik, M. Effect of Moderate Physical Exercise on Noninvasive Cardiac Autonomic Tests in Healthy Volunteers. Int. J. Cardiol. 1999, 69, 155–168. [Google Scholar] [CrossRef]
- Perez-Quilis, C.; Kingsley, J.D.; Malkani, K.; Cervellin, G.; Lippi, G.; Sanchis-Gomar, F. Modulation of Heart Rate by Acute or Chronic Aerobic Exercise. Potential Effects on Blood Pressure Control. Curr. Pharm. Des. 2017, 23, 4650–4657. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Figueroa, A. Acute and Training Effects of Resistance Exercise on Heart Rate Variability. Clin. Physiol. Funct. Imaging 2016, 36, 179–187. [Google Scholar] [CrossRef]
- Saeidi, M.; Ravanbod, R.; Pourgharib Shahi, M.H.; Navid, H.; Goosheh, B.; Baradaran, A.; Torkaman, G. The Acute Effects of 2 Different Intensities of Resistance Exercise on Autonomic Function in Heart Failure Patients: A Randomized Controlled Trial. Anatol. J. Cardiol. 2023, 27, 266. [Google Scholar] [CrossRef]
- Kirk, U.; Axelsen, J.L. Heart Rate Variability Is Enhanced during Mindfulness Practice: A Randomized Controlled Trial Involving a 10-Day Online-Based Mindfulness Intervention. PLoS ONE 2020, 15, e0243488. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Philbrook, P.; Tzika, A.A. Yoga and Mindfulness as Therapeutic Interventions for Stroke Rehabilitation: A Systematic Review. Evid. Based Complement. Altern. Med. 2013, 2013, 357108. [Google Scholar] [CrossRef] [PubMed]
- Voss, A. Longitudinal Analysis of Heart Rate Variability. J. Electrocardiol. 2007, 40, S26–S29. [Google Scholar] [CrossRef]
- Kuzemczak, M.; Białek-Ławniczak, P.; Torzyńska, K.; Janowska-Kulińska, A.; Miechowicz, I.; Kramer, L.; Moczko, J.; Siminiak, T. Comparison of Baseline Heart Rate Variability in Stable Ischemic Heart Disease Patients with and without Stroke in Long-Term Observation. J. Stroke Cerebrovasc. Dis. 2016, 25, 2526–2534. [Google Scholar] [CrossRef]
- DeLong, J.H.; Ohashi, S.N.; O’Connor, K.C.; Sansing, L.H. Inflammatory Responses After Ischemic Stroke. Semin. Immunopathol. 2022, 44, 625–648. [Google Scholar] [CrossRef]
- Przykaza, Ł. Understanding the Connection Between Common Stroke Comorbidities, Their Associated Inflammation, and the Course of the Cerebral Ischemia/Reperfusion Cascade. Front. Immunol. 2021, 12, 782569. [Google Scholar] [CrossRef]
- Dimova, V.; Welte-Jzyk, C.; Kronfeld, A.; Korczynski, O.; Baier, B.; Koirala, N.; Steenken, L.; Kollmann, B.; Tüscher, O.; Brockmann, M.A.; et al. Brain Connectivity Networks Underlying Resting Heart Rate Variability in Acute Ischemic Stroke. NeuroImage Clin. 2024, 41, 103558. [Google Scholar] [CrossRef]
- Matusik, P.S.; Zhong, C.; Matusik, P.T.; Alomar, O.; Stein, P.K. Neuroimaging Studies of the Neural Correlates of Heart Rate Variability: A Systematic Review. J. Clin. Med. 2023, 12, 1016. [Google Scholar] [CrossRef]
- Boucher, J.; Marcotte, K.; Bedetti, C.; Houzé, B.; Descoteaux, M.; Brisebois, A.; García, A.O.; Rochon, E.; Leonard, C.; Desautels, A.; et al. Longitudinal Evolution of Diffusion Metrics after Left Hemisphere Ischaemic Stroke. Brain Commun. 2023, 5, fcad313. [Google Scholar] [CrossRef]
- Attar, E.T.; Balasubramanian, V.; Subasi, E.; Kaya, M. Stress Analysis Based on Simultaneous Heart Rate Variability and EEG Monitoring. IEEE J. Transl. Eng. Health Med. 2021, 9, 2700607. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, S.-B.; Lee, K.-S. Continuous Heart Rate Variability and Electroencephalography Monitoring in Severe Acute Brain Injury: A Preliminary Study. ACC 2021, 36, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Lagerstedt, L.; Burkhard, P.R.; Debouverie, M.; Turck, N.; Sanchez, J.-C. E-Selectin and Vascular Cell Adhesion Molecule-1 as Biomarkers of 3-Month Outcome in Cerebrovascular Diseases. J Inflamm. 2015, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Wildi, K.S.; Nigro, N.; Mueller, C.; Schuetz, P.; Mueller, B.; Fluri, F.; Christ-Crain, M.; Katan, M. BNP but Not Troponin Is Associated with Cardioembolic Aetiology and Poor Outcome after Acute Ischemic Stroke or TIA. Eur. Heart J. 2013, 34, P405. [Google Scholar] [CrossRef]
- Orgianelis, I.; Merkouris, E.; Kitmeridou, S.; Tsiptsios, D.; Karatzetzou, S.; Sousanidou, A.; Gkantzios, A.; Christidi, F.; Polatidou, E.; Beliani, A.; et al. Exploring the Utility of Autonomic Nervous System Evaluation for Stroke Prognosis. Neurol. Int. 2023, 15, 661–696. [Google Scholar] [CrossRef]
- Lees, T.; Shad-Kaneez, F.; Simpson, A.M.; Nassif, N.T.; Lin, Y.; Lal, S. Heart Rate Variability as a Biomarker for Predicting Stroke, Post-Stroke Complications and Functionality. Biomark. Insights 2018, 13, 1177271918786931. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Castaldo, R.; Melillo, P.; Bracale, U.; Caserta, M.; Triassi, M.; Pecchia, L. Acute Mental Stress Assessment via Short Term HRV Analysis in Healthy Adults: A Systematic Review with Meta-Analysis. Biomed. Signal Process. Control 2015, 18, 370–377. [Google Scholar] [CrossRef]
- Besson, C.; Baggish, A.L.; Monteventi, P.; Schmitt, L.; Stucky, F.; Gremeaux, V. Assessing the Clinical Reliability of Short-Term Heart Rate Variability: Insights from Controlled Dual-Environment and Dual-Position Measurements. Sci. Rep. 2025, 15, 5611. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lu, W.-A.; Pagaduan, J.C.; Kuo, C.-D. A Novel Smartphone App for the Measurement of Ultra-Short-Term and Short-Term Heart Rate Variability: Validity and Reliability Study. JMIR Mhealth Uhealth 2020, 8, e18761. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lago, S.; de Beukelaar, T.T.; Casetta, I.; Arcara, G.; Mantini, D. Heart Rate Variability and Autonomic Dysfunction After Stroke: Prognostic Markers for Recovery. Biomedicines 2025, 13, 1659. https://doi.org/10.3390/biomedicines13071659
Lago S, de Beukelaar TT, Casetta I, Arcara G, Mantini D. Heart Rate Variability and Autonomic Dysfunction After Stroke: Prognostic Markers for Recovery. Biomedicines. 2025; 13(7):1659. https://doi.org/10.3390/biomedicines13071659
Chicago/Turabian StyleLago, Sara, Toon T. de Beukelaar, Ilaria Casetta, Giorgio Arcara, and Dante Mantini. 2025. "Heart Rate Variability and Autonomic Dysfunction After Stroke: Prognostic Markers for Recovery" Biomedicines 13, no. 7: 1659. https://doi.org/10.3390/biomedicines13071659
APA StyleLago, S., de Beukelaar, T. T., Casetta, I., Arcara, G., & Mantini, D. (2025). Heart Rate Variability and Autonomic Dysfunction After Stroke: Prognostic Markers for Recovery. Biomedicines, 13(7), 1659. https://doi.org/10.3390/biomedicines13071659






