Abstract
This review explores the potential role of topical insulin drops in corneal regeneration by analyzing the mechanism of action and clinical outcomes. Corneal integrity restoration is crucial for ocular surface healing. This review synthesizes the current literature on topical insulin for neurotrophic keratopathy (NK), highlighting its mechanism of action, therapeutic potential, and clinical outcomes. Recent studies report high rates of epithelial regeneration, suggesting that topical insulin may be an effective adjunct or alternative to conventional treatments. Further randomized controlled trials are needed to confirm its long-term efficacy and optimal dosing. Methods: Considering the limited regenerative capacity of the corneal epithelium in NK and the increasing interest in novel therapy, we review the existing literature to evaluate the role and extent of topical insulin’s contribution to corneal healing by applying the PICO framework, which allows for a clear and systematic approach to literature selection and evaluation. The literature search and study selection were conducted manually following PRISMA guidelines. Conclusions: Most of the studies resulting from the selection have small samples, and there is a lack of large, randomized clinical trials. The evidence reviewed in this study suggests that topical insulin is a promising therapy for promoting corneal healing in neurotrophic keratopathy. While clinical trials have demonstrated significant epithelial regeneration, optimal dosing and long-term safety require further investigation. Compared to conventional treatments such as autologous serum or growth factor therapy, insulin eye drops provide a cost-effective alternative. Additional research through controlled trials is needed to formulate standardized therapeutic protocols and verify long-term outcomes.
1. Introduction
The cornea exhibits dense sensory innervation, predominantly supplied by the ophthalmic branch of the trigeminal nerve, which contributes to epithelial turnover, neurotrophic support, reflex lacrimation, and the orchestration of wound healing mechanisms [1]. As seen in neurotrophic keratopathy (NK), damage to corneal nerves disrupts these mechanisms, leading to persistent epithelial defects and potential corneal perforation [2]. Emerging evidence suggests that topical insulin may facilitate corneal healing by stimulating epithelial proliferation, reducing inflammation, and restoring cellular homeostasis.
1.1. Anatomy of the Cornea
The anterior segment is composed of the conjunctiva, cornea, lens, ciliary body, iris, and aqueous humor. The cornea and sclera form the outer protective layer of the eye. The cornea is a transparent, avascular tissue that contributes approximately two-thirds of the eye’s refractive power (40–44 D) and has a 1.376 refractive index. The cornea is horizontally round, convex, and aspheric. The confines of the anterior curve are 7.8 mm, and the posterior curve is roughly 6.5 mm [3].
1.2. Corneal Histology
The corneal surface is composed of a multi-layered squamous epithelium that does not undergo keratinization, particularly at the periphery. Basal epithelial cells exhibit a polygonal morphology, transitioning into progressively flattened cells as they approach the superficial strata. Within the epithelial layers, immune-responsive Langerhans cells can be observed, typically identifiable by CD1a expression. Bowman’s layer contributes significantly to the anterior stroma. The central stroma, which constitutes approximately 90% of the corneal thickness, is acellular and contains uniformly spaced collagen fibrils interspersed with mucoproteins and glycoproteins—essential components responsible for maintaining corneal transparency. This layer measures approximately 8 to 14 microns in thickness and lacks regenerative capacity. Descemet’s membrane, synthesized by corneal endothelial cells, measures around 10–12 microns in adults and also does not regenerate after injury. The innermost layer, the endothelium, is composed of a single layer of flattened cells that play a vital role in maintaining corneal deturgescence through active ionic transport mechanisms, thereby ensuring tissue transparency [4].
1.3. Corneal Innervation and Sensation
The cornea receives extensive sensory innervation, accompanied by a smaller proportion of autonomic fibers. These nerve fibers originate primarily from the ophthalmic branch of the trigeminal nerve. At the corneal periphery, both unmyelinated C fibers and myelinated Aδ fibers penetrate the stroma. As they extend toward the central cornea, they lose their myelin sheath, a physiological adaptation that contributes to maintaining corneal transparency [2]. Corneal innervation plays a vital role in promoting tear secretion, blinking reflexes, and the release of trophic factors necessary for epithelial maintenance. When corneal nerves are damaged, these processes are disrupted, leading to reduced corneal sensitivity, persistent epithelial defects, and, in severe cases, corneal perforation [5]. Approximately 20% of the sensory fibers are mechanoreceptors that detect mechanical stimuli and transmit sharp pain signals through thin myelinated Aδ fibers. Around 70% are polymodal nociceptors that respond to chemical mediators (e.g., acetylcholine, prostaglandins, bradykinin), thermal, and mechanical stimuli via slow-conducting unmyelinated C fibers [6]. The remaining 10% are cold thermoreceptors that are activated by tear film evaporation or exposure to cold air or solutions and signal through both Aδ and C fibers [7].
1.4. The Involvement of Insulin in the Corneal Healing Process
Insulin is a hormone with an essential role in cellular metabolism, closely related to the insulin growth factor (IGF), which is capable of stimulating keratinocyte migration. At the ocular surface, insulin stimulates the proliferation and migration of corneal epithelial cells and the synthesis of growth factors involved in tissue regeneration and significantly reduces inflammatory biomarkers [8]. The mechanism by which insulin contributes to the regeneration of the corneal epithelium is not fully understood, and the optimal dose has not yet been established in studies. Bartlett et al. applied eye drops of insulin in concentrations of 100 IU/mL to eight healthy eyes and demonstrated the safety of topical insulin in the form of drops [9].
The Mackie classification (Table 1) stages the severity of the lesions as follows [10].

Table 1.
The Mackie classification [10].
2. Materials and Methods
For this review, a systematic search was performed in PubMed, Embase, Scopus, and Web of Science Core Collection using the terms “Insulin Eye Drops” OR “Topical Insulin” AND “Neurotrophic Keratopathy” OR “Corneal Ulcer”. A time restriction was applied to include only studies published between 1 January 2020 and 1 January 2025. This period was chosen to capture the most recent clinical evidence and methodological advances in the use of topical insulin for neurotrophic keratopathy. The final literature search was conducted on 31 March 2025, as predefined in the registered review protocol (PROSPERO ID: CRD420251043823).
Only peer-reviewed, full-text articles published in English and involving human subjects were considered. Filters were applied to exclude animal studies, in vitro experiments, editorials, and non-peer-reviewed publications.
- PubMed: The search yielded 108 records. After removing 18 duplicates, 90 titles and abstracts were screened. Of these, 35 full-text articles were assessed, and 12 studies were included in the final qualitative synthesis based on predefined eligibility criteria.
- Embase (via Ovid): The Ovid MEDLINE search initially yielded 61 results. After applying filters and reviewing the exportable records, 30 valid articles were retrieved and included for screening based on title and abstract. The discrepancy between the initial search count and the number of records analyzed was due to platform limitations and the removal of duplicate or incomplete entries. Fifteen studies were considered.
- Scopus: The initial search identified 63 articles. After manual screening of titles and abstracts, 23 potentially relevant articles were selected. Cross-referencing with the PubMed dataset led to the exclusion of 12 duplicates, leaving 11 unique studies for consideration.
- Web of Science Core Collection: A total of 17 studies were initially retrieved. After applying the eligibility criteria, specifically targeting studies addressing topical insulin in NK, 6 studies were excluded due to a lack of direct reference to NK, focus on unrelated pathologies, or use of animal models. Ultimately, 11 studies were deemed eligible for inclusion. One case report (study no. 8) was retained despite not explicitly diagnosing NK, as the clinical context of long-standing, refractory corneal ulcer suggested a neurotrophic etiology. This inclusion aligned with the review protocol, which allowed for clinically inferred relevance even when terminology varied.
All databases were searched independently. The results were deduplicated and screened according to standardized inclusion and exclusion criteria. The overall search and selection process adhered to PRISMA guidelines (see also Table 2, PRISMA flow summary).

Table 2.
PRISMA flow summary.
2.1. Inclusion Criteria
- Clinical studies (RCTs, observational, case series) evaluating the effect of topical insulin on neurotrophic keratopathy.
- Preclinical studies (in vitro, animals) exploring the mechanism of action of insulin in corneal healing.
- Meta-analyses and systematic reviews synthesizing relevant data on ophthalmic insulin.
- Clinical guidelines mentioning insulin as a possible therapeutic option.
2.2. Exclusion Criteria
- Studies analyzing systemic insulin without ophthalmological applicability.
- Studies on other corneal conditions not relevant to neurotrophic keratopathy.
- Letters to the editor, opinions, and articles without peer review.
- The literature search and study selection followed PRISMA guidelines (see Figure 1), and no automated screening tools (e.g., Rayyan, Covidence) were used.
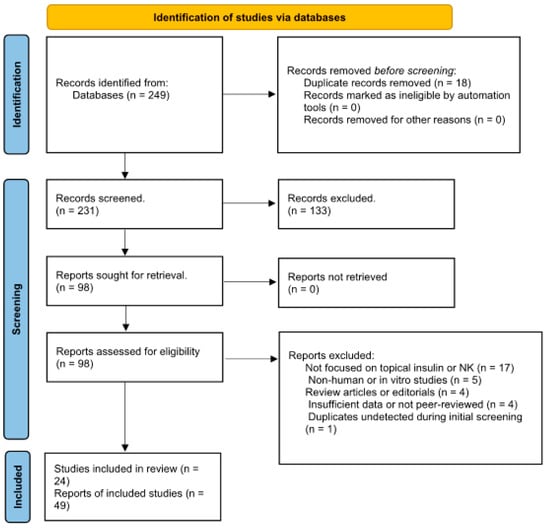 Figure 1. PRISMA flow diagram for (“Insulin Eye Drops” OR “Topical Insulin”) AND (“Neurotrophic Keratopathy” OR “Corneal Ulcer”).
Figure 1. PRISMA flow diagram for (“Insulin Eye Drops” OR “Topical Insulin”) AND (“Neurotrophic Keratopathy” OR “Corneal Ulcer”).
2.3. Study Selection Process
All search results were reviewed manually by the first author, with titles and abstracts screened for relevance. Full texts were then assessed against eligibility criteria. Duplicates were removed. Discrepancies were resolved by consensus. No automation tools (e.g., Rayyan or Covidence) were used.
2.4. Data Extraction
Key data were extracted from the included studies into structured tables. Extracted variables included the following: study design, number of patients, insulin concentration and administration frequency, treatment duration, primary outcomes (e.g., healing time, adverse effects), and conclusions.
2.5. Risk of Bias Assessment
Due to the heterogeneity and small size of most included studies, no formal risk-of-bias tool (e.g., ROBINS-I or Cochrane Risk of Bias Tool) was applied. However, study limitations, including sample size and lack of control groups, were critically discussed in Section 4.
The risk of bias in the included non-randomized studies was assessed using the ROBINS-I tool. This evaluation systematically addressed the seven key domains outlined by the ROBINS-I framework: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. Most studies demonstrated a moderate risk of bias, particularly due to small sample sizes, lack of control groups, and potential confounding variables not being adequately accounted for. The classification of interventions and outcome measurement domains generally presented low risk, indicating clear definitions of interventions and consistent reporting of clinical endpoints. However, concerns remained regarding selection bias and the potential for selective outcome reporting in studies with limited transparency or short follow-up periods. No automation tools were used in the risk of bias assessment process, and evaluations were conducted manually.
3. Results
3.1. Study Selection
Following comprehensive searches across PubMed, Embase (via Ovid), Scopus, and Web of Science, a total of 92 records were initially identified as potentially eligible, based on predefined inclusion criteria. After title and abstract screening and the removal of duplicates, 49 studies were retained and reported in the PRISMA flow diagram as eligible for full-text evaluation. However, a detailed full-text review and data extraction process revealed further duplications, overlapping patient cohorts, and instances of redundant reporting across multiple publications. Consequently, 24 unique and methodologically eligible studies were ultimately included in the final qualitative synthesis (Table 3).

Table 3.
Topical insulin studies—Structured table from PubMed, Embase, Word of Science, and Web of Science with 32 titles analyzed: one animal model study; 31 human studies, of which 14 are reviews, 6 are case reports, 10 are clinical studies, and one is a meta-analysis.
3.2. Comparison (C): Standard Treatments for Neurotrophic Keratopathy (NK)
A comparative analysis of included studies highlights the promising efficacy of topical insulin in the treatment of neurotrophic keratopathy (NK). Most of the clinical studies and case reports reviewed report high rates of re-epithelialization and improvement of corneal transparency without significant adverse effects [14]. Reported studies indicate a healing rate between 75% and 90% in patients treated with topical insulin (Table 4). No major adverse effects have been reported following the use of topical insulin [21]. The treatment duration ranged from 7 days to 8 weeks, depending on the severity of corneal lesions and response to therapy [15]. Studies suggest that the combination treatment (insulin + Hyper-CL therapeutic lenses) improves absorption and clinical effects. One possible explanation for the improved outcomes observed with insulin and therapeutic lenses is enhanced bioavailability and prolonged contact time with the corneal epithelium, leading to increased cellular uptake and sustained release of insulin over time [20]. Neurotrophic keratopathy (NK) is a challenging condition characterized by impaired corneal healing due to sensory nerve damage. Conventional treatments aim to restore corneal integrity, reduce inflammation, and promote epithelial regeneration [2,15].

Table 4.
Comparative analysis of clinical Studies on topical insulin in neurotrophic keratopathy.
Large-scale randomized clinical trials are needed to establish the optimal dose and exact duration of treatment. Future research should explore possible synergies with other therapies to maximize clinical benefits. In conclusion, topical insulin emerges as a promising and affordable treatment for NK, with a high safety profile and favorable outcomes in corneal epithelial regeneration.
3.3. Comparison of Topical Insulin vs. Standard Treatments for Neurotrophic Keratopathy
Topical insulin has emerged as a novel therapeutic option for neurotrophic keratopathy (NK), particularly in patients who have shown suboptimal response to conventional therapies such as artificial tears, punctal occlusion, therapeutic contact lenses, or autologous serum (Table 5). While standard treatments aim to restore surface hydration and reduce epithelial trauma, they often fail to stimulate regeneration of the corneal epithelium. In contrast, topical insulin appears to enhance epithelial proliferation and wound healing by activating insulin and IGF-1 receptors, promoting epithelial migration and mitogenesis.

Table 5.
Comparison of topical insulin vs. standard treatments.
The reviewed studies suggest that topical insulin may offer comparable or even superior outcomes in terms of epithelial defect resolution and time to healing, especially in moderate to severe NK (Mackie stages 2–3). However, direct comparative trials are scarce, and most included studies lacked control groups. Despite these limitations, the preliminary evidence indicates that topical insulin could complement or, in select cases, replace conventional treatments, especially when regenerative stimulation is the primary therapeutic goal.
3.4. Characteristics of Included Studies
The included studies collectively reported on a total of 94 patients treated with topical insulin for neurotrophic keratopathy. Insulin concentrations ranged from 1 IU/mL, being the most frequently used dosage, typically administered three to four times daily. Treatment durations varied from 7 days to 12 weeks, depending on the severity of the epithelial defect and the patient’s clinical response. It is important to emphasize that the 100 IU/mL concentration was used exclusively to evaluate ocular tolerability and potential adverse effects rather than therapeutic efficacy.
3.5. Efficacy Outcomes
Most clinical studies reported significant rates of re-epithelialization, ranging from 75% to 90% after 6–8 weeks of treatment. Improvements in corneal transparency, ocular surface integrity, and patient-reported symptoms were consistently noted. One study [21] found that combining insulin with Hyper-CL therapeutic contact lenses accelerated epithelial recovery compared to insulin alone.
3.6. Safety and Tolerability
No major adverse events were reported across the included studies. Mild transient discomfort upon installation was noted in two patients but did not lead to discontinuation of therapy. None of the reviewed studies documented long-term adverse effects such as corneal neovascularization, fibrosis, or decreased transparency, although most follow-up periods were limited to ≤8 weeks.
The therapeutic outcomes reported across the included studies were predominantly descriptive, reflecting the observational or exploratory nature of the available literature. Most studies involved small sample sizes and lacked control groups, limiting the use of formal effect estimates or statistical comparisons. Nevertheless, consistent improvements in corneal epithelial healing were documented in response to topical insulin, either as monotherapy or in combination with therapeutic contact lenses. The table below summarizes key findings from each study, including intervention characteristics, treatment duration, and re-epithelialization rates. While no confidence intervals or comparative statistics were available, the reported efficacy and absence of major adverse effects suggest a promising clinical utility of topical insulin in neurotrophic keratopathy.
3.7. Limitations in Evidence
While preliminary results are encouraging, the small sample sizes, lack of control groups, and absence of randomized controlled trials (RCTs) limit the generalizability of the findings. Additionally, the short duration of follow-up in all studies prevents firm conclusions regarding the long-term safety and efficacy of topical insulin therapy. No sensitivity analysis was performed due to the descriptive nature and limited number of included studies.
4. Discussion
The largest clinical trials of ophthalmic insulin have involved 37 patients, highlighting the need for research on larger sample sizes [14,15]. Case studies examine the effects of insulin on 1–4 patients [13]. The standard dose used in most clinical trials is 1 unit/mL, administered 3 to 4 times daily [15]. The duration of treatment varies between 6 and 12 weeks, with most studies reporting epithelial healing after 6–8 weeks [21]. Patients treated with insulin and therapeutic lenses had faster results (6 weeks vs. 8–10 weeks in insulin therapy alone) [15]. There are no long-term clinical studies yet (more than 3 months of follow-up) [14]. Although most studies included in this review report high rates of re-epithelialization and the absence of major adverse effects associated with the use of topical insulin, the duration of post-treatment follow-up is limited. The longest follow-up period identified was 2 months (8 weeks) in the study by Khilji et al. (2023), in which a patient with post-herpetic neurotrophic keratopathy (NK) was treated and followed for 2 months [13].
In other studies, follow-up durations ranged from 7–45 days in the study of Soares et al. (2022) [14], 6 weeks in Eleiwa et al. (2024) [15] and Eleiwa et al. (2025) [18], and 8 weeks in the studies by Mancini et al. (2024) [20] and Giannaccare et al. (2024) [21].
The lack of long-term studies (more than 3–6 months) limits the ability to assess late effects of topical insulin, such as possible corneal structural changes over time, risk of angiogenesis or corneal fibrosis, and potential decrease in efficacy with prolonged use [14,15,20].
Current data suggest that topical insulin is safe and well tolerated, but studies with extended follow-up (>6 months–1 year) are needed to confirm the safety profile and possible late adverse effects [20,21]. Future studies should include detailed clinical follow-up and histological analyses to determine long-term effects on the cornea [14,17]. The evidence reviewed in this study suggests that topical insulin is a promising therapy for promoting corneal healing in neurotrophic keratopathy [14,15,21]. While clinical studies have demonstrated significant epithelial regeneration, optimal dosing and long-term safety require further investigation [14,17]. Compared to conventional treatments such as autologous serum or growth factor therapy, insulin eye drops provide a cost-effective alternative with the potential for widespread application [11,21]. Large-scale, randomized controlled trials are necessary to establish optimal dosing, assess long-term safety, and determine the role of topical insulin as a standardized therapy for neurotrophic keratopathy [43].
5. Study Limitations and Future Research Prospects
Although the current results suggest that topical insulin may represent a promising therapeutic strategy for neurotrophic keratopathy (NK), there are still insufficiently explored aspects, especially regarding the safety of long-term use and potential adverse effects [14,17]. One of the main concerns is related to the possibility of corneal angiogenesis and stromal fibrosis, phenomena that could compromise corneal transparency and, implicitly, visual function [11,43].
5.1. Possibility of Corneal Angiogenesis
The cornea is naturally an avascular tissue, and maintaining this status is essential for visual function. Insulin is known as an anabolic factor with an essential role in cellular metabolism and, in certain pathological contexts, has been associated with the regulation of the expression of vascular endothelial growth factor (VEGF), a cytokine essential in neovascularization processes [44]. In diabetic patients, hyperinsulinemia has been correlated with increased levels of VEGF, thus contributing to proliferative diabetic retinopathy [45]. This observation raises the question of whether prolonged use of insulin at the corneal level could indirectly stimulate angiogenesis, thus affecting corneal transparency. However, in the clinical studies analyzed, no such effects were reported. It should be noted, however, that the maximum duration of patient monitoring was approximately 8 weeks, which does not allow sufficient assessment of the risk of neovascularization in the long term. Therefore, prospective studies with extensive monitoring are needed to determine whether topical insulin can have an angiogenic effect in the cornea or whether it remains safe for chronic use [11,17].
5.2. Possibility of Corneal Fibrosis
Another aspect that requires further investigation is the potential risk of stromal fibrosis. Insulin plays a fundamental role in cellular proliferation and migration and interacts with key modulators such as transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF), both of which are deeply involved in extracellular matrix remodeling and fibrogenesis [8,46]. If topical insulin excessively stimulates the activity of corneal fibroblasts, it could result in irregular collagen deposition, compromising corneal transparency and reducing visual acuity. Although no direct clinical evidence currently supports this hypothesis, the absence of detailed histological assessments in current studies restricts our understanding of insulin’s effects on the stromal layer. Therefore, future investigations should include advanced histological and imaging techniques such as in vivo confocal microscopy and anterior segment OCT to monitor stromal remodeling after prolonged insulin use [17].
5.3. Limitations of the Current Studies
Although current data support the efficacy of topical insulin in promoting corneal epithelial regeneration, it is important to acknowledge several methodological limitations of the reviewed studies. The maximum post-treatment follow-up reported was 8 weeks, as seen in studies such as those by Khilji et al. (2023) [13] and Mancini et al. (2024) [20], which is insufficient to evaluate potential long-term outcomes. Most clinical studies included relatively small cohorts, typically ranging from 8 to 21 patients, which reduces the statistical power and generalizability of findings [14,21]. Furthermore, much of the current evidence is derived from observational and retrospective analyses, with a notable lack of randomized controlled trials to validate the efficacy and safety of insulin therapy for neurotrophic keratopathy [17,19]. In addition, considerable heterogeneity exists in insulin dosing regimens (ranging from 1 IU/mL to 100 IU/mL), frequency of administration, and treatment duration, making it challenging to establish a standardized therapeutic protocol [15,20].
5.4. Directions for Future Research
The review of the therapeutic potential of insulin eye drops in neurotrophic keratopathy adds significant value to the existing literature by providing an in-depth overview of the current state of insulin eye drop research. While several narrative and systematic reviews have addressed the role of growth factors and autologous serum in the management of neurotrophic keratopathy (NK), few have focused exclusively on topical insulin as a therapeutic agent. This review adds value to the existing literature by providing a focused and updated synthesis of clinical and preclinical evidence published between 2020 and 2025, examining all mechanisms of action as well as therapeutic outcomes associated with topical insulin in NK. The review critically discusses methodological limitations of current studies, such as small sample sizes, lack of control groups, and the absence of randomized controlled trials (RCTs). This critical perspective is essential for guiding future research directions and creating study designs in this area. Highlighting the short duration of follow-up in existing studies, the review raises important questions about the long-term safety and efficacy of topical insulin therapy. This focus on long-term outcomes is often overlooked in other reviews, making this work particularly valuable to clinicians and researchers. The review examines variations in dose, frequency of administration, and treatment duration across studies. Addressing these inconsistencies paves the way for the development of standardized treatment protocols, which are crucial for clinical practice and future research. The review not only summarizes existing knowledge but also identifies gaps in the literature, encouraging further investigation. This is vital for advancing the field and ensuring that future studies build on the findings and limitations highlighted in this review. In summary, this review contributes to the existing body of literature by providing a comprehensive, critical, and forward-looking review of the therapeutic potential of insulin eye drops in neurotrophic keratopathy, thereby increasing understanding and guiding future research efforts. Thus, the present review contributes a targeted and critical perspective to the evolving field of ocular surface regeneration, with the aim of supporting the development of standardized therapeutic protocols involving topical insulin.
To strengthen the position of topical insulin as a safe and effective therapy for NK, future studies should include randomized clinical trials (RCTs) with larger patient samples to validate the efficacy and safety of the therapy [17]. Extended follow-up (>6 months–1 year) is also necessary to detect possible long-term adverse effects, including the risk of neovascularization or fibrosis [15]. Histological and imaging studies are recommended to evaluate the effects of insulin on corneal stromal architecture [20]. Additionally, comparison with standard therapies—such as nerve growth factor (NGF), autologous serum, or corneal neurotization—should be pursued to identify the specific advantages and limitations of each therapeutic approach [21].
6. Conclusions
In conclusion, topical insulin represents an innovative and affordable therapeutic option for neurotrophic keratopathy, offering a cure rate between 75% and 90%, according to available studies [14]. However, the safety of long-term use remains uncertain, and potential effects on angiogenesis and fibrogenesis need to be explored further [44]. Until these findings are confirmed through randomized controlled trials, topical insulin should be applied in clinical practice with caution and close patient monitoring to ensure maximum therapeutic benefit and minimize risk [20].
Author Contributions
Paper conceptualization R.S., M.A.M., and S.I.; methodology E.U. and Ș.O.; resources M.B. and N.A.; review and editing, A.-G.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| BCVA | best corrected visual acuity |
| CIPA | congenital insensitivity to pain |
| IGF | insulin growth factor |
| NGF | nerve growth factor |
| NK | neurotrophic keratitis |
| OCT | optical coherence tomography |
| PDGF | platelet-derived growth factor |
| RCTs | randomized clinical trials |
| TGF-β | transforming growth factor-beta |
| VEGF | vascular endothelial growth factor |
References
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Bonini, S.; Lambiase, A.; Rama, P.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000, 107, 1347–1351; discussion 1351–1342. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef]
- MacIver, M.B.; Tanelian, D.L. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J. Neurosci. 1993, 13, 4511–4524. [Google Scholar] [CrossRef]
- Zagon, I.S.; Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch. Ophthalmol. 2007, 125, 1082–1088. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Slusser, T.G.; Turner-Henson, A.; Singh, K.P.; Atchison, J.A.; Pillion, D.J. Toxicity of insulin administered chronically to human eye in vivo. J. Ocul. Pharmacol. 1994, 10, 101–107. [Google Scholar] [CrossRef]
- Gurnani, B.; Feroze, K.B.; Patel, B.C. Neurotrophic Keratitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Krolo, I.; Behaegel, J.; Termote, K.; de Bruyn, B.; De Schepper, M.; Oellerich, S.; Dhubhghaill, S.N. The role of topical insulin in ocular surface restoration: A review. Surv. Ophthalmol. 2024, 69, 805–817. [Google Scholar] [CrossRef]
- Jaworski, M.; Lorenc, A.; Leszczyński, R.; Mrukwa-Kominek, E. Topical Insulin in Neurotrophic Keratopathy: A Review of Current Understanding of the Mechanism of Action and Therapeutic Approach. Pharmaceutics 2024, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Khilji, M.; Tanveer, S.; Khan, F.Z.; Yazdan, D.A.; Khilji, A. Neurotrophic Keratopathy and Topical Insulin Therapy: A Case Report. Cureus 2023, 15, e46242. [Google Scholar] [CrossRef]
- Soares, R.; Arêde, C.; Sousa Neves, F.; da Silva Fernandes, J.; Cunha Ferreira, C.; Sequeira, J. Topical Insulin-Utility and Results in Refractory Neurotrophic Keratopathy in Stages 2 and 3. Cornea 2022, 41, 990–994. [Google Scholar] [CrossRef]
- Eleiwa, T.K.; Khater, A.A.; Elhusseiny, A.M. Topical insulin in neurotrophic keratopathy after diabetic vitrectomy. Sci. Rep. 2024, 14, 10986. [Google Scholar] [CrossRef]
- Le Nguyen, M.H.; Naoum, M.S.; Andre, C.; Lethier, L.; Limat, S.; Fagnoni-Legat, C.; Guillaume, Y.; Gauthier, A.S. Physicochemical and microbiological stability of insulin eye drops in an artificial tear vehicle used in the treatment of refractory neurotrophic keratopathy. J. Fr. Ophtalmol. 2022, 45, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Wouters, C.; Saelens, I.; Delbeke, H. Topical Insulin for Neurotrophic-Related Epithelial Defects: Where do We Stand? A Systematic Review. J. Curr. Ophthalmol. 2024, 36, 9–22. [Google Scholar] [CrossRef]
- Eleiwa, T.; Farid, M.F.; Elhusseiny, A.M. Topical insulin in pediatric neurotrophic keratopathy associated with CIPA syndrome. J. Aapos 2025, 29, 104120. [Google Scholar] [CrossRef] [PubMed]
- Moin, K.A.; Pandiri, S.; Manion, G.N.; Brown, A.H.; Moshirfar, M.; Hoopes, P.C. The Utilization of Topical Insulin for Ocular Surface Diseases: A Narrative Review. Cureus 2024, 16, e62065. [Google Scholar] [CrossRef]
- Mancini, A.; Mancini, M.; Taloni, A.; Bifezzi, L.; Romeo, M.A.; Rijillo, L.; Verdiglione, M.; Scorcia, V.; Aragona, P.; Giannaccare, G. Topical insulin used alone or in combination with drug-depository contact lens for refractory cases of neurotrophic keratopathy. Am. J. Ophthalmol. Case Rep. 2024, 36, 102227. [Google Scholar] [CrossRef]
- Giannaccare, G.; Coco, G.; Rossi, C.; Borselli, M.; Lucisano, A.; Vaccaro, S.; Verdiglione, M.; Scorcia, V. Combined Use of Therapeutic Hyper-CL Soft Contact Lens and Insulin Eye Drops for the Treatment of Recalcitrant Neurotrophic Keratopathy. Cornea 2024, 43, 120–124. [Google Scholar] [CrossRef]
- Serrano-Giménez, R.; Contreras-Macías, E.; García-Bernal, A.; Fobelo-Lozano, M.J. Insulin eye drops for treating corneal ulcer in a non-diabetic patient: Regarding a case. Farm. Hosp. 2020, 44, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Font, C.S.; Cortina, M.S. Neurotrophic keratopathy: Update in diagnosis and management. Indian J. Ophthalmol. 2025, 73, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Arias, D.; Locatelli, E.V.T.; Sepulveda-Beltran, P.A.; Mangwani-Mordani, S.; Navia, J.C.; Galor, A. Recent United States Developments in the Pharmacological Treatment of Dry Eye Disease. Drugs 2024, 84, 549–563. [Google Scholar] [CrossRef]
- Mehrez, S.M.; Ghoraba, M.E.; Hasby, H.A.; Khater, M.M. Comparative study between topical insulin, autologous serum, and honey-based eye drops as adjunctive treatment in resistant corneal ulcer. Delta J. Ophthalmol. 2024, 25, 7–13. [Google Scholar] [CrossRef]
- Vera-Duarte, G.R.; Jimenez-Collado, D.; Kahuam-López, N.; Ramirez-Miranda, A.; Graue-Hernandez, E.O.; Navas, A.; Rosenblatt, M.I. Neurotrophic keratopathy: General features and new therapies. Surv. Ophthalmol. 2024, 69, 789–804. [Google Scholar] [CrossRef]
- Antunes-Foschini, R.; Adriano, L.; Murashima, A.d.A.B.; Barbosa, A.P.; Nominato, L.F.; Dias, L.C.; Fantucci, M.Z.; Garcia, D.M.; Alves, M.; Rocha, E.M. Limitations and advances in new treatments and future perspectives of corneal blindness. Arq. Bras. Oftalmol. 2021, 84, 282–296. [Google Scholar] [CrossRef]
- Moreker, M.R.; Thakre, N.; Gogoi, A.; Bhandari, R.P.; Patel, R.C. Insulin eye drops for neurotrophic keratitis. Indian J. Ophthalmol. 2023, 71, 2911–2912. [Google Scholar] [CrossRef]
- Diaz-Valle, D.; Burgos-Blasco, B.; Gegundez-Fernandez, J.A.; Garcia-Caride, S.; Puebla-Garcia, V.; Peña-Urbina, P.; Benitez-Del-Castillo, J.M. Topical insulin for refractory persistent corneal epithelial defects. Eur. J. Ophthalmol. 2021, 31, 2280–2286. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Song, W.; Cheng, Y.; Gao, Y.; Xie, L.; Huang, M.; Yan, X. Insulin eye drops improve corneal wound healing in STZ-induced diabetic mice by regulating corneal inflammation and neuropeptide release. BMC Ophthalmol. 2024, 24, 155. [Google Scholar] [CrossRef]
- Singh, M.; Pawar, M. Efficacy of Topical Insulin Therapy for Chronic Trophic Ulcers in Patients with Leprosy: A Randomized Interventional Pilot Study. Adv. Ski. Wound Care 2020, 33, 1–6. [Google Scholar] [CrossRef]
- Lacorzana, J.; Neo, Y.N.; Maubon, L.; Sibley, D.; Ahmad, S. Current and Emerging Therapeutic Strategies for the Management of Neurotrophic Keratitis. Drugs 2025, 85, 283–291. [Google Scholar] [CrossRef]
- Jabbour, S.; Ashton, C.; Balal, S.; Kaye, A.; Ahmad, S. The management of neurotrophic keratitis. Curr. Opin. Ophthalmol. 2021, 32, 362–368. [Google Scholar] [CrossRef]
- Atikah, A.; Suzana, M.; Wan Haslina, W.A.H.; Norshamsiah, M.D.; Mushawiahti, M.; Birinder, K.S.S.; Tang, S.F.; Bastion, M.L.C. Randomised controlled trial on effects of topical insulin compared to artificial tears and normal saline on tear inflammatory mediator levels and clinical parameters in diabetics with dry eye disease. Cont. Lens Anterior Eye 2025, 48, 102346. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Singh, R.B.; Dana, R. Advances in the Medical Management of Neurotrophic Keratitis. Semin. Ophthalmol. 2021, 36, 335–340. [Google Scholar] [CrossRef]
- Ruiz-Lozano, R.E.; Hernandez-Camarena, J.C.; Loya-Garcia, D.; Merayo-Lloves, J.; Rodriguez-Garcia, A. The molecular basis of neurotrophic keratopathy: Diagnostic and therapeutic implications. A review. Ocul. Surf. 2021, 19, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Neurotrophic keratopathy: An updated understanding. Ocul. Surf. 2023, 30, 129–138. [CrossRef] [PubMed]
- Mester, A.; Gabriel, M.; Boldin, I.; Aminfar, H.; Woltsche, N.; Heidinger, A.; Guttmann, A.; Wintersteller, P.; Horwath-Winter, J. The Performance of Topical Insulin in Persistent Corneal Epithelial Defects and Persistent Corneal Ulcers—A Case Series. Curr. Eye Res. 2025, 50, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Roumeau, S.; Dutheil, F.; Sapin, V.; Baker, J.S.; Watson, S.L.; Pereira, B.; Chiambaretta, F.; Navel, V. Efficacy of treatments for neurotrophic keratopathy: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2623–2637. [Google Scholar] [CrossRef]
- Saucedo, J.; Lozano Feria, F.S.; Ramírez, A. Neurotrophic Keratitis Following Vitrectomy Surgery: A Case Report. Cureus 2025, 17, e77372. [Google Scholar] [CrossRef]
- Wajnsztajn, D.; Faraj, L.A.; Sanchez-Tabernero, S.; Solomon, A. Neurotrophic keratitis: Inflammatory pathogenesis and novel therapies. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 520–528. [Google Scholar] [CrossRef]
- Koay, S.Y.; Larkin, D.F.P. New Pharmacological Approaches for the Treatment of Neurotrophic Keratitis. Front. Pharmacol. 2022, 13, 796854. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zeppieri, M. Corneal Neurotization. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Escudero, C.A.; Herlitz, K.; Troncoso, F.; Guevara, K.; Acurio, J.; Aguayo, C.; Godoy, A.S.; González, M. Pro-angiogenic Role of Insulin: From Physiology to Pathology. Front. Physiol. 2017, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Ross, C.; Baker, J.; Alfuraih, S.; Shamloo, K.; Sharma, A. Diabetes-Associated Hyperglycemia Causes Rapid-Onset Ocular Surface Damage. Invest. Ophthalmol. Vis. Sci. 2023, 64, 11. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, N.; Horch, R.E.; Schmid, R.; Ostendorf, D.; Peddi, A.; Promny, T.; Boos, A.M.; Kengelbach-Weigand, A. Growth factors IGF-1 and KGF and adipose-derived stem cells promote migration and viability of primary human keratinocytes in an in vitro wound model. Front. Med. 2025, 12, 1516116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).