Omega-3 Polyunsaturated Fatty Acids (PUFAs) and Diabetic Peripheral Neuropathy: A Pre-Clinical Study Examining the Effect of Omega-3 PUFAs from Fish Oil, Krill Oil, Algae or Pharmaceutical-Derived Ethyl Esters Using Type 2 Diabetic Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Animals, Diets and Experimental Design
2.2. Endpoints Related to Nerve and Vascular Reactivity
2.3. Fatty Acid Composition
2.4. Data Analysis
3. Results
3.1. Status of Animal Weight, Blood Glucose, Omega-3 Index and Fatty Acid Composition in Serum and Liver
3.2. Effect on Motor and Sensory Nerve Conduction Velocity, Thermal and Cornea Sensitivity, Intraepidermal and Corneal Nerve Fiber Density
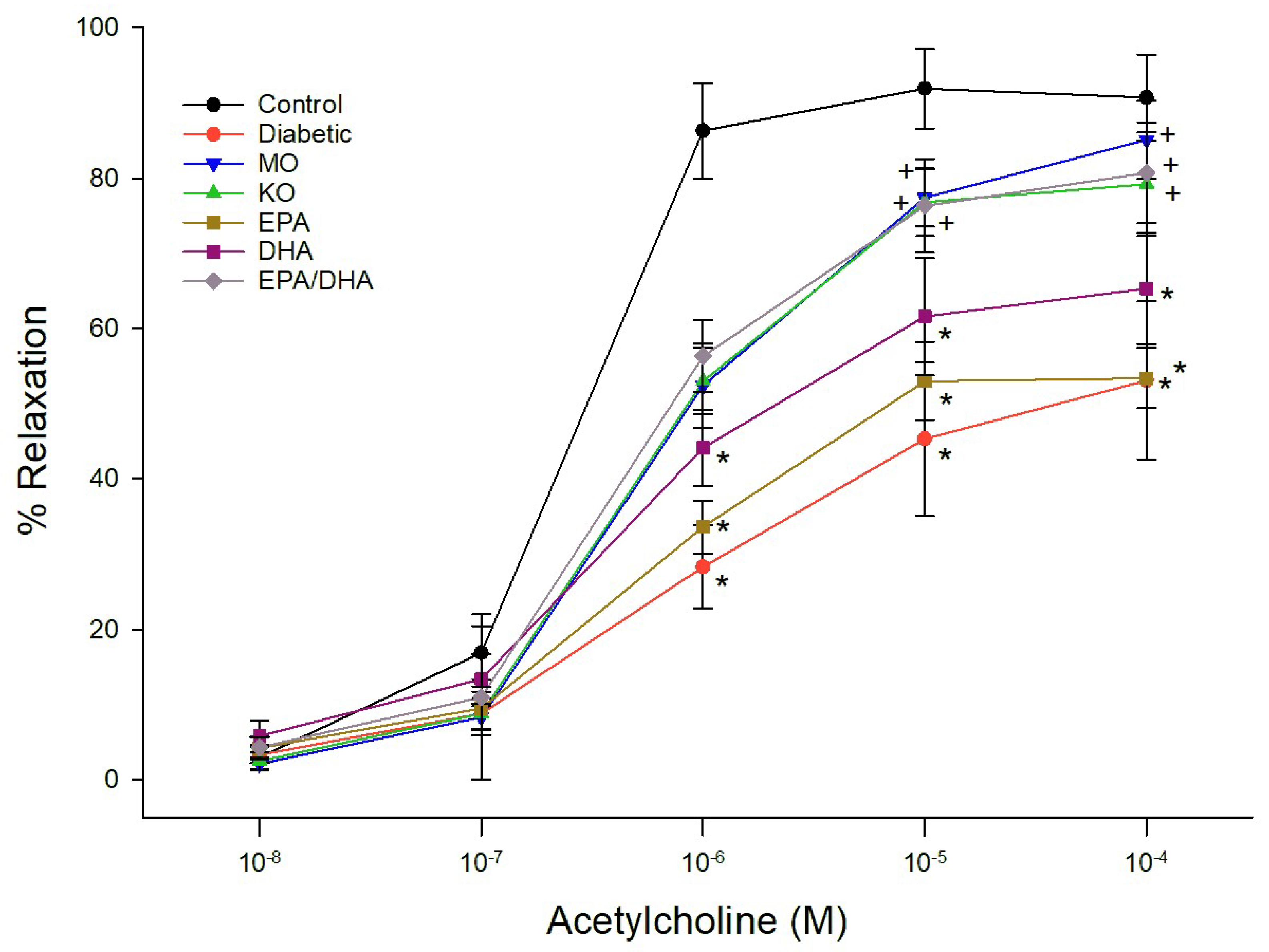
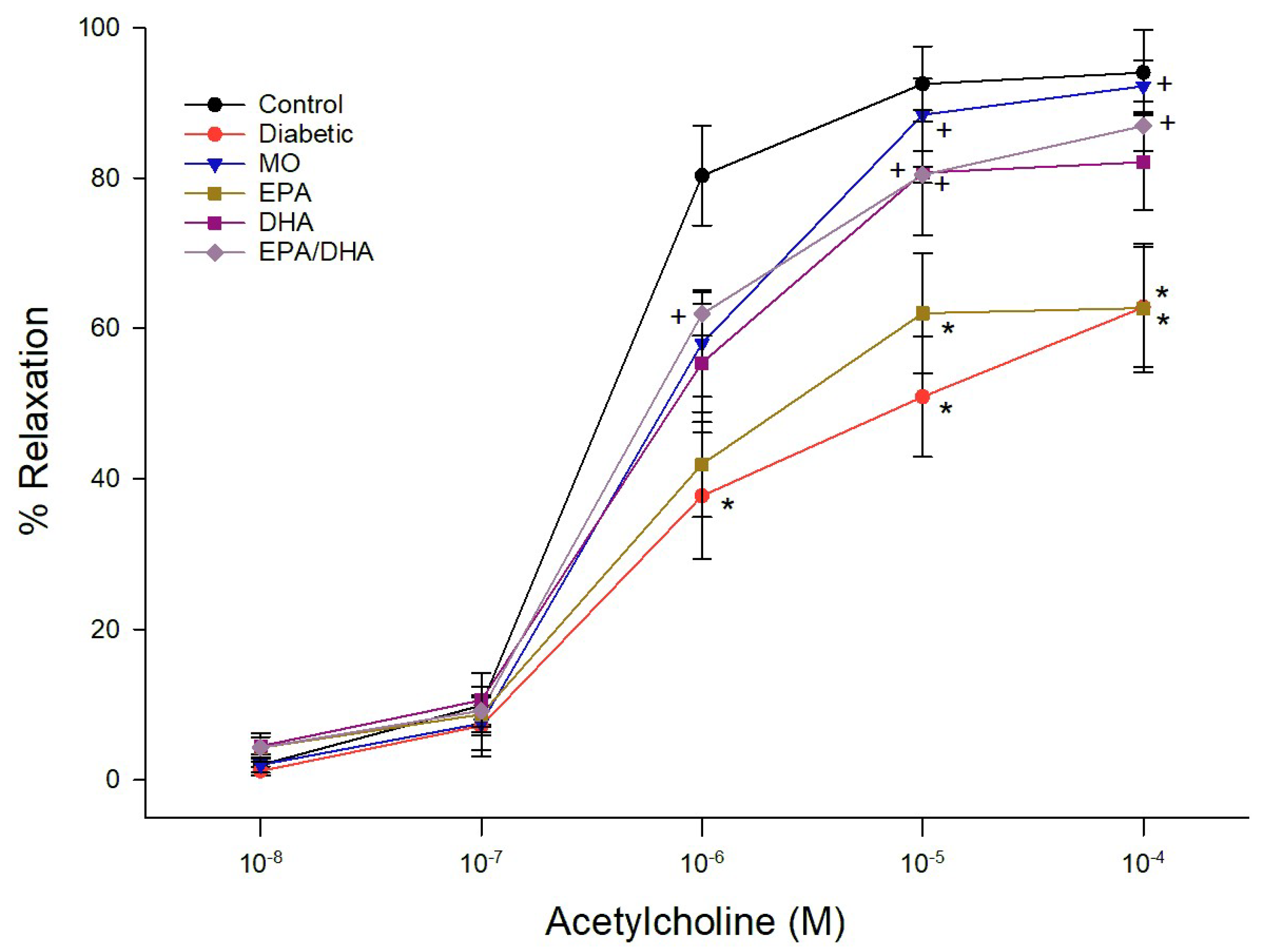
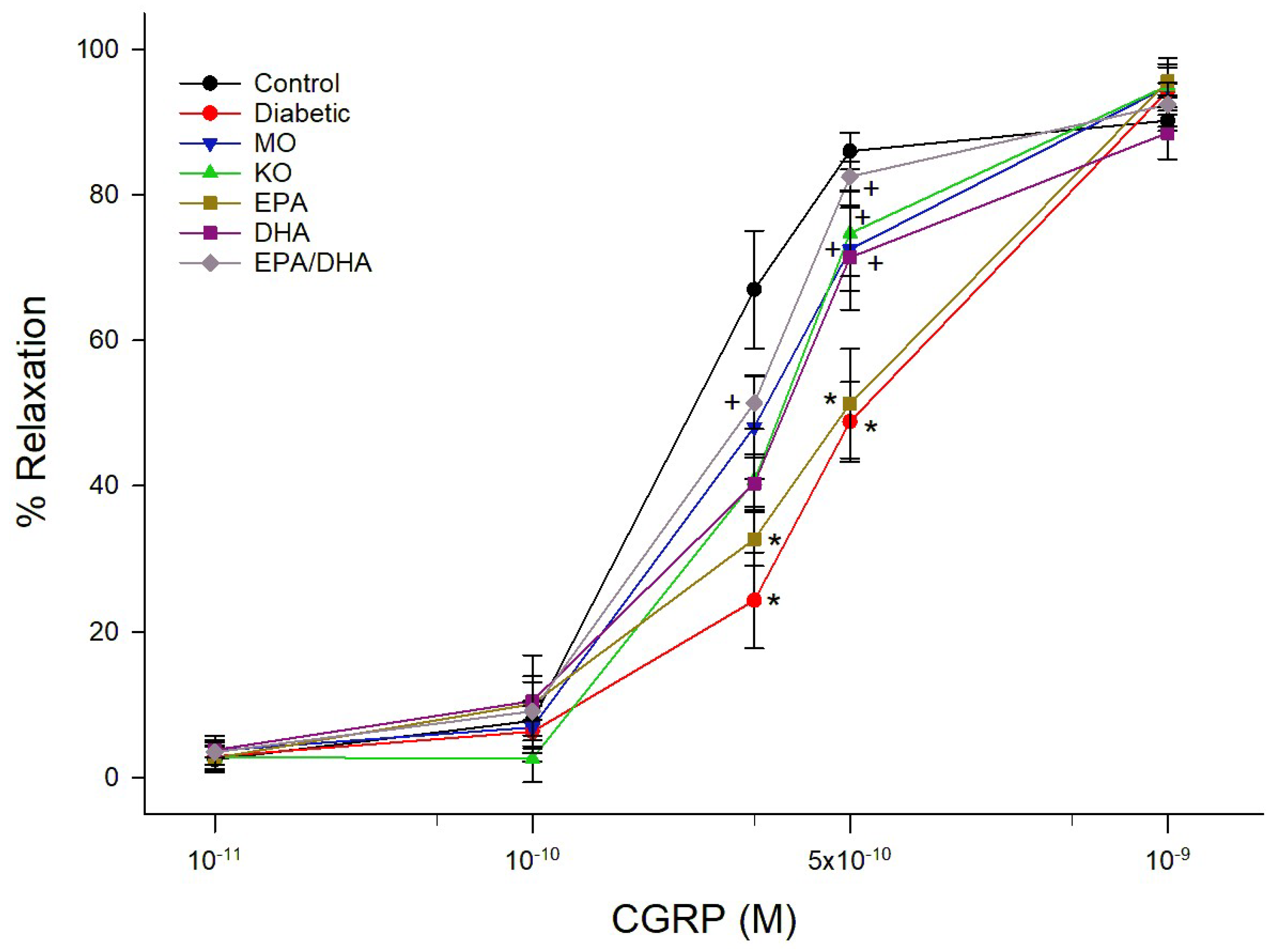
3.3. Effect on Vascular Reactivity to Acetylcholine and CGRP by Epineurial Arterioles Providing Blood Flow to the Sciatic Nerve
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dillon, B.R.; Ang, L.; Pop-Busui, R. Spectrum of diabetic neuropathy: New insights in diagnosis and treatment. Ann. Rev. Med. 2024, 75, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Sharma, R.; Kumar, R. An overview on diabetic neuropathy. Curr. Diabetes Rev. 2025, 21, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Lawal, Y.; Mshelia-Reng, R.; Omonua, S.O.; Odumodu, K.; Shuaibu, R.; Itanyi, U.D.; Abubakar, A.I.; Kolade-Yunusa, H.O.; David, Z.S.; Ogunlana, B.; et al. Predictors of peripheral neuropathy among persons with diabetes mellitus: A multicenter cross-sectional study. J. Am. Podiatr. Med. Assoc. 2025, 115, l22-053. [Google Scholar] [CrossRef]
- Yorek, M. Combination therapy is it in the future for successfully treating peripheral diabetic neuropathy? Front. Endocrinol. 2024, 15, 1357859. [Google Scholar] [CrossRef] [PubMed]
- Coppey, L.; Davidson, E.; Shevalye, H.; Obrosov, A.; Yorek, M. Effect of Early and Late Interventions with Dietary Oils on Vascular and Neural Complications in a Type 2 Diabetic Rat Model. J. Diabetes Res. 2019, 2019, 5020465. [Google Scholar] [CrossRef]
- Yorek, M.A. The Potential Role of Fatty Acids in Treating Diabetic Neuropathy. Curr. Diabetes Rep. 2018, 18, 86. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Shevalye, H.; Obrosov, A.; Yorek, M.A. Effect of Dietary Content of Menhaden Oil with or without Salsalate on Neuropathic Endpoints in High-Fat-Fed/Low-Dose Streptozotocin-Treated Sprague Dawley Rats. J. Diabetes Res. 2018, 2018, 2967127. [Google Scholar] [CrossRef]
- Coppey, L.; Davidson, E.; Shevalye, H.; Torres, M.E.; Yorek, M.A. Effect of dietary oils on peripheral neuropathy-related endpoints in dietary obese rats. Diabetes Metab. Syndr. Obes. 2018, 11, 117–127. [Google Scholar] [CrossRef]
- Qin, J.; Kurt, E.L.; Bassi, T.; Sa, L.; Xie, D. Biotechnological production of omega-3 fatty acids: Current status and future perspectives. Front. Microbiol. 2023, 14, 1280296. [Google Scholar] [CrossRef]
- Davidson, E.P.; Coppey, L.J.; Calcutt, N.A.; Oltman, C.L.; Yorek, M.A. Diet-induced obesity in Sprague-Dawley rats causes microvascular and neural dysfunction. Diabetes Metab. Res. Rev. 2010, 26, 306–318. [Google Scholar] [CrossRef]
- Coppey, L.J.; Shevalye, H.; Obrosov, A.; Davidson, E.P.; Yorek, A. Determination of peripheral neuropathy in high-fat diet fed low-dose streptozotocin-treated female C57Bl/6J mice and Sprague-Dawley rats. J. Diabetes Investig. 2018, 9, 1033–1040. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Christodoulides, S.; Christodoulou, P.; Kyriakou, T.C.; Patrikios, I.; Stephanou, A. Variability in the clinical effects of the omega-3 polyunsaturated fatty acids DHA and EPA in cardiovascular disease—Possible causes and future considerations. Nutrients 2023, 15, 4830. [Google Scholar] [CrossRef]
- Yorek, M.A.; Bohnker, R.R.; Dudley, D.D.; Spector, A.A. Comparative utilization of n-3 polyunsaturated fatty acids by cultured human Y-79 retinoblastoma cells. Biochim. Biophys. Acta-Lipids Lipid Metab. 1984, 795, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Yorek, M.A.; Coppey, L.J.; Gellett, J.S.; Davidson, E.P. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin gene-related peptide: Effect of streptozotocin-induced diabetes. Exp. Diabesity Res. 2004, 5, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert opinion on benefits of long-chain omega-3 fatty acids (DHA and EPA) in aging and clinical nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef]
- Gareri, P. Omega-3 long-chain polyunsaturated fatty acids in the elderly: A review. OBM Geriatrics 2022, 6, 198. [Google Scholar] [CrossRef]
- Weintraub, H. Update on marine omega-3 fatty acids: Management of dyslipidemia and current omega-3 treatment options. Atherosclerosis 2013, 230, 381–389. [Google Scholar] [CrossRef]
- Barmaki, H.; Nourazarian, A.; Yousefi, H.; Khalilnezhad, A.; Shahriyari, E.; Khaki-Khatibi, F. Dual mechanism of docosahexaenoic acid (DHA) in Alzheimer’s disease: PAD4 inhibition and autophagy stimulation. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef]
- Rezanka, T.; Kolouchova, I.; Matatkova, O. Alternative sources of omega-3 polyunsaturated fatty acids. Stud. Nat. Prod. Chem. 2020, 67, 123–159. [Google Scholar]
- Harris, W.S. The omega-3 index: Clinical utility for therapeutic intervention. Curr. Cardiol. Rep. 2010, 12, 503–508. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Jassal, D.S.; Ravandi, A.; Lehoczki, A. Dietary flaxseed: Cardiometabolic benefits and its role in promoting healthy aging. GeroScience 2025, 47, 2895–2923. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Sharma, J.; Khare, P. Recent advancements and strategies for omega-3 fatty acid production in yeast. J. Basic Microbiol. 2025, 12, e2400491. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic and meta-analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Bogi, L.H.; Kaprio, J.; Pietilainen, K.H. Dietary n-6 to n-3 fatty acid ratio is related to liver fat content independent of genetic effects: Evidence from the monozygotic co-twin control design. Clin. Nutr. 2020, 39, 2311–2314. [Google Scholar]
- Colletti, A.; Cravotto, G.; Citi, V.; Martelli, A.; Testai, L.; Cicero, A.F.G. Advances in technologies for highly active omega-3 fatty acids from krill oil: Clinical applications. Mar. Drugs 2021, 19, 306. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Yan, J.; Liu, M.; Yang, D.; Zhang, Y.; An, F. Efficacy and safety of omega-3 fatty acids in the prevention of cardiovascular disease: A systematic review and meta-analysis. Cardiovasc. Drugs Ther. 2024, 38, 799–817. [Google Scholar] [CrossRef]
- Gutstein, A.S.; Copple, T. Cardiovascular disease and omega-3s: Prescription products and fish oil dietary supplements are not the same. J. Am. Assoc. Nurse Pract. 2017, 29, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of omega-3 fatty acids in cardiovascular disease: The debate continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Effects of icosapent ethyl on total ischemic events: From REDUCE-IT. J. Am. Coll. Cardiol. 2019, 73, 2791–2802. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Libby, P.; Dawoud, H.; Bhatt, D.L.; Mason, R.P. Eicosapentaenoic acid improves nitric oxide bioavailability via changes in protein expression during inflammation. J. Am. Heart Assoc. 2024, 13, e034076. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.M.M.; Oliveira, M.V.B.; Quesada, K.; dos Santos Haber, J.F.; Tofano, R.J.; Rubira, C.J.; Zutin, T.L.M.; Direito, R.; Pereira, E.S.B.M.; de Oliveira, C.M.; et al. Assessing omega- therapy and its cardiovascular benefits: What about icosapent ethyl? A systematic review and meta-analysis. Pharmaceuticals 2025, 18, 601. [Google Scholar] [CrossRef]
- Coppey, L.J.; Davidson, E.P.; Obrosov, A.; Yorek, M.A. Enriching the diet with menhaden oil improves peripheral neuropathy in streptozotocin-induced type 1 diabetic rats. J. Neurophysiol. 2015, 113, 701–708. [Google Scholar] [CrossRef]
- Lewis, E.J.H.; Perkins, B.A.; Lovblom, L.E.; Bazinet, R.P.; Wolever, T.M.S.; Bril, V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot study. Neurology 2017, 88, 2294–2301. [Google Scholar] [CrossRef]
- Lewis, E.J.H.; Lovblom, L.E.; Cisbani, G.; Chen, D.K.; Bazinet, R.P.; Wolever, T.M.S.; Perkins, B.A.; Bril, V. Baseline omega-3 level is associated with nerve regeneration following 12-months of omega-3 nutrition therapy in patients with type 1 diabetes. J. Diabetes Complicat. 2021, 35, 107798. [Google Scholar] [CrossRef]
- Coppey, L.J.; Davidson, E.P.; Dunlap, J.A.; Lund, D.D.; Yorek, M.A. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int. J. Exp. Diabetes Res. 2000, 1, 131–143. [Google Scholar] [CrossRef]
- Tian, S.; Guo, T.; Qian, F. Fish oil, plasma n-3 PUFAs, and risk of macro- and microvascular complications among individuals with type 2 diabetes. J. Clin. Endocrinol. Metab. 2025, 110, e1687–e1696. [Google Scholar] [CrossRef]
- Silva, J.A.P.; Fabre, M.E.S.; Waitzberg, D.L. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin. Nutr. 2015, 34, 359–366. [Google Scholar] [CrossRef]
- Melato, J.; Goldoni, F.C.; Benvenutti, L.; Corrêa, T.P.; Remor, A.P.; Varela, K.G.; Stoeberl, L.C.; Fernandes, G.G.; de Lima Rasga, G.; Passos, G.F.; et al. Omega-3-enriched fish oil reduces the chemotherapy-induced peripheral neuropathy in mice. Neuropharmacology 2025, 271, 110384. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Kim, Y.U.; Kim, J.K.; Chun, Y.S.; Kwon, Y.S.; Ku, S.K.; Song, C.H. Preventive and therapeutic effects of krill oil on obesity and obesity-induced metabolic syndromes in high-fat diet-fed mice. Mar. Drugs 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- Hals, P.A.; Wang, X.; Xiao, Y.F. Effects of a purified krill oil phospholipid rich in long-chain omega-3 fatty acids on cardiovascular disease risk factors in non-human primates with naturally occurring diabetes type-2 and dyslipidemia. Lipids Health Dis. 2017, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Lobraico, J.; DiLello, L.C.; Butler, A.D. Effects of krill oil on endothelial function and other cardiovascular risk factors in participants with type 2 diabetes, a randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, e000107. [Google Scholar] [CrossRef]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, K.W.; Wong, R.T.Y.; Chen, F. Fatty acid composition and squalene of the marine microalgae Schizochytrium mangrovei. J. Agric. Food Chem. 2004, 52, 1196–1200. [Google Scholar] [CrossRef]
- Tur, J.A.; Bibiloni, M.M.; Sureda, A.; Pons, A. Dietary sources of omega-3 fatty acids: Public health risks and benefits. Br. J. Nutr. 2012, 107, S23–S52. [Google Scholar] [CrossRef]
- Joardar, A.; Chakraborty, H. Differential Behavior of Eicosapentaenoic and Docosahexaenoic Acids on the Organization, Dynamics, and Fusion of Homogeneous and Heterogeneous Membranes. Langmuir 2023, 39, 4439–4449. [Google Scholar] [CrossRef]
- Lo Van, A.; Bernoud-Hubac, N.; Lagarde, M. Esterification of docosahexaenoic acid enhances its transport to the brain and its potential therapeutic use in brain disease. Nutrients 2022, 14, 4550. [Google Scholar] [CrossRef]
- Bolat, R.; Yazgan, B. The emerging roles of resolvins: Potential diagnostic biomarkers for cardiovascular disease. Curr. Cardiol. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zia, B.; Elmeky, M.; Azimullah, S.; Jha, N.K.; Meeran, M.F.N.; Ojha, S.K. The multifaceted role of neuroprotectin D1: Physiological, pathophysiological, and pharmacological insights in neurodegenerative diseases. Curr. Neuropharmacol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bang, S.; Chen, O.; McGinnis, A.; Zhang, Q.; Ji, R.R. Neuroprotectin D1 and GPR37 protect against chemotherapy-induced peripheral neuropathy and the transition from acute to chronic pain. Pharmacol. Res. 2025, 216, 107746. [Google Scholar] [CrossRef] [PubMed]

| Diet | 16:0 | 18:0 | 18:1 | 18:2 | 20:4 | 20:5 | 22:5 | 22:6 | ∑ EPA, DPA, DHA |
|---|---|---|---|---|---|---|---|---|---|
| Control | 28.3 ± 2.7 | 16.2 ± 1.1 | 8.6 ± 0.6 | 7.8 ± 0.9 | 20.2 ± 1.4 | <1 | 1.4 ± 0.3 | 1.2 ± 0.2 | 2.7 ± 0.1 |
| Diabetic | 25.6 ± 1.9 | 18.4 ± 1.5 | 9.6 ± 0.7 | 9.4 ± 0.9 | 20.9 ± 1.7 | <1 | 1.3 ± 0.1 | 1.7 ± 0.3 | 3.4 ± 0.2 |
| Diabetic + Menhaden oil | 23.2 ± 1.2 | 17.5 ± 0.8 | 9.1 ± 0.5 | 8.7 ± 0.6 | 14.2 ± 0.9 | 4.8 ± 0.3 | 3.9 ± 0.1 | 4.0 ± 0.1 | 12.7 ± 0.6 a,b |
| Diabetic + Krill oil | 29.9 ± 1.7 | 14.8 ± 1.0 | 9.2 ± 0.6 | 8.9 ± 0.6 | 10.0 ± 0.8 | 6.8 ± 0.4 | 3.4 ± 0.2 | 4.1 ± 0.2 | 14.3 ± 0.6 a,b |
| Diabetic + Algal oil EPA | 22.9 ± 1.4 | 14.8 ± 1.4 | 6.2 ± 0.3 | 8.8 ± 0.6 | 15.0 ± 1.0 | 4.0 ± 0.2 | 4.8 ± 0.7 | <1 | 9.4 ± 0.5 a,b |
| Diabetic + Algal oil DHA | 25.2 ± 1.7 | 12.9 ± 0.8 | 6.1 ± 0.3 | 7.9 ± 0.6 | 16.5 ± 1.3 | <1 | 1.0 ± 0.2 | 7.1 ± 0.3 | 8.9 ± 0.2 a,b |
| Diabetic + Algal oil EPA/DHA | 26.1 ± 1.1 | 16.6 ± 1.1 | 8.3 ± 0.4 | 7.8 ± 0.4 | 12.9 ± 0.9 | 5.0 ± 0.3 | 2.0 ± 0.3 | 6.0 ± 0.3 | 13.0 ± 0.4 a,b |
| Diabetic + Ethyl Ester EPA | 23.9 ± 0.6 | 15.8 ± 0.7 | 8.2 ± 0.5 | 10.1 ± 0.9 | 17.2 ± 1.0 | 4.6 ± 0.2 | 4.0 ± 0.7 | <1 | 8.8 ± 0.2 a,b |
| Diabetic + Ethyl Ester DHA | 27.5 ± 1.6 | 15.9 ± 1.0 | 7.7 ± 0.4 | 10.2 ± 0.7 | 14.0 ± 1.0 | <1 | 2.4 ± 0.1 | 5.6 ± 0.2 | 8.8 ± 0.3 a,b |
| Diabetic + Ethyl Ester EPA/DHA | 23.7 ± 1.8 | 16.1 ± 1.3 | 8.8 ± 0.7 | 9.0 ± 0.4 | 14.6 ± 1.0 | 2.6 ± 0.2 | 6.8 ± 0.2 | 4.8 ± 0.4 | 14.2 ± 0.6 a,b |
| Conditions | MNCV (m/s) | SNCV (m/s) | Heat Sensitivity (s) | IENF (Profiles/mm) | Cornea Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 53.1 ± 1.5 | 39.2 ± 0.7 | 11.6 ± 0.7 | 24.1 ± 0.9 | 7.5 ± 0.4 | 5.94 ± 0.04 | 27 ± 9 |
| Diabetic | 40.2 ± 1.2 a | 32.5 ± 0.9 a | 20.6 ± 1.7 a | 14.0 ± 0.6 a | 3.2 ± 0.2 a | 4.66 ± 0.21 a | 118 ± 8 a |
| Diabetic + MO | 52.2 ± 1.4 b | 37.2 ± 0.6 b | 12.7 ± 0.8 b | 17.2 ± 0.6 a,b | 6.9 ± 0.5 b | 5.84 ± 0.08 b | 34 ± 9 b |
| Diabetic + KO | 49.6 ± 1.6 b | 37.2 ± 1.1 b | 13.1 ± 0.9 b | 18.4 ± 0.3 a,b | 6.9 ± 0.5 b | 5.72 ± 0.07 b | 47 ± 13 b |
| Diabetic + EPA | 44.6 ± 1.1 | 36.1 ± 0.9 | 11.1 ± 1.2 b | 19.5 ± 1.0 a,b | 4.4 ± 0.2 a | 5.72 ± 0.10 b | 70 ± 15 |
| Diabetic + DHA | 49.7 ± 1.9 b | 37.6 ± 0.5 b | 12.2 ± 0.8 b | 19.1 ± 0.8 a,b | 6.4 ± 0.3 b | 5.88 ± 0.07 b | 64 ± 7 b |
| Diabetic + EPA + DHA | 53.2 ± 1.8 b | 38.1 ± 0.7 b | 12.1 ± 0.9 b | 21.8 ± 0.6 b | 6.3 ± 0.5 b | 5.94 ± 0.04 b | 65 ± 4 b |
| Conditions | MNCV (m/s) | SNCV (m/s) | Heat Sensitivity (s) | IENF (Profiles/mm) | Corneal Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 56.9 ± 1.8 | 39.0 ± 0.4 | 12.2 ± 0.7 | 22.5 ± 0.9 | 7.5 ± 0.3 | 5.88 ± 0.06 | 54 ± 4 |
| Diabetic | 40.0 ± 1.0 a | 32.8 ± 0.8 a | 20.8 ± 1.2 a | 15.0 ± 0.2 a | 3.2 ± 0.3 a | 4.98 ± 0.15 a | 155 ± 15 a |
| Diabetic + MO | 50.5 ± 1.0 b | 40.1 ± 0.7 b | 14.4 ± 0.8 b | 19.0 ± 0.4 a,b | 6.8 ± 0.3 b | 5.80 ± 0.07 b | 58 ± 10 |
| Diabetic + KO | 52.3 ± 1.9 b | 38.0 ± 0.9 b | 12.3 ± 0.8 b | 17.8 ± 0.5 a,b | 5.7 ± 0.6 b | 5.80 ± 0.07 b | 63 ± 7 |
| Diabetic + EPA | 45.8 ± 2.1 a | 36.2 ± 0.6 a | 12.1 ± 0.6 b | 21.0 ± 0.6 b | 4.6 ± 0.4 a | 5.57 ± 0.08 b | 85 ± 12 |
| Diabetic + DHA | 50.9 ± 1.8 b | 38.7 ± 0.6 b | 12.7 ± 0.5 b | 20.9 ± 0.9 b | 6.4 ± 0.6 b | 5.81 ± 0.06 b | 61 ± 8 |
| Diabetic + EPA + DHA | 49.4 ± 1.1 b | 39.5 ± 0.8 b | 12.3 ± 0.5 b | 21.6 ± 0.7 b | 6.5 ± 0.4 b | 5.82 ± 0.06 b | 58 ± 12 |
| Conditions | MNCV (m/s) | SNCV (m/s) | Heat Sensitivity (s) | IENF (Profiles/mm) | Corneal Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 56.1 ± 2.4 | 38.8 ± 0.6 | 12.3 ± 0.3 | 24.1 ± 0.9 | 7.5 ± 0.3 | 5.86 ± 0.05 | 35 ± 5 |
| Diabetic | 42.6 ± 1.2 a | 32.9 ± 1.0 a | 22.6 ± 0.8 a | 14.0 ± 0.6 a | 3.2 ± 0.2 a | 4.53 ± 0.15 a | 150 ± 4 a |
| Diabetic + MO | 52.6 ± 1.4 | 39.1 ± 0.6 b | 12.8 ± 0.8 b | 17.2 ± 0.6 a,b | 6.4 ± 0.3 b | 5.84 ± 0.06 b | 33 ± 9 b |
| Diabetic + EPA | 43.7 ± 2.2 a | 36.4 ± 1.2 | 11.9 ± 0.7 b | 18.6 ± 0.8 a,b | 4.2 ± 0.7 a | 5.53 ± 0.12 b | 52 ± 14 b |
| Diabetic + DHA | 53.4 ± 1.7 | 39.0 ± 0.7 b | 13.5 ± 0.7 b | 18.9 ± 0.5 a,b | 5.5 ± 0.6 a,b | 5.83 ± 0.08 b | 45 ± 9 b |
| Diabetic + EPA + DHA | 51.4 ± 1.5 | 38.7 ± 0.7 b | 12.9 ± 0.7 b | 20.9 ± 1.0 b | 6.2 ± 0.2 b | 5.84 ± 0.08 b | 40 ± 11 b |
| Conditions | MNCV (m/s) | SNCV (m/s) | Heat Sensitivity (s) | IENF (Profiles/mm) | Corneal Nerve Fiber Length (mm/mm2) | Cornea Sensitivity (cm) | Cornea Sensitivity (AUC) |
|---|---|---|---|---|---|---|---|
| Control | 55.2 ± 1.8 | 40.0 ± 1.0 | 12.2 ± 0.4 | 22.5 ± 0.9 | 8.5 ± 0.4 | 5.88 ± 0.06 | 47 ± 9 |
| Diabetic | 39.7 ± 1.7 a | 32.3 ± 1.4 a | 21.4 ± 1.2 a | 15.0 ± 0.2 a | 3.4 ± 0.3 a | 4.92 ± 0.14 a | 116 ± 8 a |
| Diabetic + MO | 52.8 ± 1.7 b | 37.5 ± 0.3 b | 12.0 ± 0.5 b | 19.0 ± 0.3 a,b | 6.3 ± 0.4 a,b | 5.83 ± 0.07 b | 28 ± 6 b |
| Diabetic + EPA | 46.8 ± 1.7 a,b | 36.0 ± 1.1 | 17.8 ± 1.5 a | 21.2 ± 0.2 b | 4.4 ± 0.4 a | 5.60 ± 0.10 b | 77 ± 18 |
| Diabetic + DHA | 50.8 ± 0.9 b | 37.5 ± 0.3 b | 14.8 ± 0.7 b | 19.1 ± 0.4 b | 7.6 ± 0.5 b | 5.78 ± 0.07 b | 45 ± 11 b |
| Diabetic + EPA+ DHA | 50.5 ± 1.3 b | 39.9 ± 1.5 b | 12.5 ± 0.8 b | 22.0 ± 0.7 b | 7.2 ± 0.4 b | 5.79 ± 0.09 b | 57 ± 14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson, E.; Obrosov, O.; Coppey, L.; Yorek, M. Omega-3 Polyunsaturated Fatty Acids (PUFAs) and Diabetic Peripheral Neuropathy: A Pre-Clinical Study Examining the Effect of Omega-3 PUFAs from Fish Oil, Krill Oil, Algae or Pharmaceutical-Derived Ethyl Esters Using Type 2 Diabetic Rats. Biomedicines 2025, 13, 1607. https://doi.org/10.3390/biomedicines13071607
Davidson E, Obrosov O, Coppey L, Yorek M. Omega-3 Polyunsaturated Fatty Acids (PUFAs) and Diabetic Peripheral Neuropathy: A Pre-Clinical Study Examining the Effect of Omega-3 PUFAs from Fish Oil, Krill Oil, Algae or Pharmaceutical-Derived Ethyl Esters Using Type 2 Diabetic Rats. Biomedicines. 2025; 13(7):1607. https://doi.org/10.3390/biomedicines13071607
Chicago/Turabian StyleDavidson, Eric, Oleksandr Obrosov, Lawrence Coppey, and Mark Yorek. 2025. "Omega-3 Polyunsaturated Fatty Acids (PUFAs) and Diabetic Peripheral Neuropathy: A Pre-Clinical Study Examining the Effect of Omega-3 PUFAs from Fish Oil, Krill Oil, Algae or Pharmaceutical-Derived Ethyl Esters Using Type 2 Diabetic Rats" Biomedicines 13, no. 7: 1607. https://doi.org/10.3390/biomedicines13071607
APA StyleDavidson, E., Obrosov, O., Coppey, L., & Yorek, M. (2025). Omega-3 Polyunsaturated Fatty Acids (PUFAs) and Diabetic Peripheral Neuropathy: A Pre-Clinical Study Examining the Effect of Omega-3 PUFAs from Fish Oil, Krill Oil, Algae or Pharmaceutical-Derived Ethyl Esters Using Type 2 Diabetic Rats. Biomedicines, 13(7), 1607. https://doi.org/10.3390/biomedicines13071607





