Nanotechnology in Osteogenesis and Inflammation Management: Metal–Organic Frameworks, Metal Complexes, and Biomaterials for Bone Restoration
Abstract
1. Introduction
2. Materials and Methods
3. Synergistic Flavonoid–Metal Combinations: An Approach
3.1. Copper and Flavonoid Complexes
3.2. Zinc and Flavonoid Complexes
3.3. Additional Metal Complexes
3.4. Biomaterials Made from Flavonoid Electrospun Nanofibers
3.5. Functionally Modified HA
3.6. Chitosan-Based Systems
3.7. Immunomodulatory Effects
4. Flavonoid Synergism Using MOFs
4.1. Flavonoid Coatings in Implantology
4.2. MOFs and Injectable Gels
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| BIC | Bone–Implant Contact |
| BV/TV | Bone-Volume-to-Total-Volume Ratio |
| ECM | Extracellular Matrix |
| EGCG | Epigallocatechin Gallate |
| HA | Hydroxyapatite |
| hMSC | Human Mesenchymal Stem Cell |
| MOFs | Metal–Organic Frameworks |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| PCL | Polycaprolactone |
| ROS | Reactive Oxygen Species |
| RUNX2 | Runt-Related Transcription Factor 2 |
| TNF-α | Tumor Necrosis Factor Alpha |
References
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, P.; Wu, Y.; Wu, Y.; Tan, Z.; Ling, J.; Ma, J.; Zhang, J.; Zhu, W.; Liu, X. Sex Specific Global Burden of Osteoporosis in 204 Countries and Territories, from 1990 to 2030: An Age-Period-Cohort Modeling Study. J. Nutr. Health Aging 2023, 27, 767–774. [Google Scholar] [CrossRef]
- Lorentzon, M.; Johansson, H.; Harvey, N.C.; Liu, E.; Vandenput, L.; McCloskey, E.V.; Kanis, J.A. Osteoporosis and Fractures in Women: The Burden of Disease. Climacteric 2022, 25, 4–10. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.; Li, B.; Zhan, S.; Wang, S.; Song, C. Global Burden of Hip Fracture: The Global Burden of Disease Study. Osteoporos. Int. 2024, 35, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Rashidi, M.-M.; Saeedi Moghaddam, S.; Azadnajafabad, S.; Heidari-Foroozan, M.; Hashemi, S.M.; Mohammadi, E.; Esfahani, Z.; Ebrahimi, N.; Shobeiri, P.; Malekpour, M.-R.; et al. Low Bone Mineral Density, a Neglected Condition in North Africa and Middle East: Estimates from the Global Burden of Disease Study, 1990–2019. Osteoporos. Int. 2023, 34, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Harvey, N.C.; Cooper, C. The Burden of Osteoporosis. In Osteoporosis; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-23462-7. [Google Scholar]
- Dong, Y.; Peng, R.; Kang, H.; Song, K.; Guo, Q.; Zhao, H.; Zhu, M.; Zhang, Y.; Guan, H.; Li, F. Global Incidence, Prevalence, and Disability of Vertebral Fractures: A Systematic Analysis of the Global Burden of Disease Study 2019. Spine J. 2022, 22, 857–868. [Google Scholar] [CrossRef]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Marcellusi, A.; Rotundo, M.A.; Nardone, C.; Sciattella, P.; Gazzillo, S.; Rossini, M.; Barbagallo, M.; Antenori, A.; Valle, D.; Mennini, F.S. Osteoporosis: Economic Burden of Disease in Italy. Clin. Drug Investig. 2020, 40, 449–458. [Google Scholar] [CrossRef]
- Agrawal, A.C.; Garg, A.K. Epidemiology of Osteoporosis. Indian J. Orthop. 2023, 57, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Bruyère, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to Manage Osteoporosis before the Age of 50. Maturitas 2020, 138, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary Osteoporosis. Endocr. Rev. 2022, 43, 240–313. [Google Scholar] [CrossRef] [PubMed]
- Foessl, I.; Dimai, H.P.; Obermayer-Pietsch, B. Long-Term and Sequential Treatment for Osteoporosis. Nat. Rev. Endocrinol. 2023, 19, 520–533. [Google Scholar] [CrossRef]
- Gao, Y.; Patil, S.; Jia, J. The Development of Molecular Biology of Osteoporosis. Int. J. Mol. Sci. 2021, 22, 8182. [Google Scholar] [CrossRef]
- Lungu, I.I.; Stefanache, A.; Crivoi, F.; BUREC, A.-F.; Belei, D.; Cioanca, O.; Hancianu, M. Innovative Synthesis of Zinc and Selenium Complexes with Gallic Acid: Exploring Their Antioxidant Potential. Med.-Surg. J. 2024, 128, 177–188. [Google Scholar] [CrossRef]
- Andia, I.; Maffulli, N. Platelet-Rich Plasma for Managing Pain and Inflammation in Osteoarthritis. Nat. Rev. Rheumatol. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Bone Regeneration: A Review of Current Treatment Strategies. J. Clin. Med. 2025, 14, 1838. [Google Scholar] [CrossRef]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Ding, Q.; Zhang, S.; Sun, S.; Liu, W.; Liu, J.; Han, X.; Ding, C. Flavonoid-Loaded Biomaterials in Bone Defect Repair. Molecules 2023, 28, 6888. [Google Scholar] [CrossRef]
- Mei, H.; Liu, H.; Sha, C.; Lv, Q.; Song, Q.; Jiang, L.; Tian, E.; Gao, Z.; Li, J.; Zhou, J. Multifunctional Metal–Phenolic Composites Promote Efficient Periodontitis Treatment via Antibacterial and Osteogenic Properties. ACS Appl. Mater. Interfaces 2024, 16, 13573–13584. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghomi, M.; Padil, V.V.T.; Shalchy, F.; Ashrafizadeh, M.; Askarinejad, S.; Pourreza, N.; Zarrabi, A.; Nazarzadeh Zare, E.; Kooti, M.; et al. Biofabricated Nanostructures and Their Composites in Regenerative Medicine. ACS Appl. Nano Mater. 2020, 3, 6210–6238. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of Bioavailability and Bioactivity of Diet-Derived Flavonoids by Application of Nanotechnology: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 378–393. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research Progress of the Antiviral Bioactivities of Natural Flavonoids. Nat. Prod. Bioprospect. 2020, 10, 271–283. [Google Scholar] [CrossRef]
- Rédai, E.-M.; Antonoaea, P.; Todoran, N.; Vlad, R.A.; Bîrsan, M.; Tătaru, A.; Ciurba, A. Development and Evaluation of Fluoxetine Fast Dissolving Films: An Alternative for Noncompliance in Pediatric Patients. Processes 2021, 9, 778. [Google Scholar] [CrossRef]
- Shanmugavadivu, A.; Balagangadharan, K.; Selvamurugan, N. Angiogenic and Osteogenic Effects of Flavonoids in Bone Regeneration. Biotechnol. Bioeng. 2022, 119, 2313–2330. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Luo, Y.; Li, X.; Huang, G.; Chen, H.; Li, A.; Qin, S. The Role of Flavonoids in the Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Pharmacol. 2022, 13, 849513. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Wang, Y.; Lu, C.; Zheng, D.; Zhang, J. Isoquercitrin, a Flavonoid Glucoside, Exerts a Positive Effect on Osteogenesis In Vitro and In Vivo. Chem.-Biol. Interact. 2019, 297, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Nam, J.-S. Kaempferol Stimulates WNT/β-Catenin Signaling Pathway to Induce Differentiation of Osteoblasts. J. Nutr. Biochem. 2019, 74, 108228. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.J.Y.; Choi, R.C.Y.; Cheung, A.W.H.; Chen, V.P.; Xu, S.L.; Dong, T.T.X.; Chen, J.J.; Tsim, K.W.K. Baicalin, a Flavone, Induces the Differentiation of Cultured Osteoblasts: An Action via the Wnt/β-CATENIN Signaling Pathway * ♦. J. Biol. Chem. 2011, 286, 27882–27893. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Hu, C.; Yang, J.; Ye, J.; Zhou, Y.; Li, Z.; Chen, L.; Zhou, Q. Total Flavonoids of Rhizoma drynariae Promotes Differentiation of Osteoblasts and Growth of Bone Graft in Induced Membrane Partly by Activating Wnt/β-Catenin Signaling Pathway. Front. Pharmacol. 2021, 12, 675470. [Google Scholar] [CrossRef]
- Sharma, A.R.; Lee, Y.-H.; Bat-Ulzii, A.; Chatterjee, S.; Bhattacharya, M.; Chakraborty, C.; Lee, S.-S. Bioactivity, Molecular Mechanism, and Targeted Delivery of Flavonoids for Bone Loss. Nutrients 2023, 15, 919. [Google Scholar] [CrossRef]
- Li, Z. Investigation of the Molecular Mechanism of Quercetin in Inhibiting Ankylosing Spondylitis Ossification via the Bone Morphogenetic Protein/Smad Signaling Pathway. Med. Mol. Morphol. 2024, 58, 114–125. [Google Scholar] [CrossRef]
- Sheibani, M.; Shayan, M.; Jafari-Sabet, M.; Sharifi, A.M. Applications of Phytomedicines in Chondrocytes and Osteocytes Regeneration Therapy: Pre-Clinical and Clinical Studies. Tradit. Integr. Med. 2023, 8, 299–315. [Google Scholar] [CrossRef]
- Su, H.; Liu, L.; Yan, Z.; Guo, W.; Huang, G.; Zhuang, R.; Pan, Y. Therapeutic Potential of Total Flavonoids of Rhizoma drynariae: Inhibiting Adipogenesis and Promoting Osteogenesis via MAPK/HIF-1α Pathway in Primary Osteoporosis. J. Orthop. Surg. Res. 2025, 20, 260. [Google Scholar] [CrossRef]
- Zha, X.; Xu, Z.; Liu, Y.; Xu, L.; Huang, H.; Zhang, J.; Cui, L.; Zhou, C.; Xu, D. Amentoflavone Enhances Osteogenesis of Human Mesenchymal Stem Cells through JNK and P38 MAPK Pathways. J. Nat. Med. 2016, 70, 634–644. [Google Scholar] [CrossRef]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, W.; Chen, L.; Liao, J.; Yang, X. Bioflavonoid Tangeretin Regulates RANK/RANKL/OPG Signaling Proteins via Stimulating Estrogenic Activity in Ovariectomized Rats. Pharmacogn. Mag. 2024, 83, 09731296241296201. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; Luca, A.D.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, N.; Xu, W.; Zhang, Z.; Yang, Y.; Zhang, J.; Xu, H. Effect of Flavonoids from Rhizoma drynariae on Osteoporosis Rats and Osteocytes. Biomed. Pharmacother. 2022, 153, 113379. [Google Scholar] [CrossRef]
- Rodríguez, V.; Rivoira, M.; Picotto, G.; de Barboza, G.D.; Collin, A.; Tolosa de Talamoni, N. Analysis of the Molecular Mechanisms by Flavonoids with Potential Use for Osteoporosis Prevention or Therapy. Curr. Med. Chem. 2022, 29, 2913–2936. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Wu, C.; Zhang, X.; Zhang, X.; Xu, X. Targeting Bone Homeostasis Regulation: Potential of Traditional Chinese Medicine Flavonoids in the Treatment of Osteoporosis. Front. Pharmacol. 2024, 15, 1361864. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Rajalakshmi, S.; Raj Preeth, D.; Vinoth Kumar, S.; Deepak, T.; Gopinath, V.; Murugan, K.; Chatterjee, S. Mixed-Ligand Copper(II) Complex of Quercetin Regulate Osteogenesis and Angiogenesis. Mater. Sci. Eng. C 2018, 83, 187–194. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Al-Garawi, Z.S.; Al-Qaisi, A.H.I.; Al-Shamari, K.A.; Öztürkkan, F.E.; Necefoğlu, H. The Utility of Hibiscus sabdariffa L. to Prepare Metal Oxides NPs for Clinical Application on Osteoporosis Supported by Theoretical Study. Bioprocess. Biosyst. Eng. 2024, 47, 753–766. [Google Scholar] [CrossRef]
- Gaddi, G.M.; Caro-Ramírez, J.Y.; Parente, J.E.; Williams, P.A.M.; Ferrer, E.G. Copper-Flavonoid Family of Complexes Involved in Alkaline Phosphatase Activation. Biometals 2023, 36, 1221–1239. [Google Scholar] [CrossRef]
- Walencik, P.K.; Choińska, R.; Gołębiewska, E.; Kalinowska, M. Metal–Flavonoid Interactions—From Simple Complexes to Advanced Systems. Molecules 2024, 29, 2573. [Google Scholar] [CrossRef]
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal Complexes of Flavonoids: Their Synthesis, Characterization and Enhanced Antioxidant and Anticancer Activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arce, E.; Saldías, M. Antioxidant Properties of Flavonoid Metal Complexes and Their Potential Inclusion in the Development of Novel Strategies for the Treatment against Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Sang, X.; Liu, Q.; Yu, H.; Hu, S.; Mao, Y.; Zhang, L. Spatiotemporal Regulation of the Bone Immune Microenvironment via a ‘Zn2+-Quercetin’ Hierarchical Delivery System for Bone Regeneration. Regen. Biomater. 2025, 12, rbaf006. [Google Scholar] [CrossRef] [PubMed]
- Raj Preeth, D.; Saravanan, S.; Shairam, M.; Selvakumar, N.; Selestin Raja, I.; Dhanasekaran, A.; Vimalraj, S.; Rajalakshmi, S. Bioactive Zinc(II) Complex Incorporated PCL/Gelatin Electrospun Nanofiber Enhanced Bone Tissue Regeneration. Eur. J. Pharm. Sci. 2021, 160, 105768. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Chen, E.; Zhang, W.; Gao, X.; Wang, S.; Zheng, Q.; Pan, Z.; Li, H.; Liu, L. The Role of Hesperetin on Osteogenesis of Human Mesenchymal Stem Cells and Its Function in Bone Regeneration. Oncotarget 2017, 8, 21031–21043. [Google Scholar] [CrossRef]
- Mancim-Imbriani, M.J.; Duarte, J.L.; Di Filippo, L.D.; Durão, L.P.L.; Chorilli, M.; Palomari Spolidorio, D.M.; Maquera-Huacho, P.M. Formulation of a Novel Hesperetin-Loaded Nanoemulsion and Its Promising Effect on Osteogenesis. Pharmaceutics 2024, 16, 698. [Google Scholar] [CrossRef]
- Ortiz, A.D.C.; Fideles, S.O.M.; Reis, C.H.B.; Bellini, M.Z.; Pereira, E.d.S.B.M.; Pilon, J.P.G.; de Marchi, M.Â.; Detregiachi, C.R.P.; Flato, U.A.P.; Trazzi, B.F.d.M.; et al. Therapeutic Effects of Citrus Flavonoids Neohesperidin, Hesperidin and Its Aglycone, Hesperetin on Bone Health. Biomolecules 2022, 12, 626. [Google Scholar] [CrossRef]
- Li, W.; Pi, J.; Zhang, Y.; Ma, X.; Zhang, B.; Wang, S.; Qi, D.; Li, N.; Guo, P.; Liu, Z. A Strategy to Improve the Oral Availability of Baicalein: The Baicalein-Theophylline Cocrystal. Fitoterapia 2018, 129, 85–93. [Google Scholar] [CrossRef]
- Guan, D.; Xuan, B.; Wang, C.; Long, R.; Jiang, Y.; Mao, L.; Kang, J.; Wang, Z.; Chow, S.F.; Zhou, Q. Improving the Physicochemical and Biopharmaceutical Properties of Active Pharmaceutical Ingredients Derived from Traditional Chinese Medicine through Cocrystal Engineering. Pharmaceutics 2021, 13, 2160. [Google Scholar] [CrossRef]
- You, G.; Feng, T.; Zhang, G.; Chen, M.; Liu, F.; Sun, L.; Wang, M.; Ren, X. Preparation, Optimization, Characterization and In Vitro Release of Baicalein-Solubilizing Glycyrrhizic Acid Nano-Micelles. Int. J. Pharm. 2021, 601, 120546. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Dai, Y.; Shen, H.; Ju, J.; Zhao, Z. Application of Soluplus to Improve the Flowability and Dissolution of Baicalein Phospholipid Complex. Molecules 2017, 22, 776. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, L.; Tian, F.; Ding, Q.; Hu, Z.; Wang, J.-R.; Mei, X. Rutin Cocrystals with Improved Solubility, Bioavailability, and Bioactivities. Cryst. Growth Des. 2024, 24, 5637–5647. [Google Scholar] [CrossRef]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of Quercetin with Improved Solubility and Oral Bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Chen, S.-C.; Lai, W.-F.T.; Chen, Y.-C.; Tsai, Y.-H. Screening of Flavonoids for Effective Osteoclastogenesis Suppression. Anal. Biochem. 2013, 433, 48–55. [Google Scholar] [CrossRef]
- Huh, J.-E.; Jung, I.-T.; Choi, J.; Baek, Y.-H.; Lee, J.-D.; Park, D.-S.; Choi, D.-Y. The Natural Flavonoid Galangin Inhibits Osteoclastic Bone Destruction and Osteoclastogenesis by Suppressing NF-κB in Collagen-Induced Arthritis and Bone Marrow-Derived Macrophages. Eur. J. Pharmacol. 2013, 698, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Li, G.; Meng, C.-L.; Dong, Q.; Chan, C.-Y.; He, M.-L.; Leung, P.-C.; Zhang, Y.-O.; Kung, H.-F. Total Flavonoids of Herba Epimedii Improves Osteogenesis and Inhibits Osteoclastogenesis of Human Mesenchymal Stem Cells. Phytomedicine 2009, 16, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Saravanan, S.; Hariprabu, G.; Yuvashree, R.; Ajieth Kanna, S.K.; Sujoy, K.; Anjali, D. Kaempferol-Zinc(II) Complex Synthesis and Evaluation of Bone Formation Using Zebrafish Model. Life Sci. 2020, 256, 117993. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Saravanan, S.; Thirumalai, D.; Kadarkarai, M.; Rajkumar, A.V.; Dhanasekaran, A. Zinc Chelated Morin Promotes Osteoblast Differentiation over Its Uncomplexed Counterpart. Process Biochem. 2019, 82, 167–172. [Google Scholar] [CrossRef]
- Vimalraj, S.; Saravanan, S.; Subramanian, R. Rutin-Zn(II) Complex Promotes Bone Formation—A Concise Assessment in Human Dental Pulp Stem Cells and Zebrafish. Chem.-Biol. Interact. 2021, 349, 109674. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Vimalraj, S.; Saravanan, S.; Raj Preeth, D.; Shairam, M.; Anuradha, D. Synthesis and Characterization of Silibinin/Phenanthroline/Neocuproine Copper(II) Complexes for Augmenting Bone Tissue Regeneration: An In Vitro Analysis. J. Biol. Inorg. Chem. 2018, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Guo, M.; Bai, J.; Zhao, L.; Wang, L.; Song, W.; Zhang, P. Quercetin-Loaded Nanocomposite Microspheres for Chronologically Promoting Bone Repair via Synergistic Immunoregulation and Osteogenesis. Mater. Des. 2022, 222, 111045. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Wang, Y.; Wang, N.; Wei, H.; Zhang, S.; Ding, Q.; Sun, S.; Ding, C.; Liu, W. Dihydromyricetin-Loaded Oxidized Polysaccharide/L-Arginine Chitosan Adhesive Hydrogel Promotes Bone Regeneration by Regulating PI3K/AKT Signaling Pathway and MAPK Signaling Pathway. Carbohydr. Polym. 2024, 346, 122614. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yin, W.; Xu, C.; Feng, Y.; Huang, X.; Hao, J.; Zhu, C. Rutin Promotes Osteogenic Differentiation of Mesenchymal Stem Cells (MSCs) by Increasing ECM Deposition and Inhibiting P53 Expression. Aging 2024, 16, 3583–3595. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Subramanian, R.; Sekaran, S.; Veeraiyan, D.N.; Thangavelu, L. Ferulic Acid-Cu(II) and Zn(II) Complexes Promote Bone Formation. Process Biochem. 2021, 107, 145–152. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Europium–Tannic Acid Nanocomplexes Devised for Bone Regeneration under Oxidative or Inflammatory Environments. J. Mater. Chem. B 2024, 12, 7153–7170. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Knowles, J.C. Electrospinning Biomedical Nanocomposite Fibers of Hydroxyapatite/Poly(Lactic Acid) for Bone Regeneration. J. Biomed. Mater. Res. 2006, 79A, 643–649. [Google Scholar] [CrossRef]
- Shetty, K.; Bhandari, A.; Yadav, K.S. Nanoparticles Incorporated in Nanofibers Using Electrospinning: A Novel Nano-in-Nano Delivery System. J. Control. Release 2022, 350, 421–434. [Google Scholar] [CrossRef]
- Shin, S.-H.; Purevdorj, O.; Castano, O.; Planell, J.A.; Kim, H.-W. A Short Review: Recent Advances in Electrospinning for Bone Tissue Regeneration. J. Tissue Eng. 2012, 3, 2041731412443530. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical Electrospinning and 3D Printing Scaffold Design for Bone Regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Raja, I.S.; Preeth, D.R.; Vedhanayagam, M.; Hyon, S.-H.; Lim, D.; Kim, B.; Rajalakshmi, S.; Han, D.-W. Polyphenols-Loaded Electrospun Nanofibers in Bone Tissue Engineering and Regeneration. Biomater. Res. 2021, 25, 29. [Google Scholar] [CrossRef]
- Hoveidaei, A.H.; Sadat-Shojai, M.; Mosalamiaghili, S.; Salarikia, S.R.; Roghani-shahraki, H.; Ghaderpanah, R.; Ersi, M.H.; Conway, J.D. Nano-Hydroxyapatite Structures for Bone Regenerative Medicine: Cell-Material Interaction. Bone 2024, 179, 116956. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone Regeneration with Hydroxyapatite-Based Biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Abere, D.V.; Ojo, S.A.; Oyatogun, G.M.; Paredes-Epinosa, M.B.; Niluxsshun, M.C.D.; Hakami, A. Mechanical and Morphological Characterization of Nano-Hydroxyapatite (nHA) for Bone Regeneration: A Mini Review. Biomed. Eng. Adv. 2022, 4, 100056. [Google Scholar] [CrossRef]
- Florea, D.A.; Chircov, C.; Grumezescu, A.M. Hydroxyapatite Particles—Directing the Cellular Activity in Bone Regeneration Processes: An Up-To-Date Review. Appl. Sci. 2020, 10, 3483. [Google Scholar] [CrossRef]
- Costache, A.-D.; Leon-Constantin, M.-M.; Roca, M.; Maștaleru, A.; Anghel, R.-C.; Zota, I.-M.; Drugescu, A.; Costache, I.-I.; Chetran, A.; Moisă, Ș.-M.; et al. Cardiac Biomarkers in Sports Cardiology. J. Cardiovasc. Dev. Dis. 2022, 9, 453. [Google Scholar] [CrossRef]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Rubini, K.; Fini, M.; Bigi, A. Antioxidant and Bone Repair Properties of Quercetin-Functionalized Hydroxyapatite: An In Vitro Osteoblast–Osteoclast–Endothelial Cell Co-Culture Study. Acta Biomater. 2016, 32, 298–308. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Kudiyarasu, S.; Karuppan Perumal, M.K.; Rajan Renuka, R.; Manickam Natrajan, P. Chitosan Composite with Mesenchymal Stem Cells: Properties, Mechanism, and Its Application in Bone Regeneration. Int. J. Biol. Macromol. 2024, 275, 133502. [Google Scholar] [CrossRef]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and Anti-Inflammatory Properties of Chitosan-Based Scaffolds for Bone Regeneration. Mater. Sci. Eng. C 2019, 105, 110046. [Google Scholar] [CrossRef]
- Dmour, B.-A.; Costache, A.D.; Dmour, A.; Huzum, B.; Duca, Ș.T.; Chetran, A.; Miftode, R.Ș.; Afrăsânie, I.; Tuchiluș, C.; Cianga, C.M.; et al. Could Endothelin-1 Be a Promising Neurohormonal Biomarker in Acute Heart Failure? Diagnostics 2023, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, W.; Fu, Q.; Wang, Z.; Zhao, H.; Wang, Z.; Gao, Y.; Wang, J. Fabrication and Evaluation of Zn-EGCG-Loaded Chitosan Scaffolds for Bone Regeneration: From Cellular Responses to in Vivo Performance. Int. J. Biol. Macromol. 2024, 283, 137695. [Google Scholar] [CrossRef]
- Li, Y.; Selvaraj, V.; Saravanan, S.; Abullais, S.S.; Wankhade, V. Exploring the Osteogenic Potential of Chitosan-Quercetin Bio-Conjugate: In Vitro and In Vivo Investigations in Osteoporosis Models. Int. J. Biol. Macromol. 2024, 274, 133492. [Google Scholar] [CrossRef] [PubMed]
- Valentino, A.; Di Cristo, F.; Bosetti, M.; Amaghnouje, A.; Bousta, D.; Conte, R.; Calarco, A. Bioactivity and Delivery Strategies of Phytochemical Compounds in Bone Tissue Regeneration. Appl. Sci. 2021, 11, 5122. [Google Scholar] [CrossRef]
- Lungu, I.I.; Cioanca, O.; Mircea, C.; Tuchilus, C.; Stefanache, A.; Huzum, R.; Hancianu, M. Insights into Catechin–Copper Complex Structure and Biologic Activity Modulation. Molecules 2024, 29, 4969. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, H.; Wang, Z.; Zhu, D.; Li, Y.; Zhang, Y.; Wang, D.; Chen, S.; Liu, H.; Kang, X. Macrophage Efferocytosis as a Therapeutic Strategy in Intervertebral Disc Degeneration. Cell Prolif. 2025, e70068. [Google Scholar] [CrossRef]
- Radandish, M.; Khalilian, P.; Esmaeil, N. The Role of Distinct Subsets of Macrophages in the Pathogenesis of MS and the Impact of Different Therapeutic Agents on These Populations. Front. Immunol. 2021, 12, 667705. [Google Scholar] [CrossRef]
- Tang, M.; Wang, G.; Li, J.; Wang, Y.; Peng, C.; Chang, X.; Guo, J.; Gui, S. Flavonoid Extract from Propolis Alleviates Periodontitis by Boosting Periodontium Regeneration and Inflammation Resolution via Regulating TLR4/MyD88/NF-κB and RANK/NF-κB Pathway. J. Ethnopharmacol. 2024, 319, 117324. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Jiang, W.; Hu, M.; Meng, Y.; Li, W.; Zhou, X.; Wang, C. Naringenin Is a Potential Anabolic Treatment for Bone Loss by Modulating Osteogenesis, Osteoclastogenesis, and Macrophage Polarization. Front. Pharmacol. 2022, 13, 872188. [Google Scholar] [CrossRef]
- Ge, Y.-W.; Feng, K.; Liu, X.-L.; Zhu, Z.-A.; Chen, H.-F.; Chang, Y.-Y.; Sun, Z.-Y.; Wang, H.-W.; Zhang, J.-W.; Yu, D.-G.; et al. Quercetin Inhibits Macrophage Polarization through the P-38α/β Signalling Pathway and Regulates OPG/RANKL Balance in a Mouse Skull Model. J. Cell. Mol. Med. 2020, 24, 3203–3216. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Wu, C.; Zhu, L.; Chen, O.; Wang, X. Flavonoid Compound Icariin Enhances BMP-2 Induced Differentiation and Signalling by Targeting to Connective Tissue Growth Factor (CTGF) in SAMP6 Osteoblasts. PLoS ONE 2018, 13, e0200367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Pan, H.; Xu, H.; Deng, W.; Sun, X. Panicum miliaceum L. and Bone Healing Properties in Rat Model of Femur Fracture by Activating Phosphate Stimulating Macrophages via BMP2 and RANK Signaling Pathway. Pharmacogn. Mag. 2024, 20, 16–29. [Google Scholar] [CrossRef]
- Krasnikov, A.; Krasnikova, E.; Morozova, D.; Spirkina, N. Study of Osseointegration Properties of Multilayer Coatings with a Biodegradable Film of Flavonoid Nanoaggregates. J. Phys. Conf. Ser. 2022, 2373, 032026. [Google Scholar] [CrossRef]
- Llopis-Grimalt, M.A.; Arbós, A.; Gil-Mir, M.; Mosur, A.; Kulkarni, P.; Salito, A.; Ramis, J.M.; Monjo, M. Multifunctional Properties of Quercitrin-Coated Porous Ti-6Al-4V Implants for Orthopaedic Applications Assessed In Vitro. J. Clin. Med. 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lan, X.; Chen, X.; Dai, S.; Wang, Z.; Zhao, A.; Lu, L.; Huang, N.; Chen, J.; Yang, P.; et al. Multi-Functional Plant Flavonoids Regulate Pathological Microenvironments for Vascular Stent Surface Engineering. Acta Biomater. 2023, 157, 655–669. [Google Scholar] [CrossRef]
- Arias-Mainer, C.; Romero-Gavilán, F.; Cerqueira, A.; Peñarrocha-Oltra, D.; García-Arnáez, I.; Amorrortu, O.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goni, I.; et al. Quercetin-Doped Sol-Gel Coatings on Titanium Implants: A Promising Approach for Enhanced Immune Response and Cell Adhesion. J. Mater. Chem. B 2025, 13, 7048–7061. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, H.; Zhang, Z.; Chen, L.; Li, J. Research Progress on the Application of Natural Medicines in Biomaterial Coatings. Materials 2024, 17, 5607. [Google Scholar] [CrossRef]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Monjo, M.; Ramis, J.M. Flavonoid Coated Titanium Surfaces for Bioactive Bone Implants. Stem Cell Transl. Investig. 2015, 2, 10.14800. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Chen, F.; Wang, B. Loading Rutin on Surfaces by the Layer-by-Layer Assembly Technique to Improve the Oxidation Resistance and Osteogenesis of Titanium Implants in Osteoporotic Rats. Biomed. Mater. 2024, 19, 045011. [Google Scholar] [CrossRef]
- Córdoba, A.; Manzanaro-Moreno, N.; Colom, C.; Rønold, H.J.; Lyngstadaas, S.P.; Monjo, M.; Ramis, J.M. Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3319. [Google Scholar] [CrossRef]
- Wang, A.; Yuan, W.; Song, Y.; Zang, Y.; Yu, Y. Osseointegration Effect of Micro-Nano Implants Loaded With Kaempferol in Osteoporotic Rats. Front. Bioeng. Biotechnol. 2022, 10, 842014. [Google Scholar] [CrossRef]
- Wang, B.; Chen, L.; Xie, J.; Tang, J.; Hong, C.; Fang, K.; Jin, C.; Huang, C.; Xu, T.; Yang, L. Coating Polyelectrolyte Multilayers Loaded with Quercetin on Titanium Surfaces by Layer-By-Layer Assembly Technique to Improve Surface Osteogenesis Under Osteoporotic Condition. J. Biomed. Nanotechnol. 2021, 17, 1392–1403. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, T.; Wen, L.; Li, R.; Zhang, Y.; Bi, W.; Feng, X.; Qi, M. Osteogenic Capability of Strontium and Icariin-Loaded TiO2 Nanotube Coatings in Vitro and in Osteoporotic Rats. J. Biomater. Appl. 2021, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, H.; Fu, Z.; Zhang, C.; Hui, W.; Wu, J.; Zhang, Y.; Zhang, S. Quercetin-Coating Promotes Osteogenic Differentiation, Osseointegration and Anti-Inflammatory Properties of Nano-Topographic Modificated 3D-Printed Ti6Al4V Implant. Front. Bioeng. Biotechnol. 2022, 10, 933135. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Luo, S.; Shan, C.; Geng, Y.; Zhang, T.; Sheng, S.; Zan, X. Building Polyphenol and Gelatin Films as Implant Coating, Evaluating from in Vitro and in Vivo Performances. Colloids Surf. B Biointerfaces 2019, 181, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M. Potentials of Polyphenols in Bone-Implant Devices; IntechOpen: London, UK, 2018; pp. 1–28. [Google Scholar]
- Weber, F. Development of Multifunctional Polyphenolic Coatings for Improved Peri-Implant Healing. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2021. [Google Scholar]
- Bjelič, D.; Finšgar, M. Bioactive Coatings with Anti-Osteoclast Therapeutic Agents for Bone Implants: Enhanced Compliance and Prolonged Implant Life. Pharmacol. Res. 2022, 176, 106060. [Google Scholar] [CrossRef]
- Córdoba, A.; Satué, M.; Gómez-Florit, M.; Hierro-Oliva, M.; Petzold, C.; Lyngstadaas, S.P.; González-Martín, M.L.; Monjo, M.; Ramis, J.M. Flavonoid-Modified Surfaces: Multifunctional Bioactive Biomaterials with Osteopromotive, Anti-Inflammatory, and Anti-Fibrotic Potential. Adv. Healthc. Mater. 2015, 4, 540–549. [Google Scholar] [CrossRef]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, 2001369. [Google Scholar] [CrossRef]
- Chen, M.; Wang, D.; Li, M.; He, Y.; He, T.; Chen, M.; Hu, Y.; Luo, Z.; Cai, K. Nanocatalytic Biofunctional MOF Coating on Titanium Implants Promotes Osteoporotic Bone Regeneration through Cooperative Pro-Osteoblastogenesis MSC Reprogramming. ACS Nano 2022, 16, 15397–15412. [Google Scholar] [CrossRef]
- Yu, S.; Wu, T.; Xu, K.; Liu, R.; Yu, T.; Wang, Z.; Zhang, Z. 3D Bioprinted Biomimetic MOF-Functionalized Hydrogel Scaffolds for Bone Regeneration: Synergistic Osteogenesis and Osteoimmunomodulation. Mater. Today Bio 2025, 32, 101740. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Yu, H.; Jiang, X.; Sun, D. MOF-Derived CuO@ZnO Modified Titanium Implant for Synergistic Antibacterial Ability, Osteogenesis and Angiogenesis. Colloids Surf. B Biointerfaces 2022, 219, 112840. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, Y.; Ji, C.; Zhu, H.; Lao, A.; Zhao, R.; Hu, Y.; Zhou, Y.; Zhou, J.; Lin, K.; et al. A Five-in-One Novel MOF-Modified Injectable Hydrogel with Thermo-Sensitive and Adhesive Properties for Promoting Alveolar Bone Repair in Periodontitis: Antibacterial, Hemostasis, Immune Reprogramming, pro-Osteo-/Angiogenesis and Recruitment. Bioact. Mater. 2024, 41, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Niu, Y.; Zhang, H.; Ouyang, H.; Zhang, G.; Fu, Y. Baicalin Nanocomplexes with an In Situ-Forming Biomimetic Gel Implant for Repair of Calvarial Bone Defects via Localized Sclerostin Inhibition. ACS Appl. Mater. Interfaces 2023, 15, 9044–9057. [Google Scholar] [CrossRef] [PubMed]
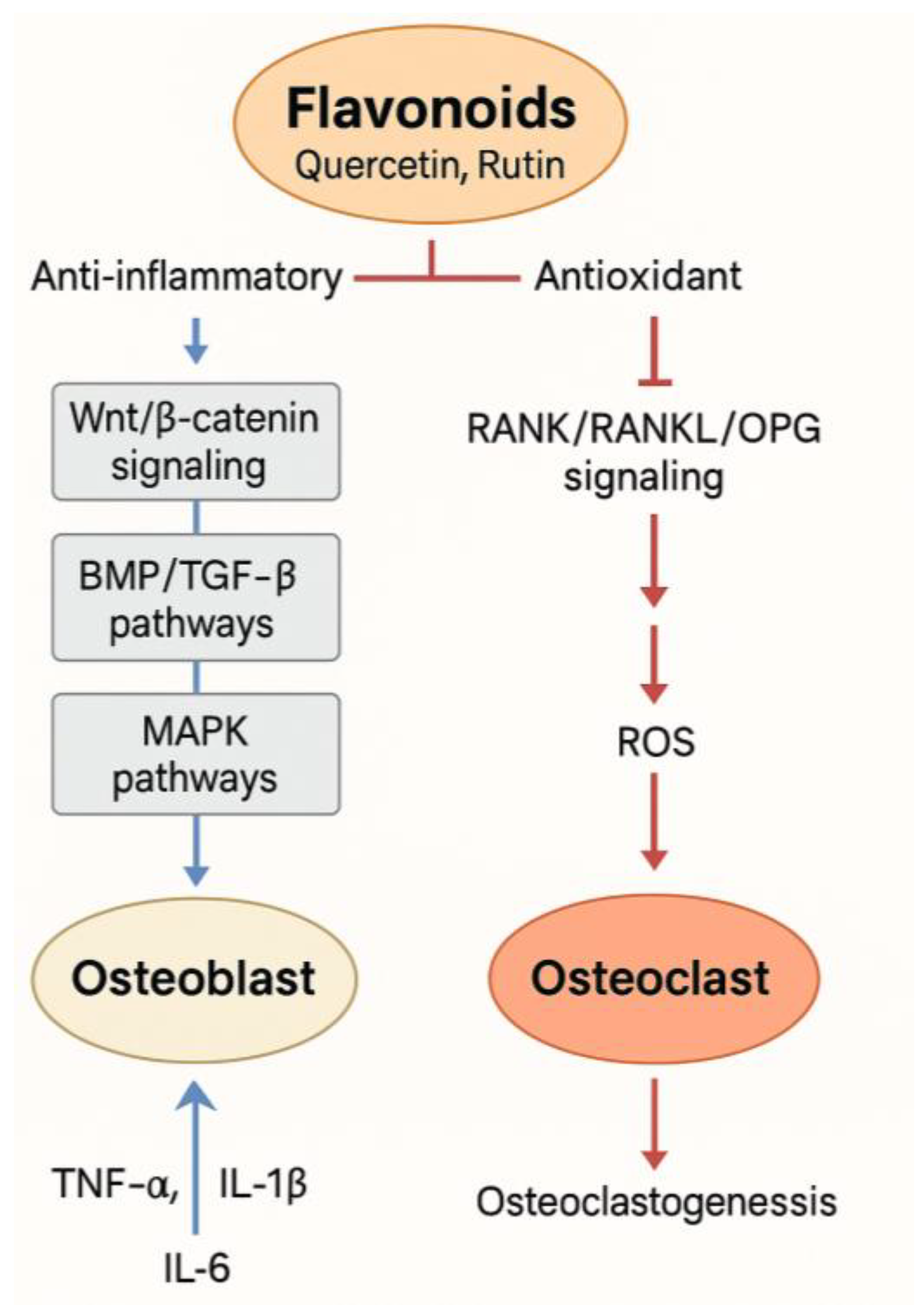
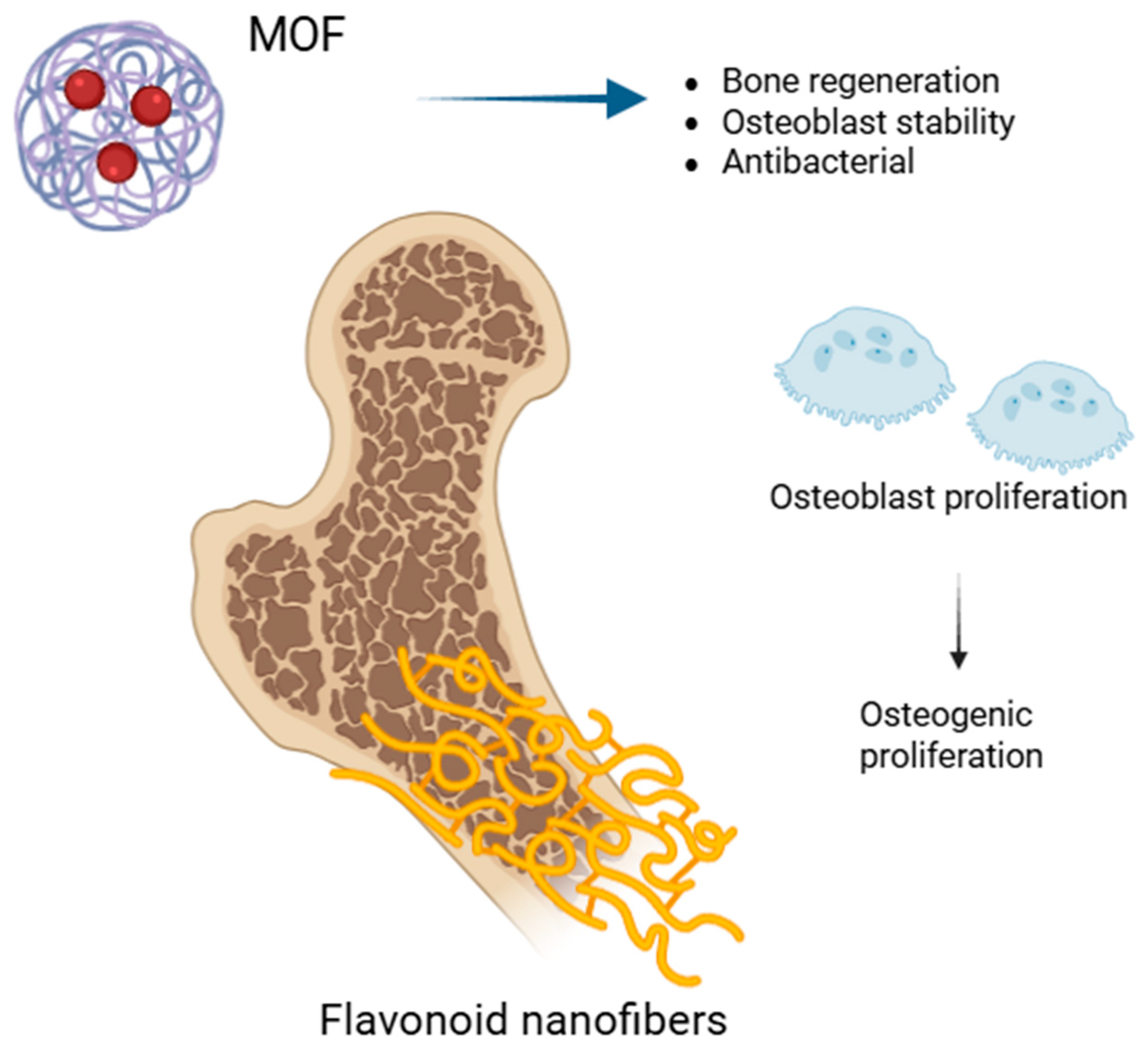
| Flavonoid | Metal Ion | System Type | Key Findings | Reference |
|---|---|---|---|---|
| Quercetin | Cu(II) | Hydrogel/Complex | ↑ VEGF, ↑ ALP (45%), ↑ OCN (osteogenesis) | [48] |
| Kaempferol | Zn(II) | Zebrafish Model | ↑ Bone mineralization, enhanced vertebral regeneration | [69] |
| Morin | Zn(II) | In Vitro Culture | ↑ ALP, ↑ COL1A1, enhanced osteogenic differentiation | [70] |
| Rutin | Zn(II) | hDPSC Culture | ↑ Mineralization, osteogenic gene expression | [71] |
| Silibinin | Cu(II) | In Vitro Culture | ↑ Osteogenesis (1.6×), increased OCN, ALP expression | [72] |
| Flavonoid/Agent | Experimental Models | Main Quantitative Outcomes | Limitations/Comments | Reference |
|---|---|---|---|---|
| Flavonoid derivatives | In vitro (osteoblasts, fibroblasts) | +40% ALP activity; −60% IL-6/TNF-α secretion | No in vivo data; only short-term assays | [120] |
| Quercitrin | In vitro (osteoblasts) | +35% osteoblast viability; −50% bacterial adhesion | Lacked mechanical evaluation | [105] |
| Rutin | In vivo (osteoporotic rats) | +32% BV/TV; +28% BIC | Layer stability over time uncertain | [110] |
| Quercitrin | In vitro and in vivo (rat tibia) | −50% osteoclast activity; −45% bone resorption | No large animal models | [111] |
| Kaempferol | In vivo (osteoporotic rats) | +27% pull-out force; +20% BV/TV | Lack of mechanistic pathway elucidation | [112] |
| Quercetin | In vitro (osteoporotic-like conditions) | +48% ALP activity; 2.3-fold Runx2 increase | No in vivo confirmation | [113] |
| Strontium + icariin | In vitro and in vivo (osteoporotic rats) | +35% BV/TV; +30% push-out strength | Manufacturing complexity | [114] |
| Quercetin | In vitro and in vivo | +40% bone volume; −50% IL-1β, TNF-α levels | High cost and technical complexity | [115] |
| Polyphenol + gelatin | In vitro and in vivo | +30% ALP; +25% BIC | Mechanical resilience questionable | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huzum, B.; Lungu, I.I.; Alexa, O.; Sirbu, P.D.; Cionca, V.D.; Corciova, A.; Lungu, A.; Hancianu, M.; Serban, I.L.; Cioanca, O. Nanotechnology in Osteogenesis and Inflammation Management: Metal–Organic Frameworks, Metal Complexes, and Biomaterials for Bone Restoration. Biomedicines 2025, 13, 1597. https://doi.org/10.3390/biomedicines13071597
Huzum B, Lungu II, Alexa O, Sirbu PD, Cionca VD, Corciova A, Lungu A, Hancianu M, Serban IL, Cioanca O. Nanotechnology in Osteogenesis and Inflammation Management: Metal–Organic Frameworks, Metal Complexes, and Biomaterials for Bone Restoration. Biomedicines. 2025; 13(7):1597. https://doi.org/10.3390/biomedicines13071597
Chicago/Turabian StyleHuzum, Bogdan, Ionut Iulian Lungu, Ovidiu Alexa, Paul Dan Sirbu, Viorel Dan Cionca, Andreia Corciova, Andreea Lungu, Monica Hancianu, Ionela Lacramioara Serban, and Oana Cioanca. 2025. "Nanotechnology in Osteogenesis and Inflammation Management: Metal–Organic Frameworks, Metal Complexes, and Biomaterials for Bone Restoration" Biomedicines 13, no. 7: 1597. https://doi.org/10.3390/biomedicines13071597
APA StyleHuzum, B., Lungu, I. I., Alexa, O., Sirbu, P. D., Cionca, V. D., Corciova, A., Lungu, A., Hancianu, M., Serban, I. L., & Cioanca, O. (2025). Nanotechnology in Osteogenesis and Inflammation Management: Metal–Organic Frameworks, Metal Complexes, and Biomaterials for Bone Restoration. Biomedicines, 13(7), 1597. https://doi.org/10.3390/biomedicines13071597








