Immunohistochemical Analysis of Mastocyte Inflammation: A Comparative Study of COPD Associated with Tobacco Smoking and Wood Smoke Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Selection of Histological Samples
2.2. Tissue Procurement and Processing
2.3. Definition of Lung Regions and Morphometric Analysis
2.4. Measurements by Pathologists
- (1)
- Inflammation: inflammatory infiltrate of lymphocytes in the airway wall.
- (2)
- Peribronchiolar fibrosis: the amount of fibroconnective tissue deposited in the wall of the airway
- (3)
- Goblet cell metaplasia: replacement of a mature epithelial cell by another mature cell different from the original
- (4)
- Intensity of the affinity of anti-CD34+ for the microvascular endothelium.
- (5)
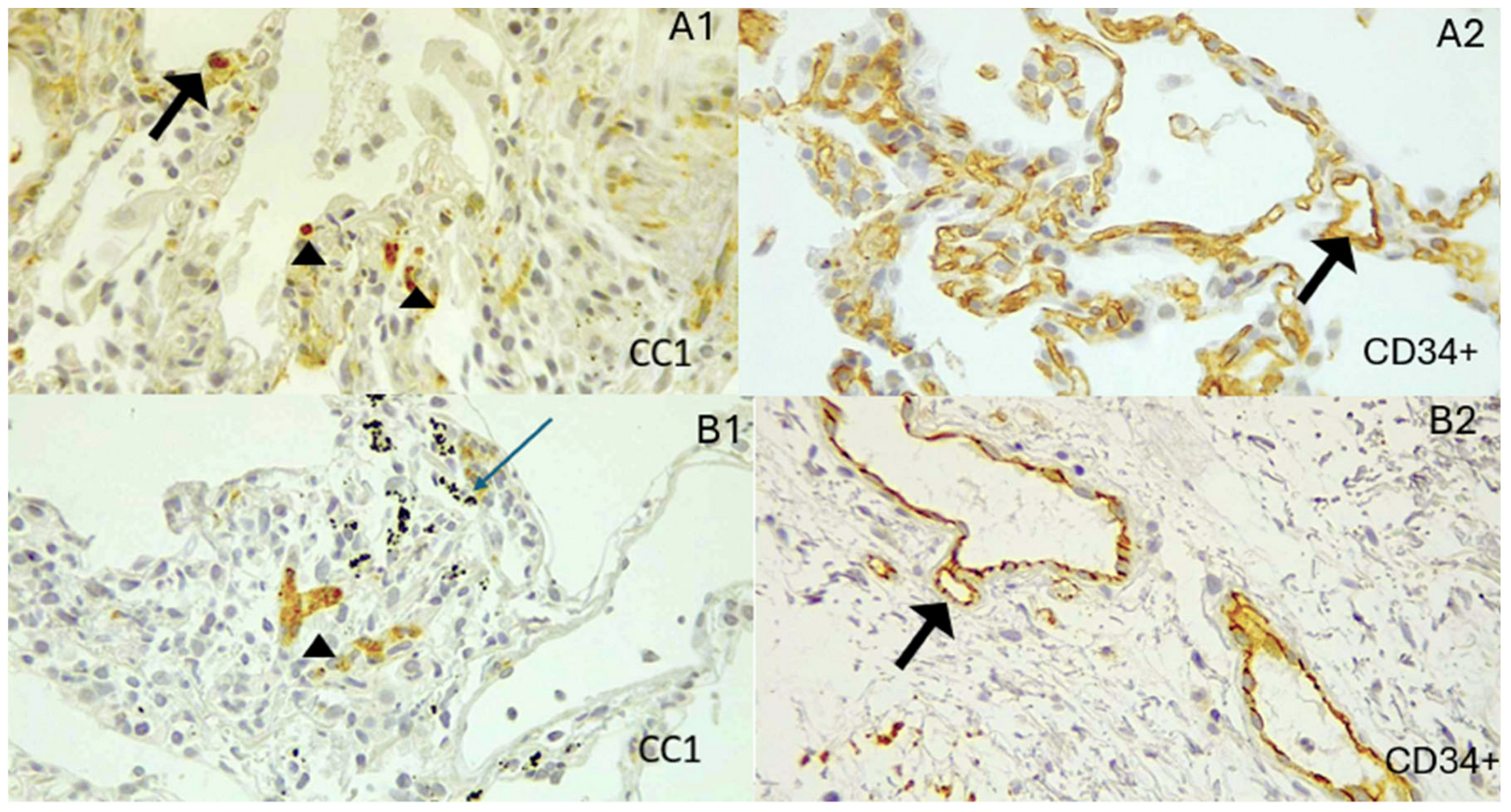
- A single pathologist counted mast cells marked by CC1 in the peribronchial region of the small airway. The pathologist performed two counts, separated by a 14-days, and averaged them to produce a repeatable measurement.
2.5. Statistical Analysis
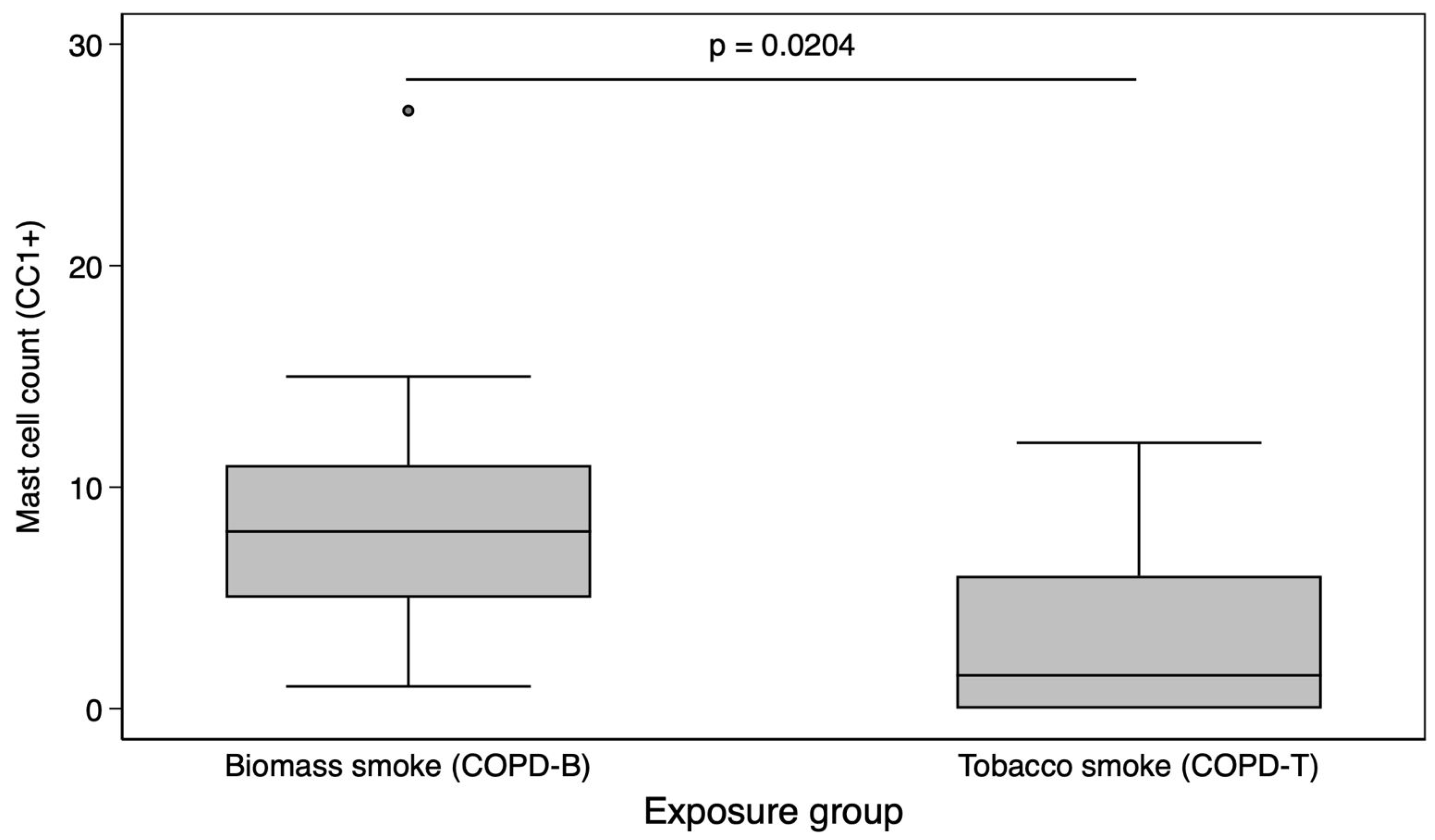
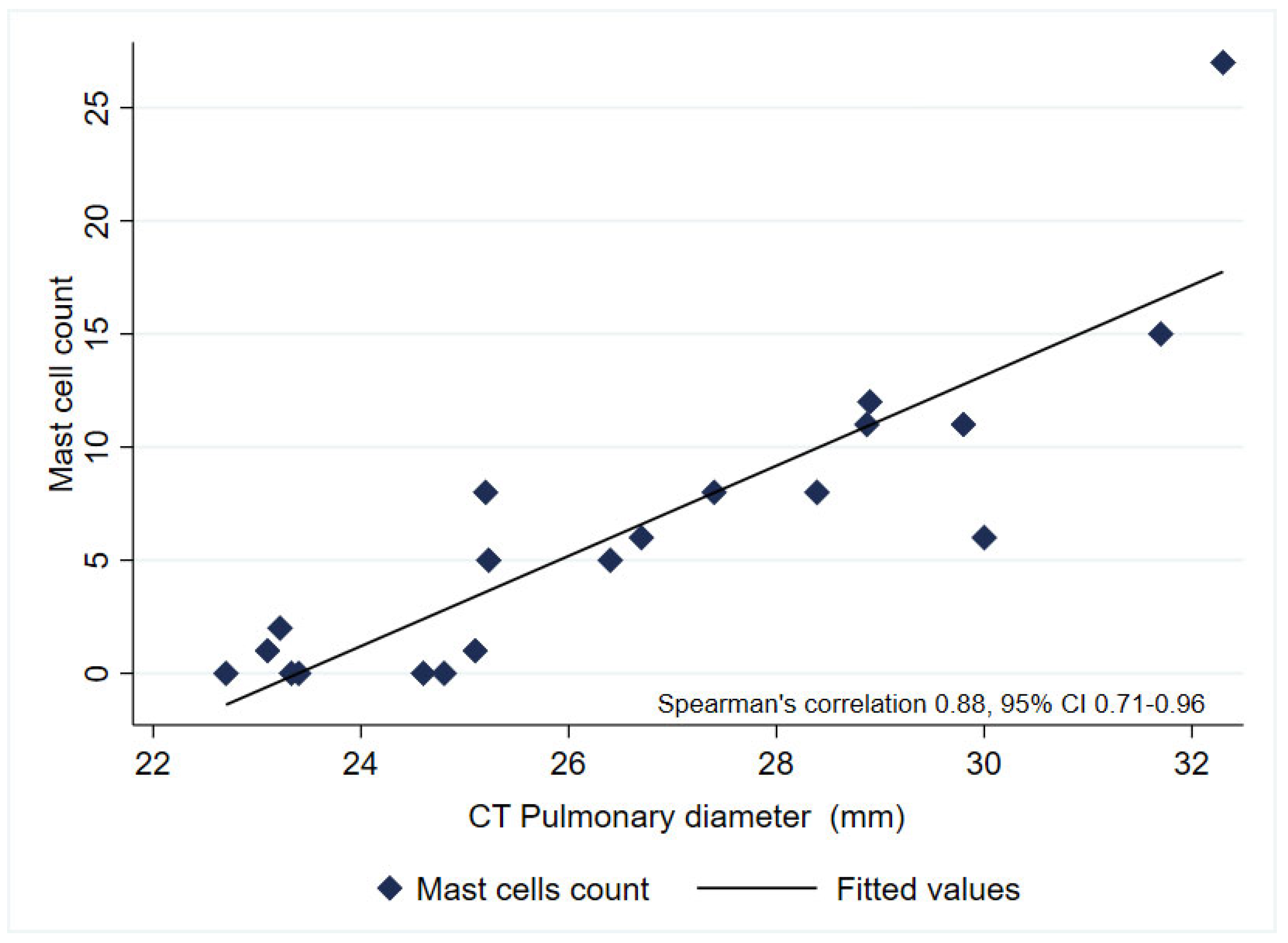
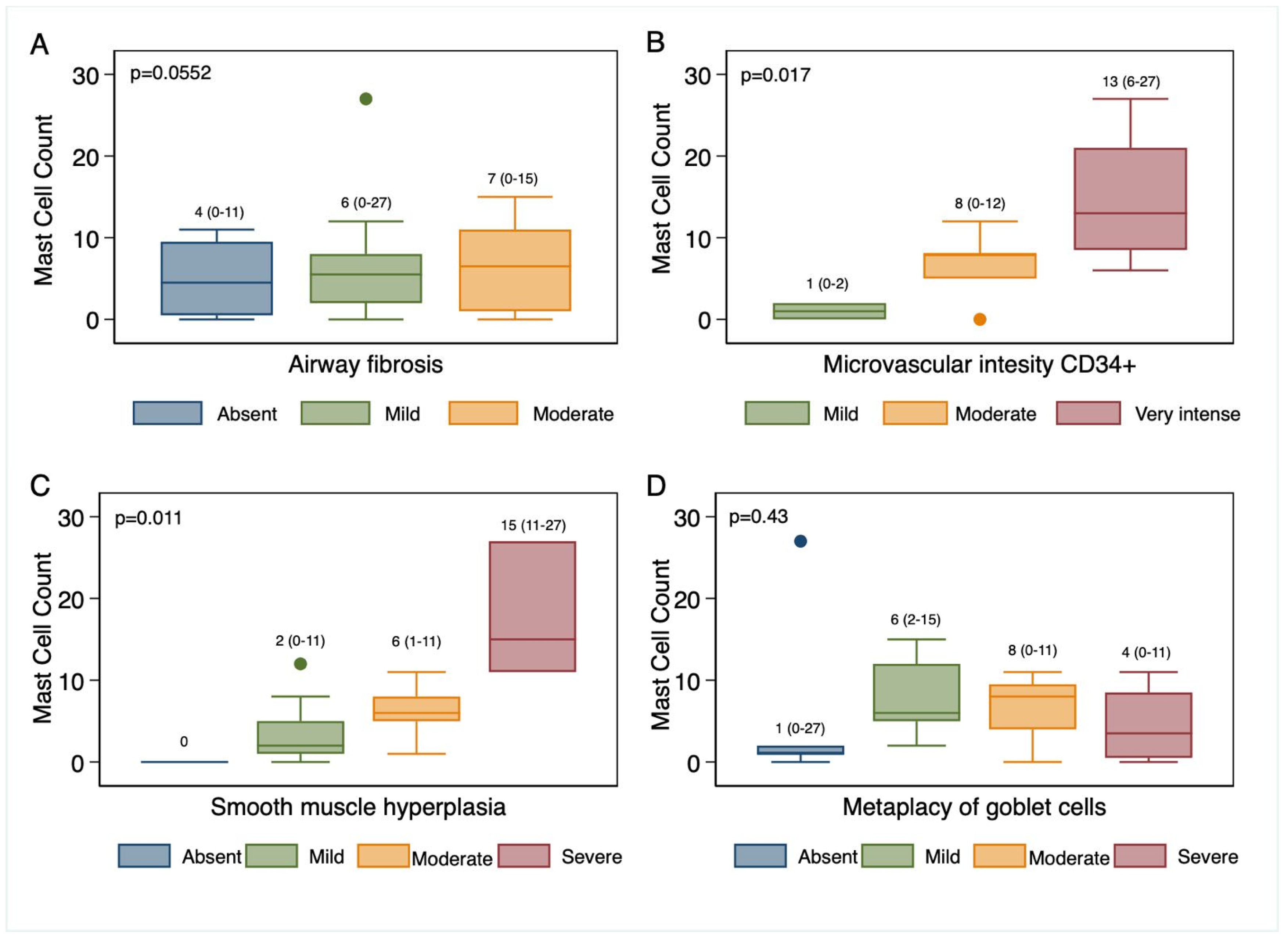
3. Results
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Padilla, J.; Thirión-Romero, I.; Robles-Hernández, R.; Cagney, J.; Razo, C.; Rios-Blancas, M. Enfermedades respiratorias en México. Análisis del estudio Global Burden of Disease 2021. Gac. Méd. Méx. 2023, 159, 599–613. [Google Scholar] [CrossRef]
- Fernandes, L.; Rane, S.; Mandrekar, S.; Mesquita, A.M. Eosinophilic Airway Inflammation in Patients with Stable Biomass Smoke- versus Tobacco Smoke-Associated Chronic Obstructive Pulmonary Disease. J. Health Pollut. 2019, 9, 191209. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, T.; Li, X.; Li, D.; Liao, Z.; Shen, Y.; Xu, D.; Chen, L.; Wen, F. Clinical Predictors of High Blood Eosinophils in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon. Dis. 2021, 16, 2467–2474. [Google Scholar] [CrossRef]
- Han, M.; Jin, Z.; Zhao, Y.; Zhang, Y.; Han, W.; Zhang, M. Indoor Thermal Environment Evaluation for Emergency Medical Tents in Heating Season: Onsite Testing and Case Study in China. Atmosphere 2024, 15, 388. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory Mechanisms in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Salvi, S.S.; Brashier, B.B.; Londhe, J.; Pyasi, K.; Vincent, V.; Kajale, S.S.; Tambe, S.; Mandani, K.; Nair, A.; Mak, S.M.; et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 50. [Google Scholar] [CrossRef]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Paré, P.D.; Müller, N.L.; Silva, C.I.S.; Rojas, C.E.; et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef]
- Rivera, R.M.; Cosio, M.G.; Ghezzo, H.; Salazar, M.; Pérez-Padilla, R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int. J. Tuberc. Lung Dis. 2008, 12, 972–977. [Google Scholar]
- Ramírez-Venegas, A.; Torres-Duque, C.A.; Guzmán-Bouilloud, N.E.; González-García, M.; Sansores, R.H.; Ramírez-Venegas, A.; Torres-Duque, C.A.; Guzmán-Bouilloud, N.E.; González-García, M.; Sansores, R.H. Small Airway Disease in COPD Associated to Biomass Exposure. Rev. Investig. Clín. 2019, 71, 70–78. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, Y.; Jiang, C.; Zhao, Z.; He, F.; Ran, P. Small airway disease: A different phenotype of early stage COPD associated with biomass smoke exposure. Respirology 2018, 23, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.; Salas, J.; Martinez-Guerra, M.L.; Gómez, A.; Martinez, C.; Portales, A.; Palomar, A.; Villegas, M.; Barrios, R. Pulmonary Arterial Hypertension and Cor Pulmonale Associated with Chronic Domestic Woodsmoke Inhalation. Chest 1993, 103, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sertogullarindan, B.; Gumrukcuoglu, H.A.; Sezgi, C.; Akil, M.A. Frequency of Pulmonary Hypertension in Patients with COPD due to Biomass Smoke and Tobacco Smoke. Int. J. Med. Sci. 2012, 9, 406–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Padmavati, S.; Pathak, S.N. Chronic cor pulmonale in Delhi: A study of 127 cases. Circulation 1959, 20, 343–352. [Google Scholar] [CrossRef]
- Soltani, A.; Ewe, Y.P.; Lim, Z.S.; Sohal, S.S.; Reid, D.; Weston, S.; Wood-Baker, R.; Walters, E.H. Mast cells in COPD airways: Relationship to bronchodilator responsiveness and angiogenesis. Eur Respir J. 2012, 39, 1361–1367. [Google Scholar] [CrossRef]
- Gosman, M.M.; Postma, D.S.; Vonk, J.M.; Rutgers, B.; Lodewijk, M.; Smith, M.; Luinge, M.A.; ten Hacken, N.H.; Timens, W. Association of mast cells with lung function in chronic obstructive pulmonary disease. Respir. Res. 2008, 9, 64. [Google Scholar] [CrossRef]
- Kosanovic, D.; Dahal, B.K.; Peters, D.M.; Seimetz, M.; Wygrecka, M.; Hoffmann, K.; Antel, J.; Reiss, I.; Ghofrani, H.A.; Weissmann, N.; et al. Histological Characterization of Mast Cell Chymase in Patients with Pulmonary Hypertension and Chronic Obstructive Pulmonary Disease. Pulm. Circ. 2014, 4, 128–136. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, Y.; Tian, J.; Yao, W.; Li, J.; Li, B.; Ran, P. Risk of COPD From Exposure to Biomass Smoke: A Metaanalysis. Chest 2010, 138, 20–31. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Farha, S.; Asosingh, K.; Xu, W.; Sharp, J.; George, D.; Comhair, S.; Park, M.; Wilson Tang, W.H.; Loyd, J.E.; Theil, K.; et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: A link to the intrinsic myeloid abnormalities. Blood 2011, 117, 3485–3493. [Google Scholar] [CrossRef]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Venegas, A.; Sansores, R.H.; Quintana-Carrillo, R.H.; Velázquez-Uncal, M.; Hernandez-Zenteno, R.J.; Sánchez-Romero, C.; Velazquez-Montero, A.; Flores-Trujillo, F. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am. J. Respir. Crit. Care Med. 2014, 190, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Quintero, B.; Martínez-Espinosa, I.; Pérez-Padilla, R. Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Faiz, A.; Pavlidis, S.; Kuo, C.-H.; Rowe, A.; Hiemstra, P.S.; Timens, W.; Berg, M.; Wisman, M.; Guo, Y.-K.; Djukanović, R.; et al. Th2 high and mast cell gene signatures are associated with corticosteroid sensitivity in COPD. Thorax 2023, 78, 335–343. [Google Scholar] [CrossRef]
- Winter, N.A.; Gibson, P.G.; McDonald, V.M.; Fricker, M. Sputum Gene Expression Reveals Dysregulation of Mast Cells and Basophils in Eosinophilic COPD. Int. J. Chron. Obs. Pulm. Dis. 2021, 16, 2165–2179. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Mortaz, E.; Amani, S.; Tiotiu, A.; Folkerts, G.; Adcock, I.M. The Role of Mast Cells in IgE-Independent Lung Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 377–387. [Google Scholar] [CrossRef]
- Roos, A.B.; Mori, M.; Gura, H.K.; Lorentz, A.; Bjermer, L.; Hoffmann, H.J.; Erjefält, J.S.; Stampfli, M.R. Increased IL-17RA and IL-17RC in End-Stage COPD and the Contribution to Mast Cell Secretion of FGF-2 and VEGF. Respir. Res. 2017, 18, 48. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef]
- Shyam Prasad Shetty, B.; Chaya, S.K.; Kumar, V.S.; Mahendra, M.; Jayaraj, B.S.; Lokesh, K.S.; Ganguly, K.; Mahesh, P.A. Inflammatory Biomarkers Interleukin 1 Beta (IL-1β) and Tumour Necrosis Factor Alpha (TNF-α) Are Differentially Elevated in Tobacco Smoke Associated COPD and Biomass Smoke Associated COPD. Toxics 2021, 9, 72. [Google Scholar] [CrossRef]
- Silva, R.; Oyarzún, M.; Olloquequi, J. Mecanismos patogénicos en la enfermedad pulmonar obstructiva crónica causada por exposición a humo de biomasa. Arch. Bronconeumol. 2015, 51, 285–292. [Google Scholar] [CrossRef]
- Eapen, M.S.; Lu, W.; Dey, S.; Chia, C.; Hardikar, A.; Hassan, M.I.; Bhattarai, P.; Gaikwad, A.V.; Das, S.; Hansbro, P.M.; et al. Differential expression of mast cells in the small airways and alveolar septa of current smokers and patients with small airway disease and COPD. ERJ Open Res. 2024, 10, 00579–2023. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; McNagny, K.M. CD34 is a key regulator of hematopoietic stem cell trafficking to bone marrow and mast cell progenitor trafficking in the periphery. Microcirculation 2009, 16, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.R.S.; Gonzalez, N.C.; Wood, J.G. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J. Appl. Physiol. 2003, 94, 325–334. [Google Scholar] [CrossRef]
- Gulliksson, M.; Carvalho, R.F.S.; Ullerås, E.; Nilsson, G. Mast cell survival and mediator secretion in response to hypoxia. PLoS ONE 2010, 5, e12360. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Mayar, M.E. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am. J. Respir. Crit. Care Med. 2006, 173, 393–397. [Google Scholar] [CrossRef]
- Meneghini, A.C.; Koenigkam-Santos, M.; Pereira, M.C.; Tonidandel, P.R.; Terra-Filho, J.; Cunha, F.Q.; de Menezes, M.B.; Vianna, E.O. Biomass smoke COPD has fewer tomographic abnormalities but worse hypoxemia compared with tobacco COPD. Braz. J. Med. Biol. Res. 2019, 52, e8233. [Google Scholar] [CrossRef]
- Ballarin, A.; Bazzan, E.; Zenteno, R.H.; Turato, G.; Baraldo, S.; Zanovello, D.; Mutti, E.; Hogg, J.C.; Saetta, M.; Cosio, M.G. Mast cell infiltration discriminates between histopathological phenotypes of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 233–239. [Google Scholar] [CrossRef]
- Liu, G.; Jarnicki, A.G.; Paudel, K.R.; Lu, W.; Wadhwa, R.; Philp, A.M.; Van Eeckhoutte, H.; Marshall, J.E.; Malyla, V.; Katsifis, A.; et al. Adverse roles of mast cell chymase-1 in COPD. Eur. Respir. J. 2022, 60, 2101431. [Google Scholar] [CrossRef]
- Li, S.; Qu, L.; Zhou, L.; Zhan, N.; Liu, L.; Ling, Y.; Chen, Q.; Lai, W.; Lin, N.; Li, J. Biomass fuels related-PM2.5 promotes lung fibroblast-myofibroblast transition through the PI3K/AKT/TRPC1 pathway. Ecotoxicol. Environ. Saf. 2024, 276, 116309. [Google Scholar] [CrossRef]
- Huang, L.; Xu, J.; Zhou, H.; Li, H.; Cao, W.; Pu, J. NR1D1 mitigates IL-17a-induced small airway remodeling in biomass smoke-induced COPD. Toxicol. Lett. 2025, 409, 74–86. [Google Scholar] [CrossRef]
- Catana, O.M.; Nemes, A.F.; Cioboata, R.; Toma, C.L.; Mitroi, D.M.; Calarasu, C.; Streba, C.T. Leptin and Insulin in COPD: Unveiling the Metabolic-Inflammatory Axis—A Narrative Review. J. Clin. Med. 2025, 14, 2611. [Google Scholar] [CrossRef]
| COPD–Biomass (n = 10) | COPD–Tobacco (n = 10) | p Value | |
|---|---|---|---|
| Female, n (%) ¥ | 8 (80) | 1 (10) | 0.003 |
| Age, median (p25-75) * | 63 (46–78) | 68.5 (55–76) | 0.382 |
| Pack-years, median (p25-75) * | - | 45 (37.5–68) | - |
| Hour–years of biomass exposure, median (p25-75) | 238 (180–308) | - | - |
| Diabetes, n (%) ¥ | 4 (40) | 2 (20) | 0.314 |
| Previous COPD diagnosis, n (%) | 7 (70) | 9 (90) | 0.291 |
| Arterial hypertension, n (%) ¥ | 5 (50) | 5 (50) | 0.672 |
| Ischemic heart disease, n (%) ¥ | 1 (10) | 5 (50) | 0.070 |
| Lung cancer biopsy, n (%) ¥ | 7 (70) | 6 (60) | 0.5 |
| Non specific inflammation biopsy, n (%) ¥ | 3 (30) | 4 (40) | |
| FEV1/FVC, median (p25-75) * | 0.53 (0.47–0.63) | 0.61 (0.55–0.87) | 0.158 |
| FEV1 (L), median (p25-75) * | 1.02 (0.97–1.31) | 1.8 (0.88–2.58) | 0.408 |
| FEV1 %, median (p25-75) * | 60 (49–75) | 65 (39–78) | 0.796 |
| FVC (L), median (p25-75) * | 2.01 (1.9–2.1) | 2.66 (1.4–4.2) | 0.814 |
| FVC %, median (p25-75) * | 75 (64–89) | 78.5 (45–101) | 0.10 |
| Pulmonary artery diameter (mm), median (p25-75) * | 27.9 (25.2–29.8) | 24.9 (23.3–29.4) | 0.143 |
| Kappa Coefficient | Agreement (%) | |
|---|---|---|
| Small airway inflammation * | 0.869 | 90 |
| Peribronchiolar fibrosis * | 0.771 | 85 |
| Pigment deposit * | 0.921 | 95 |
| Smooth muscle hyperplasia * | 0.773 | 85 |
| Goblet cell metaplasia * | 0.855 | 95 |
| Microvascular intensity of CD 34+ * | 0.719 | 80 |
| COPD–Biomass (n = 10) | COPD–Tobacco (n = 10) | |
|---|---|---|
| Small airway inflammation ∂ | ||
| Mild + | 5 | 4 |
| Moderate ++ | 4 | 5 |
| Severe +++ | 1 | 1 |
| Peribronchiolar fibrosis | ||
| Absent − | 0 | 3 |
| Mild+ | 4 | 6 |
| Moderate++ β | 6 | 1 |
| Pigment deposit ∂ | ||
| Mild + | 6 | 5 |
| Moderate ++ | 3 | 3 |
| Severe +++ | 1 | 2 |
| Smooth muscle hyperplasia ∂ | ||
| Absent − | 1 | 3 |
| Mild + | 0 | 2 |
| Moderate ++ | 6 | 4 |
| Severe +++ | 3 | 1 |
| Goblet cell metaplasia ∂ | ||
| Absent − | 1 | 3 |
| Mild + | 0 | 2 |
| Moderate ++ | 6 | 4 |
| Severe +++ | 3 | 1 |
| Microvascular intensity of CD34+ ∂ | ||
| Absent − | 0 | 2 |
| Mild + | 2 | 4 |
| Moderate ++ | 4 | 3 |
| Severe +++ | 4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-Hernández, R.; Rivera, R.M.; Páramo-Pérez, M.; Quiroz-Camacho, D.M.; Centeno-Saenz, G.I.; Bedolla-Tinoco, A.; Maya-García, M.C.; Pérez-Padilla, R. Immunohistochemical Analysis of Mastocyte Inflammation: A Comparative Study of COPD Associated with Tobacco Smoking and Wood Smoke Exposure. Biomedicines 2025, 13, 1593. https://doi.org/10.3390/biomedicines13071593
Robles-Hernández R, Rivera RM, Páramo-Pérez M, Quiroz-Camacho DM, Centeno-Saenz GI, Bedolla-Tinoco A, Maya-García MC, Pérez-Padilla R. Immunohistochemical Analysis of Mastocyte Inflammation: A Comparative Study of COPD Associated with Tobacco Smoking and Wood Smoke Exposure. Biomedicines. 2025; 13(7):1593. https://doi.org/10.3390/biomedicines13071593
Chicago/Turabian StyleRobles-Hernández, Robinson, Rosa María Rivera, Marcos Páramo-Pérez, Dulce Mariana Quiroz-Camacho, Gustavo I. Centeno-Saenz, Alan Bedolla-Tinoco, María C. Maya-García, and Rogelio Pérez-Padilla. 2025. "Immunohistochemical Analysis of Mastocyte Inflammation: A Comparative Study of COPD Associated with Tobacco Smoking and Wood Smoke Exposure" Biomedicines 13, no. 7: 1593. https://doi.org/10.3390/biomedicines13071593
APA StyleRobles-Hernández, R., Rivera, R. M., Páramo-Pérez, M., Quiroz-Camacho, D. M., Centeno-Saenz, G. I., Bedolla-Tinoco, A., Maya-García, M. C., & Pérez-Padilla, R. (2025). Immunohistochemical Analysis of Mastocyte Inflammation: A Comparative Study of COPD Associated with Tobacco Smoking and Wood Smoke Exposure. Biomedicines, 13(7), 1593. https://doi.org/10.3390/biomedicines13071593








