Advances in Cerebellar TMS Therapy: An Updated Systematic Review on Multi-Session Interventions
Abstract
1. Introduction
Cerebellar Anatomy and Functions
2. Materials and Methods
2.1. Identifying the Review Questions
2.2. Inclusion Criteria
2.3. Search Strategy
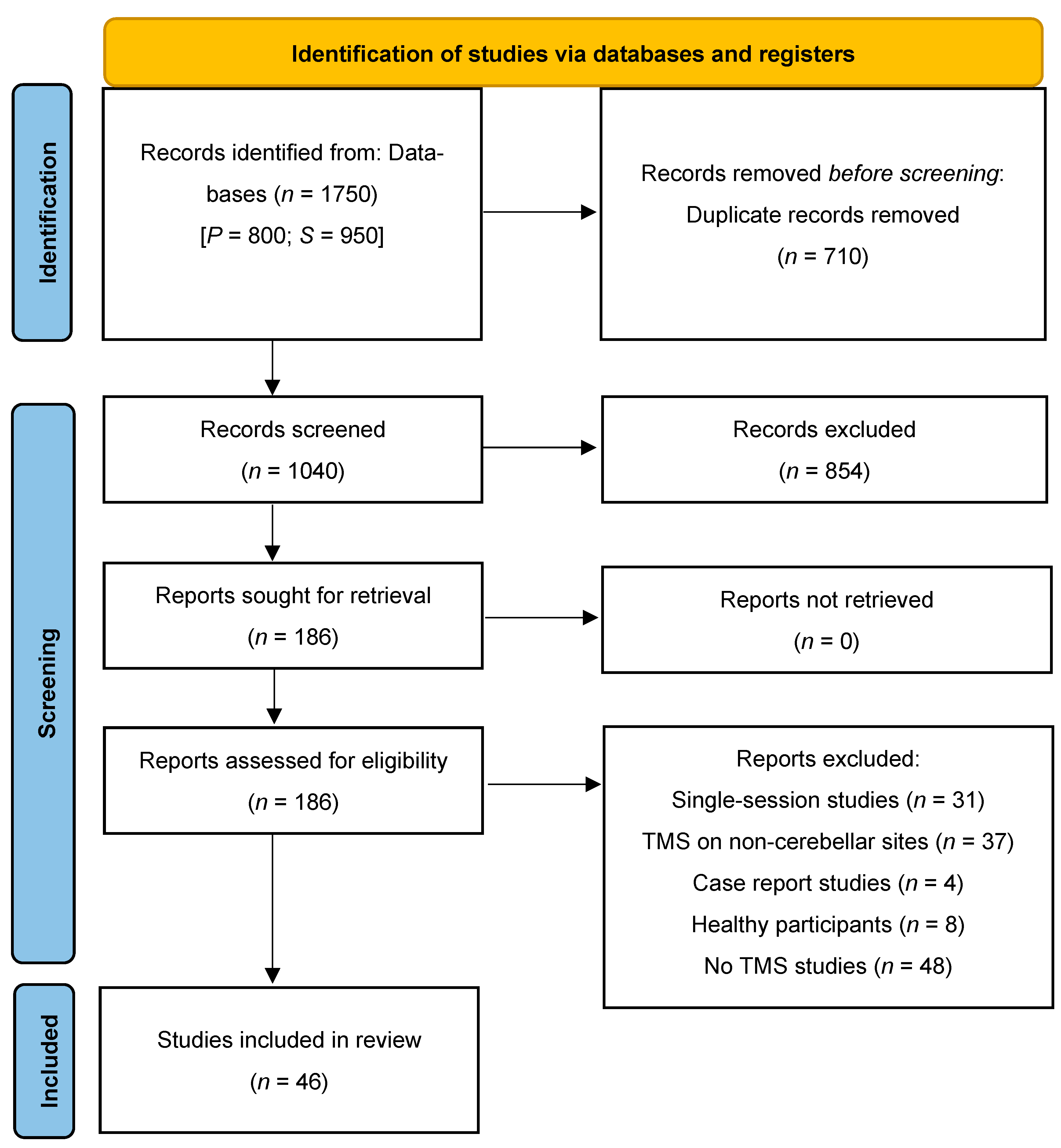
2.4. Evidence Screening and Selection
2.5. Data Extraction and Charting
2.6. Risk of Bias Assessment
3. Results
3.1. Study Selection for Review
3.2. TMS Protocols
3.3. Studies Methodology and Characteristics
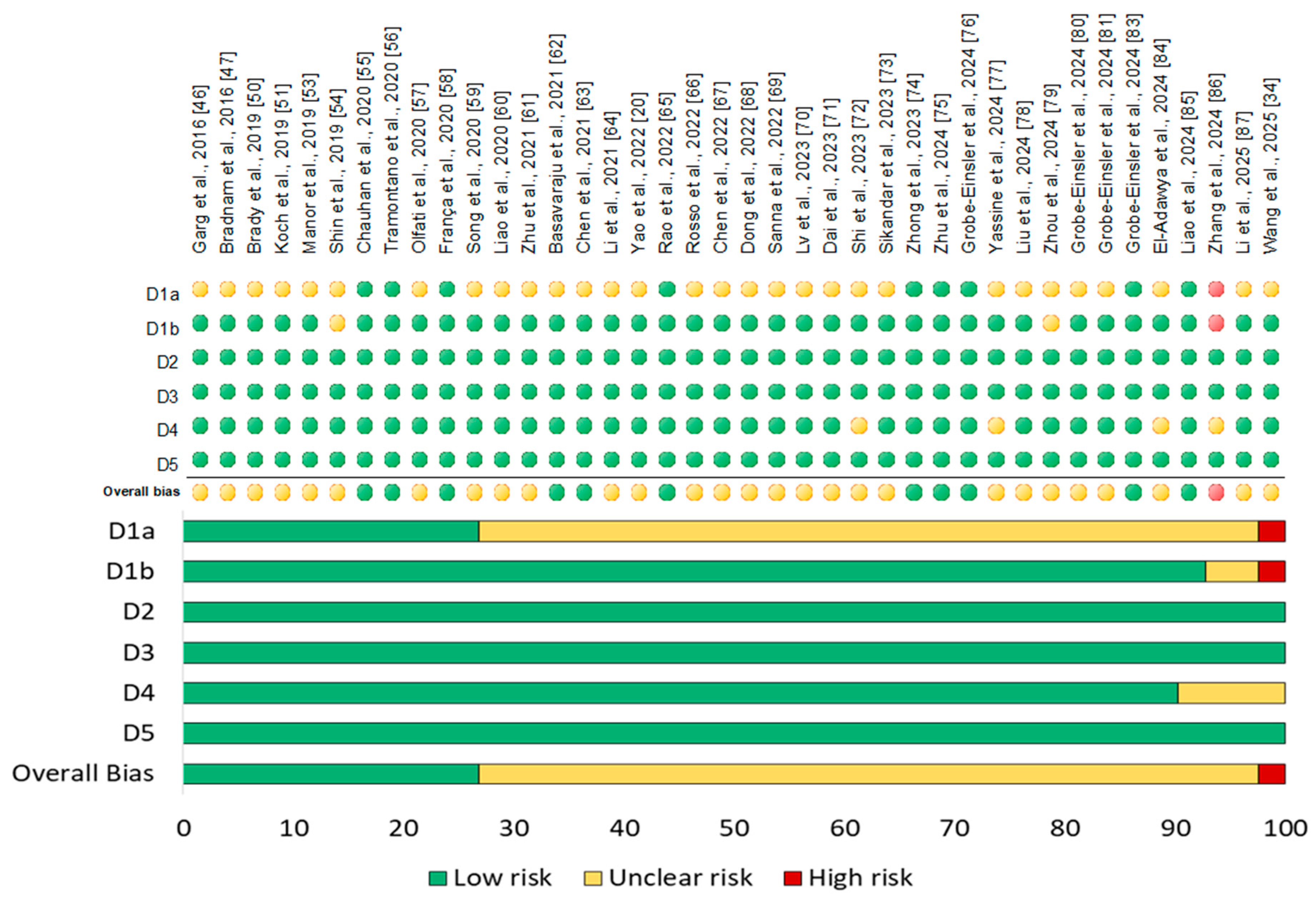
3.4. Risk of Bias
4. Discussion
4.1. Movement Disorders
4.2. Post-Stroke Recovery
4.3. Non-Motor Neurological Disorders
4.4. Psychiatric Disorders
4.5. Technical Challenges and Recommendations
4.6. Tolerability, Safety and Ethical Concerns
4.7. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adamaszek, M.; Cattaneo, Z.; Ciricugno, A.; Chatterjee, A. The Cerebellum and Beauty: The Impact of the Cerebellum in Art Experience and Creativity. Adv. Exp. Med. Biol. 2022, 1378, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, Z.; Ferrari, C.; Ciricugno, A.; Heleven, E.; Schutter, D.J.L.G.; Manto, M.; Van Overwalle, F. New Horizons on Non-invasive Brain Stimulation of the Social and Affective Cerebellum. Cerebellum 2022, 21, 482–496. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The Cerebellar Cognitive Affective Syndrome and the Neuropsychiatry of the Cerebellum. In Handbook of the Cerebellum and Cerebellar Disorders; Manto, M.U., Gruol, D.L., Schmahmann, J.D., Koibuchi, N., Sillitoe, R.V., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1955–1993. [Google Scholar] [CrossRef]
- van den Berg, N.S.; Huitema, R.B.; Spikman, J.M.; Luijckx, G.-J.; de Haan, E.H.F. Impairments in Emotion Recognition and Risk-Taking Behavior After Isolated, Cerebellar Stroke. Cerebellum 2020, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Pallarès-Sastre, M.; García, M.; Rouco-Axpe, I.; Amayra, I. A systematic review of social cognition in hereditary ataxia patients: Evidence from neuroimaging studies. Brain Res. 2024, 1827, 148765. [Google Scholar] [CrossRef] [PubMed]
- Ajith, M.; Aycock, D.M.; Tone, E.B.; Liu, J.; Misiura, M.B.; Ellis, R.; Plis, S.M.; King, T.Z.; Dotson, V.M.; Calhoun, V. A deep learning approach for mental health quality prediction using functional network connectivity and assessment data. Brain Imaging Behav. 2024, 18, 630–645. [Google Scholar] [CrossRef]
- Romer, A.L.; Knodt, A.R.; Houts, R.; Brigidi, B.D.; Moffitt, T.E.; Caspi, A.; Hariri, A.R. Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol. Psychiatry 2018, 23, 1084–1090. [Google Scholar] [CrossRef]
- Kulkarni, M.; Kent, J.S.; Park, K.; Guell, X.; Anteraper, S. Resting-state functional connectivity-based parcellation of the human dentate nucleus: New findings and clinical relevance. Brain Struct. Funct. 2023, 228, 1799–1810. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Somaa, F.A.; de Graaf, T.A.; Sack, A.T. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef]
- Kallioniemi, E.; Lei, Y.; Jo, H.J. Editorial: Transcranial Magnetic Stimulation (TMS) in motor control and motor rehabilitation: Current trends and future directions. Front. Hum. Neurosci. 2025, 19, 1595762. [Google Scholar] [CrossRef]
- Gong, C.; Hu, H.; Peng, X.-M.; Li, H.; Xiao, L.; Liu, Z.; Zhong, Y.-B.; Wang, M.-Y.; Luo, Y. Therapeutic effects of repetitive transcranial magnetic stimulation on cognitive impairment in stroke patients: A systematic review and meta-analysis. Front. Hum. Neurosci. 2023, 17, 1177594. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.C.; Green Bernacki, C.; Helmer, A.; Pinninti, N.; O’reardon, J.P. Efficacy of Transcranial Magnetic Stimulation (TMS) in the Treatment of Schizophrenia: A Review of the Literature to Date. Innov. Clin. Neurosci. 2015, 12, 12–19. [Google Scholar] [PubMed]
- Lorentzen, R.; Nguyen, T.D.; McGirr, A.; Hieronymus, F.; Østergaard, S.D. The efficacy of transcranial magnetic stimulation (TMS) for negative symptoms in schizophrenia: A systematic review and meta-analysis. Schizophrenia 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- De Risio, L.; Borgi, M.; Pettorruso, M.; Miuli, A.; Ottomana, A.M.; Sociali, A.; Martinotti, G.; Nicolò, G.; Macrì, S.; di Giannantonio, M.; et al. Recovering from depression with repetitive transcranial magnetic stimulation (rTMS): A systematic review and meta-analysis of preclinical studies. Transl. Psychiatry 2020, 10, 393. [Google Scholar] [CrossRef]
- Cohen, S.L.; Bikson, M.; Badran, B.W.; George, M.S. A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices. Brain Stimul. 2021, 15, 73–75. [Google Scholar] [CrossRef]
- Ciricugno, A.; Oldrati, V.; Cattaneo, Z.; Leggio, M.; Urgesi, C.; Olivito, G. Cerebellar Neurostimulation for Boosting Social and Affective Functions: Implications for the Rehabilitation of Hereditary Ataxia Patients. Cerebellum 2024, 23, 1651–1677. [Google Scholar] [CrossRef]
- Gatti, D.; Rinaldi, L.; Vecchi, T.; Ferrari, C. Understanding cerebellar cognitive and social functions: Methodological challenges and new directions for future transcranial magnetic stimulation studies. Curr. Opin. Behav. Sci. 2023, 53, 101300. [Google Scholar] [CrossRef]
- França, C.; de Andrade, D.C.; Teixeira, M.J.; Galhardoni, R.; Silva, V.; Barbosa, E.R.; Cury, R.G. Effects of cerebellar neuromodulation in movement disorders: A systematic review. Brain Stimul. 2017, 11, 249–260. [Google Scholar] [CrossRef]
- Yao, Q.; Tang, F.; Wang, Y.; Yan, Y.; Dong, L.; Wang, T.; Zhu, D.; Tian, M.; Lin, X.; Shi, J. Effect of cerebellum stimulation on cognitive recovery in patients with Alzheimer disease: A randomized clinical trial. Brain Stimul. 2022, 15, 910–920. [Google Scholar] [CrossRef]
- Basavaraju, R.; Kaur, S.; Mehta, U.M. Cerebellar Transcranial Magnetic Stimulation in Psychiatric Disorders: A Systematic Review. Curr. Behav. Neurosci. Rep. 2024, 11, 23–32. [Google Scholar] [CrossRef]
- Barmack, N.H.; Yakhnitsa, V. Vestibulocerebellar Functional Connections. In Handbook of the Cerebellum and Cerebellar Disorders; Springer: Cham, Switzerland, 2021; pp. 1–30. [Google Scholar] [CrossRef]
- Saadon-Grosman, N.; Du, J.; Kosakowski, H.L.; Angeli, P.A.; DiNicola, L.M.; Eldaief, M.C.; Buckner, R.L. Within-individual organization of the human cognitive cerebellum: Evidence for closely juxtaposed, functionally specialized regions. Sci. Adv. 2024, 10, eadq4037. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Tsai, P.T. Adaptive Prediction for Social Contexts: The Cerebellar Contribution to Typical and Atypical Social Behaviors. Annu. Rev. Neurosci. 2021, 44, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, G.P.D.; van Dun, K.; Adamaszek, M.; Leggio, M.; Manto, M.; Masciullo, M.; Molinari, M.; Stoodley, C.J.; Van Overwalle, F.; Ivry, R.B.; et al. The Cerebellar Cognitive Affective/Schmahmann Syndrome: A Task Force Paper. Cerebellum 2020, 19, 102–125. [Google Scholar] [CrossRef]
- Buckner, R.L.; Krienen, F.M.; Castellanos, A.; Diaz, J.C.; Yeo, B.T.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 2322–2345. [Google Scholar] [CrossRef]
- Nettekoven, C.; Diedrichsen, J. Chapter 23—Cerebellar asymmetries. In Handbook of Clinical Neurology; Papagno, C., Corballis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; Volume 208, pp. 369–378. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Ferrari, C.; Ciricugno, A.; Arioli, M.; Cattaneo, Z. Functional Segregation of the Human Cerebellum in Social Cognitive Tasks Revealed by TMS. J. Neurosci. 2023, 43, 3708–3717. [Google Scholar] [CrossRef]
- Prati, J.M.; Pontes-Silva, A.; Gianlorenço, A.C.L. The cerebellum and its connections to other brain structures involved in motor and non-motor functions: A comprehensive review. Behav. Brain Res. 2024, 465, 114933. [Google Scholar] [CrossRef]
- Doron, K.W.; Funk, C.M.; Glickstein, M. Fronto-cerebellar circuits and eye movement control: A diffusion imaging tractography study of human cortico-pontine projections. Brain Res. 2010, 1307, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Habas, C. Functional Connectivity of the Cognitive Cerebellum. Front. Syst. Neurosci. 2021, 15, 642225. [Google Scholar] [CrossRef]
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Shi, X.; Liu, Y.; Wu, D.; Hao, J.; Leng, X.; Jin, L.; Yuan, F.; Sun, Z.; et al. Cerebellar transcranial magnetic stimulation to treat drug-resistant epilepsy: A randomized, controlled, crossover clinical trial. Epilepsia 2025, 66, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The Cerebro-Cerebellum as a Locus of Forward Model: A Review. Frontiers in Systems Neuroscience 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef]
- Imamizu, H.; Kawato, M. Brain mechanisms for predictive control by switching internal models: Implications for higher-order cognitive functions. Psychol. Res. 2009, 73, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Bases and implications of learning in the cerebellum—Adaptive control and internal model mechanism. Prog. Brain Res. 2005, 148, 95–109. [Google Scholar] [CrossRef]
- Ito, M. Control of mental activities by internal models in the cerebellum. Nat. Reviews. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef]
- Diedrichsen, J.; King, M.; Hernandez-Castillo, C.; Sereno, M.; Ivry, R.B. Universal Transform or Multiple Functionality? Understanding the Contribution of the Human Cerebellum across Task Domains. Neuron 2019, 102, 918–928. [Google Scholar] [CrossRef] [PubMed]
- de Xivry, J.-J.O.; Diedrichsen, J. Diversity of the nature of input and output signals in the cerebellum suggests a diversity of function. Curr. Opin. Behav. Sci. 2024, 57, 101386. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Colquhoun, H.; Garritty, C.M.; Hempel, S.; Horsley, T.; Langlois, E.V.; Lillie, E.; O’bRien, K.K.; Tunçalp, Ö.; et al. Scoping reviews: Reinforcing and advancing the methodology and application. Syst. Rev. 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, N.; Hartling, L.; Vandermeer, B.; Tjosvold, L.; Klassen, T.P. Single data extraction generated more errors than double data extraction in systematic reviews. J. Clin. Epidemiol. 2006, 59, 697–703. [Google Scholar] [CrossRef]
- Tikka, S.K.; Garg, S.; Sinha, V.K.; Nizamie, S.H.; Goyal, N. Resting State Dense Array Gamma Oscillatory Activity as a Response Marker for Cerebellar-Repetitive Transcranial Magnetic Stimulation (rTMS) in Schizophrenia. J. ECT 2015, 31, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sinha, V.K.; Tikka, S.K.; Mishra, P.; Goyal, N. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: A randomized rater blind-sham controlled study. Psychiatry Res. 2016, 243, 413–420. [Google Scholar] [CrossRef]
- Bradnam, L.; McDonnell, M.; Ridding, M. Cerebellar Intermittent Theta-Burst Stimulation and Motor Control Training in Individuals with Cervical Dystonia. Brain Sci. 2016, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Johkura, K.; Kudo, Y.; Sugawara, E.; Watanabe, K.; Nakamizo, T.; Yamamoto, M.; Amari, K.; Takahashi, K.; Tanaka, O. Effects of cerebellar magnetic stimulation on chronic post-lateral medullary infarction dizziness: A proof-of-principle cohort study. J. Neurol. Sci. 2018, 392, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, H.; Poole, V.; Wang, X.; Wang, Z.; Yang, Y.; Meng, L.; Manor, B.; Zhou, J.; Feng, T. Effects of Multi-Session Repetitive Transcranial Magnetic Stimulation on Motor Control and Spontaneous Brain Activity in Multiple System Atrophy: A Pilot Study. Front. Behav. Neurosci. 2018, 12, 90. [Google Scholar] [CrossRef]
- Brady, R.O.; Gonsalvez, I.; Lee, I.; Öngür, D.; Seidman, L.J.; Schmahmann, J.D.; Eack, S.M.; Keshavan, M.S.; Pascual-Leone, A.; Halko, M.A. Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am. J. Psychiatry 2019, 176, 512–520. [Google Scholar] [CrossRef]
- Koch, G.; Bonnì, S.; Casula, E.P.; Iosa, M.; Paolucci, S.; Pellicciari, M.C.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Effect of Cerebellar Stimulation on Gait and Balance Recovery in Patients with Hemiparetic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 170. [Google Scholar] [CrossRef]
- Cha, Y.-H.; Gleghorn, D.; Doudican, B. Occipital and Cerebellar Theta Burst Stimulation for Mal De Debarquement Syndrome. Otol. Neurotol. 2019, 40, e928–e937. [Google Scholar] [CrossRef]
- Manor, B.; Greenstein, P.E.; Davila-Perez, P.; Wakefield, S.; Zhou, J.; Pascual-Leone, A. Repetitive Transcranial Magnetic Stimulation in Spinocerebellar Ataxia: A Pilot Randomized Controlled Trial. Front. Neurol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Shin, H.-W.; Hallett, M.; Sohn, Y.H. Cerebellar repetitive transcranial magnetic stimulation for patients with essential tremor. Park. Relat. Disord. 2019, 64, 304–307. [Google Scholar] [CrossRef]
- Chauhan, P.; Garg, S.; Tikka, S.K.; Khattri, S. Efficacy of Intensive Cerebellar Intermittent Theta Burst Stimulation (iCiTBS) in Treatment-Resistant Schizophrenia: A Randomized Placebo-Controlled Study. Cerebellum 2020, 20, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Grasso, M.G.; Soldi, S.; Casula, E.P.; Bonnì, S.; Mastrogiacomo, S.; D’Acunto, A.; Porrazzini, F.; Caltagirone, C.; Koch, G. Cerebellar Intermittent Theta-Burst Stimulation Combined with Vestibular Rehabilitation Improves Gait and Balance in Patients with Multiple Sclerosis: A Preliminary Double-Blind Randomized Controlled Trial. Cerebellum 2020, 19, 897–901. [Google Scholar] [CrossRef]
- Olfati, N.; Shoeibi, A.; Abdollahian, E.; Ahmadi, H.; Hoseini, A.; Akhlaghi, S.; Vakili, V.; Foroughipour, M.; Rezaeitalab, F.; Farzadfard, M.-T.; et al. Cerebellar repetitive transcranial magnetic stimulation (rTMS) for essential tremor: A double-blind, sham-controlled, crossover, add-on clinical trial. Brain Stimul. 2020, 13, 190–196. [Google Scholar] [CrossRef]
- França, C.; De Andrade, D.C.; Silva, V.; Galhardoni, R.; Barbosa, E.R.; Teixeira, M.J.; Cury, R.G. Effects of cerebellar transcranial magnetic stimulation on ataxias: A randomized trial. Park. Relat. Disord. 2020, 80, 1–6. [Google Scholar] [CrossRef]
- Song, P.; Li, S.; Wang, S.; Wei, H.; Lin, H.; Wang, Y. Repetitive transcranial magnetic stimulation of the cerebellum improves ataxia and cerebello-fronto plasticity in multiple system atrophy: A randomized, double-blind, sham-controlled and TMS-EEG study. Aging 2020, 12, 20611–20622. [Google Scholar] [CrossRef]
- Liao, L.-Y.; Xie, Y.-J.; Chen, Y.; Gao, Q. Cerebellar Theta-Burst Stimulation Combined with Physiotherapy in Subacute and Chronic Stroke Patients: A Pilot Randomized Controlled Trial. Neurorehabilit. Neural Repair 2020, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, W.; Zhu, Y.; Mu, X.; Zhang, Q.; Wang, Y.; Cai, J.; Xie, B. Cerebellar theta burst stimulation for the treatment of negative symptoms of schizophrenia: A multicenter, double-blind, randomized controlled trial. Psychiatry Res. 2021, 305, 114204. [Google Scholar] [CrossRef]
- Basavaraju, R.; Ithal, D.; Thanki, M.V.; Ramalingaiah, A.H.; Thirthalli, J.; Reddy, R.P.; Brady, R.O.; Halko, M.A.; Bolo, N.R.; Keshavan, M.S.; et al. Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: A randomized controlled trial. Schizophr. Res. 2021, 238, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, Q.-C.; Zhang, M.-Z.; Xie, Y.-J.; Liao, L.-Y.; Tan, H.-X.; Guo, Q.-F.; Gao, Q. Cerebellar Intermittent Theta-Burst Stimulation Reduces Upper Limb Spasticity After Subacute Stroke: A Randomized Controlled Trial. Front. Neural Circuits 2021, 15, 655502. [Google Scholar] [CrossRef]
- Li, D.; Cheng, A.; Zhang, Z.; Sun, Y.; Liu, Y. Effects of low-frequency repetitive transcranial magnetic stimulation combined with cerebellar continuous theta burst stimulation on spasticity and limb dyskinesia in patients with stroke. BMC Neurol. 2021, 21, 369. [Google Scholar] [CrossRef]
- Rao, J.; Li, F.; Zhong, L.; Wang, J.; Peng, Y.; Liu, H.; Wang, P.; Xu, J. Bilateral Cerebellar Intermittent Theta Burst Stimulation Combined with Swallowing Speech Therapy for Dysphagia After Stroke: A Randomized, Double-Blind, Sham-Controlled, Clinical Trial. Neurorehabilit. Neural Repair 2022, 36, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Moulton, E.J.; Kemlin, C.; Leder, S.; Corvol, J.-C.; Mehdi, S.; Obadia, M.A.; Obadia, M.; Yger, M.; Meseguer, E.; et al. Cerebello-Motor Paired Associative Stimulation and Motor Recovery in Stroke: A Randomized, Sham-Controlled, Double-Blind Pilot Trial. Neurotherapeutics 2022, 19, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Lian, Y.-H.; Liu, X.-H.; Sikandar, A.; Li, M.-C.; Xu, H.-L.; Hu, J.-P.; Chen, Q.-L.; Gan, S.-R. Effects of Repetitive Transcranial Magnetic Stimulation on Cerebellar Metabolism in Patients with Spinocerebellar Ataxia Type 3. Front. Aging Neurosci. 2022, 14, 827993. [Google Scholar] [CrossRef]
- Dong, L.; Pan, X.; Wang, Y.; Bai, G.; Han, C.; Wang, Q.; Meng, P. High-Frequency Cerebellar rTMS Improves the Swallowing Function of Patients with Dysphagia after Brainstem Stroke. Neural Plast. 2022, 2022, 6259693. [Google Scholar] [CrossRef]
- Sanna, A.; Follesa, P.; Tacconi, P.; Serra, M.; Pisu, M.G.; Cocco, V.; Figorilli, M.; Defazio, G.; Puligheddu, M. Therapeutic Use of Cerebellar Intermittent Theta Burst Stimulation (iTBS) in a Sardinian Family Affected by Spinocerebellar Ataxia 38 (SCA 38). Cerebellum 2022, 21, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, M.; Yang, J.; Shi, J.; Xuan, T.; Zhang, J.; Du, D.; Cheng, J.; Li, H. Cerebellar repetitive transcranial magnetic stimulation versus propranolol for essential tremor. Brain Behav. 2023, 13, e2926. [Google Scholar] [CrossRef]
- Dai, M.; Qiao, J.; Shi, Z.; Wei, X.; Chen, H.; Shen, L.; Wen, H.; Dou, Z. Effect of cerebellar transcranial magnetic stimulation with double-cone coil on dysphagia after subacute infratentorial stroke: A randomized, single-blinded, controlled trial. Brain Stimul. 2023, 16, 1012–1020. [Google Scholar] [CrossRef]
- Shi, Y.; Zou, G.; Chen, Z.; Wan, L.; Peng, L.; Peng, H.; Shen, L.; Xia, K.; Qiu, R.; Tang, B.; et al. Efficacy of cerebellar transcranial magnetic stimulation in spinocerebellar ataxia type 3: A randomized, single-blinded, controlled trial. J. Neurol. 2023, 270, 5372–5379. [Google Scholar] [CrossRef]
- Sikandar, A.; Liu, X.-H.; Xu, H.-L.; Li, Y.; Lin, Y.-Q.; Chen, X.-Y.; Li, G.-H.; Lin, M.-T.; Wang, N.; Chen, W.-J.; et al. Short-term efficacy of repetitive transcranial magnetic stimulation in SCA3: A prospective, randomized, double-blind, sham-controlled study. Park. Relat. Disord. 2023, 106, 105236. [Google Scholar] [CrossRef]
- Zhong, L.; Wen, X.; Liu, Z.; Li, F.; Ma, X.; Liu, H.; Chen, H. Effects of bilateral cerebellar repetitive transcranial magnetic stimulation in poststroke dysphagia: A randomized sham-controlled trial. NeuroRehabilitation 2023, 52, 227–234. [Google Scholar] [CrossRef]
- Zhu, P.-A.; Li, Z.-L.; Lu, Q.-Q.; Nie, Y.-Y.; Liu, H.; Kiernan, E.; Yuan, J.; Zhang, L.-J.; Bao, X. Can cerebellar theta-burst stimulation improve balance function and gait in stroke patients? A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2024, 60, 391–399. [Google Scholar] [CrossRef]
- Grobe-Einsler, M.; Baljasnikowa, V.; Faikus, A.; Schaprian, T.; Kaut, O. Cerebellar transcranial magnetic stimulation improves motor function in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2024, 11, 2673–2684. [Google Scholar] [CrossRef]
- Yassine, I.A.; Shehata, H.; Hamdy, S.; Abdel-Naseer, M.; Hassan, T.; Sherbiny, M.; Magdy, E.; Elmazny, A.; Shalaby, N.; ElShebawy, H. Effect of high frequency repetitive transcranial magnetic stimulation (rTMS) on the balance and the white matter integrity in patients with relapsing-remitting multiple sclerosis: A long-term follow-up study. Mult. Scler. Relat. Disord. 2024, 83, 105471. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Xu, H.-L.; Liu, X.-H.; Sikandar, A.; Li, M.-C.; Xia, X.-Y.; Huang, Z.-Q.; Chen, N.-P.; Tu, Y.-Q.; et al. Effect of Regional Brain Activity Following Repeat Transcranial Magnetic Stimulation in SCA3: A Secondary Analysis of a Randomized Clinical Trial. Cerebellum 2024, 23, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Qiu, M.; Jin, Y.; Li, D.; Tao, C.; Lou, D.; Hu, Z.; Wang, Y.; You, Z.; Shao, Y.; et al. Effectiveness of High-Frequency Repetitive Transcranial Magnetic Stimulation in Patients with Spinocerebellar Ataxia Type 3. J. ECT 2024, 40, 15–19. [Google Scholar] [CrossRef]
- Grobe-Einsler, M.; Bork, F.; Faikus, A.; Hurlemann, R.; Kaut, O. Effects of cerebellar repetitive transcranial magnetic stimulation plus physiotherapy in spinocerebellar ataxias—A randomized clinical trial. CNS Neurosci. Ther. 2024, 30, e14797. [Google Scholar] [CrossRef] [PubMed]
- Grobe-Einsler, M.; Bork, F.; Faikus, A.; Neggers, S.F.W.; Kaut, O. Feasibility of a randomized, sham-controlled pilot study for accelerated rTMS-treatment of the cerebellum plus physiotherapy in CANVAS patients. NeuroRehabilitation 2024, 54, 691–698. [Google Scholar] [CrossRef]
- He, R.; Shi, X.; Jiang, L.; Zhu, Y.; Pei, Z.; Zhu, L.; Su, X.; Yao, D.; Xu, P.; Guo, Y.; et al. Prediction of rTMS Efficacy in Patients with Essential Tremor: Biomarkers from Individual Resting-State EEG Network. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 3719–3728. [Google Scholar] [CrossRef]
- Grobe-Einsler, M.; Lupa, A.; Weller, J.; Kaut, O. RTMS of the Cerebellum Using an Accelerated Stimulation Protocol Improved Gait in Parkinson’s Disease. Neurorehabilit. Neural Repair 2024, 38, 539–550. [Google Scholar] [CrossRef]
- El-Adawy, A.F.I.; Reda, M.A.-B.M.G.; Ahmed, A.M.; Rashad, M.H.; Zaki, M.A.; Mohamed, M.T.; Hassan, M.A.S.; Abdulsalam, M.F.; Hassan, A.M.; Mohamed, A.F.; et al. Efficacy of Cerebellar Transcranial Magnetic Stimulation in Treating Essential Tremor: A Randomized, Sham-Controlled Trial. J. Clin. Neurol. 2024, 20, 378. [Google Scholar] [CrossRef]
- Liao, L.-Y.; Zhu, Y.; Peng, Q.-Y.; Gao, Q.; Liu, L.; Wang, Q.-H.; Gao, S.-H.; Tao, Y.; Huang, H.; Xu, P.-D.; et al. Intermittent Theta-Burst Stimulation for Stroke: Primary Motor Cortex Versus Cerebellar Stimulation: A Randomized Sham-Controlled Trial. Stroke 2024, 55, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, X.; Yao, L.; Liu, Q.; Lu, Y.; Chen, X.; Wang, T. EEG microstates analysis after TMS in patients with subacute stroke during the resting state. Cereb. Cortex 2024, 34, bhad480. [Google Scholar] [CrossRef]
- Li, D.; Jiang, C.; Liu, J.; Fan, Y.; Hao, X.; Fu, M.; Xu, Y.; Chen, X.; Zhang, J.; Liu, G. Repeated transcranial magnetic stimulation on the bilateral cerebellum to improve symptoms of ataxia with multiple system atrophy: A prospective, randomized, sham-controlled pilot study. Neurol. Sci. 2025, 46, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, W.; Zhang, Y.; Song, W.; Xie, H.; Cao, L. Exploring Neurobiological Effects of Intermittent Theta-Burst Stimulation on the Left Cerebellum for Post-stroke Unilateral Neglect: A Preliminary Transcranial Magnetic Stimulation—Electroencephalography Investigation. Cerebellum 2025, 24, 103. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, K.A.; Fleischmann, H.H.; Cox, C.E.; Wolf, J.P.; George, M.S.; McTeague, L.M. Neuronavigation maximizes accuracy and precision in TMS positioning: Evidence from 11,230 distance, angle, and electric field modeling measurements. Brain Stimul. 2022, 15, 1192–1205. [Google Scholar] [CrossRef]
- Matosin, N.; Frank, E.; Engel, M.; Lum, J.S.; Newell, K.A. Negativity towards negative results: A discussion of the disconnect between scientific worth and scientific culture. Dis. Models Mech. 2014, 7, 171–173. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, M.; Zhu, Y. The Effect of Cerebellar rTMS on Modulating Motor Dysfunction in Neurological Disorders: A Systematic Review. Cerebellum 2023, 22, 954–972. [Google Scholar] [CrossRef]
- Trujillo, P.; Darby, R.R. The Cerebellum as the Central Hub of a Widespread Network in Essential Tremor. Neurology 2023, 101, 639–640. [Google Scholar] [CrossRef]
- Parmar, K.; Stadelmann, C.; Rocca, M.A.; Langdon, D.; D’Angelo, E.; D’Souza, M.; Burggraaff, J.; Wegner, C.; Sastre-Garriga, J.; Barrantes-Freer, A.; et al. The role of the cerebellum in multiple sclerosis-150 years after Charcot. Neurosci. Biobehav. Rev. 2018, 89, 85–98. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Liesirova, K.; Broeg-Morvay, A.; Meisterernst, J.; Schlager, M.; Mono, M.-L.; El-Koussy, M.; Kägi, G.; Jung, S.; Sarikaya, H. Dysphagia in Acute Stroke: Incidence, Burden and Impact on Clinical Outcome. PLoS ONE 2016, 11, e0148424. [Google Scholar] [CrossRef]
- Cha, Y.-H. Mal de debarquement. Semin. Neurol. 2009, 29, 520–527. [Google Scholar] [CrossRef]
- Bernard, J.A.; McOwen, K.M.; Huynh, A.T. New frontiers for the understanding of aging: The power and possibilities of studying the cerebellum. Curr. Opin. Behav. Sci. 2023, 54, 101311. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Marino, M.; Mantini, D.; Spironelli, C. Unveiling altered connectivity between cognitive networks and cerebellum in schizophrenia. Schizophr. Res. 2024, 271, 47–58. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ha, M.; Choi, S.; Moon, S.-Y.; Park, S.; Kim, M.; Kwon, J.S. Altered intrinsic cerebellar-cerebral functional connectivity is related to negative symptoms in patients with first-episode psychosis. Schizophr. Res. 2023, 252, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Chung, S.; Lee, S.-H.; Bang, M. Cerebro-cerebellar gray matter abnormalities associated with cognitive impairment in patients with recent-onset and chronic schizophrenia. Schizophrenia 2024, 10, 11. [Google Scholar] [CrossRef]
- Bottmer, C.; Bachmann, S.; Pantel, J.; Essig, M.; Amann, M.; Schad, L.R.; Magnotta, V.; Schröder, J. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005, 140, 239–250. [Google Scholar] [CrossRef]
- Okugawa, G.; Nobuhara, K.; Takase, K.; Kinoshita, T. Cerebellar posterior superior vermis and cognitive cluster scores in drug-naive patients with first-episode schizophrenia. Neuropsychobiology 2007, 56, 216–219. [Google Scholar] [CrossRef]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef]
- Hussain, S.J.; Freedberg, M.V. Debunking the Myth of Excitatory and Inhibitory Repetitive Transcranial Magnetic Stimulation in Cognitive Neuroscience Research. J. Cogn. Neurosci. 2025, 37, 1009–1022. [Google Scholar] [CrossRef]
- Cho, S.S.; Yoon, E.J.; Bang, S.A.; Park, H.S.; Kim, Y.K.; Strafella, A.P.; Kim, S.E. Metabolic Changes of Cerebrum by Repetitive Transcranial Magnetic Stimulation over Lateral Cerebellum: A Study with FDG PET. Cerebellum 2012, 11, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Oldrati, V.; Schutter, D.J.L.G. Targeting the Human Cerebellum with Transcranial Direct Current Stimulation to Modulate Behavior: A Meta-Analysis. Cerebellum 2018, 17, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Conte, D.; Roman, A.; Beorchia, Y.; Pinzini, C.; Castriotta, L.; Valente, M. The effects of transcranial magnetic stimulation in motor symptoms of Parkinson’s disease: An overview of systematic reviews with meta-analysis. Neurol. Sci. 2025. advance online publication. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, C. Assessment of noninvasive brain stimulation interventions in Parkinson’s disease: A systematic review and network meta-analysis. Sci. Rep. 2024, 14, 14219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lu, Z.; Jin, Y.; Duan, X.; Teng, J.; Duan, D. Low-frequency repetitive transcranial magnetic stimulation on Parkinson motor function: A meta-analysis of randomised controlled trials. Acta Neuropsychiatr. 2015, 27, 82–89. [Google Scholar] [CrossRef]
- Du, X.; Rowland, L.M.; Summerfelt, A.; Choa, F.-S.; Wittenberg, G.F.; Wisner, K.; Wijtenburg, A.; Chiappelli, J.; Kochunov, P.; Hong, L.E. Cerebellar-Stimulation Evoked Prefrontal Electrical Synchrony Is Modulated by GABA. Cerebellum 2018, 17, 550–563. [Google Scholar] [CrossRef]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical utility and prospective of TMS-EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef]
- Koch, G. Repetitive transcranial magnetic stimulation: A tool for human cerebellar plasticity. Funct. Neurol. 2010, 25, 159–163. [Google Scholar]
- King, M.; Hernandez-Castillo, C.R.; Poldrack, R.A.; Ivry, R.B.; Diedrichsen, J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci. 2019, 22, 1371–1378. [Google Scholar] [CrossRef]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Habas, C.; Manto, M. Probing the neuroanatomy of the cerebellum using tractography. Handb. Clin. Neurol. 2018, 154, 235–249. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Fernandez, L.; Major, B.P.; Teo, W.-P.; Byrne, L.K.; Enticott, P.G. The Impact of Stimulation Intensity and Coil Type on Reliability and Tolerability of Cerebellar Brain Inhibition (CBI) via Dual-Coil TMS. Cerebellum 2018, 17, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Lesage, E.; Miall, R.C. Cerebellar Transcranial Magnetic Stimulation: The Role of Coil Geometry and Tissue Depth. Brain Stimul. 2014, 7, 643–649. [Google Scholar] [CrossRef]
- Basavaraju, R.; Ithal, D.; Ramalingaiah, A.H.; Thirthalli, J.; Mehta, U.M.; Kesavan, M. «Apathetic to hypomanic/manic»: A case series-illustration of emergent mood symptoms during intermittent theta burst stimulation (iTBS) of cerebellar vermis in schizophrenia with predominant negative symptoms. Schizophr. Res. 2020, 222, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.; Ciricugno, A.; Urgesi, C.; Cattaneo, Z. Cerebellar contribution to emotional body language perception: A TMS study. Soc. Cogn. Affect. Neurosci. 2022, 17, 81–90. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Pezzetta, R.; Gambarota, F.; Tarantino, V.; Devita, M.; Cattaneo, Z.; Arcara, G.; Mapelli, D.; Masina, F. A meta-analysis of non-invasive brain stimulation (NIBS) effects on cerebellar-associated cognitive processes. Neurosci. Biobehav. Rev. 2024, 157, 105509. [Google Scholar] [CrossRef]
- Stanca, S.; Rossetti, M.; Bongioanni, P. The Cerebellum’s Role in Affective Disorders: The Onset of Its Social Dimension. Metabolites 2023, 13, 1113. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, L.; Olivito, G.; Leggio, M. The cerebellum gains weight: A systematic review of alterations in cerebellar volume and cerebro-cerebellar functional alterations in individuals with eating disorders. Neurosci. Biobehav. Rev. 2022, 141, 104863. [Google Scholar] [CrossRef] [PubMed]
- Huggins, A.A.; Baird, C.L.; Briggs, M.; Laskowitz, S.; Hussain, A.; Fouda, S.; Haswell, C.; Sun, D.; Salminen, L.E.; Jahanshad, N.; et al. Smaller total and subregional cerebellar volumes in posttraumatic stress disorder: A mega-analysis by the ENIGMA-PGC PTSD workgroup. Mol. Psychiatry 2024, 29, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Klaus, J.; Wolfs, E.M.; Schutter, D.J. Cerebellar roots of aggression in violent psychopathic offenders: Evidence from structural neuroimaging studies. Curr. Opin. Behav. Sci. 2024, 55, 101333. [Google Scholar] [CrossRef]
- Pavon, J.H.; Schneider-Garces, N.; Begnoche, J.; Raij, T. Effects of paired associative stimulation asynchrony on modulating cortico-cortical connectivity. Brain Stimul. 2019, 12, 582. [Google Scholar] [CrossRef]
| Author and Year | Sample Size, Mean Age (SD) | Diagnosis | Study Design | Sham-Controlled | Blinding | Follow-Up | Medications | Primary Outcome | Outcome Domain | Modulation Effect |
|---|---|---|---|---|---|---|---|---|---|---|
| Tikka et al., 2015 [44] | 11, 24.63 (3.35) | Schizophrenia | Within-subject | No | Single-blind | No | Psychotropic Medication | PANSS, CDSS | Physiological/Socio-affective | Yes (+) |
| Garg et al., 2016 [45] | 40, 32.40 (8.44) | Schizophrenia | Between-subject | Yes | Double-blind | Yes | Psychotropic Medication | PANSS | Socio-affective | Yes (+) |
| Bradnam et al., 2016 [46] | 16, 51.95 (11.55) | Cervical Dystonia | Between-subject | Yes | Double-blind | Yes | Not Reported | TWSTRS | Motor | Yes (+) |
| Johkura et al., 2018 [47] | 6, 53.5 (9.33) | Post-stroke Dizziness | Within-subject | No | Not reported | Yes | No Medication That Could Affect the Dizziness | VOR, DHI, Nystagmus | Motor | Yes (+) |
| Liu et al., 2018 [48] | 9, 58.0 (7.0) | MSA | Within-subject | No | Not Reported | No | Dopaminergic Treatments | UMSARS, rs-fMRI | Motor | Yes (+) |
| Brady et al., 2019 [49] | 11, 35.55 (10.50) | Schizophrenia | Between-subject | Yes | Double-blind | Yes | Not Reported | PANSS, rs-fMRI | Physiological/Socio-affective | Yes (+) |
| Koch et al., 2019 [50] | 34, 64 (11.3) | Post-stroke Hemiparesis | Between-subject | Yes | Double-blind | No | Not Reported | BBS | Motor | Yes (+) |
| Cha et al., 2019 [51] | 26, 51.3 (12.4) | Mal de Débarquement Syndrome | Within-subject | No | Single-blind | Yes | Not reported | DHI, MBRS, HADS | Motor | Yes (+) |
| Manor et al., 2019 [52] | 20, 51 (6,5) | SCA | Between-subject | Yes | Double-blind | Yes | Not reported | SARA | Motor | Yes (+) |
| Shin et al., 2019 [53] | 21, 67 (9.25) | Essential tremor | Between-subject | Yes | Single-blind | Yes | Yes, Not reported | TRS, MEPs | Motor | No |
| Chauhan et al., 2020 [54] | 30, 40.05 (8,54) | Schizophrenia | Between-subject | Yes | Double-blind | Yes | Yes, Psychotropic Medication | PANSS | Socio-affective | No |
| Tramontano et al., 2020 [55] | 20, 51.75 (7.37) | Multiple Sclerosis | Between-subject | Yes | Double-blind | No | Not Reported | TBG | Motor | Yes (+) |
| Olfati et al., 2020 [56] | 23, 60 (15.38) | Essential Tremors | Cross-over | Yes | Double-blind | Yes | Not Reported | FTM | Motor | No |
| França et al., 2020 [57] | 24, 49 (13,8) | Cerebellar Ataxia (MSA-c, Post-lesion Ataxia, SCA3) | Cross-over | Yes | Double-blind | Yes | Yes, Not Reported | SARA | Motor | Yes (+) |
| Song et al., 2020 [58] | 20, 51.1 (9.2) | MSA-C | Between-subject | Yes | Double-blind | No | Not reported | SARA, EEG activity | Physiological/Motor | Yes (+) |
| Liao et al., 2020 [59] | 30, 54.2 (8.66) | Post-stroke | Between-subject | Yes | Double-blind | Yes | Not Reported | BBS | Motor | Yes (+) |
| Zhu et al., 2021 [60] | 64, 35.25 (6.63) | Schizophrenia | Between-subject | Yes | Double-blind | Yes | Antipsychotic Medication | PANSS | Socio-affective | Yes (+) |
| Basavaraju et al., 2021 [61] | 30, 32.67 (8.98) | Schizophrenia | Between-subject | Yes | Double-blind | Yes | Antipsychotic Medication | SANS, rs-fMRI | Physiological/Socio-affective | Yes (+) |
| Chen et al., 2021 [62] | 32, 54.41 (8.62) | Post-stroke Upper Limb Spasticity | Between-subject | Yes | Double-blind | No | Not Reported | MAS, MTS, SWV | Motor | Yes (+) |
| Li et al., 2021 [63] | 90, 56.5 (7.96) | Post-stroke Hemiplegia | Between-subject | No | Not Reported | No | Not Reported | MAS, FMA, BI | Motor | Yes (+) |
| Yao et al., 2022 [20] | 27, 65.74 (7.37) | Alzheimer’s Disease | Between-subject | Yes | Double-blind | Yes | Acetylcholinesterase Inhibitors | MMSE, MoCA, CDR, ADAS, Cog, RAVLT, CDT, BNT, VFT, TMT, SDMT, DST, HAMD, HAMA, PSQI, ADL | Cognitive | Yes (+) |
| Rao et al., 2022 [64] | 70, 64.66 (10.89) | Post-stroke Dysphagia | Between-subject | Yes | Double-blind | Yes | Benzodiazepine and Antidepressants | FEDSS | Motor | Yes (+) |
| Rosso et al., 2022 [65] | 27, 61.5 (12.5) | Post-stroke | Between-subject | Yes | Double-blind | Yes | Not Reported | JTT, Grip Strength | Motor | Yes (+) |
| Chen et al., 2022 [66] | 18, 39.78 (9.23) | SCA3 | Between-subject | Yes | Double-blind | No | Not Reported | ICARS | Motor | Yes (+) |
| Dong et al., 2022 [67] | 36, 53.8 (10.13) | Post-stroke Dysphagia | Between-subject | Yes | Not Reported | No | Not Reported | PAS, FDS | Motor | Yes (+) |
| Sanna et al., 2022 [68] | 6, 50.33 (4.68) | SCA38 | Cross-over | Yes | Double-blind | No | Not Reported | MICARS, MEPs, DNA analysis | Motor | Yes (+) |
| Lv et al., 2023 [69] | 38, 55.45 (6.86) | Essential Tremors | Between-subject | No | Single-blind | Yes | Yes, Propranolol | FTM | Motor | No |
| Dai et al., 2023 [70] | 42, 60.09 (2.84) | Post-stroke | Between-subject | Yes | Single-blind | Yes | Not Reported | FOIS | Motor | Yes (+) |
| Shi et al., 2023 [71] | 120, 42.29 (9.49) | SCA3 | Between-subject | Yes | Single-blind | Yes | Yes, Neuroprotective Medications | SARA, ICARS | Motor | Yes (+) |
| Sikandar et al., 2023 [72] | 44, 39.42 (9.67) | SCA3 | Between-subject | Yes | Double-blind | No | Yes, Stop Ataxia Medications | ICARS | Motor | Yes (+) |
| Zhong et al., 2023 [73] | 84, 63.21 (10.58) | Post-stroke Dysphagia | Between-subject | Yes | Double-blind | Yes | Not Reported | FEDSS | Motor | Yes (+) |
| Zhu et al., 2024 [74] | 36, 60.5 (8.01) | Post-stroke Hemiplegia | Between-subject | Yes | Double-blind | No | Not Reported | BBS | Motor | Yes (+) |
| Grobe-Einsler et al., 2024 [75] | 35, 68.24 (10.04) | Parkinson’s Disease | Between-subject | Yes | Double-blind | Yes | Levodopa | Dynamic Posturography | Motor | Yes (+) |
| Yassine et al., 2024 [76] | 39, 39.9 (6.1) | Relapsing-remitting Multiple Sclerosis | Phase 1: Between-Subject Phase 2: Within-subject | Yes | Single-blind | Yes | Disease-modifying Medications | 10MWT, TUG, BBS, DTI | Motor | Yes (+) |
| Liu et al., 2024 [77] | 22, 42.09 (10.9) | SCA3 | Between-subject | Yes | Double-blind | No | Not Reported | ICARS, rs-fMRI | Physiological/Motor | Yes (+) |
| Zhou et al., 2024 [78] | 16, 39.72 (10.14) | SCA3 | Between-subject | No | Double-blind | No | Yes, Not Reported | SARA, ICARS | Motor | Yes (+) |
| Grobe-Einsler et al., 2024 [79] | 33, 48.5 (13.8) | SCA | Between-subject | Yes | Double-blind | Yes | Yes, Not Reported | SARA | Motor | Yes (+) |
| Grobe-Einsler et al., 2024 [80] | 8, 65.1 (6.1) | CANVAS | Between-subject | Yes | Double-blind | No | Yes, Not Reported | SARA | Motor | Yes (+) |
| He et al., 2024 [81] | 20, 64.7 (8.40) | Essential Tremor | Within-subject | No | Not Reported | No | Yes, Anti-tremor Medication discontinued | TETRAS, rs-EEG | Physiological/Motor | Yes (+) |
| Grobe-Einsler et al., 2024 [82] | 36, 69.5 (10.0) | Parkinson’s disease | Between-subject | Yes | Double-blind | Yes | Yes, Antiparkinsonian Medication | UPDRSIII | Motor | Yes (+) |
| El-Adawy et al., 2024 [83] | 45, 38.77 (4.8) | Essential Tremor | Between-subject | Yes | Single-blind | No | Yes, Not Reported | FTM | Motor | Yes (+) |
| Liao et al., 2024 [84] | 36, 57.5 (12.51) | Post-stroke Lower Limb Dysfunction | Between-subject | Yes | Double-blind | Yes | Not Reported | BBS | Motor | Yes (+) |
| Zhang et al., 2024 [85] | 24, 56.99 (7.21) | Post-stroke | Between-subject | No | Not reported | No | No Psychotropic Medications | EEG activity, BBS, HMDS, HAMA, MADRS, IDSSR, MMSE, MoCA, WMT, BNT | Physiological/Motor, Cognitive, affective | Yes (+) |
| Li et al., 2025 [86] | 26, 66.81 (4.08) | MSA-C | Between-subject | Yes | Double-blind | Yes | Yes, Not Reported | SARA | Motor and Affective | Yes (+) |
| Wang et al., 2025 [34] | 38, 31.0 (11.64) | Drug-resistant Epilepsy | Cross-over | Yes | Double-blind | Yes | Antiseizure Medications | % of Seizure Reduction | Epilepsy | Yes (+) |
| Ye et al., 2025 [87] | 20, 50.60 (4.72) | Post-Stroke Unilateral Neglect | Between-subject | Yes | Not Reported | No | Not Reported | Line Cancellation Task, Star Cancellation Task, Line Bisection Task, rs-EEG | Cognition | Yes (+) |
| Author and Year | TMS Protocol | Coil (Diameter) | Target Site (s) | MRI-guided | Frequency and Intensity | Training | N Sessions and Duration | Safety and Tolerability | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Tikka et al., 2015 [44] | iTBS | Double-cone | Vermis | No | 5, 6, and 7 Hz 100% MT | No | 10 (2 weeks) | Not Reported | Not Reported |
| Garg et al., 2016 [45] | HF-rTMS | Double-cone | Vermis | No | 5, 6, and 7 Hz 100% MT | No | 10 (2 weeks) | Yes | Headache and Sleepiness |
| Bradnam et al., 2016 [46] | iTBS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 50 Hz bursts at 5 Hz 80% MT | Active Exercise Training | 10 (2 weeks) | Yes | None |
| Johkura et al., 2018 [47] | iTBS | Double-cone | Vermis | No | 50 Hz Bursts at 5 Hz 80% MT | No | 5 (5 days) | Yes | Discomfort from Posterior Neck Muscle Contraction |
| Liu et al., 2018 [48] | HF-rTMS | Circular (50 mm) | L M1 R M1 L Cerebellum R Cerebellum | No | 5 Hz 100% MT | No | 5 (5 days) | Yes | None |
| Brady et al., 2019 [49] | iTBS | Figure 8 (70 mm) | Vermis | Yes | 50 Hz Bursts at 5 Hz 100% MT | No | 10 (5 days) | Not Reported | Not Reported |
| Koch et al., 2019 [50] | iTBS | Figure 8 (70 mm) | Contralesional Lateral Cerebellum | Yes | 50 Hz bursts at 5 Hz 80% MT | Physiotherapy | 15 (3 weeks) | Yes | None |
| Cha et al., 2019 [51] | cTBS | Double-cone | Occipital Cortex Vermis L Cerebellum R Cerebellum | Yes | 50 Hz Bursts at 5 Hz Maximum Tolerated Level | No | 10-12 (5 days) | Yes | Neck Muscle Contraction |
| Manor et al., 2019 [52] | LF-rTMS | Circular (140 mm) | Vermis, L Cerebellum R Cerebellum | Yes | 1 Hz 100% MSO | No | 20 (4 weeks) | Yes | None |
| Shin et al., 2019 [53] | LF-rTMS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 1 Hz 90% MT | No | 5 (5 days) | Yes | None |
| Chauhan et al., 2020 [54] | iTBS | Figure 8 (70 mm) | Vermis | No | 50 Hz bursts at 5 Hz 80% MT | No | 10 (5 days) | Yes | Headache |
| Tramontano et al., 2020 [55] | iTBS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | Yes | 50 Hz Bursts at 5 Hz 80% MT | Gaze Stability and Postural Stability | 10 (2 weeks) | Not Reported | Not Reported |
| Olfati et al., 2020 [56] | LF-rTMS | Figure 8 (100 mm) | L Cerebellum R Cerebellum | No | 1 Hz 90% MT | No | 5 (5 days) | Yes | Headache and Local Pain |
| França et al., 2020 [57] | LF-rTMS | Double-cone | Contralesional Lateral Cerebellum | Yes | 1 Hz 90% MT | No | 5 (5 days) | Yes | Headache and Short-lasting Worsening of Left Leg Pain |
| Song et al., 2020 [58] | iTBS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | No | 10 (2 weeks) | Yes | None |
| Liao et al., 2020 [59] | iTBS | Figure 8 (70 mm) | Contralesional Lateral Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | Physiotherapy | 10 (2 weeks) | Yes | None |
| Zhu et al., 2021 [60] | iTBS | Figure 8 (Not reported) | Vermis | No | 50 Hz Bursts at 5 Hz 100% MT | No | 10 (2 weeks) | Not Reported | Not Reported |
| Basavaraju et al., 2021 [61] | iTBS | Figure 8 (70 mm) | Vermis | Yes | 50 Hz Bursts at 5 Hz 100% MT | No | 10 (5 days) | Yes | Neck Muscle Contraction and Hypomania |
| Chen et al., 2021 [62] | iTBS | Figure 8 (70 mm) | Ipsilesional Lateral Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | Physical Therapy | 10 (2 weeks) | Yes | None |
| Li et al., 2021 [63] | LF-rTMS cTBS LF-rTMS + cTBS | Circular (Not Reported) | M1 R Cerebellum | No | 1 Hz 50 Hz Bursts at 5 Hz 80% MT | Rehabilitative Training and Acupuncture Therapy | 24 (4 weeks) | Not Reported | Not Reported |
| Yao et al., 2022 [20] | HF-rTMS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 5 Hz 90% MT | No | 20 (4 weeks) | Yes | None |
| Rao et al., 2022 [64] | iTBS | Figure 8 (90 mm) | L Cerebellum R Cerebellum | No | 50 Hz Bursts at 5 Hz 100% MT | Dysphagia Therapy | 10 (2 weeks) | Yes | None |
| Rosso et al., 2022 [65] | Cerebellum-M1 PAS | Double-cone | Contralesional Lateral Cerebellum | No | 120 pairs of pulses at 0.2 Hz 90% MT | Physical Therapy | 5 (1 week) | Yes | Headache, Reflex Syncope and Discomfort |
| Chen et al., 2022 [66] | LF-rTMS | Figure 8 (Not Reported) | L Cerebellum R Cerebellum | No | 1 Hz Not Reported | No | 15 (15 days) | Yes | None |
| Dong et al., 2022 [67] | HF-rTMS | Circular (70 mm) | L Cerebellum R Cerebellum | No | 10 Hz 80% MT | Swallowing Rehabilitation | 10 (2 weeks) | Not Reported | Not Reported |
| Sanna et al., 2022 [68] | iTBS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | No | 10 (2 weeks) | Not Reported | Not Reported |
| Lv et al., 2023 [69] | LF-rTMS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 1 Hz 90% MT | No | 10 (10 days) | Yes | None |
| Dai et al., 2023 [70] | iTBS | Double-cone | L Cerebellum R Cerebellum | No | 50 Hz Bursts at 5 Hz 90% MT | No | 10 (2 weeks) | Yes | Twitching Feeling |
| Shi et al., 2023 [71] | LF-rTMS iTBS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 1 Hz 50 Hz Bursts at 5 Hz 100% MT | No | 10 (2 weeks) | Yes | None |
| Sikandar et al., 2023 [72] | LF-rTMS | Circular (140 mm) | L Cerebellum R Cerebellum | No | 1 Hz 100% MT | No | 15 (15 days) | Yes | Nausea |
| Zhong et al., 2023 [73] | HF-rTMS | Figure 8 (90 mm) | L Cerebellum R Cerebellum | No | 10 Hz 80% MT | Swallowing Rehabilitation | 10 (10 days) | Yes | Neck Twitching |
| Zhu et al., 2024 [74] | iTBS | Figure 8 (Not Reported) | Ipsilesional Lateral Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | Physical Therapy | 10 (2 weeks) | Yes | None |
| Grobe-Einsler et al., 2024 [75] | iTBS | Figure 8 (70 mm) | Vermis L Cerebellum R Cerebellum | No | 48 Hz bursts at 5 Hz 50% MSO | No | 10 (5 days) | Yes | Headache, Fatigue and Head/Neck Muscle Tension |
| Yassine et al., 2024 [76] | iTBS | Figure 8 (70 mm) | Vermis L Cerebellum R Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | No | Phase I: 12 (4 Weeks) Phase II: 48 (1 Year) | Yes | Nausea, Vomiting, Headache and Neck Stiffness. |
| Liu et al., 2024 [77] | LF-rTMS | Figure 8 (Not Reported) | L Cerebellum R Cerebellum | No | 1 Hz 100% MT | No | 15 (2 weeks) | Yes | None |
| Zhou et al., 2024 [78] | HF-rTMS | Double-cone | Vermis L Cerebellum R Cerebellum | No | 10 Hz 100% MT | No | 10 (2 weeks) | Yes | Not Reported |
| Grobe-Einsler et al., 2024 [79] | iTBS | Figure 8 (70 mm) | Vermis L Cerebellum R Cerebellum | Yes | 48 Hz Bursts at 5 Hz 50% MSO | Physiotherapy | 15 (5 days) | Yes | Headache |
| Grobe-Einsler et al., 2024 [80] | iTBS | Figure 8 (70 mm) | Vermis L Cerebellum R Cerebellum | Yes | 48 Hz Bursts at 5 Hz 50% MSO | Speech and Physiotherapy | 15 (5 days) | Yes | Local Pain over the Stimulation Area |
| He et al., 2024 [81] | LF-rTMS | Not Reported | L Cerebellum R Cerebellum | No | 1 Hz 90% MT | No | 20 (4 weeks) | Not Reported | Not Reported |
| Grobe-Einsler, et al., 2024 [82] | iTBS | Figure 8 (70 mm) | Vermis L Cerebellum R Cerebellum | No | 48 Hz Bursts at 5 Hz 50% MSO | No | 15 (5 days) | Yes | Headache and Subjective Worsening of Gait Disturbance |
| El-Adawy et al., 2024 [83] | LF-rTMS | Figure 8 (100 mm) | L Cerebellum R Cerebellum | No | 1 Hz 90% MT | No | 12 (4 weeks) | Not Reported | Not Reported |
| Liao et al., 2024 [84] | iTBS | Figure 8 (70 mm) | Contralesional Lateral Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | No | 15 (5 days) | Yes | Vertigo |
| Zhang et al., 2024 [85] | iTBS | Double-cone | Contralesional Lateral Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | Routine Rehabilitation | 10 (2 weeks) | Not Reported | Not Reported |
| Li et al., 2025 [86] | iTBS | Figure 8 (70 mm) | L Cerebellum R Cerebellum | No | 50 Hz Bursts at 5 Hz 80% MT | No | 10 (2 weeks) | Yes | None |
| Wang et al., 2025 [34] | cTBS | Double-cone | L Cerebellum R Cerebellum | Yes | 50 Hz Bursts at 5 Hz 80% MT | No | 10 (2 weeks) | Yes | Headache, Tinnitus and Dizziness |
| Ye et al., 2025 [87] | iTBS | Figure 8 (92 mm) | L Cerebellum | No | 50 Hz Bursts at 5 Hz 90% MT | Physical Therapy | 20 (10 days) | Not Reported | Not Reported |
| NHLBI Quality Assessment Tool | Tikka et al., 2015 [44] | Johkura et al., 2018 [47] | Liu et al., 2018 [48] | Cha et al., 2019 [51] | He et al., 2024 [81] |
|---|---|---|---|---|---|
| 1. Was the study question or objective clearly stated? | Y | Y | Y | Y | Y |
| 2. Were eligibility/selection criteria for the study population prespecified and clearly described? | Y | Y | Y | Y | Y |
| 3. Were the participants in the study representative of those who would be eligible for the test/service/intervention in the general or clinical population of interest? | Y | Y | Y | Y | Y |
| 4. Were all eligible participants that met the prespecified entry criteria enrolled? | Y | Y | Y | Y | Y |
| 5. Was the sample size sufficiently large to provide confidence in the findings? | N | N | N | N | Y |
| 6. Was the test/service/intervention clearly described and delivered consistently across the study population? | Y | Y | Y | Y | Y |
| 7. Were the outcome measures prespecified, clearly defined, valid, reliable, and assessed consistently across all study participants? | Y | Y | Y | Y | Y |
| 8. Were the people assessing the outcomes blinded to the participants’ exposures/interventions? | Y | N | N | NR | NR |
| 9. Was the loss to follow-up after baseline 20% or less? Were those lost to follow-up accounted for in the analysis? | N | N | Y | Y | NR |
| 10. Did the statistical methods examine changes in outcome measures from before to after the intervention? Were statistical tests done that provided p values for the pre-to-post changes? | Y | Y | Y | Y | Y |
| 11. Were outcome measures of interest taken multiple times before the intervention and multiple times after the intervention (i.e., did they use an interrupted time-series design)? | N | N | N | N | N |
| 12. If the intervention was conducted at a group level (e.g., a whole hospital, a community, etc.) did the statistical analysis take into account the use of individual-level data to determine effects at the group level? | NA | NA | NA | NA | NA |
| Overall Bias | Fair | Poor | Fair | Fair | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciricugno, A.; Paternò, S.; Barbati, N.; Borgatti, R.; Cattaneo, Z.; Ferrari, C. Advances in Cerebellar TMS Therapy: An Updated Systematic Review on Multi-Session Interventions. Biomedicines 2025, 13, 1578. https://doi.org/10.3390/biomedicines13071578
Ciricugno A, Paternò S, Barbati N, Borgatti R, Cattaneo Z, Ferrari C. Advances in Cerebellar TMS Therapy: An Updated Systematic Review on Multi-Session Interventions. Biomedicines. 2025; 13(7):1578. https://doi.org/10.3390/biomedicines13071578
Chicago/Turabian StyleCiricugno, Andrea, Sonia Paternò, Nicole Barbati, Renato Borgatti, Zaira Cattaneo, and Chiara Ferrari. 2025. "Advances in Cerebellar TMS Therapy: An Updated Systematic Review on Multi-Session Interventions" Biomedicines 13, no. 7: 1578. https://doi.org/10.3390/biomedicines13071578
APA StyleCiricugno, A., Paternò, S., Barbati, N., Borgatti, R., Cattaneo, Z., & Ferrari, C. (2025). Advances in Cerebellar TMS Therapy: An Updated Systematic Review on Multi-Session Interventions. Biomedicines, 13(7), 1578. https://doi.org/10.3390/biomedicines13071578






