The Effect of Dapagliflozin, a Sodium–Glucose Co-Transporter 2 Inhibitor, on Vancomycin-Induced Nephrotoxicity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Rats
2.2. Ethics Committee
2.3. Drugs, Rationale for Dose Selection, Administration Route, and Duration
2.4. Animals and Experimental Groups
- Group 1 (Control): Rats received an IP injection of saline for seven days.
- Group 4 (VA+DAPA): Rats received DAPA (10 mg/kg) by oral gavage for seven days and VA (200 mg/kg) via an IP injection twice daily for the same period.
2.5. Operation Procedures and Measurements
2.5.1. Measurement of Serum Urea and Creatinine Levels
2.5.2. Measurement of MDA and CAT Levels
2.5.3. Measurement of Bax, Bcl-2, and Caspase-3 Levels
2.5.4. Measurement of SOD, GPx, and MPO Levels
2.5.5. Measurement of TAS and TOS Levels
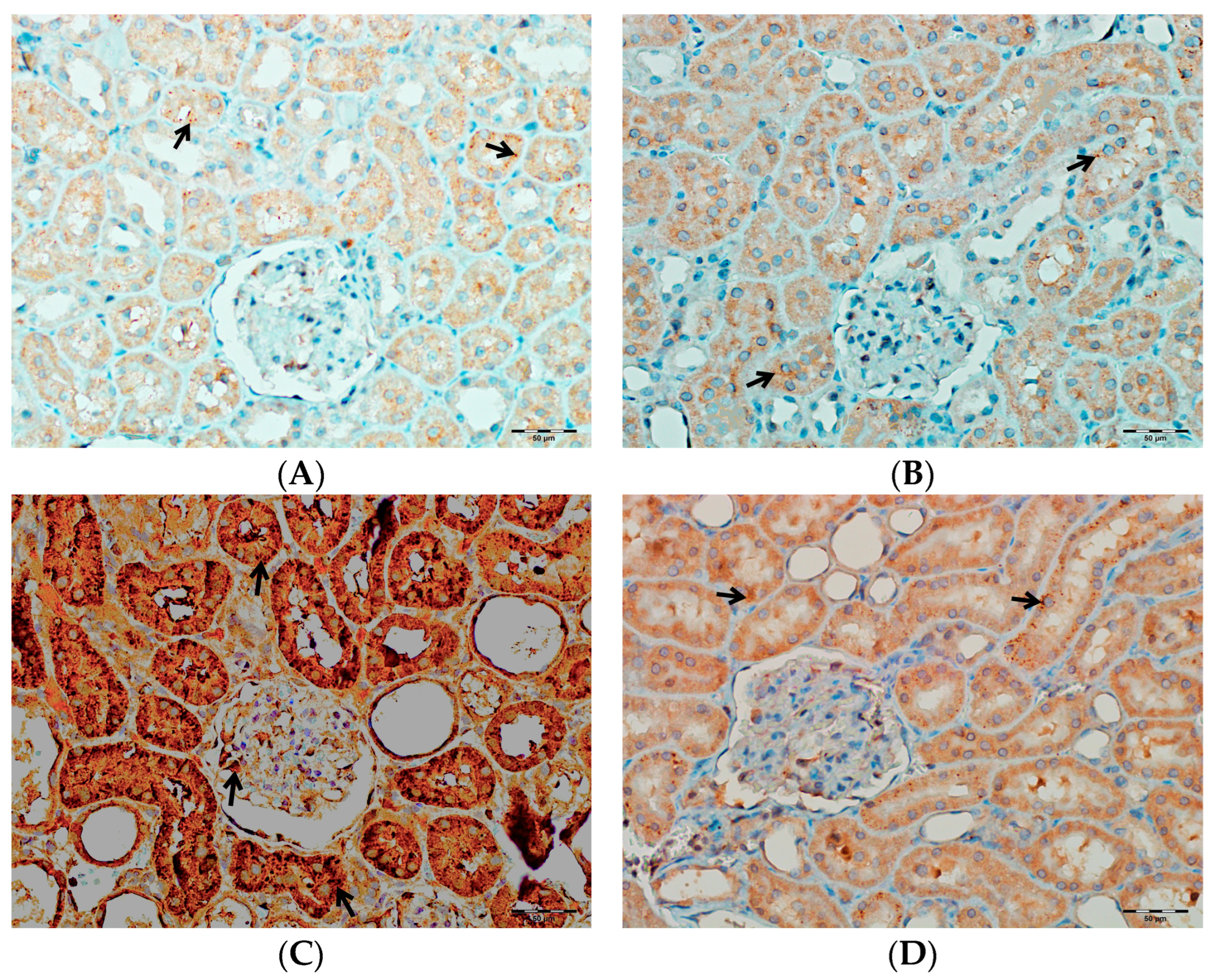
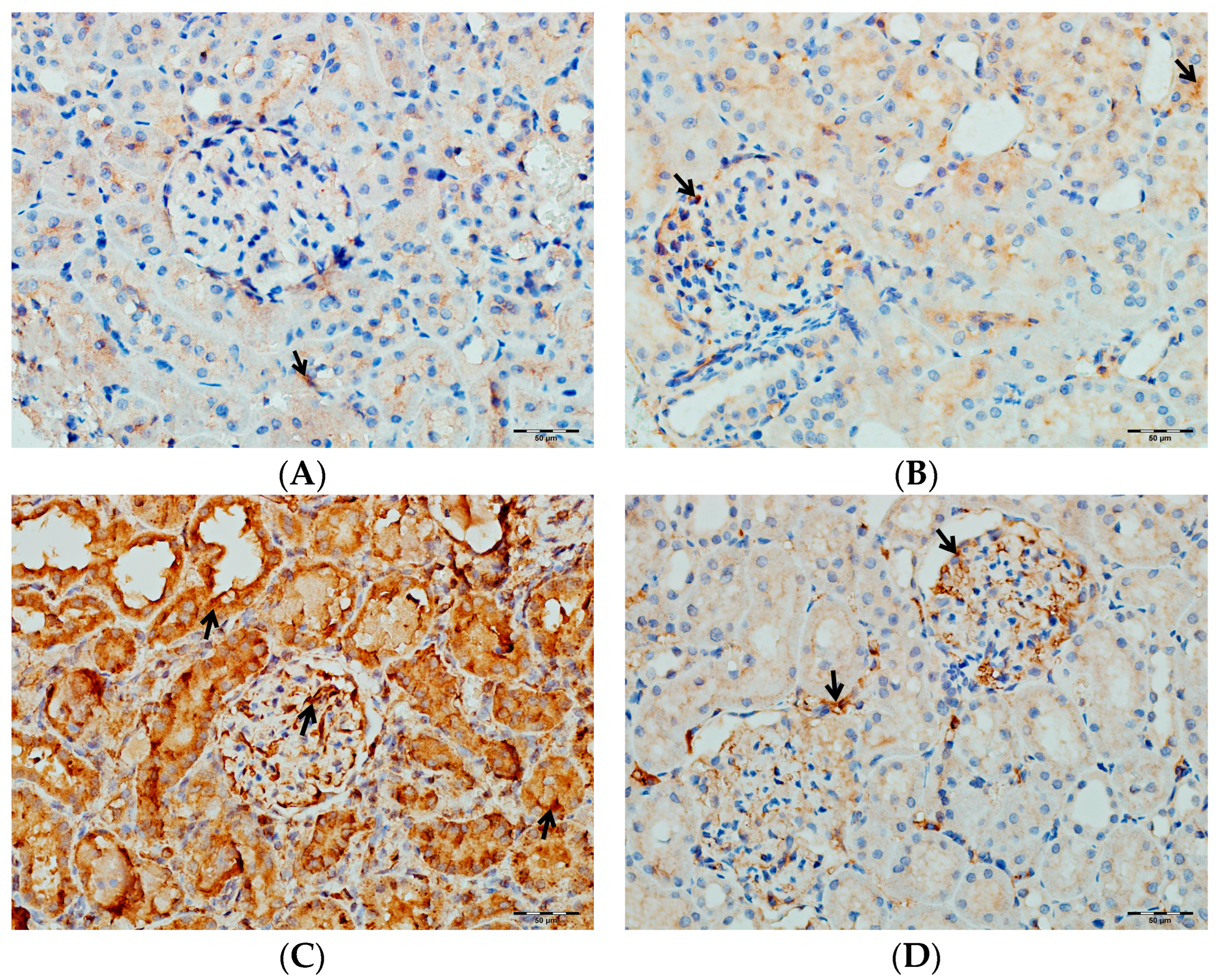
2.5.6. Immunohistochemical Evaluation of TNF-α, IL-1β, and IL-6 in Kidney Tissues
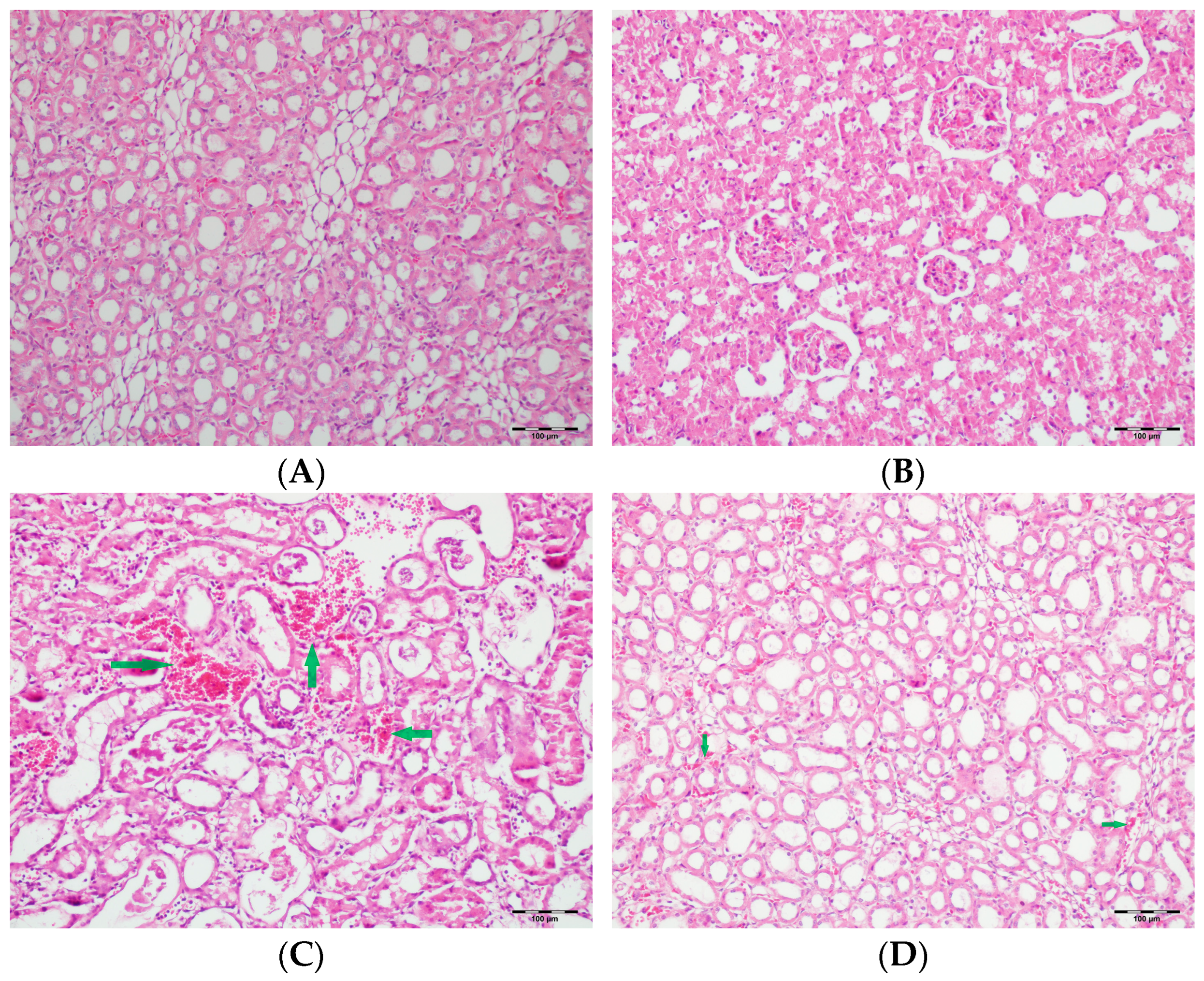
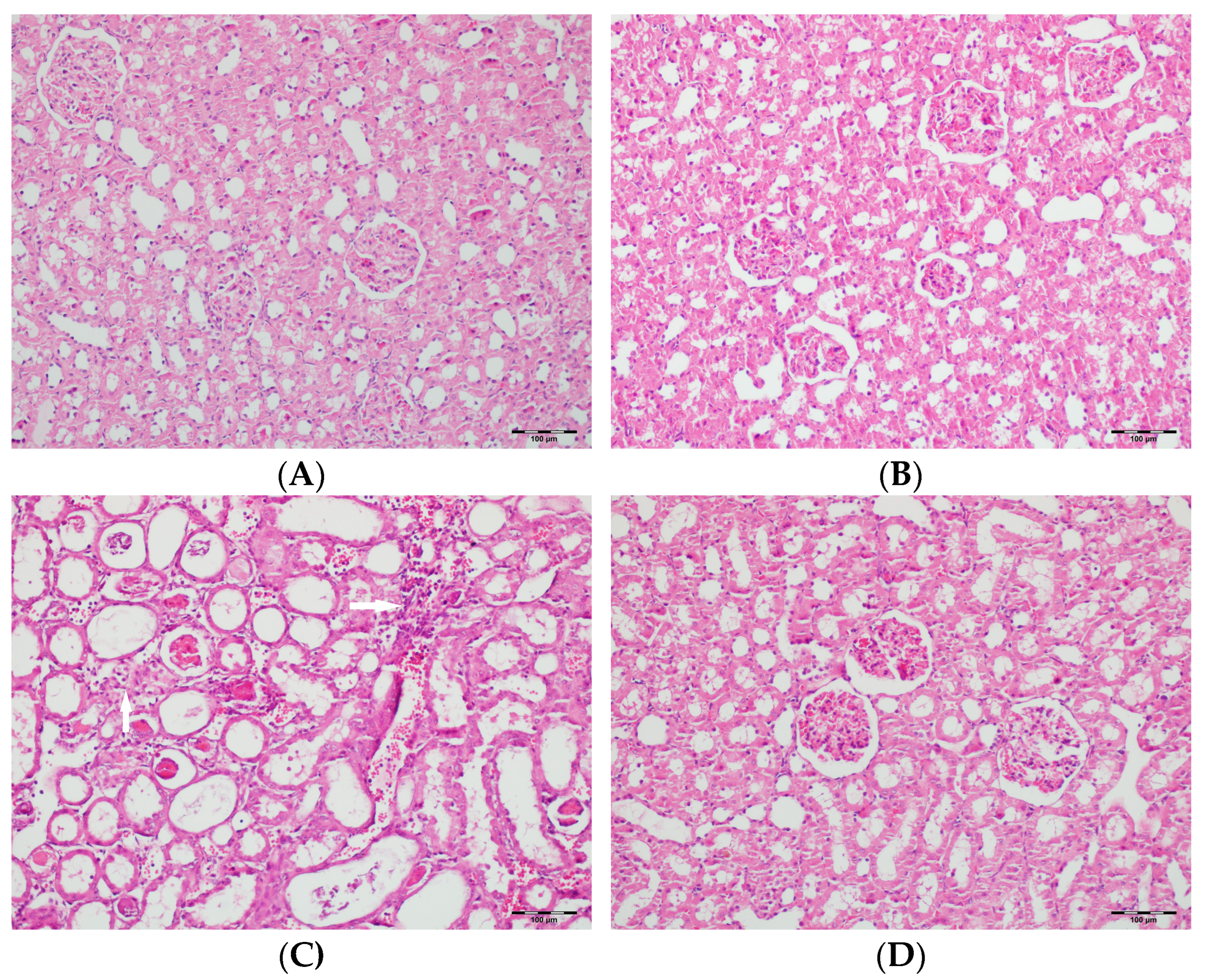
2.6. Histopathological Examination of Renal Tissues
2.7. Statistical Analyses
3. Results
3.1. Serum Urea and Creatinine Levels
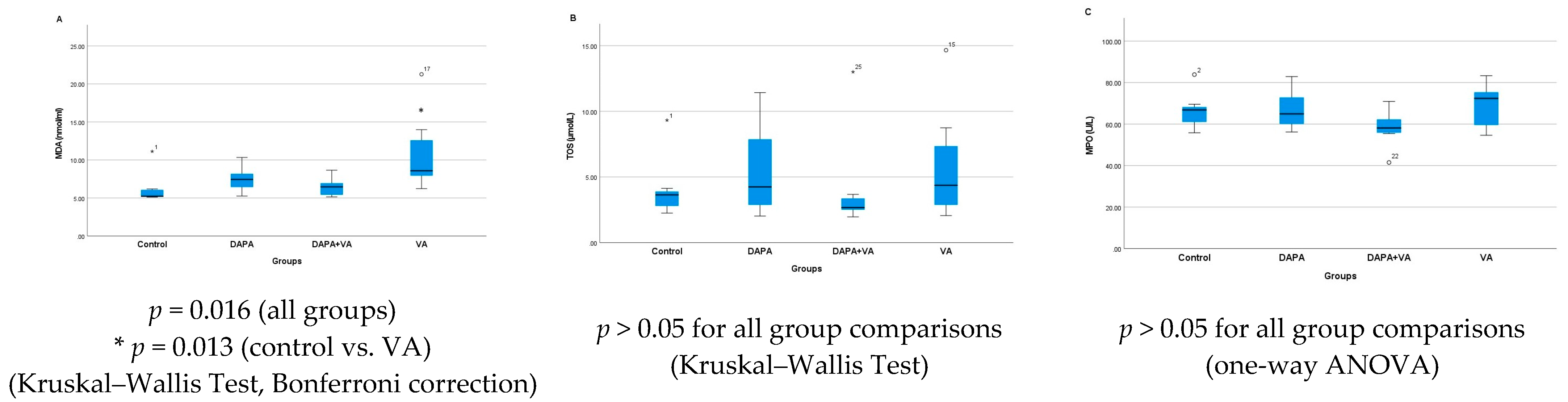
3.2. MDA, TOS, and MPO Levels
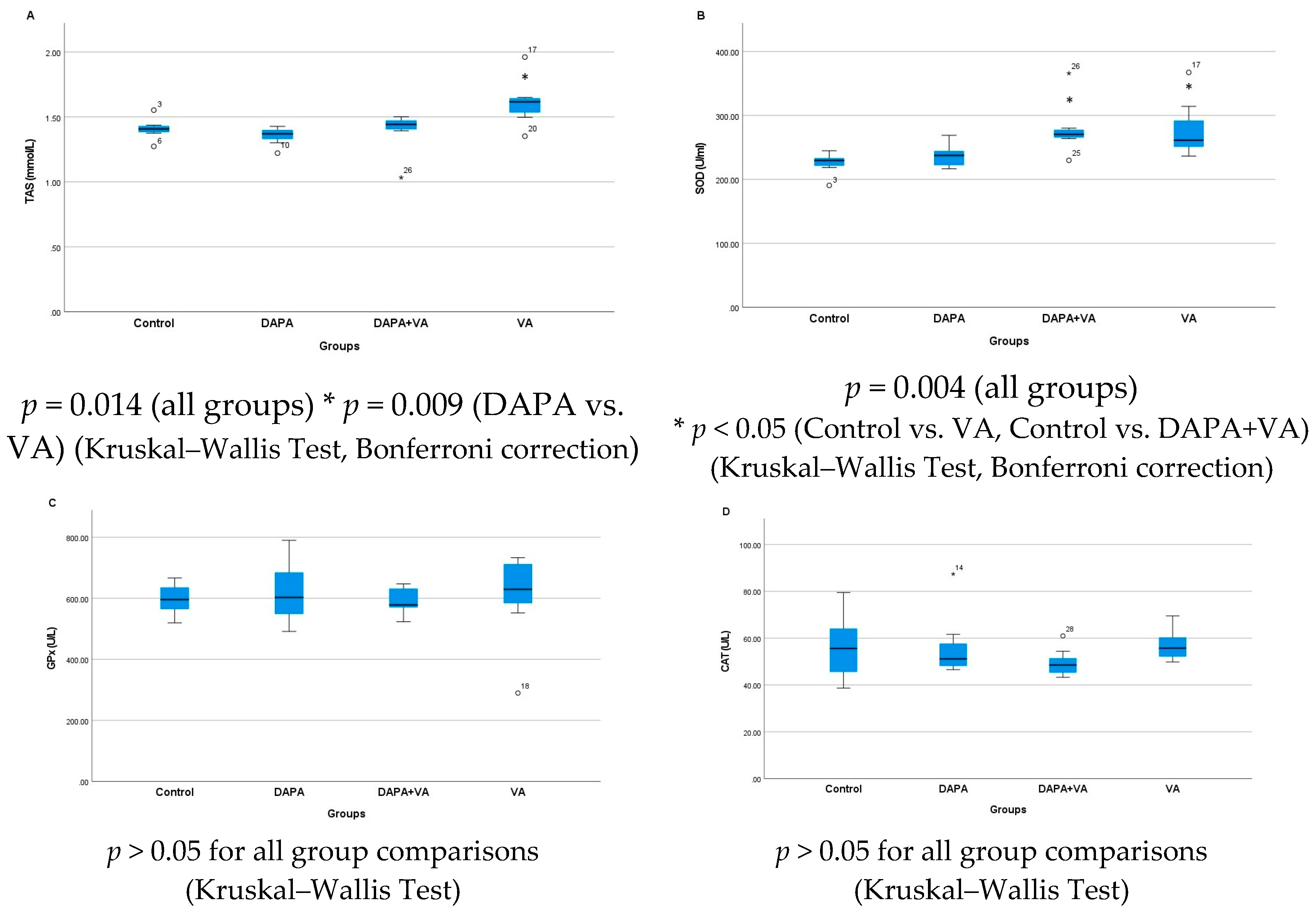
3.3. TAS, GPx, CAT, and SOD Levels
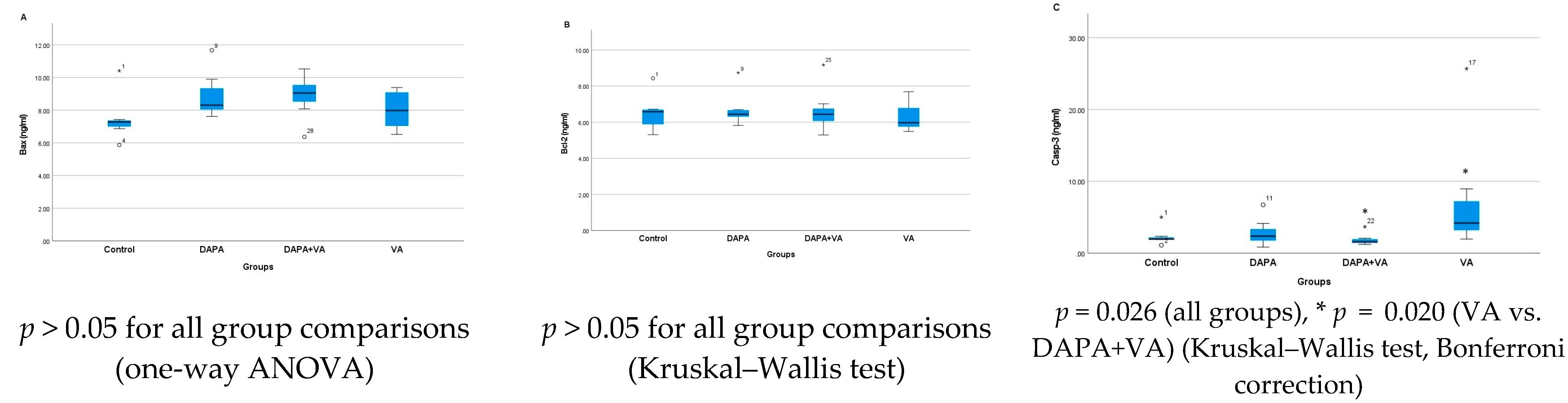
3.4. Bax, Bcl-2, and Casp-3 Enzyme Activities
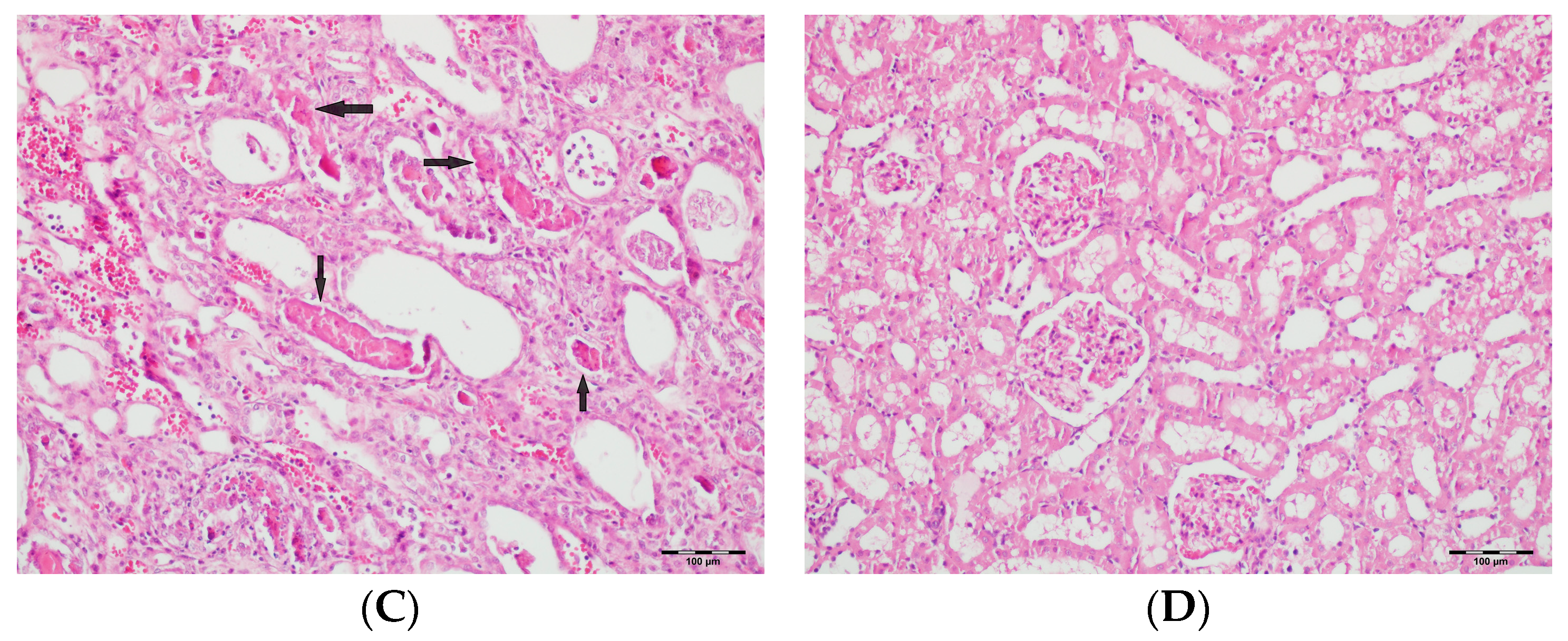
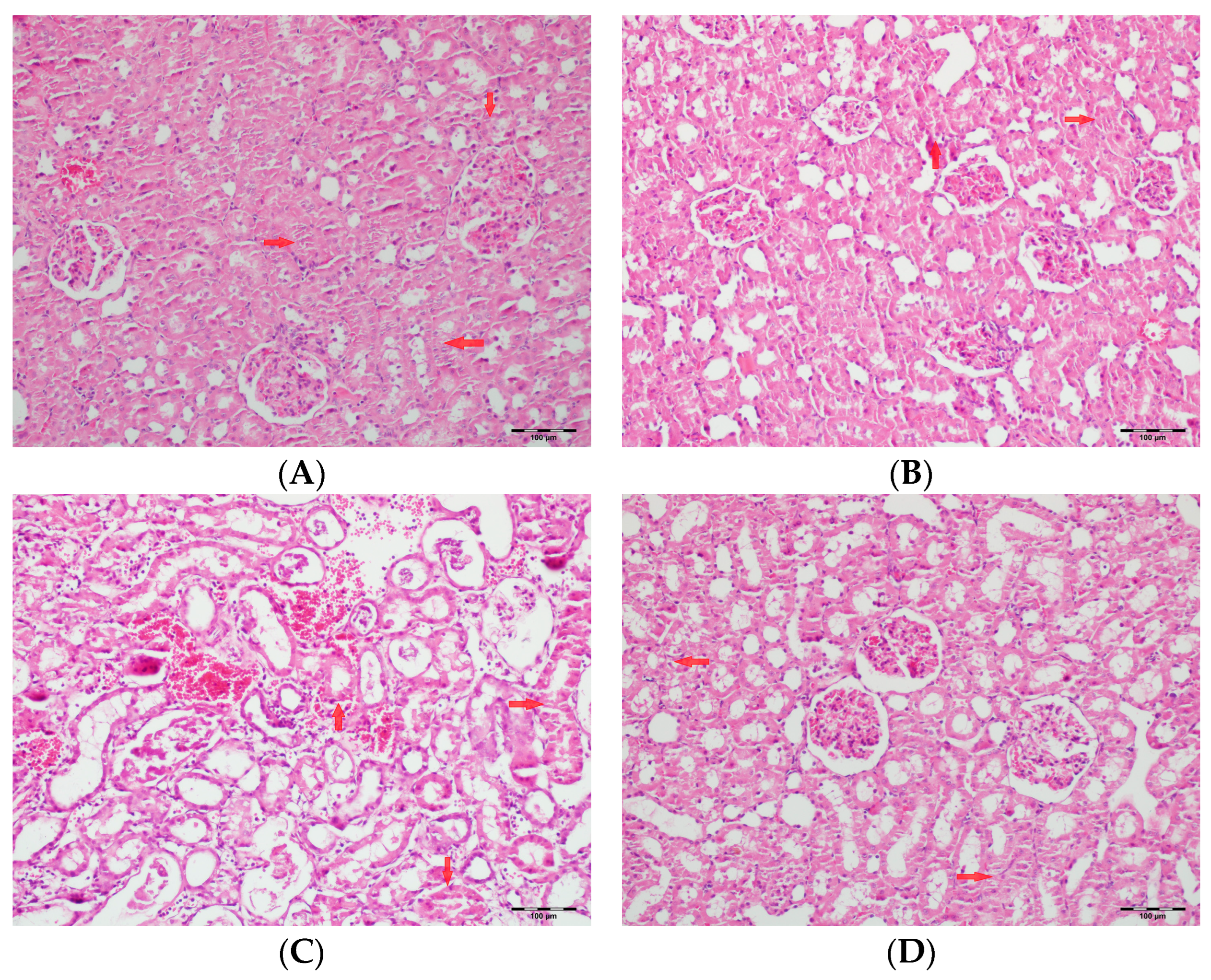
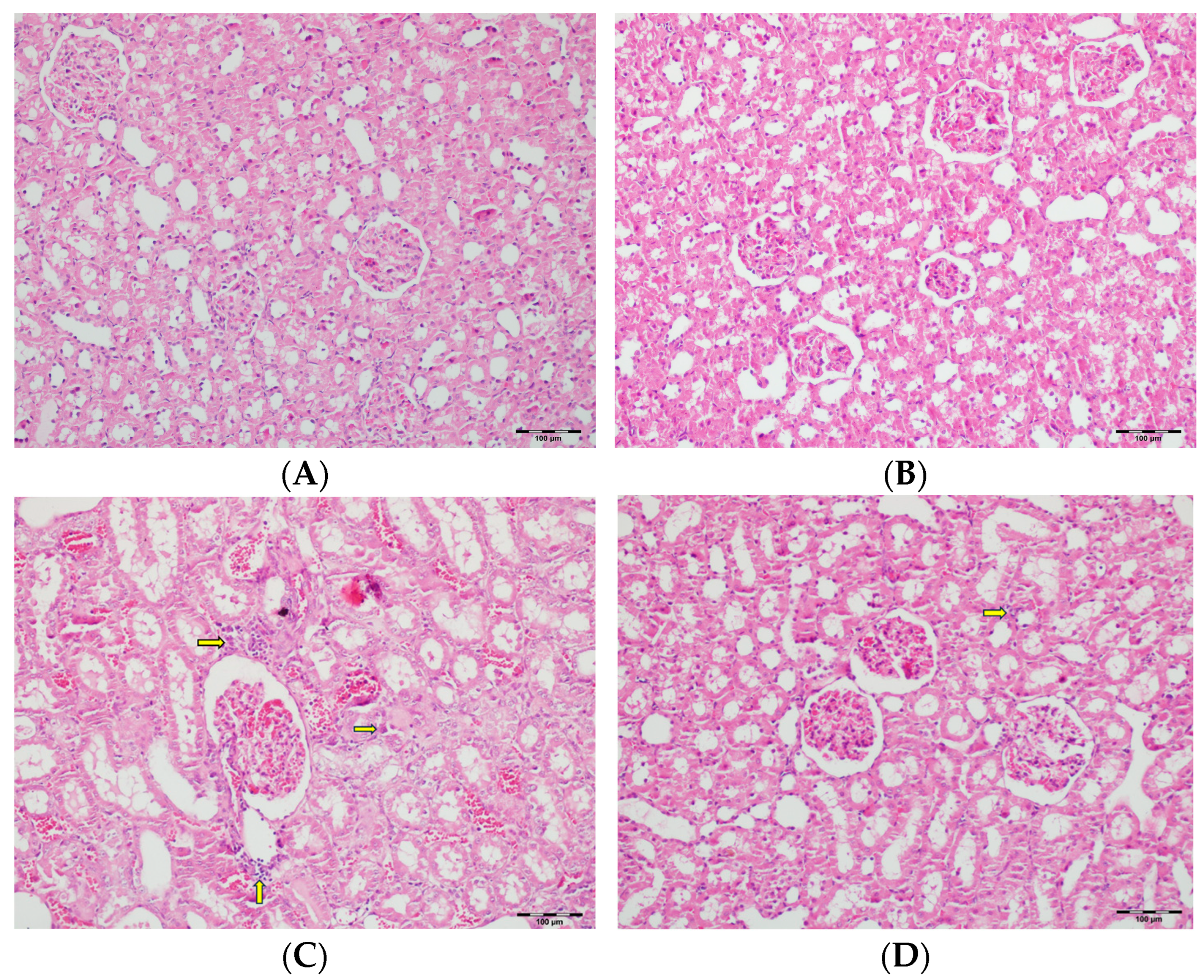
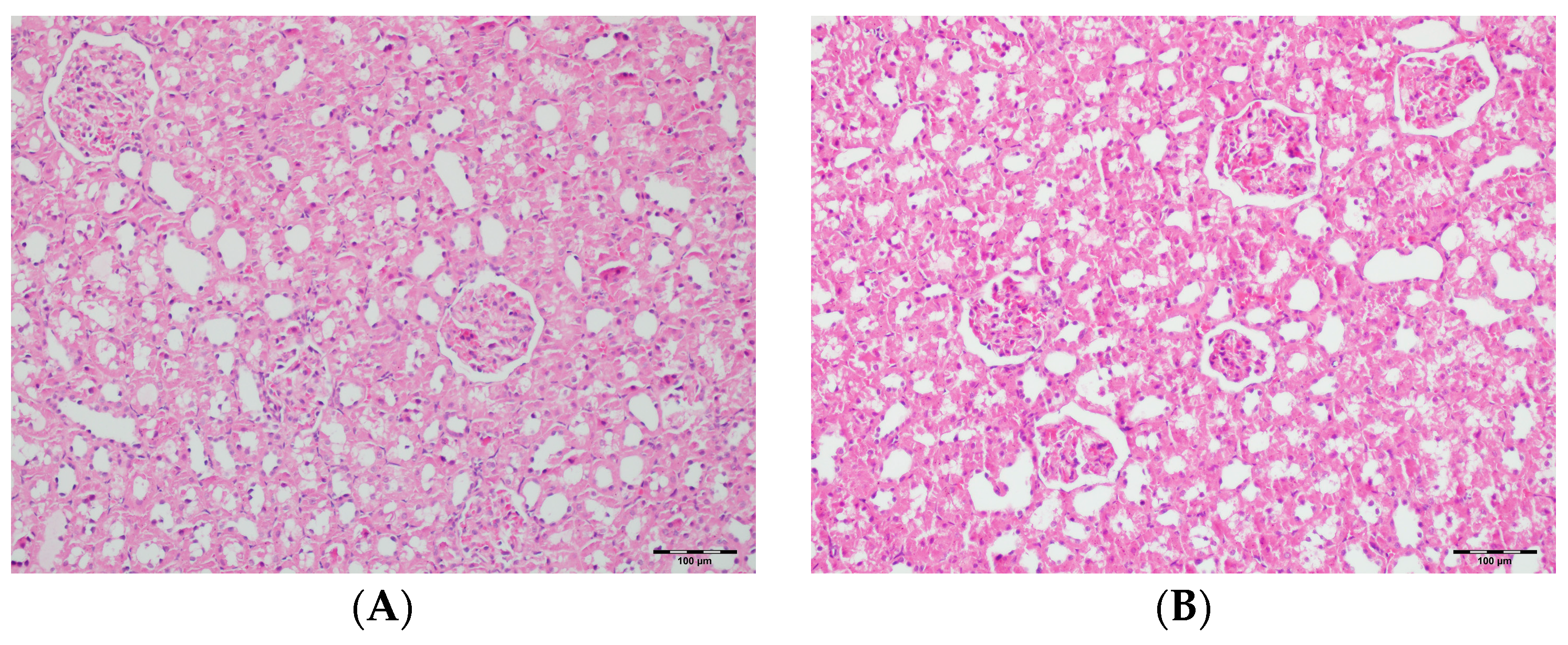
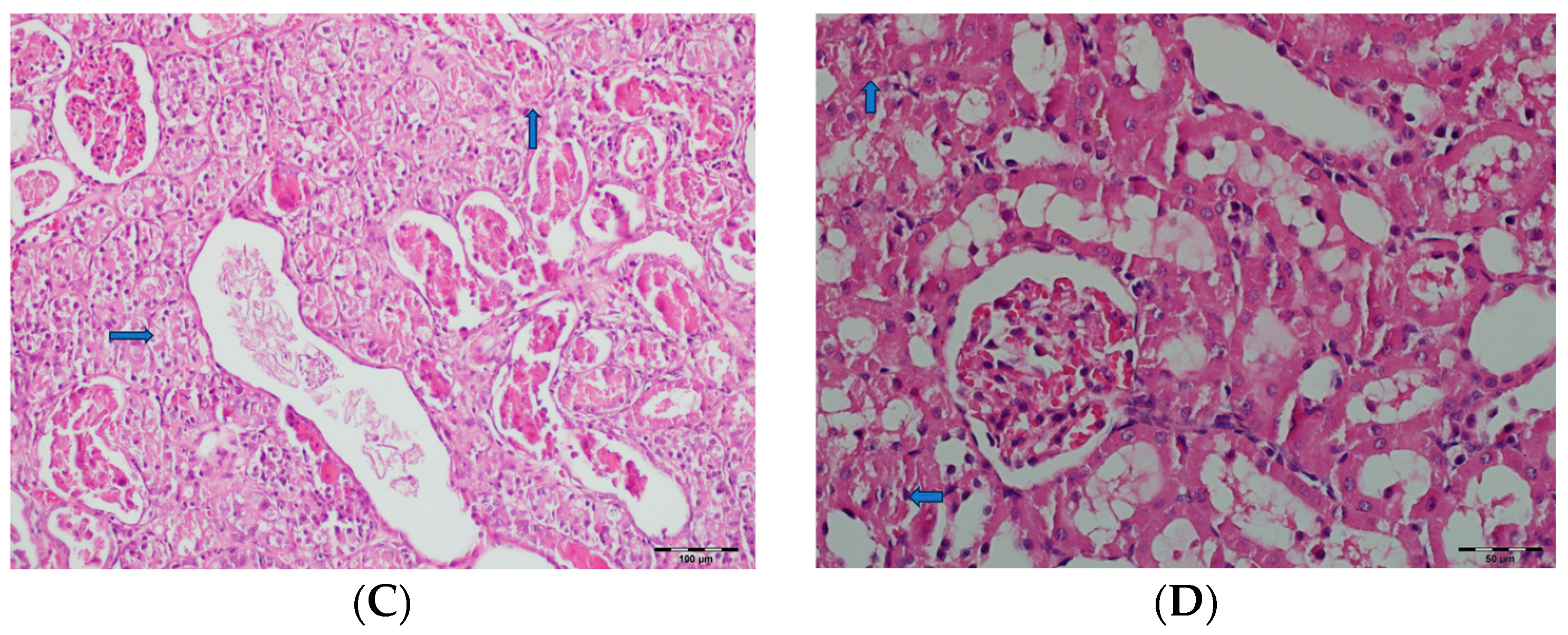
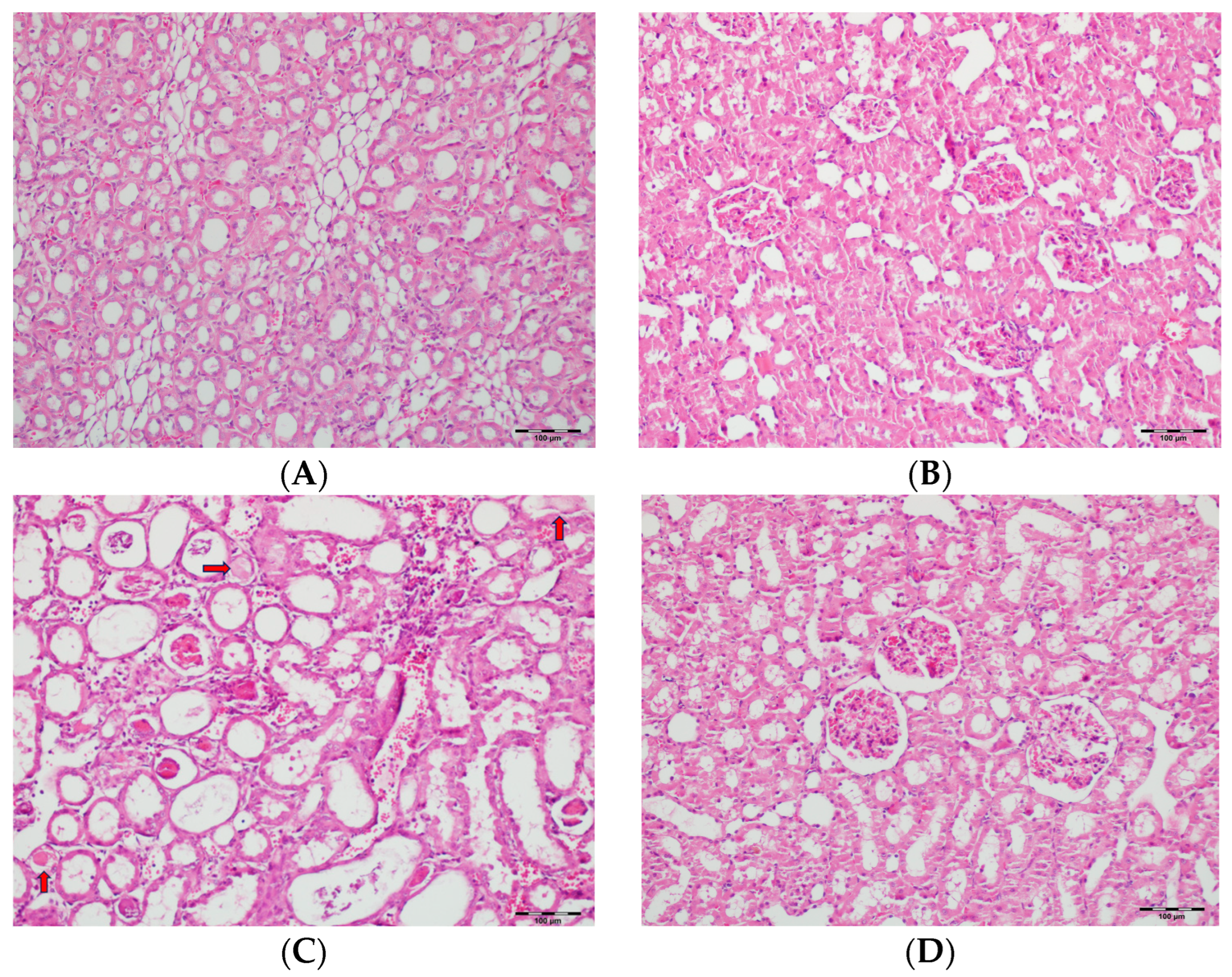
3.5. Histopathological Studies
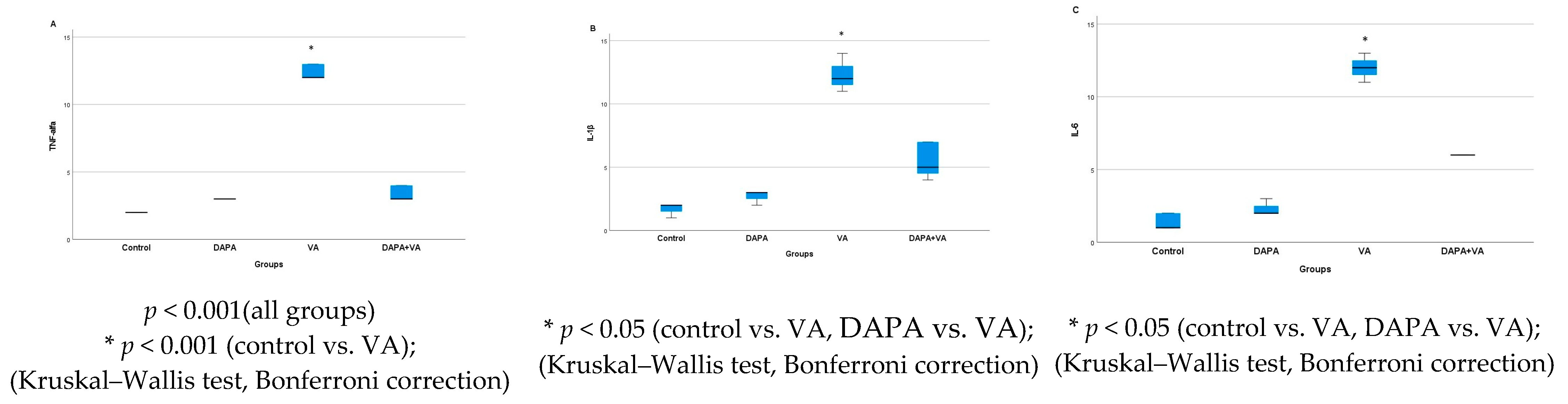
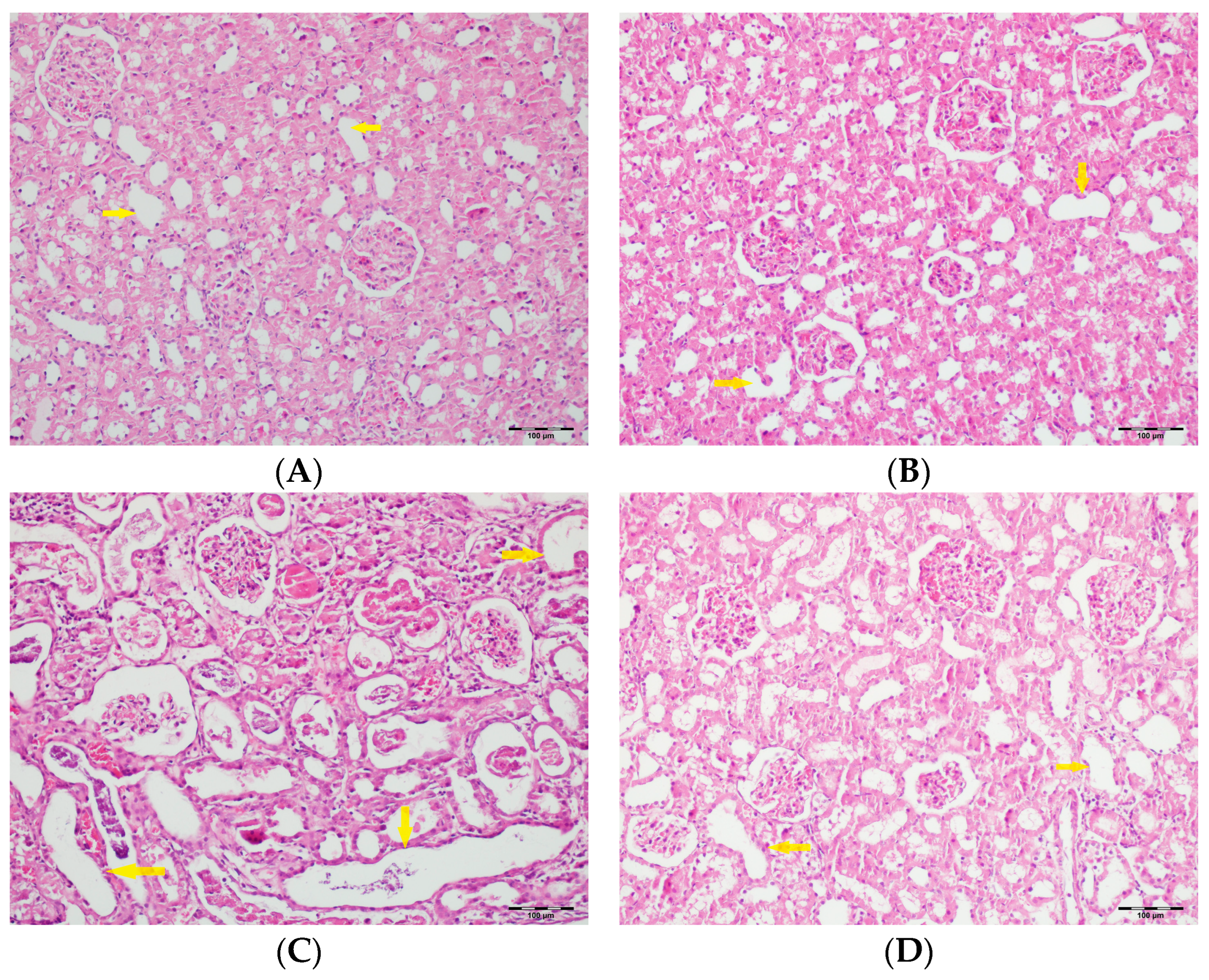
3.6. Immunohistochemical Evaluation of IL-1β, IL-6, and TNF-α in Kidney Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2-associated X protein |
| CAT | Catalase |
| Casp-3 | Caspase-3 |
| DAPA | Dapagliflozin |
| GPx | Glutathione peroxidase |
| Il-6 | Interleukin-6 |
| IL-1β | Interleukin-1 βeta |
| MPO | Myeloperoxidase |
| MDA | Malondialdehyde |
| SGLT2 | Sodium–glucose co-transporter |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor-alpha |
| TOS | Total oxidant status |
| TAS | Total antioxidant status |
| VA | Vancomycin |
| VIN | Vancomycin-associated nephrotoxicity |
References
- Zamoner, W.; Prado, I.R.S.; Balbi, A.L.; Ponce, D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin. Exp. Pharmacol. Physiol. 2019, 46, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E.; Domanski, L.; Dziedziejko, V.; Kajdy, A.; Stefanska, K.; Kwiatkowski, S. The Mechanism of Drug Nephrotoxicity and the Methods for Preventing Kidney Damage. Int. J. Mol. Sci. 2021, 22, 6109. [Google Scholar] [CrossRef] [PubMed]
- Mergenhagen, K.A.; Borton, A.R. Vancomycin nephrotoxicity: A review. J. Pharm. Pract. 2014, 27, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Awdishu, L.; Le, A.; Amato, J.; Jani, V.; Bal, S.; Mills, R.H.; Carrillo-Terrazas, M.; Gonzalez, D.J.; Tolwani, A.; Acharya, A.; et al. Urinary Exosomes Identify Inflammatory Pathways in Vancomycin Associated Acute Kidney Injury. Int. J. Mol. Sci. 2021, 22, 2784. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Murray, K.P.; Lagnf, A.M.; Melvin, S.; Bhatia, S.; Shamim, M.D.; Smith, J.R.; Brade, K.D.; Simon, S.P.; Nagel, J.; et al. A Multicenter Evaluation of Vancomycin-Associated Acute Kidney Injury in Hospitalized Patients with Acute Bacterial Skin and Skin Structure Infections. Infect. Dis. Ther. 2020, 9, 89–106. [Google Scholar] [CrossRef]
- Rahmani, H.; Khalili, H. Prevention of Vancomycin-Induced Nephrotoxicity; An Updated Review of Clinical and Preclinical Studies. Infect. Disord. Drug Targets 2022, 22, e310321192584. [Google Scholar] [CrossRef]
- Oktem, F.; Arslan, M.K.; Ozguner, F.; Candir, O.; Yilmaz, H.R.; Ciris, M.; Uz, E. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: Protection by erdosteine. Toxicology 2005, 215, 227–233. [Google Scholar] [CrossRef]
- Kahraman, M.; Ertekin, Y.H.; Satman, I. The Effects of Kefir on Kidney Tissues and Functions in Diabetic Rats. Probiotics Antimicrob. Proteins 2021, 13, 375–382. [Google Scholar] [CrossRef]
- Ocak, S.; Gorur, S.; Hakverdi, S.; Celik, S.; Erdogan, S. Protective effects of caffeic acid phenethyl ester, vitamin C, vitamin E and N-acetylcysteine on vancomycin-induced nephrotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 2007, 100, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Luo, J.; Song, H.; Qian, T.; He, X.; Fang, J.; Dong, W.; Bian, X. N-acetylcysteine Ameliorates Vancomycin-induced Nephrotoxicity by Inhibiting Oxidative Stress and Apoptosis in the in vivo and in vitro Models. Int. J. Med. Sci. 2022, 19, 740–752. [Google Scholar] [CrossRef]
- Ahmida, M.H. Protective role of curcumin in nephrotoxic oxidative damage induced by vancomycin in rats. Exp. Toxicol. Pathol. 2012, 64, 149–153. [Google Scholar] [CrossRef]
- Uckun, Z.; Guzel, S.; Canacankatan, N.; Yalaza, C.; Kibar, D.; Coskun Yilmaz, B. Potential protective effects of naringenin against vancomycin-induced nephrotoxicity via reduction on apoptotic and oxidative stress markers in rats. Drug Chem. Toxicol. 2020, 43, 104–111. [Google Scholar] [CrossRef]
- Cetin, H.; Olgar, S.; Oktem, F.; Ciris, M.; Uz, E.; Aslan, C.; Ozguner, F. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1181–1185. [Google Scholar] [CrossRef]
- Celik, I.; Cihangiroglu, M.; Ilhan, N.; Akpolat, N.; Akbulut, H.H. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin. Pharmacol. Toxicol. 2005, 97, 325–332. [Google Scholar] [CrossRef]
- Choi, M.R.; Fernandez, B.E. Protective Renal Effects of Atrial Natriuretic Peptide: Where Are We Now? Front. Physiol. 2021, 12, 680213. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, S.; Zhang, Y.; Wang, Q.; Kong, L.; Zhou, L. Effect of N-acetylcysteine on antimicrobials induced nephrotoxicity: A meta-analysis. BMC Nephrol. 2025, 26, 128. [Google Scholar] [CrossRef]
- Hong, T.S.; Briscese, K.; Yuan, M.; Deshpande, K.; Aleksunes, L.M.; Brunetti, L. Renoprotective Effects of Melatonin against Vancomycin-Related Acute Kidney Injury in Hospitalized Patients: A Retrospective Cohort Study. Antimicrob. Agents Chemother. 2021, 65, e0046221. [Google Scholar] [CrossRef]
- He, J.; Mao, E.; Xu, W.; Zhao, B.; Jing, F.; Bian, X.; Chen, E. High dose vitamin C significantly reduces the nephrotoxicity of vancomycin in critically ill patients. Chin. J. Crit. Care Med. 2020, 32, 468–472. [Google Scholar] [CrossRef]
- Soltani, R.; Khorvash, F.; Meidani, M.; Badri, S.; Alaei, S.; Taheri, S. Vitamin E in the prevention of vancomycin-induced nephrotoxicity. Res. Pharm. Sci. 2020, 15, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; Dong, Z. Renoprotective approaches and strategies in acute kidney injury. Pharmacol. Ther. 2016, 163, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Papaetis, G. SGLT2 Inhibitors and Diabetic Kidney Disease: Targeting Multiple and Interrelated Signaling Pathways for Renal Protection. Curr. Mol. Pharmacol. 2023, 17, e18761429261105. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.N.; da Conceicao, R.A.; do Couto Maia, R.; de Castro Barbosa, M.L. Sodium-glucose cotransporter 2 (SGLT-2) inhibitors: A new antidiabetic drug class. Medchemcomm 2018, 9, 1273–1281. [Google Scholar] [CrossRef]
- Alsereidi, F.R.; Khashim, Z.; Marzook, H.; Gupta, A.; Al-Rawi, A.M.; Ramadan, M.M.; Saleh, M.A. Targeting inflammatory signaling pathways with SGLT2 inhibitors: Insights into cardiovascular health and cardiac cell improvement. Curr. Probl. Cardiol. 2024, 49, 102524. [Google Scholar] [CrossRef]
- Schonberger, E.; Mihaljevic, V.; Steiner, K.; Saric, S.; Kurevija, T.; Majnaric, L.T.; Bilic Curcic, I.; Canecki-Varzic, S. Immunomodulatory Effects of SGLT2 Inhibitors-Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Oraby, M.A.; El-Yamany, M.F.; Safar, M.M.; Assaf, N.; Ghoneim, H.A. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed. Pharmacother. 2019, 109, 910–920. [Google Scholar] [CrossRef]
- Certikova Chabova, V.; Zakiyanov, O. Sodium Glucose Cotransporter-2 Inhibitors: Spotlight on Favorable Effects on Clinical Outcomes beyond Diabetes. Int. J. Mol. Sci. 2022, 23, 2812. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H. Sodium-glucose Cotransporter Type 2 Inhibitors: A New Insight into the Molecular Mechanisms of Diabetic Nephropathy. Curr. Pharm. Des. 2022, 28, 2131–2139. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, S.S.; He, Y.X.; Yan, L.J.; Lv, F.; Liang, Q.M.; Gan, Y.H.; Han, L.P.; Xu, H.D.; Li, Y.C.; et al. SGLT2 inhibitors alleviated podocyte damage in lupus nephritis by decreasing inflammation and enhancing autophagy. Ann. Rheum. Dis. 2023, 82, 1328–1340. [Google Scholar] [CrossRef]
- Salah, H.M.; Fudim, M. Sodium-glucose Cotransporter 2 Inhibitors and Nonalcoholic Fatty Liver Disease. Heart Fail. Clin. 2022, 18, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.M.; Al Suleimani, Y.; Shalaby, A.; Ashique, M.; Manoj, P.; Nemmar, A.; Ali, B.H. Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on cisplatin-induced nephrotoxicity in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Oe, Y.; Kim, Y.C.; Sidorenko, V.S.; Zhang, H.; Kanoo, S.; Lopez, N.; Goodluck, H.A.; Crespo-Masip, M.; Vallon, V. SGLT2 inhibitor dapagliflozin protects the kidney in a murine model of Balkan nephropathy. Am. J. Physiol. Renal Physiol. 2024, 326, F227–F240. [Google Scholar] [CrossRef]
- Deger, M.; Kaya, B.; Akdogan, N.; Kaplan, H.M.; Bagir, E.; Izol, V.; Aridogan, I.A. Protective effect of dapagliflozin against cyclosporine A-induced nephrotoxicity. Drug Chem. Toxicol. 2022, 45, 2637–2643. [Google Scholar] [CrossRef]
- Farrokh-Eslamlou, N.; Momtaz, S.; Niknejad, A.; Hosseini, Y.; Mahdaviani, P.; Ghasemnejad-Berenji, M.; Abdolghaffari, A.H. Empagliflozin protective effects against cisplatin-induced acute nephrotoxicity by interfering with oxidative stress and inflammation in Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 7061–7070. [Google Scholar] [CrossRef]
- Kabel, A.M.; Salama, S.A. Effect of taxifolin/dapagliflozin combination on colistin-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2021, 40, 1767–1780. [Google Scholar] [CrossRef]
- Chang, Y.K.; Choi, H.; Jeong, J.Y.; Na, K.R.; Lee, K.W.; Lim, B.J.; Choi, D.E. Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PLoS ONE 2016, 11, e0158810. [Google Scholar] [CrossRef]
- Ma, Q.; Steiger, S.; Anders, H.J. Sodium glucose transporter-2 inhibition has no renoprotective effects on non-diabetic chronic kidney disease. Physiol. Rep. 2017, 5, e13228. [Google Scholar] [CrossRef]
- Zhang, Y.; Thai, K.; Kepecs, D.M.; Gilbert, R.E. Sodium-Glucose Linked Cotransporter-2 Inhibition Does Not Attenuate Disease Progression in the Rat Remnant Kidney Model of Chronic Kidney Disease. PLoS ONE 2016, 11, e0144640. [Google Scholar] [CrossRef]
- Darwish, S.F.; Mahmoud, A.M.A.; Abdel Mageed, S.S.; Sallam, A.M.; Oraby, M.A. Dapagliflozin improves early acute kidney injury induced by vancomycin in rats: Insights on activin A/miRNA-21 signaling and FOXO3a expression. Eur. J. Pharmacol. 2023, 955, 175908. [Google Scholar] [CrossRef]
- Tantranont, N.; Luque, Y.; Hsiao, M.; Haute, C.; Gaber, L.; Barrios, R.; Adrogue, H.E.; Niasse, A.; Truong, L.D. Vancomycin-Associated Tubular Casts and Vancomycin Nephrotoxicity. Kidney Int. Rep. 2021, 6, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research; Division on Earth and Life Studies. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Rhodes, N.J.; Prozialeck, W.C.; Lodise, T.P.; Venkatesan, N.; O’Donnell, J.N.; Pais, G.; Cluff, C.; Lamar, P.C.; Neely, M.N.; Gulati, A.; et al. Evaluation of Vancomycin Exposures Associated with Elevations in Novel Urinary Biomarkers of Acute Kidney Injury in Vancomycin-Treated Rats. Antimicrob. Agents Chemother. 2016, 60, 5742–5751. [Google Scholar] [CrossRef]
- Bamgbola, O. Review of vancomycin-induced renal toxicity: An update. Ther. Adv. Endocrinol. Metab. 2016, 7, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Pais, G.M.; Liu, J.; Zepcan, S.; Avedissian, S.N.; Rhodes, N.J.; Downes, K.J.; Moorthy, G.S.; Scheetz, M.H. Vancomycin-Induced Kidney Injury: Animal Models of Toxicodynamics, Mechanisms of Injury, Human Translation, and Potential Strategies for Prevention. Pharmacotherapy 2020, 40, 438–454. [Google Scholar] [CrossRef]
- Nishino, Y.; Takemura, S.; Minamiyama, Y.; Hirohashi, K.; Ogino, T.; Inoue, M.; Okada, S.; Kinoshita, H. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic. Res. 2003, 37, 373–379. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kawada, K.; Shimada, T.; Mori, M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am. J. Obstet. Gynecol. 1979, 135, 372–376. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005, 42, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant effect of p-coumaric acid on interleukin 1-beta and tumor necrosis factor-alpha in rats with renal ischemic reperfusion. Nefrologia 2020, 40, 311–319. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Ozkaraca, M.; Yildirim, B.A.; Hanedan, B.; Kirbas, A.; Kilic, K.; Aktas, E.; Benzer, F. Rutin attenuates gentamicin-induced renal damage by reducing oxidative stress, inflammation, apoptosis, and autophagy in rats. Ren. Fail. 2015, 37, 518–525. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009, 29, 1275–1279. [Google Scholar] [CrossRef]
- Elyasi, S.; Khalili, H.; Hatamkhani, S.; Dashti-Khavidaki, S. Prevention of vancomycin induced nephrotoxicity: A review of preclinical data. Eur. J. Clin. Pharmacol. 2013, 69, 747–754. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Lang, F.; Hu, L.; Tang, Q.; Wang, H.; Zhang, Y.; Hao, Z. Rutin Attenuates Vancomycin-Induced Nephrotoxicity by Ameliorating Oxidative Stress, Apoptosis, and Inflammation in Rats. Antimicrob. Agents Chemother. 2018, 63, e01545-18. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yano, T.; Hanada, Y.; Takeshita, A.; Inagaki, F.; Masuda, S.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur. J. Pharmacol. 2017, 800, 48–56. [Google Scholar] [CrossRef]
- Arimura, Y.; Yano, T.; Hirano, M.; Sakamoto, Y.; Egashira, N.; Oishi, R. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic. Biol. Med. 2012, 52, 1865–1873. [Google Scholar] [CrossRef]
- Humanes, B.; Jado, J.C.; Camano, S.; Lopez-Parra, V.; Torres, A.M.; Alvarez-Sala, L.A.; Cercenado, E.; Tejedor, A.; Lazaro, A. Protective Effects of Cilastatin against Vancomycin-Induced Nephrotoxicity. Biomed. Res. Int. 2015, 2015, 704382. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 2015, 66, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Tsimihodimos, V.; Filippatos, T.D.; Filippas-Ntekouan, S.; Elisaf, M. Renoprotective Effects of SGLT2 Inhibitors: Beyond Glucose Reabsorption Inhibition. Curr. Vasc. Pharmacol. 2017, 15, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 (Suppl. S2), S165–S171. [Google Scholar] [CrossRef]
- Shibusawa, R.; Yamada, E.; Okada, S.; Nakajima, Y.; Bastie, C.C.; Maeshima, A.; Kaira, K.; Yamada, M. Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci. Rep. 2019, 9, 9887. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Stephens, J.W. A systematic review examining the effects of sodium-glucose cotransporter-2 inhibitors (SGLT2is) on biomarkers of inflammation and oxidative stress. Diabetes Res. Clin. Pract. 2020, 168, 108368. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int. Immunopharmacol. 2022, 111, 109080. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Fujita, T. Formation and removal of reactive oxygen species, lipid peroxides and free radicals, and their biological effects. Yakugaku Zasshi 2002, 122, 203–218. [Google Scholar] [CrossRef]
- Filippone, E.J.; Kraft, W.K.; Farber, J.L. The Nephrotoxicity of Vancomycin. Clin. Pharmacol. Ther. 2017, 102, 459–469. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Guo, H.; Wang, C.; Hao, Z.; Tang, Q.; Wang, H.; Zhang, Y. Rutin attenuates vancomycin-induced renal tubular cell apoptosis via suppression of apoptosis, mitochondrial dysfunction, and oxidative stress. Phytother. Res. 2019, 33, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Song, M.; Wang, S.; Huang, C.; Pan, T. Dapagliflozin has protective effects on palmitate-induced renal tubular epithelial cells by enhancing mitochondrial function and reducing oxidative stress. J. Diabetes Complicat. 2025, 39, 108930. [Google Scholar] [CrossRef] [PubMed]
- Dubin, R.F.; Rhee, E.P. Proteomics and Metabolomics in Kidney Disease, including Insights into Etiology, Treatment, and Prevention. Clin. J. Am. Soc. Nephrol. 2020, 15, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Faubel, S.; Edelstein, C.L. Cytokines in acute kidney injury (AKI). Clin. Nephrol. 2011, 76, 165–173. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Quercia, A.D.; Dellepiane, S.; Ferrario, S.; Camussi, G.; Biancone, L. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol. Dial. Transplant. 2014, 29, 2004–2011. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; Edelstein, C.L. Mediators of inflammation in acute kidney injury. Mediat. Inflamm. 2009, 2009, 137072. [Google Scholar] [CrossRef]
- Bellos, I.; Pergialiotis, V.; Perrea, D.N. Kidney biopsy findings in vancomycin-induced acute kidney injury: A pooled analysis. Int. Urol. Nephrol. 2022, 54, 137–148. [Google Scholar] [CrossRef]
- Nishino, Y.; Takemura, S.; Minamiyama, Y.; Hirohashi, K.; Tanaka, H.; Inoue, M.; Okada, S.; Kinoshita, H. Inhibition of vancomycin-induced nephrotoxicity by targeting superoxide dismutase to renal proximal tubule cells in the rat. Redox Rep. 2002, 7, 317–319. [Google Scholar] [CrossRef]
- Aronoff, G.R.; Sloan, R.S.; Dinwiddie, C.B., Jr.; Glant, M.D.; Fineberg, N.S.; Luft, F.C. Effects of vancomycin on renal function in rats. Antimicrob. Agents Chemother. 1981, 19, 306–308. [Google Scholar] [CrossRef]
- Kashihara, N.; Kidokoro, K.; Kanda, E. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors and underlying mechanisms. Curr. Opin. Nephrol. Hypertens. 2020, 29, 112–118. [Google Scholar] [CrossRef]
- Ashfaq, A.; Meineck, M.; Pautz, A.; Arioglu-Inan, E.; Weinmann-Menke, J.; Michel, M.C. A systematic review on renal effects of SGLT2 inhibitors in rodent models of diabetic nephropathy. Pharmacol. Ther. 2023, 249, 108503. [Google Scholar] [CrossRef]
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes. Metab. 2018, 20, 1988–1993. [Google Scholar] [CrossRef]




| Mediator | Control | DAPA | VA | VA+DAPA | p |
|---|---|---|---|---|---|
| Urea | 64.8 ± 5.6 a,b | 71.1 ± 5.5 a,d | 191.2 ± 75.1 b,d,f | 73.4 ± 7.1 f | <0.001 |
| Creatinine | 0.75 ± 0.06 c | 0.78 ± 0.09 d | 3.67 ± 2.4 c,d,f | 0.86 ± 0.11 f | <0.001 |
| MDA | 6.28 ± 2.2 b | 7.47 ± 1.8 | 11.0 ± 5.2 b,f | 6.43 ± 1.2 f | 0.016 |
| TOS | 4.09 ± 2.4 | 5.60 ± 3.9 | 5.94 ± 4.5 | 4.20 ± 3.9 | 0.609 |
| MPO | 66.4 ± 9.3 | 67.1 ± 9.7 | 68.6 ± 10.9 | 58.1 ± 9.0 | 0.256 |
| GPx | 597.5 ± 52.9 | 621.6 ± 103.9 | 606.4 ± 154.3 | 593.2 ± 45.7 | 0.809 |
| CAT | 56.2 ± 14.9 | 56.7 ± 14.5 | 57.1 ± 6.9 | 49.5 ± 6.3 | 0.311 |
| SOD | 224.9 ± 17.2 b,c | 236.6 ± 18.3 | 278.7 ± 46.5 b | 278.9 ± 41.7 c | 0.004 |
| TAS | 1.41 ± 0.08 | 1.35 ± 0.07 d | 1.61 ± 0.19 d | 1.39 ± 0.16 | 0.014 |
| Bax | 7.47 ± 1.40 | 8.91 ± 1.4 | 8.02 ± 1.2 | 8.87 ± 1.3 | 0.130 |
| Casp-3 | 2.33 ± 1.2 | 2.88 ± 1.9 | 7.53 ± 8.3 f | 1.89 ± 0.8 f | 0.026 |
| Bcl-2 | 6.50 ± 1.0 | 6.71 ± 0.9 | 6.32 ± 0.8 | 6.65 ± 1.2 | 0.873 |
| Parameters | Control | DAPA | VA | VA+DAPA | p | |
|---|---|---|---|---|---|---|
| Tubular dilatation | Normal | 5 a (71.4) | 6 a (85.7) | - | 5 a (71.4) | <0.001 |
| Light | 2 a (28.6) | 1 a (14.3) | - | 2 a (28.6) | ||
| Moderate | - | - | 1 a (14.3) | - | ||
| Severe | - | - | 6 b (85.7) | - | ||
| Tubular vacuolization | Normal | 4 a (57.1) | 2 a,b (28.6) | - | 1 a,b (14.3) | <0.001 |
| Light | 3 a,b,c (42.9) | 5 c (71.4) | - | 4 a,c (57.1) | ||
| Moderate | - | - | - | 2 a (28.6) | ||
| Severe | - | - | 7 b (100) | - | ||
| Hyaline cast | Normal | 1 a (14.3) | - | - | - | <0.001 |
| Light | 6 a (85.7) | 7 a (100) | - | 5 a (71.4) | ||
| Moderate | - | 3 a (42.9) | 2 a (28.6) | |||
| Severe | - | - | 4 b (57.1) | - | ||
| Tubular necrosis | Normal | 5 a (71.4) | 7 a (100) | - | 4 a (57.1) | <0.001 |
| Light | 2 a (28.6) | - | - | 2 a (28.6) | ||
| Moderate | - | - | 1 a (14.3) | 1 (14.3) | ||
| Severe | - | - | 6 b (85.7) | - | ||
| Tubular atrophy | Normal | 6 a,b (85.7) | 7 b (100) | - | 3 a,c (42.9) | <0.001 |
| Light | 1 a (14.3) | - | - | 3 a (42.9) | ||
| Moderate | - | - | 2 a (28.6) | 1 a (14.3) | ||
| Severe | - | - | 5 b (71,4) | |||
| Interstitial edema | Normal | 5 a (71.4) | 2 a,b (28.6) | - | 2 a,b (28.6) | <0.001 |
| Light | 2 a,b(28.6) | 5 b (71.4) | - | 3 a,b (42.9) | ||
| Moderate | - | 1 a (14.3) | 2 a (28.6) | |||
| Severe | - | - | 6 b (85.7) | - | ||
| Tubular inflammation | Normal | 7 a (100) | 7 a (100) | - | 6 a (85.7) | <0.001 |
| Light | - | - | - | - | ||
| Moderate | - | - | 1 a (14.3) | 1 a (14.3) | ||
| Severe | - | - | 6 b (85.7) | - | ||
| Mononuclear cells in the medulla | Normal | 6 a (85.7) | 7 a (100) | - | 4 a (57.1) | <0.001 |
| Light | 1 a (14.3) | - | - | 2 a (28.6) | ||
| Moderate | - | - | - | - | ||
| Severe | - | - | 7 b (100) | 1 a (14.3) | ||
| Medullary hemorrhage | Normal | 7 a (100) | 7 a (100) | - | 6 a (85.7) | <0.001 |
| Light | - | - | - | - | ||
| Moderate | - | - | 1 a (14.3) | 1 a (14.3) | ||
| Severe | - | - | 6 b (85.7) | - | ||
| Necrotic area in cortex | Normal | 7 a (100) | 7 a (100) | - | 6 a (85.7) | <0.001 |
| Light | - | - | - | - | ||
| Moderate | - | - | 4 b (57.1) | 1 a,b (14.3) | ||
| Severe | - | - | 3 a (42.9) | - | ||
| Tubular desquamation | Normal | 5 a,b(71.4) | 7 b (100) | - | 3 a,c (42.9) | <0.001 |
| Light | 2 a (28.6) | - | - | 3 a (42.9) | ||
| Moderate | - | - | - | 1 a (14.3) | ||
| Severe | - | - | 7 b (100) | - |
| Parameter | Control | DAPA | VA | VA+DAPA | p |
|---|---|---|---|---|---|
| TNF-α | 2.00 ± 0.0 a,b,c | 3.00 ± 0.0 a,d | 12.43 ± 0.53 b,d | 3.43 ± 0.53 c | <0.001 |
| IL-1β | 1.71 ± 0.48 b,c | 2.71 ± 0.48 d | 12.29 ± 1.25 b,d | 5.57 ± 1.39 c | <0.001 |
| IL-6 | 1.43 ± 0.53 b,c | 2.29 ± 0.49 d | 12.00 ± 0.82 b,d | 6.00 ± 0.00 c | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Kaya, B.; Akburak, E.; Avci, C.; Ates, K.E.; Gonlusen, G.; Ercakalli, T.S.; Mete, B. The Effect of Dapagliflozin, a Sodium–Glucose Co-Transporter 2 Inhibitor, on Vancomycin-Induced Nephrotoxicity in Rats. Biomedicines 2025, 13, 1582. https://doi.org/10.3390/biomedicines13071582
Tan S, Kaya B, Akburak E, Avci C, Ates KE, Gonlusen G, Ercakalli TS, Mete B. The Effect of Dapagliflozin, a Sodium–Glucose Co-Transporter 2 Inhibitor, on Vancomycin-Induced Nephrotoxicity in Rats. Biomedicines. 2025; 13(7):1582. https://doi.org/10.3390/biomedicines13071582
Chicago/Turabian StyleTan, Seyhmus, Bulent Kaya, Ercan Akburak, Cagri Avci, Kivilcim Eren Ates, Gulfiliz Gonlusen, Tugce Sapmaz Ercakalli, and Burak Mete. 2025. "The Effect of Dapagliflozin, a Sodium–Glucose Co-Transporter 2 Inhibitor, on Vancomycin-Induced Nephrotoxicity in Rats" Biomedicines 13, no. 7: 1582. https://doi.org/10.3390/biomedicines13071582
APA StyleTan, S., Kaya, B., Akburak, E., Avci, C., Ates, K. E., Gonlusen, G., Ercakalli, T. S., & Mete, B. (2025). The Effect of Dapagliflozin, a Sodium–Glucose Co-Transporter 2 Inhibitor, on Vancomycin-Induced Nephrotoxicity in Rats. Biomedicines, 13(7), 1582. https://doi.org/10.3390/biomedicines13071582









