Inhibition of Matrix Metalloproteinase-7 Attenuates Subpleural Fibrosis in Rheumatoid Arthritis-Associated Interstitial Lung Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.1.1. Micro-Matrix Data
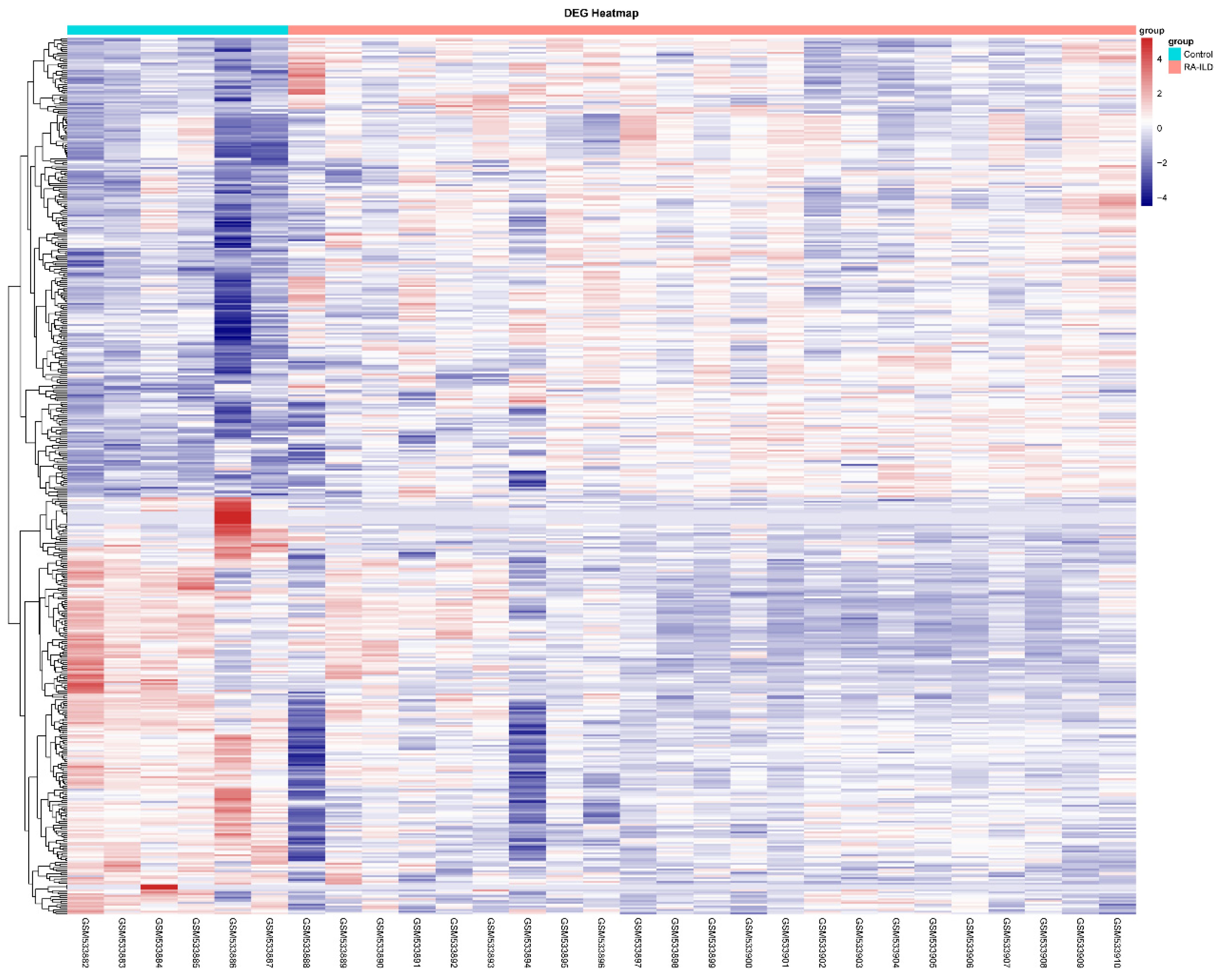
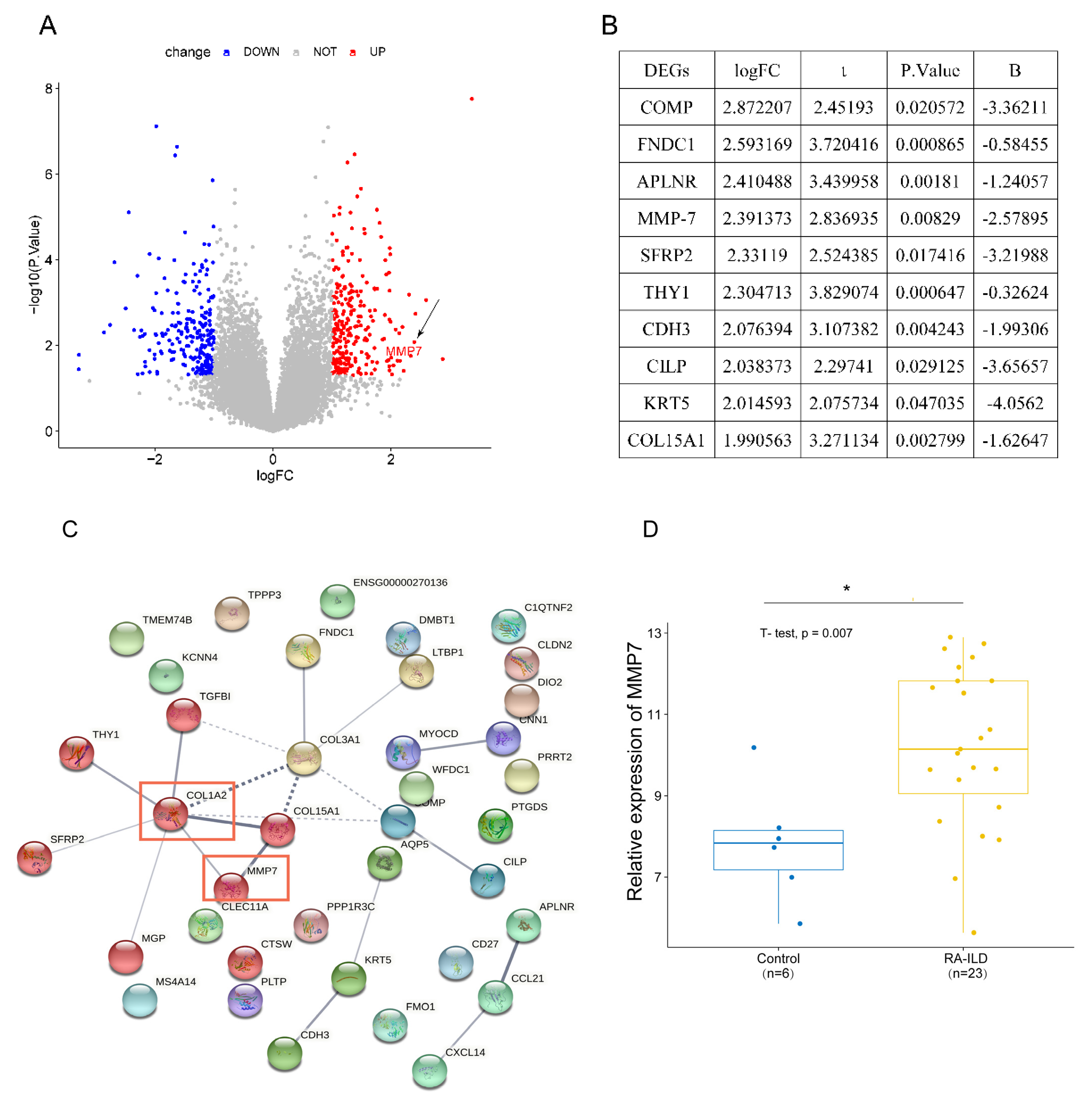
2.1.2. Identification of Differentially Expressed Genes (DEGs)
2.1.3. Construction of the PPI Network
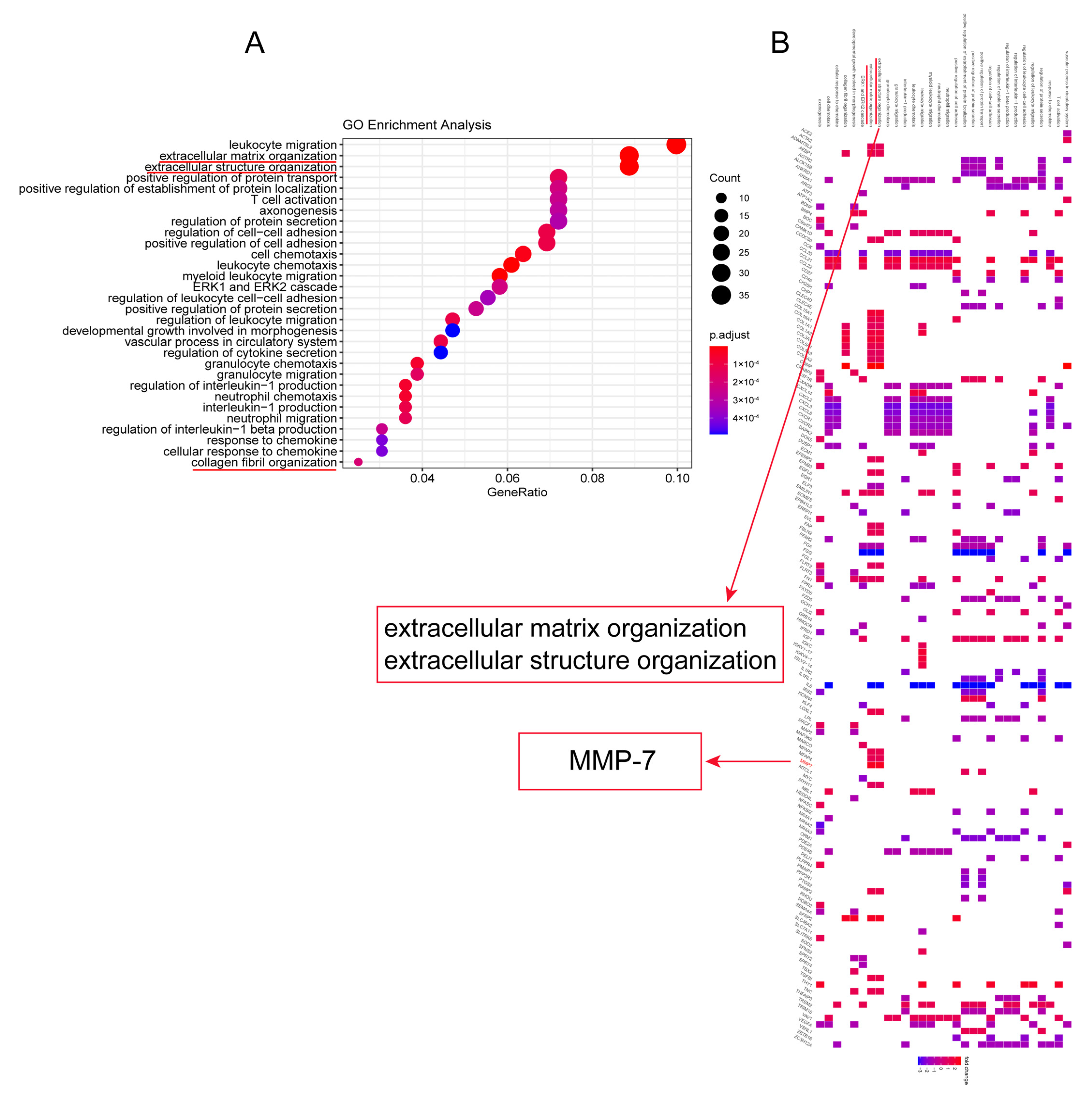
2.1.4. Gene Ontology (GO) Analysis
2.2. Materials and Reagents
2.3. Culturing and Treating Human PMCs
2.4. Cell Proliferation Testing
2.5. Patients’ Serum Samples’ Collection
2.6. siRNA and Lentivirus Plasmid Transfection
2.7. RA-ILD Animal Model
2.8. Histological Analysis
2.9. Pulmonary Fibrosis Degree Score
2.10. Western Blot
2.11. Statistical Analysis
3. Results
3.1. MMP-7 Was Involved in Formation of Extracellular Matrix Within Lung Tissue of RA-ILD
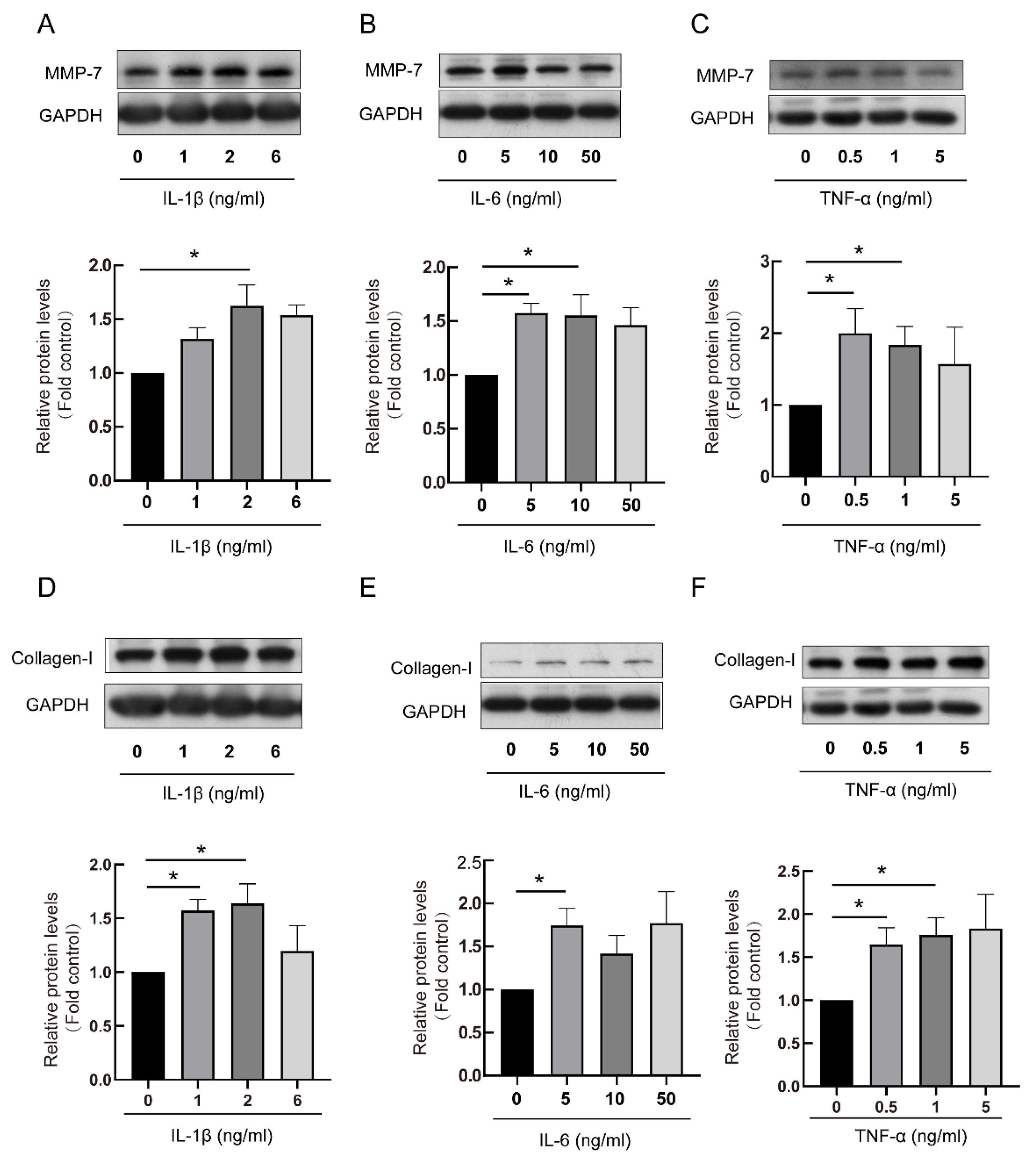
3.2. The RA-ILD-Related Inflammatory Factors Mediated Production of MMP-7 and Collagen-I in PMCs
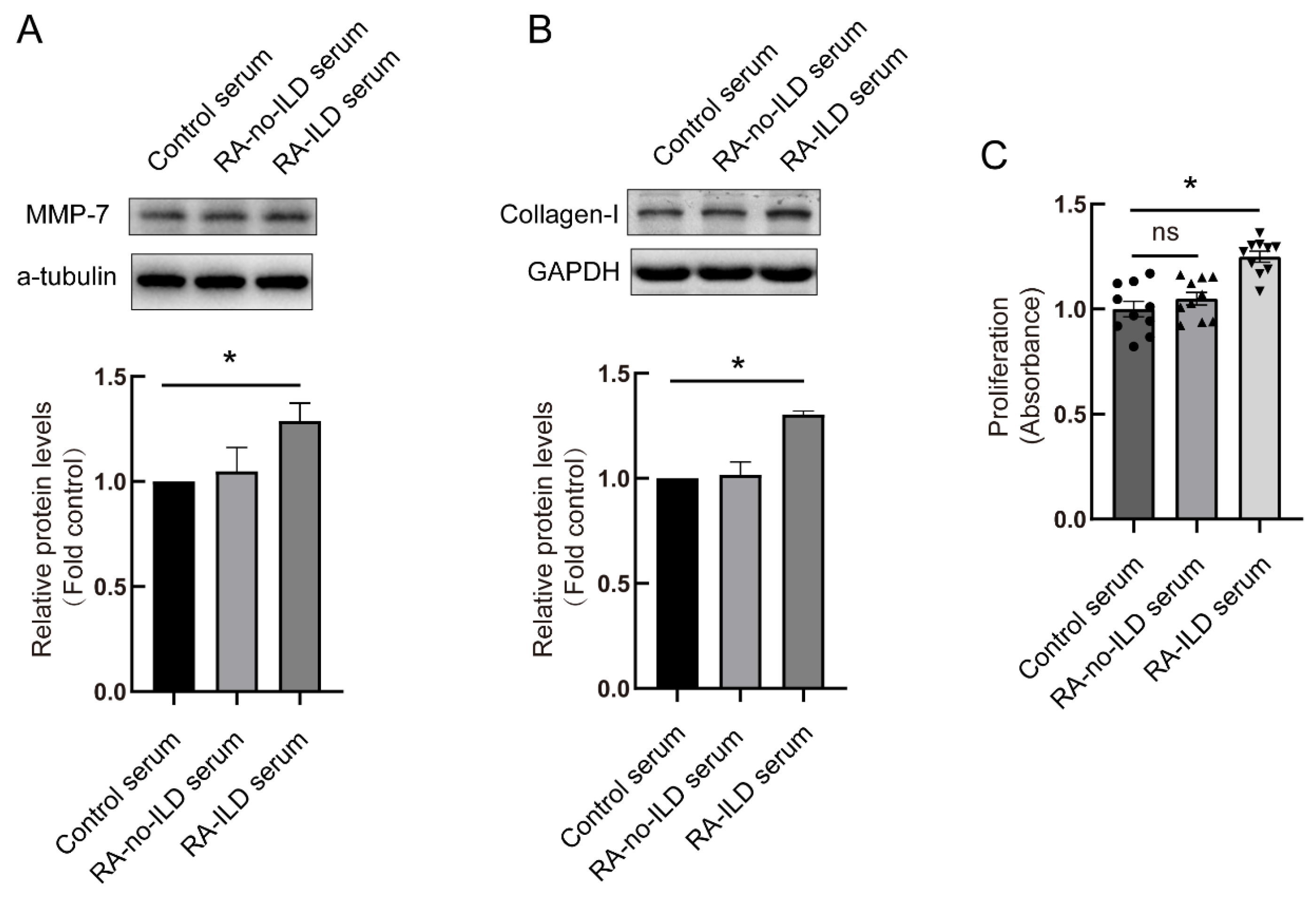
3.3. RA-ILD Patients’ Serum Induced MMP-7 and Collagen-I Overexpression and Promoted Cell Proliferation in PMCs
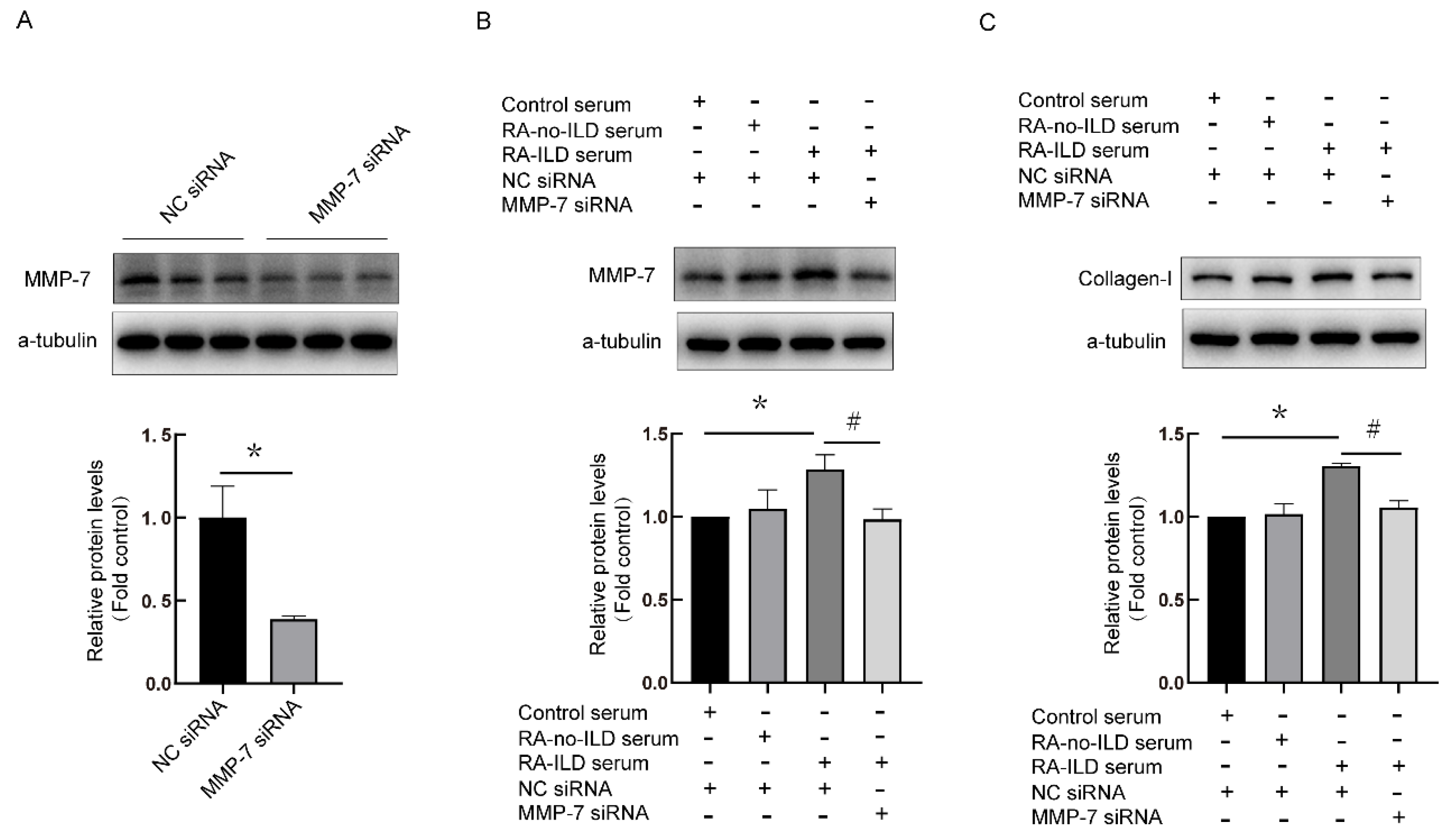
3.4. Knock-Down of MMP-7 Prevented Collagen-I Synthesis Induced by RA-ILD Patients’ Serum in PMCs
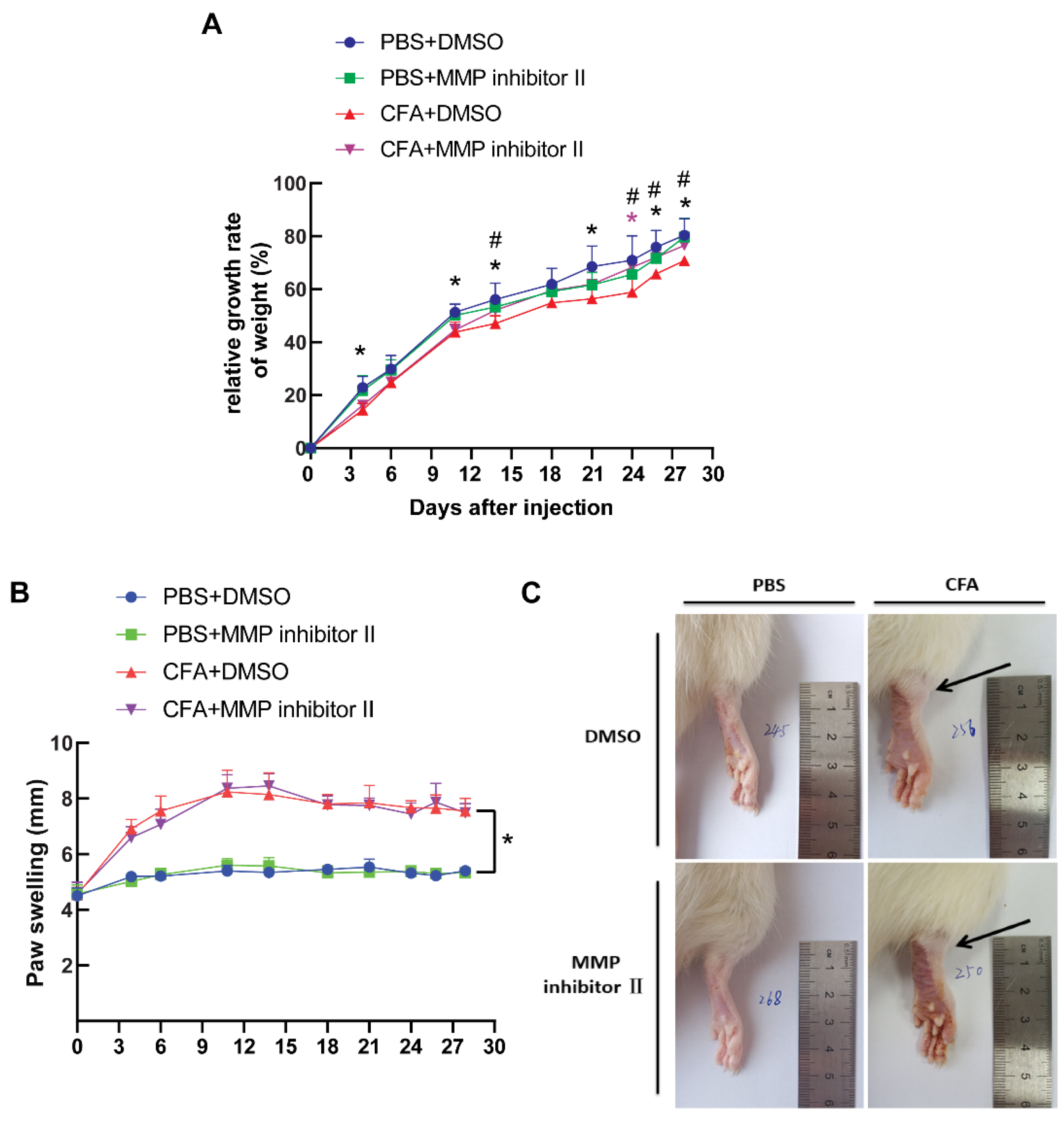
3.5. CFA Induced the Development of Arthritis In Vivo
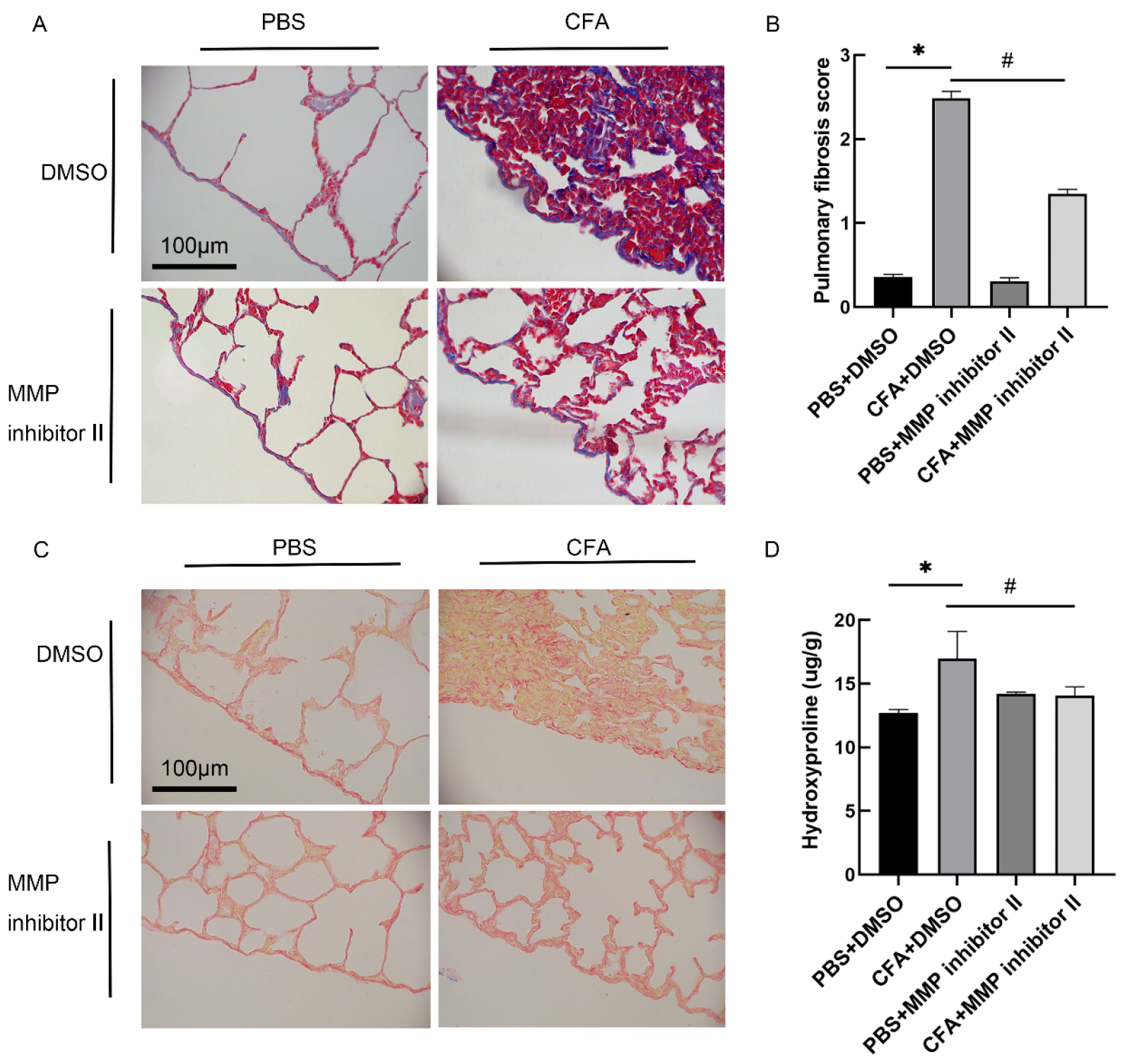
3.6. MMP-7 Inhibitor Attenuated CFA-Induced Subpleural Lung Fibrosis In Vivo
3.7. MMP-7 shRNA Alleviated CFA-Induced Subpleural Lung Fibrosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFA | Complete Freund’s adjuvant |
| DEGs | Screening of differentially expressed genes |
| MMP-7 | Matrix metalloproteinase-7 |
| PPI | Protein–protein interaction |
| PMCs | Pleural mesothelial cells |
| RA-ILD | Rheumatoid arthritis-related interstitial lung disease |
| RA | Rheumatoid arthritis |
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Hart, F.D. Extra-articular manifestations of rheumatoid arthritis. Semin. Arthritis Rheum. 1971, 1, 161–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olson, A.L.; Swigris, J.J.; Sprunger, D.B.; Fischer, A.; Fernandez-Perez, E.R.; Solomon, J.; Murphy, J.; Cohen, M.; Raghu, G.; Brown, K.K. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am. J. Respir. Crit. Care Med. 2011, 183, 372–378. [Google Scholar] [CrossRef]
- Kim, E.J.; Elicker, B.M.; Maldonado, F.; Webb, W.R.; Ryu, J.H.; Van Uden, J.H.; Lee, J.S.; King, T.E.; Collard, H.R. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2010, 35, 1322–1328. [Google Scholar] [CrossRef]
- Bongartz, T.; Nannini, C.; Medina-Velasquez, Y.F.; Achenbach, S.J.; Crowson, C.S.; Ryu, J.H.; Vassallo, R.; Gabriel, S.E.; Matteson, E.L. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2010, 62, 1583–1591. [Google Scholar] [CrossRef]
- Hakala, M. Poor prognosis in patients with rheumatoid arthritis hospitalized for interstitial lung fibrosis. Chest 1988, 93, 114–118. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Yoshinouchi, T.; Ohtsuki, Y.; Fujita, J.; Yamadori, I.; Bandoh, S.; Ishida, T.; Ueda, R. Nonspecific interstitial pneumonia pattern as pulmonary involvement of rheumatoid arthritis. Rheumatol. Int. 2005, 26, 121–125. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, D.S.; Yoo, B.; Seo, J.B.; Rho, J.Y.; Colby, T.V.; Kitaichi, M. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005, 127, 2019–2027. [Google Scholar] [CrossRef]
- Yousem, S.A.; Colby, T.V.; Carrington, C.B. Lung biopsy in rheumatoid arthritis. Am. Rev. Respir. Dis. 1985, 131, 770–777. [Google Scholar]
- Veeraraghavan, S.; Nicholson, A.G.; Wells, A.U. Lung fibrosis: New classifications and therapy. Curr. Opin. Rheumatol. 2001, 13, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Carrington, C.B.; Gaensler, E.A.; Coutu, R.E.; FitzGerald, M.X.; Gupta, R.G. Natural history and treated course of usual and desquamative interstitial pneumonia. N. Engl. J. Med. 1978, 298, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L.; Myers, J.L. Idiopathic pulmonary fibrosis: Clinical relevance of pathologic classification. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1301–1315. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Fiorelli, R.F. Nonspecific interstitial pneumonia/fibrosis: Histologic features and clinical significance. Am. J. Surg. Pathol. 1994, 18, 136–147. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Myers, J.L. Nonspecific interstitial pneumonia and the other idiopathic interstitial pneumonias: Classification and diagnostic criteria. Am. J. Surg. Pathol. 2000, 24, 1. [Google Scholar] [CrossRef]
- Clark, I.M.; Cawston, T.E. Fragments of human fibroblast collagenase. Purification and characterization. Biochem. J. 1989, 263, 201–206. [Google Scholar] [CrossRef]
- Knäuper, V.; Cowell, S.; Smith, B.; López-Otin, C.; O’Shea, M.; Morris, H.; Zardi, L.; Murphy, G. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J. Biol. Chem. 1997, 272, 7608–7616. [Google Scholar] [CrossRef]
- Yang, X.; Du, X.; Sun, L.; Zhao, X.; Zhu, J.; Li, G.; Tian, J.; Li, X.; Wang, Z. SULT2B1b promotes epithelial-mesenchymal transition through activation of the β-catenin/MMP7 pathway in hepatocytes. Biochem. Biophys. Res. Commun. 2019, 510, 495–500. [Google Scholar] [CrossRef]
- Oelusarz, A.; Nichols, L.A.; Grunz-Borgmann, E.A.; Chen, G.; Akintola, A.D.; Catania, J.M.; Burghardt, R.C.; Trzeciakowski, J.P.; Parrish, A.R. Overexpression of MMP-7 Increases Collagen 1A2 in the Aging Kidney. Physiol Rep. 2013, 1, e00090. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.; Richter, A.G.; O’Kane, C.; McAuley, D.F.; Thickett, D.R. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur. Respir. J. 2009, 33, 77–84. [Google Scholar] [CrossRef]
- Cosgrove, G.P.; Schwarz, M.I.; Geraci, M.W.; Brown, K.K.; Worthen, G.S. Overexpression of matrix metalloproteinase-7 in pulmonary fibrosis. Chest 2002, 121 (Suppl. 3), 25S–26S. [Google Scholar] [CrossRef] [PubMed]
- García-de-Alba, C.; Becerril, C.; Ruiz, V.; González, Y.; Reyes, S.; García-Alvarez, J.; Selman, M.; Pardo, A. Expression of matrix metalloproteases by fibrocytes: Possible role in migration and homing. Am. J. Respir. Crit. Care Med. 2010, 182, 1144–1452. [Google Scholar] [CrossRef]
- Doyle, T.J.; Patel, A.S.; Hatabu, H.; Nishino, M.; Wu, G.; Osorio, J.C.; Golzarri, M.F.; Traslosheros, A.; Chu, S.G.; Frits, M.L.; et al. Detection of Rheumatoid Arthritis-Interstitial Lung Disease Is Enhanced by Serum Biomarkers. Am. J. Respir. Crit. Care Med. 2015, 191, 1403–1412. [Google Scholar] [CrossRef]
- Yang, G.; Lyu, L.; Wang, X.; Bao, L.; Lyu, B.; Lin, Z. Systemic treatment with resveratrol alleviates adjuvant arthritis-interstitial lung disease in rats via modulation of JAK/STAT/RANKL signaling pathway. Pulm. Pharmacol. Ther. 2019, 56, 69–74. [Google Scholar] [CrossRef]
- Song, L.N.; Kong, X.D.; Wang, H.J.; Zhan, L.B. Establishment of a Rat Adjuvant Arthritis-Interstitial Lung Disease Model. Biomed. Res. Int. 2016, 2016, 2970783. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.M.; Xiong, L.; Cheng, P.P.; Chen, S.J.; Feng, X.; Zhou, Y.Y.; Niu, Q.; Wang, M.; Chen, Q.; Song, L.-J.; et al. Splicing factor SRSF6 mediates pleural fibrosis. JCI Insight 2021, 6, e146197. [Google Scholar] [CrossRef]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar]
- He, W.; Tan, R.J.; Li, Y.; Wang, D.; Nie, J.; Hou, F.F.; Liu, Y. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J. Am. Soc. Nephrol. 2012, 23, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.J.; Zhou, D.; Zhou, L.; Liu, Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 2014, 4, 84–90. [Google Scholar] [CrossRef]
- Srirangan, S.; Choy, E.H. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef]
- Miossec, P. The role of interleukin 1 in the pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 1987, 5, 305–308. [Google Scholar] [PubMed]
- Matsuno, H.; Yudoh, K.; Katayama, R.; Nakazawa, F.; Uzuki, M.; Sawai, T.; Yonezawa, T.; Saeki, Y.; Panayi, G.S.; Pitzalis, C.; et al. The role of TNF-α in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology 2002, 41, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Fan, C.; Yang, L.; Fang, X. Matrix Metalloproteinases-7 and Kidney Fibrosis. Front. Physiol. 2017, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tian, Y.; Sun, L.; Zhou, L.; Xiao, L.; Tan, R.J.; Tian, J.; Fu, H.; Hou, F.F.; Liu, Y. Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 598–611. [Google Scholar] [CrossRef]
- Nomden, M.; Beljaars, L.; Verkade, H.J.; Hulscher, J.B.F.; Olinga, P. Current Concepts of Biliary Atresia and Matrix Metalloproteinase-7: A Review of Literature. Front. Med. 2020, 7, 617261. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Wang, C.; Song, M.; Li, C.; Chen, M.; Zhou, J.; Mei, C. Matrix metalloproteinase-7 in platelet-activated macrophages accounts for cardiac remodeling in uremic mice. Basic Res. Cardiol. 2020, 115, 30. [Google Scholar] [CrossRef]
- Song, J.W.; Do, K.H.; Jang, S.J.; Colby, T.V.; Han, S.; Kim, D.S. Blood biomarkers MMP-7 and SP-A: Predictors of outcome in idiopathic pulmonary fibrosis. Chest 2013, 143, 1422–1429. [Google Scholar] [CrossRef]
- Richards, T.J.; Kaminski, N.; Baribaud, F.; Flavin, S.; Brodmerkel, C.; Horowitz, D.; Li, K.; Choi, J.; Vuga, L.J.; Lindell, K.O.; et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012, 185, 67–76. [Google Scholar] [CrossRef]
- Rosas, I.O.; Richards, T.J.; Konishi, K.; Zhang, Y.; Gibson, K.; Lokshin, A.E.; Lindell, K.O.; Cisneros, J.; MacDonald, S.D.; Pardo, A.; et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008, 5, e93. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Li, J.; Yao, J.; Zhao, H. Biomarkers of rheumatoid arthritis-associated interstitial lung disease: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1455346. [Google Scholar] [CrossRef]
- Colmenares, V.; Hedman, A.; Hesslow, A.; Wahlin, B.; Södergren, A. Cohort study of serological biomarkers for interstitial lung disease in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2024, 53, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, M.; Rosen, G.D.; Swigris, J.J. Connective Tissue Disease-associated Interstitial Lung Disease: A review. Curr. Respir. Care Rep. 2012, 1, 224–232. [Google Scholar] [CrossRef] [PubMed]
- White, E.S.; Xia, M.; Murray, S.; Dyal, R.; Flaherty, C.M.; Flaherty, K.R.; Moore, B.B.; Cheng, L.; Doyle, T.J.; Villalba, J.; et al. Plasma Surfactant Protein-D, Matrix Metalloproteinase-7, and Osteopontin Index Distinguishes Idiopathic Pulmonary Fibrosis from Other Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2016, 194, 1242–1251. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Liang, L.-M.; Ye, S.-Y.; Cui, X.-L.; Hu, S.-H.; Lian, C.-Y.; Sun, W.-J.; Lv, Y.-P.; Zhang, H.-D.; Wang, M.; et al. Inhibition of Matrix Metalloproteinase-7 Attenuates Subpleural Fibrosis in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Biomedicines 2025, 13, 1581. https://doi.org/10.3390/biomedicines13071581
Xiong L, Liang L-M, Ye S-Y, Cui X-L, Hu S-H, Lian C-Y, Sun W-J, Lv Y-P, Zhang H-D, Wang M, et al. Inhibition of Matrix Metalloproteinase-7 Attenuates Subpleural Fibrosis in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Biomedicines. 2025; 13(7):1581. https://doi.org/10.3390/biomedicines13071581
Chicago/Turabian StyleXiong, Li, Li-Mei Liang, Shu-Yi Ye, Xiao-Lin Cui, Shi-He Hu, Chen-Yue Lian, Wen-Jia Sun, Yang-Ping Lv, He-De Zhang, Meng Wang, and et al. 2025. "Inhibition of Matrix Metalloproteinase-7 Attenuates Subpleural Fibrosis in Rheumatoid Arthritis-Associated Interstitial Lung Disease" Biomedicines 13, no. 7: 1581. https://doi.org/10.3390/biomedicines13071581
APA StyleXiong, L., Liang, L.-M., Ye, S.-Y., Cui, X.-L., Hu, S.-H., Lian, C.-Y., Sun, W.-J., Lv, Y.-P., Zhang, H.-D., Wang, M., Xiang, F., Xiong, L., Ye, H., Ma, W.-L., & Song, L.-J. (2025). Inhibition of Matrix Metalloproteinase-7 Attenuates Subpleural Fibrosis in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Biomedicines, 13(7), 1581. https://doi.org/10.3390/biomedicines13071581





