Histological Evaluation for Collagen Expression Prior to LVAD Implantation Is Useful to Estimate Weaning Success
Abstract
1. Introduction
2. Methods
2.1. Study Groups and Clinical Characteristics
2.2. Cardiac Tissue Preparation
2.3. RNA Isolation and cDNA Synthesis
2.4. Quantitative Real-Time Polymerase Chain Reaction
2.5. Masson–Goldner Trichrome Staining
2.6. Immunohistochemistry of Collagen I and Collagen III
2.7. Statistics
3. Results
3.1. Demographic and Clinical Characteristics
3.2. mRNA Expression of Collagen Type I and III
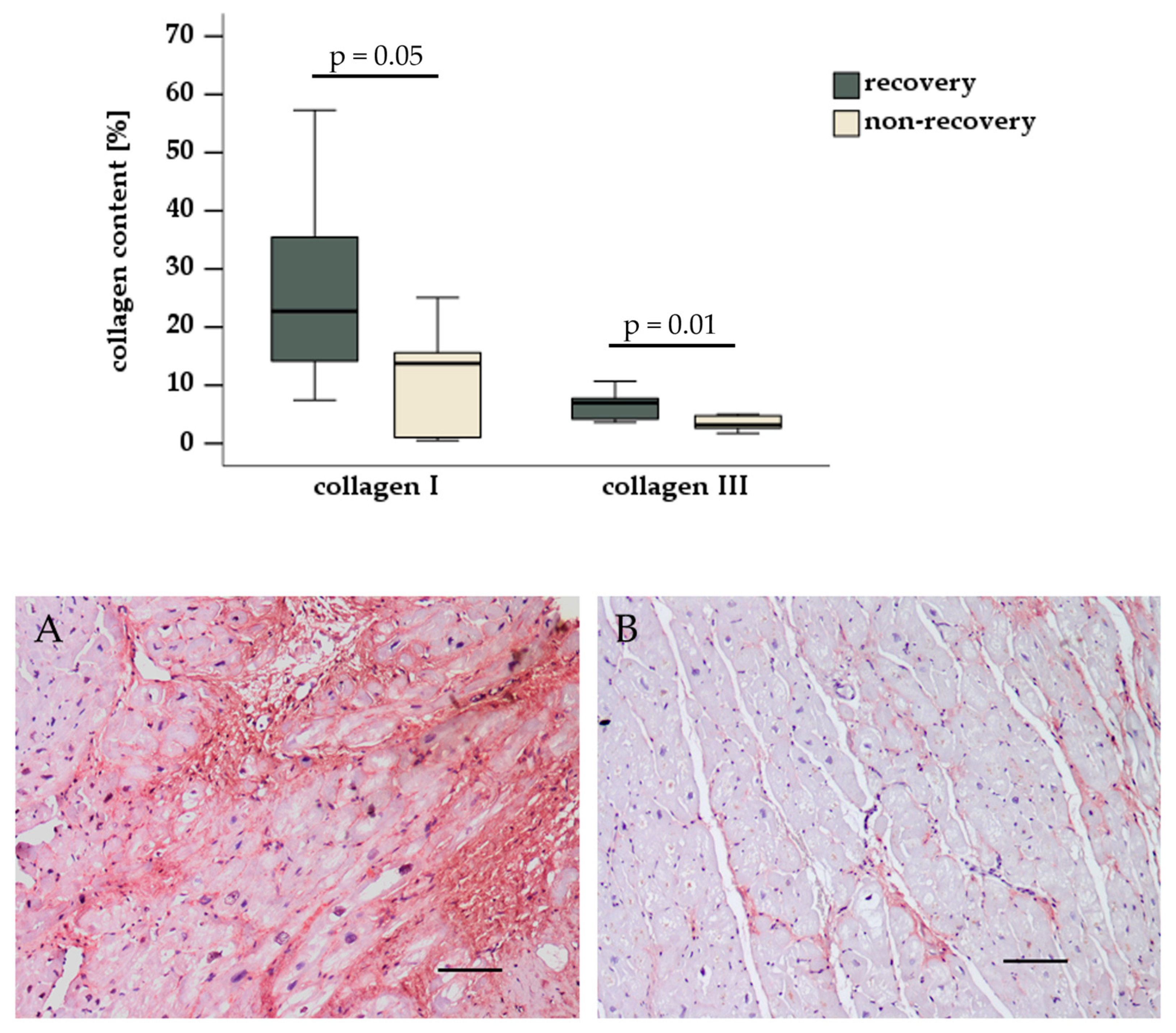
3.3. Histological and Immunohistochemical Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hrytsyna, Y.; Kneissler, S.; Kaufmann, F.; Müller, M.; Schoenrath, F.; Mulzer, J.; Sündermann, S.H.; Falk, V.; Potapov, E.; Knierim, J. Experience with a standardized protocol to predict successful explantation of left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2022, 164, 1922–1930.e2. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.; Tsyganenko, D.; Stein, J.; Mulzer, J.; Müller, M.; Hrytsyna, Y.; Schoenrath, F.; Falk, V.; Potapov, E. Results of non-elective withdrawal of continuous-flow left ventricular assist devices in selected patients. J. Heart Lung Transplant. 2023, 42, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Drakos, S.G.; Badolia, R.; Makaju, A.; Kyriakopoulos, C.P.; Wever-Pinzon, O.; Tracy, C.M.; Bakhtina, A.; Bia, R.; Parnell, T.; Taleb, I.; et al. Distinct Transcriptomic and Proteomic Profile Specifies Patients Who Have Heart Failure With Potential of Myocardial Recovery on Mechanical Unloading and Circulatory Support. Circulation 2023, 147, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, L.; Dieterlen, M.T.; Klaeske, K.; Haunschild, J.; Saeed, D.; Eifert, S.; Borger, M.A.; Jawad, K. Myostatin/AKT/FOXO Signaling Is Altered in Human Non-Ischemic Dilated Cardiomyopathy. Life 2022, 12, 1418. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, R.A.; Marin-Cuartas, M.; van Kampen, A.; Fahr, F.; Sieg, F.; Strotdrees, E.; Jahnke, C.; Klaeske, K.; Wiesner, K.; Morningstar, J.E.; et al. Left ventricular fibrosis and CMR tissue characterization of papillary muscles in mitral valve prolapse patients. Int. J. Cardiovasc. Imaging 2024, 40, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Feng, Y.; McTiernan, C.F.; Pei, W.; Moravec, C.S.; Wang, P.; Rosenblum, W.; Kormos, R.L.; Feldman, A.M. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 2001, 104, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, B.A.; Stetson, S.J.; Perez-Verdia, A.; Youker, K.A.; Radovancevic, B.; Connelly, J.H.; Koerner, M.M.; Entman, M.E.; Frazier, O.H.; Noon, G.P.; et al. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. J. Heart Lung Transplant. 2001, 20, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, B.A.; Stetson, S.J.; Farmer, J.A.; Radovancevic, B.; Frazier, O.H.; Noon, G.P.; Entman, M.L.; Torre-Amione, G.; Youker, K.A. The implications for cardiac recovery of left ventricular assist device support on myocardial collagen content. Am. J. Surg. 2000, 180, 498–501; discussion 501–502. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019, 125, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, A.H.; van Oosterhout, M.F.; de Jonge, N.; Ivangh, B.; van Kuik, J.; Voorbij, R.H.A.M.; Cleutjens, J.P.M.; Gmelig-Meyling, F.H.J.; de Weger, R.A. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J. Heart Lung Transplant. 2006, 25, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Foronjy, R.F.; Dickstein, M.L.; Gu, A.; Garrelds, I.M.; Jan Danser, A.H.; Oz, M.C.; D’Armiento, J.; Burkhoff, D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation 2005, 112, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Chaggar, P.S.; Williams, S.G.; Yonan, N.; Fildes, J.; Venkateswaran, R.; Shaw, S.M. Myocardial recovery with mechanical circulatory support. Eur. J. Heart Fail. 2016, 18, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Rypdal, K.B.; Van Linthout, S.; Diez, J.; González, A. Myocardial fibrosis from the perspective of the extracellular matrix: Mechanisms to clinical impact. Matrix Biol. 2024, 134, 1–22. [Google Scholar] [CrossRef] [PubMed]
| Recovery Group n = 7 | Non-Recovery Group n = 7 | p Value | |
|---|---|---|---|
| Male gender | 2 (29%) | 2 (29%) | 1 |
| Age at LVAD implantation [yrs] | 57.0 ± 9.0 | 61.7 ± 6.4 | 0.14 |
| BMI [kg/m2] | 31.2 ± 7.5 | 27.1 ± 6.5 | 0.15 |
| LVEF [%] | 18.4 ± 7.3 | 16.4 ± 7.9 | 0.32 |
| NT-proBNP [ng/L] | 4619 ± 4120 | 7102 ± 3319 | 0.19 |
| NYHA classification Class III Class IV | 5 (71%) 2 (29%) | 2 (29%) 5 (71%) | 0.29 |
| Nicotine abuse Smoker Non-smoker Ex-smoker | 0 (0%) 5 (71%) 2 (29%) | 0 (0%) 5 (71%) 2 (29%) | 1 |
| Arterial hypertension | 4 (57%) | 5 (71%) | 1 |
| Coronary heart disease | 2 (29%) | 0 (0%) | 0.45 |
| Type 2 diabetes | 3 (43%) | 2 (29%) | 1 |
| Renal function eGFR [mL/min/1.73 m2] Creatinine [µmol/L] | 57.4 ± 26.0 131 ± 90 | 41.1 ± 19.8 151 ± 64 | 0.11 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieterlen, M.-T.; Schreiber, L.; Klaeske, K.; Jozwiak-Nozdrzykowska, J.; Borger, M.A.; Dashkevich, A.; Eifert, S.; Nozdrzykowski, M. Histological Evaluation for Collagen Expression Prior to LVAD Implantation Is Useful to Estimate Weaning Success. Biomedicines 2025, 13, 1515. https://doi.org/10.3390/biomedicines13071515
Dieterlen M-T, Schreiber L, Klaeske K, Jozwiak-Nozdrzykowska J, Borger MA, Dashkevich A, Eifert S, Nozdrzykowski M. Histological Evaluation for Collagen Expression Prior to LVAD Implantation Is Useful to Estimate Weaning Success. Biomedicines. 2025; 13(7):1515. https://doi.org/10.3390/biomedicines13071515
Chicago/Turabian StyleDieterlen, Maja-Theresa, Lea Schreiber, Kristin Klaeske, Joanna Jozwiak-Nozdrzykowska, Michael A. Borger, Alexey Dashkevich, Sandra Eifert, and Michal Nozdrzykowski. 2025. "Histological Evaluation for Collagen Expression Prior to LVAD Implantation Is Useful to Estimate Weaning Success" Biomedicines 13, no. 7: 1515. https://doi.org/10.3390/biomedicines13071515
APA StyleDieterlen, M.-T., Schreiber, L., Klaeske, K., Jozwiak-Nozdrzykowska, J., Borger, M. A., Dashkevich, A., Eifert, S., & Nozdrzykowski, M. (2025). Histological Evaluation for Collagen Expression Prior to LVAD Implantation Is Useful to Estimate Weaning Success. Biomedicines, 13(7), 1515. https://doi.org/10.3390/biomedicines13071515






