Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A Pan-Steatotic Liver Disease Treatment?
Abstract
1. Introduction
2. Glucagon-like Peptide-1 and Glucagon-like Peptide-1 Receptor Agonists
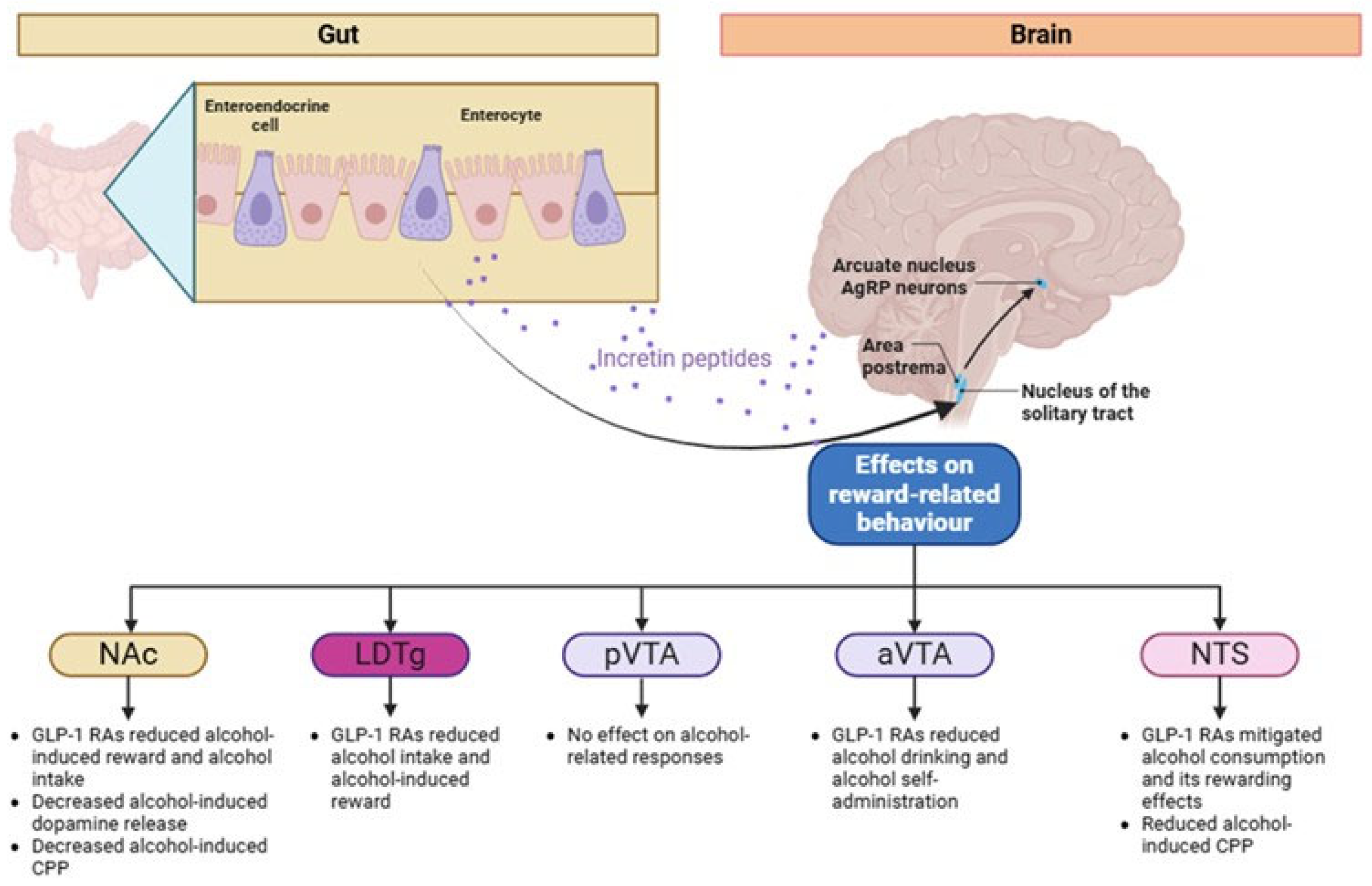
3. Possible Pathophysiological Pathways of GLP-1 RAs on AUD: Evidence from Mouse Studies
4. GLP-1 RAs in AUD: Evidence from Human Observational Studies and Randomized Clinical Trials (RCTs)
5. GLP-1 RAs in Steatotic Liver Disease (MASLD, MetALD, and ALD)
6. Applicability of Therapeutic Opportunities from MASLD to MetALD and ALD
7. Limitations and Future Challenges
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.M.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Mazzotta, A.; Longaroni, M.; Petrucciani, N. Potential role of glucagon-like peptide-1 (GLP-1) receptor agonists in substance use disorder: A systematic review of randomized trials. Drug Alcohol Depend. 2024, 264, 112424. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P.; et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Duangsonk, K.; Tham, E.K.J.; Tothanarungroj, P.; Auttapracha, T.; Prasitsumrit, V.; Sim, B.; Tung, D.; Barba, R.; Wong, R.J.; et al. Increased mortality from alcohol use disorder, alcohol-associated liver disease, and liver cancer from alcohol among older adults in the United States: 2000 to 2021. Alcohol Clin. Exp. Res. 2024, 49, 368–378. [Google Scholar] [CrossRef]
- Thomsen, M.; Holst, J.J.; Molander, A.; Linnet, K.; Ptito, M.; Fink-Jensen, A. Effects of glucagon-like peptide 1 analogs on alcohol intake in alcohol-preferring vervet monkeys. Psychopharmacology 2019, 236, 603–611. [Google Scholar] [CrossRef]
- Witkiewitz, K.; Litten, R.Z.; Leggio, L. Advances in the science and treatment of alcohol use disorder. Sci. Adv. 2019, 5, eaax4043. [Google Scholar] [CrossRef]
- Hillemacher, T.; Leggio, L.; Heberlein, A. Investigational therapies for the pharmacological treatment of alcoholism. Expert Opin. Investig. Drugs 2015, 24, 17–30. [Google Scholar] [CrossRef]
- Kranzler, H.R. Overview of Alcohol Use Disorder. Am. J. Psychiatry 2023, 180, 565–572. [Google Scholar] [CrossRef]
- Yang, W.; Singla, R.; Maheshwari, O.; Fontaine, C.J.; Gil-Mohapel, J. Alcohol Use Disorder: Neurobiology and Therapeutics. Biomedicines 2022, 10, 1192. [Google Scholar] [CrossRef]
- Dellazizzo, L.; Potvin, S.; Giguere, S.; Landry, C.; Leveille, N.; Dumais, A. Meta-review on the efficacy of psychological therapies for the treatment of substance use disorders. Psychiatry Res. 2023, 326, 115318. [Google Scholar] [CrossRef] [PubMed]
- Ray, L.A.P.; Bujarski, S.P.; Grodin, E.P.; Hartwell, E.P.; Green, R.M.; Venegas, A.B.; Lim, A.M.; Gillis, A.M.; Miotto, K.M. State-of-the-art behavioral and pharmacological treatments for alcohol use disorder. Am. J. Drug Alcohol Abus. 2019, 45, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Soyka, M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 2018, 320, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Bobashev, G.; Thomas, K.; Wines, R.; Kim, M.M.; Shanahan, E.; Gass, C.E.; Rowe, C.J.; et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA 2014, 311, 1889–1900. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Harris, R.A. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: Relationship to acamprosate actions. Int. J. Neuropsychopharmacol. 2008, 11, 775–793. [Google Scholar] [CrossRef]
- Wackernah, R.C.; Minnick, M.J.; Clapp, P. Alcohol use disorder: Pathophysiology, effects, and pharmacologic options for treatment. Subst. Abus. Rehabil. 2014, 5, 1–12. [Google Scholar]
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol use disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef]
- Subhani, M.; Dhanda, A.; King, J.A.; Warren, F.C.; Creanor, S.; Davies, M.J.; Eldeghaidy, S.; Bawden, S.; Gowland, P.A.; Bataller, R.; et al. Association between glucagon-like peptide-1 receptor agonists use and change in alcohol consumption: A systematic review. eClinicalMedicine 2024, 78, 102920. [Google Scholar] [CrossRef]
- Brunchmann, A.; Thomsen, M.; Fink-Jensen, A. The effect of glucagon-like peptide-1 (GLP-1) receptor agonists on substance use disorder (SUD)-related behavioural effects of drugs and alcohol: A systematic review. Physiol. Behav. 2019, 206, 232–242. [Google Scholar] [CrossRef]
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012, 153, 647–658. [Google Scholar] [CrossRef]
- Suchankova, P.; Yan, J.; Schwandt, M.L.; Stangl, B.L.; Caparelli, E.C.; Momenan, R.; Jerlhag, E.; Engel, J.A.; Hodgkinson, C.A.; Egli, M.; et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: Evidence from human genetic association studies and a mouse model of alcohol dependence. Transl. Psychiatry 2015, 5, e583. [Google Scholar] [CrossRef] [PubMed]
- Sofogianni, A.; Filippidis, A.; Chrysavgis, L.; Tziomalos, K.; Cholongitas, E. Glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: An update. World J. Hepatol. 2020, 12, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Das, A.; Geetha, K.M.; Hazarika, I. Contemporary Updates on the Physiology of Glucagon like Peptide-1 and Its Agonist to Treat Type 2 Diabetes Mellitus. Int. J. Pept. Res. Ther. 2020, 26, 1211–1221. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Effects of GLP-1 and Its Analogs on Gastric Physiology in Diabetes Mellitus and Obesity. Adv. Exp. Med. Biol. 2021, 1307, 171–192. [Google Scholar]
- Chrysavgis, L.G.; Kazanas, S.; Bafa, K.; Rozani, S.; Koloutsou, M.E.; Cholongitas, E. Glucagon-like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide, and Glucagon Receptor Agonists in Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Medication in New Liver Disease Nomenclature. Int. J. Mol. Sci. 2024, 25, 3832. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Wajcberg, E.; Amarah, A. Liraglutide in the management of type 2 diabetes. Drug Des. Dev. Ther. 2010, 4, 279–290. [Google Scholar] [CrossRef]
- Zaazouee, M.S.; Hamdallah, A.; Helmy, S.K.; Hasabo, E.A.; Sayed, A.K.; Gbreel, M.I.; Elmegeed, A.A.; Aladwan, H.; Elshanbary, A.A.; Abdel-Aziz, W.; et al. Semaglutide for the treatment of type 2 Diabetes Mellitus: A systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes Metab. Syndr. 2022, 16, 102511. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhang, Y.; Tong, N. Efficacy and safety of dulaglutide in patients with type 2 diabetes: A meta-analysis and systematic review. Sci. Rep. 2016, 6, 18904. [Google Scholar] [CrossRef] [PubMed]
- Konwar, M.; Bose, D.; Jaiswal, S.K.; Maurya, M.K.; Ravi, R. Efficacy and Safety of Liraglutide 3.0 mg in Patients with Overweight and Obese with or without Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1201977. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Heerspink, H.J.L.; Cuthbertson, D.J.; Wilding, J.P.H. SGLT2 inhibitors and GLP-1 receptor agonists: Established and emerging indications. Lancet 2021, 398, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jodar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Ryden, L.; et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Taal, M.W.; Selby, N.M. Glucagon-like Peptide-1 Receptor Agonists: New Evidence of Kidney and Cardiovascular Protection From the FLOW and SELECT Trials. Am. J. Kidney Dis. 2025, 85, 115–118. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Catarig, A.M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: A substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef]
- Dahiya, S.; Tisch, S.; Greenfield, J. The effect of GLP-1 receptor agonists in pre-clinical rodent models of Parkinson’s disease: A systematic review and meta-analysis. Clin. Park. Relat. Disord. 2022, 6, 100133. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Wang, L.; Wang, W. Evaluating the effects of glucagon-like peptide-1 receptor agonists on cognitive function in Alzheimer’s disease: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2023, 32, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sanyal, A.J.; Engebretsen, K.A.; Kliers, I.; Ostergaard, L.; Vanni, D.; Bugianesi, E.; Rinella, M.E.; Roden, M.; Ratziu, V. Semaglutide 2.4 mg in Participants With Metabolic Dysfunction-Associated Steatohepatitis: Baseline Characteristics and Design of the Phase 3 ESSENCE Trial. Aliment. Pharmacol. Ther. 2024, 60, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Ozburn, A.R.; Spencer, S.M. Repurposing anti-inflammatory medications for alcohol and substance use disorders. Neuropsychopharmacology 2024, 49, 317–318. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Ronne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Salinas, C.B.G.; Lu, T.T.; Gabery, S.; Marstal, K.; Alanentalo, T.; Mercer, A.J.; Cornea, A.; Conradsen, K.; Hecksher-Sorensen, J.; Dahl, A.B.; et al. Integrated Brain Atlas for Unbiased Mapping of Nervous System Effects Following Liraglutide Treatment. Sci. Rep. 2018, 8, 10310. [Google Scholar] [CrossRef]
- Aranas, C.; Edvardsson, C.E.; Shevchouk, O.T.; Zhang, Q.; Witley, S.; Blid Skoldheden, S.; Zentveld, L.; Vallof, D.; Tufvesson-Alm, M.; Jerlhag, E. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. eBioMedicine 2023, 93, 104642. [Google Scholar] [CrossRef]
- Richard, J.E.; Anderberg, R.H.; Goteson, A.; Gribble, F.M.; Reimann, F.; Skibicka, K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS ONE 2015, 10, e0119034. [Google Scholar] [CrossRef]
- Chuong, V.; Farokhnia, M.; Khom, S.; Pince, C.L.; Elvig, S.K.; Vlkolinsky, R.; Marchette, R.C.; Koob, G.F.; Roberto, M.; Vendruscolo, L.F.; et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 2023, 8, e170671. [Google Scholar] [CrossRef]
- Aranas, C.; Blid Skoldheden, S.; Jerlhag, E. Antismoking agents do not contribute synergistically to semaglutide’s ability to reduce alcohol intake in rats. Front. Pharmacol. 2023, 14, 1180512. [Google Scholar] [CrossRef] [PubMed]
- Vallof, D.; Maccioni, P.; Colombo, G.; Mandrapa, M.; Jornulf, J.W.; Egecioglu, E.; Engel, J.A.; Jerlhag, E. The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict. Biol. 2016, 21, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Vallof, D.; Vestlund, J.; Jerlhag, E. Glucagon-like peptide-1 receptors within the nucleus of the solitary tract regulate alcohol-mediated behaviors in rodents. Neuropharmacology 2019, 149, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Howell, E.; Currie, P.J. Accumbal ghrelin and glucagon-like peptide 1 signaling in alcohol reward in female rats. Neuroreport 2018, 29, 1046–1053. [Google Scholar] [CrossRef]
- Eren-Yazicioglu, C.Y.; Yigit, A.; Dogruoz, R.E.; Yapici-Eser, H. Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front. Behav. Neurosci. 2020, 14, 614884. [Google Scholar] [CrossRef]
- Dixon, T.N.; McNally, G.P.; Ong, Z.Y. Glucagon-Like Peptide-1 Receptor Signaling in the Ventral Tegmental Area Reduces Alcohol Self-Administration in Male Rats. Alcohol Clin. Exp. Res. 2020, 44, 2118–2129. [Google Scholar] [CrossRef]
- Volkow, N.D.; Kim, S.W.; Wang, G.J.; Alexoff, D.; Logan, J.; Muench, L.; Shea, C.; Telang, F.; Fowler, J.S.; Wong, C.; et al. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage 2013, 64, 277–283. [Google Scholar] [CrossRef]
- Yin, S.J.; Liao, C.S.; Wu, C.W.; Li, T.T.; Chen, L.L.; Lai, C.L.; Tsao, T.Y. Human stomach alcohol and aldehyde dehydrogenases: Comparison of expression pattern and activities in alimentary tract. Gastroenterology 1997, 112, 766–775. [Google Scholar] [CrossRef]
- de Wit, H.; Bodker, B.; Ambre, J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology 1992, 107, 352–358. [Google Scholar] [CrossRef]
- Quddos, F.; Hubshman, Z.; Tegge, A.; Sane, D.; Marti, E.; Kablinger, A.S.; Gatchalian, K.M.; Kelly, A.L.; DiFeliceantonio, A.G.; Bickel, W.K. Semaglutide and Tirzepatide reduce alcohol consumption in individuals with obesity. Sci. Rep. 2023, 13, 20998. [Google Scholar] [CrossRef]
- Klausen, M.K.; Jensen, M.E.; Moller, M.; Le Dous, N.; Jensen, A.O.; Zeeman, V.A.; Johannsen, C.F.; Lee, A.; Thomsen, G.K.; Macoveanu, J.; et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 2022, 7, e159863. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Probst, L.; Monnerat, S.; Vogt, D.R.; Lengsfeld, S.; Burkard, T.; Meienberg, A.; Bathelt, C.; Christ-Crain, M.; Winzeler, B. Effects of dulaglutide on alcohol consumption during smoking cessation. JCI Insight 2023, 8, e170419. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, C.S.; Bremmer, M.P.; Paladino, M.B.; Kostantinis, G.; Gilmore, T.A.; Sullivan, N.R.; Tow, A.C.; Dermody, S.S.; Prince, M.A.; Jordan, R.; et al. Once-Weekly Semaglutide in Adults With Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2025, 82, 395–405. [Google Scholar] [CrossRef]
- Richards, J.R.; Dorand, M.F.; Royal, K.; Mnajjed, L.; Paszkowiak, M.; Simmons, W.K. Significant Decrease in Alcohol Use Disorder Symptoms Secondary to Semaglutide Therapy for Weight Loss: A Case Series. J. Clin. Psychiatry 2023, 85, 50515. [Google Scholar] [CrossRef]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Orskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef]
- Patel Chavez, C.; Cusi, K.; Kadiyala, S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2022, 107, 29–38. [Google Scholar] [CrossRef]
- Yabut, J.M.; Drucker, D.J. Glucagon-like Peptide-1 Receptor-based Therapeutics for Metabolic Liver Disease. Endocr. Rev. 2023, 44, 14–32. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Marchesini, G. Time for Glucagon like peptide-1 receptor agonists treatment for patients with NAFLD? J. Hepatol. 2016, 64, 262–264. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Hull, D.; Guo, K.; Barton, D.; Hazlehurst, J.M.; Gathercole, L.L.; Nasiri, M.; Yu, J.; Gough, S.C.; Newsome, P.N.; et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016, 64, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lin, C.; Zhuo, X.; Wang, J.; Rao, S.; Xu, W.; Cheng, Y.; Yang, L. Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating AMP-activated protein kinase-mammalian target of rapamycin pathway. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E1019–E1030. [Google Scholar] [CrossRef] [PubMed]
- Milani, I.; Codini, M.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. Int. J. Mol. Sci. 2024, 25, 10795. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.K.; Liu, Y.C.; Shi, L.L.; Lu, K.D. Glucagon-like peptide-1 receptor agonists inhibit hepatic stellate cell activation by blocking the p38 MAPK signaling pathway. Genet. Mol. Res. 2015, 14, 19087–19093. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Houlihan, D.D.; Rowe, I.A.; Clausen, W.H.; Elbrond, B.; Gough, S.C.; Tomlinson, J.W.; Newsome, P.N. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment. Pharmacol. Ther. 2013, 37, 234–242. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN Trial Team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K.; Fernandez Lando, L.; Bray, R.; Brouwers, B.; Rodriguez, A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022, 10, 393–406. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Ostergaard, L.H.; Long, M.T.; Kjaer, M.S.; Cali, A.M.G.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Hou, M.C.; Cheng-Chung Wei, J.; Shih, Y.H.; Hsu, C.Y.; Hsu, C.C.; Hwu, C.M. Glucagon-like Peptide-1 Receptor Agonist Use in Patients With Liver Cirrhosis and Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2024, 22, 1255–1264.e18. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Malhi, H.; Gores, G.J.; Shah, V.H. Therapeutic opportunities for alcoholic steatohepatitis and nonalcoholic steatohepatitis: Exploiting similarities and differences in pathogenesis. JCI Insight 2017, 2, e95354. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Feng, Y.; Wang, X.; Feng, Y. Recent Insights Into the Role of Immune Cells in Alcoholic Liver Disease. Front. Immunol. 2019, 10, 1328. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D.A. The Crosstalk between Hepatocytes, Hepatic Macrophages, and Hepatic Stellate Cells Facilitates Alcoholic Liver Disease. Cell Metab. 2019, 30, 850–852. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef]
- Miyata, T.; Nagy, L.E. Programmed cell death in alcohol-associated liver disease. Clin. Mol. Hepatol. 2020, 26, 618–625. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Gores, G.J. Non-alcoholic steatohepatitis pathogenesis: Sublethal hepatocyte injury as a driver of liver inflammation. Gut 2018, 67, 963–972. [Google Scholar] [CrossRef]
- Benede-Ubieto, R.; Estevez-Vazquez, O.; Guo, F.; Chen, C.; Singh, Y.; Nakaya, H.I.; Gomez Del Moral, M.; Lamas-Paz, A.; Moran, L.; Lopez-Alcantara, N.; et al. An Experimental DUAL Model of Advanced Liver Damage. Hepatol. Commun. 2021, 5, 1051–1068. [Google Scholar] [CrossRef]
- Boyle, M.; Masson, S.; Anstee, Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Tampaki, M.; Tsochatzis, E.; Lekakis, V.; Cholongitas, E. Prevalence, characteristics and outcomes of patients with metabolic and alcohol related/associated liver disease (MetALD): A systematic review and meta-analysis. Metabolism 2025, 163, 156101. [Google Scholar] [CrossRef] [PubMed]
- Marti-Aguado, D.; Calleja, J.L.; Vilar-Gomez, E.; Iruzubieta, P.; Rodriguez-Duque, J.C.; Del Barrio, M.; Puchades, L.; Rivera-Esteban, J.; Perello, C.; Puente, A.; et al. Low-to-moderate alcohol consumption is associated with increased fibrosis in individuals with metabolic dysfunction-associated steatotic liver disease. J. Hepatol. 2024, 81, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.; Fryers, P.T.; Sharpe, C.; Clarke, Z.; Henn, C.; Hydes, T.; Marsden, J.; Pearce-Smith, N.; Sheron, N. The independent and joint risks of alcohol consumption, smoking, and excess weight on morbidity and mortality: A systematic review and meta-analysis exploring synergistic associations. Public Health 2024, 226, 39–52. [Google Scholar] [CrossRef]
- Mahalingam, S.; Bellamkonda, R.; Arumugam, M.K.; Perumal, S.K.; Yoon, J.; Casey, C.; Kharbanda, K.; Rasineni, K. Glucagon-like peptide 1 receptor agonist, exendin-4, reduces alcohol-associated fatty liver disease. Biochem. Pharmacol. 2023, 213, 115613. [Google Scholar] [CrossRef]
- Kuo, C.C.; Li, C.H.; Chuang, M.H.; Huang, P.Y.; Kuo, H.T.; Lai, C.C. Impact of GLP-1 Receptor Agonists on Alcohol-Related Liver Disease Development and Progression in Alcohol Use Disorder. Aliment. Pharmacol. Ther. 2025, 61, 1343–1356. [Google Scholar] [CrossRef]
- Klausen, M.K.; Kuzey, T.; Pedersen, J.N.; Justesen, S.K.; Rasmussen, L.; Knorr, U.B.; Mason, G.; Ekstrom, C.T.; Holst, J.J.; Koob, G.; et al. Does semaglutide reduce alcohol intake in Danish patients with alcohol use disorder and comorbid obesity? Trial protocol of a randomised, double-blinded, placebo-controlled clinical trial (the SEMALCO trial). BMJ Open 2025, 15, e086454. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607 e592. [Google Scholar] [CrossRef]
- Hsu, C.; Caussy, C.; Imajo, K.; Chen, J.; Singh, S.; Kaulback, K.; Le, M.D.; Hooker, J.; Tu, X.; Bettencourt, R.; et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin. Gastroenterol. Hepatol. 2019, 17, 630–637.e8. [Google Scholar] [CrossRef]
- Thanapirom, K.; Suksawatamnuay, S.; Tanpowpong, N.; Chaopathomkul, B.; Sriphoosanaphan, S.; Thaimai, P.; Srisoonthorn, N.; Treeprasertsuk, S.; Komolmit, P. Non-invasive tests for liver fibrosis assessment in patients with chronic liver diseases: A prospective study. Sci. Rep. 2022, 12, 4913. [Google Scholar] [CrossRef]
- European Association for Study of the Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Frias, J.P.; Neff, G.; Abrams, G.A.; Lucas, K.J.; Sanchez, W.; Gogia, S.; Sheikh, M.Y.; Behling, C.; Bedossa, P.; et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): A multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1080–1093. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J.; Kowdley, K.V.; Bhatt, D.L.; Alkhouri, N.; Frias, J.P.; Bedossa, P.; Harrison, S.A.; Lazas, D.; Barish, R.; et al. Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH. N. Engl. J. Med. 2023, 389, 998–1008. [Google Scholar] [CrossRef]
- Flippo, K.H.; Trammell, S.A.J.; Gillum, M.P.; Aklan, I.; Perez, M.B.; Yavuz, Y.; Smith, N.K.; Jensen-Cody, S.O.; Zhou, B.; Claflin, K.E.; et al. FGF21 suppresses alcohol consumption through an amygdalo-striatal circuit. Cell Metab. 2022, 34, 317–328 e316. [Google Scholar] [CrossRef]
- Desai, B.N.; Singhal, G.; Watanabe, M.; Stevanovic, D.; Lundasen, T.; Fisher, F.M.; Mather, M.L.; Vardeh, H.G.; Douris, N.; Adams, A.C.; et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol. Metab. 2017, 6, 1395–1406. [Google Scholar] [CrossRef]
- Sun, L.; Cai, J.; Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 335–347. [Google Scholar] [CrossRef]
- Mekontso, J.G.K.; Nnang, J.Y.B.; Tembi, T.B.T.; Kortim, A.B.; Nguefang, G.L.; Wagner, J.; Bernstein, M. Efficacy, Safety, and Tolerability of Farnesoid X Receptor Agonists in the Treatment of Metabolic Dysfunction-associated Steatotic Liver Disease: A Systematic Review and Meta-analysis. J. Clin. Exp. Hepatol. 2025, 15, 102563. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, B.; Peng, X.; Zhou, M.; Jia, D.; Gu, J. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem. Biophys. Res. Commun. 2014, 443, 68–73. [Google Scholar] [CrossRef]
- Kong, L.; Dong, R.; Huang, K.; Wang, X.; Wang, D.; Yue, N.; Wang, C.; Sun, P.; Gu, J.; Luo, H.; et al. Yangonin modulates lipid homeostasis, ameliorates cholestasis and cellular senescence in alcoholic liver disease via activating nuclear receptor FXR. Phytomedicine 2021, 90, 153629. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Noureddin, M.; Kowdley, K.V.; Kohli, A.; Sheikh, A.; Neff, G.; Bhandari, B.R.; Gunn, N.; Caldwell, S.H.; Goodman, Z.; et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatology 2021, 73, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Calle, R.A.; Amin, N.B.; Carvajal-Gonzalez, S.; Ross, T.T.; Bergman, A.; Aggarwal, S.; Crowley, C.; Rinaldi, A.; Mancuso, J.; Aggarwal, N.; et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: Two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 2021, 27, 1836–1848. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Mohseni, R.; Lucas, K.J.; Gutierrez, J.A.; Perry, R.G.; Trotter, J.F.; Rahimi, R.S.; Harrison, S.A.; Ajmera, V.; Wayne, J.D.; et al. TVB-2640 (FASN Inhibitor) for the Treatment of Nonalcoholic Steatohepatitis: FASCINATE-1, a Randomized, Placebo-Controlled Phase 2a Trial. Gastroenterology 2021, 161, 1475–1486. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The interplay between thyroid and liver: Implications for clinical practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. TG68, a Novel Thyroid Hormone Receptor-beta Agonist for the Treatment of NAFLD. Int. J. Mol. Sci. 2021, 22, 13105. [Google Scholar] [CrossRef]
- Wang, W.; Volkow, N.D.; Berger, N.A.; Davis, P.B.; Kaelber, D.C.; Xu, R. Associations of semaglutide with incidence and recurrence of alcohol use disorder in real-world population. Nat. Commun. 2024, 15, 4548. [Google Scholar] [CrossRef]
- Wang, W.; Volkow, N.D.; Berger, N.A.; Davis, P.B.; Kaelber, D.C.; Xu, R. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 2024, 30, 168–176. [Google Scholar] [CrossRef]
- Rizk, M.M.; Herzog, S.; Dugad, S.; Stanley, B. Suicide Risk and Addiction: The Impact of Alcohol and Opioid Use Disorders. Curr. Addict. Rep. 2021, 8, 194–207. [Google Scholar] [CrossRef]
- Leza, L.; Haro, B.; Lopez-Goni, J.J.; Fernandez-Montalvo, J. Substance use disorder and lifetime suicidal behaviour: A scoping review. Psychiatry Res. 2024, 334, 115830. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, M.; Rezaeianzadeh, R.; Kezouh, A.; Etminan, M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA 2023, 330, 1795–1797. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, J.; Ping, F.; Yang, N.; Huang, J.; Li, Y.; Xu, L.; Li, W.; Zhang, H. Association of Glucagon-Like Peptide-1 Receptor Agonist Use With Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.C.; Falcetta, M.R.; Rados, D.V.; Leitao, C.B.; Gross, J.L. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: A meta-analysis with trial sequential analysis. Sci. Rep. 2019, 9, 2375. [Google Scholar] [CrossRef]
- Dankner, R.; Murad, H.; Agay, N.; Olmer, L.; Freedman, L.S. Glucagon-Like Peptide-1 Receptor Agonists and Pancreatic Cancer Risk in Patients With Type 2 Diabetes. JAMA Netw. Open 2024, 7, e2350408. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Kaelber, D.C.; Xu, R.; Berger, N.A. GLP-1 Receptor Agonists and Colorectal Cancer Risk in Drug-Naive Patients With Type 2 Diabetes, With and Without Overweight/Obesity. JAMA Oncol. 2024, 10, 256–258. [Google Scholar] [CrossRef]
- Vallof, D.; Kalafateli, A.L.; Jerlhag, E. Long-term treatment with a glucagon-like peptide-1 receptor agonist reduces ethanol intake in male and female rats. Transl. Psychiatry 2020, 10, 238. [Google Scholar] [CrossRef]
- Faerch, K.; Torekov, S.S.; Vistisen, D.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; Sandbaek, A.; et al. GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes 2015, 64, 2513–2525. [Google Scholar] [CrossRef]
- Michaud, A.; Vainik, U.; Garcia-Garcia, I.; Dagher, A. Overlapping Neural Endophenotypes in Addiction and Obesity. Front. Endocrinol. 2017, 8, 127. [Google Scholar] [CrossRef]
- De Ridder, D.; Manning, P.; Leong, S.L.; Ross, S.; Sutherland, W.; Horwath, C.; Vanneste, S. The brain, obesity and addiction: An EEG neuroimaging study. Sci. Rep. 2016, 6, 34122. [Google Scholar] [CrossRef]
- Brown, R.M.; Kupchik, Y.M.; Spencer, S.; Garcia-Keller, C.; Spanswick, D.C.; Lawrence, A.J.; Simonds, S.E.; Schwartz, D.J.; Jordan, K.A.; Jhou, T.C.; et al. Addiction-like Synaptic Impairments in Diet-Induced Obesity. Biol. Psychiatry 2017, 81, 797–806. [Google Scholar] [CrossRef]
- AlKalbani, S.R.; Murrin, C. The association between alcohol intake and obesity in a sample of the Irish adult population, a cross-sectional study. BMC Public Health 2023, 23, 2075. [Google Scholar] [CrossRef]

| GLP-1 RA | Dose | Disease of Approval | Date of Approval | RCTs | Main Outcomes |
|---|---|---|---|---|---|
| Exenatide | 5 μg or 10 μg b.i.d. sc | T2DM | 2005 | NCT00039026 https://clinicaltrials.gov/study/NCT00039026 (accessed on 3 May 2025) NCT00039013 https://clinicaltrials.gov/study/NCT00039013 (accessed on 3 May 2025) NCT00035984 https://clinicaltrials.gov/study/NCT00035984 (accessed on 3 May 2025) NCT00082381 https://clinicaltrials.gov/study/NCT00082381 (accessed on 3 May 2025) NCT00082407 https://clinicaltrials.gov/study/NCT00082407 (accessed on 3 May 2025) NCT00381342 https://clinicaltrials.gov/study/NCT00381342 (accessed on 3 May 2025) NCT00360334 https://clinicaltrials.gov/study/NCT00360334 (accessed on 3 May 2025) NCT00375492 https://clinicaltrials.gov/study/NCT00375492 (accessed on 3 May 2025) NCT00603239 https://clinicaltrials.gov/study/NCT00603239 (accessed on 3 May 2025) NCT00765817 https://clinicaltrials.gov/study/NCT00765817 (accessed on 3 May 2025) NCT00577824 https://clinicaltrials.gov/study/NCT00577824 (accessed on 3 May 2025) NCT00434954 https://clinicaltrials.gov/study/NCT00434954 (accessed on 3 May 2025) | ↓ HbA1c, HOMA-IR, body weight, blood pressure, and TC and LDL-C vs. placebo |
| Liraglutide | 0.6 mg and 1.2 or 1.8 mg q.d sc | T2DM and obesity | 2010 | NCT00318461 https://clinicaltrials.gov/study/NCT00318461 (accessed on 3 May 2025) NCT00318422 https://clinicaltrials.gov/study/NCT00318422 (accessed on 3 May 2025) NCT00331851 https://clinicaltrials.gov/study/NCT00331851 (accessed on 3 May 2025) NCT00333151 https://clinicaltrials.gov/study/NCT00333151 (accessed on 3 May 2025) NCT00294723 https://clinicaltrials.gov/study/NCT00294723 (accessed on 3 May 2025) | ↓ HbA1c, body weight, FPG, and blood pressure and improved beta cell function vs. placebo ↓ HbA1c vs. rosiglitazone |
| Lixisenatide | 10 μg then 20 μg q.d sc | T2DM | 2016 | NCT00715624 https://clinicaltrials.gov/study/NCT00715624 (accessed on 4 May 2025) NCT00713830 https://clinicaltrials.gov/study/NCT00713830 (accessed on 4 May 2025) NCT00866658 https://clinicaltrials.gov/study/NCT00866658 (accessed on 4 May 2025) NCT01768559 https://clinicaltrials.gov/study/NCT01768559 (accessed on 4 May 2025) NCT00707031 https://clinicaltrials.gov/study/NCT00707031 (accessed on 4 May 2025) NCT00763815 https://clinicaltrials.gov/study/NCT00763815 (accessed on 4 May 2025) NCT00975286 https://clinicaltrials.gov/study/NCT00975286 (accessed on 4 May 2025) NCT01169779 https://clinicaltrials.gov/study/NCT01169779 (accessed on 4 May 2025) | ↓ HbA1c, body weight, FPG, PPG, and blood pressure and improved beta cell function vs. placebo |
| Albiglutide | 30 or 50 mg q.w sc | T2DM | 2014 | NCT00849017 https://clinicaltrials.gov/study/NCT00849017 (accessed on 4 May 2025) NCT01098539 https://clinicaltrials.gov/study/NCT01098539 (accessed on 4 May 2025) NCT00976391 https://clinicaltrials.gov/study/NCT00976391 (accessed on 4 May 2025) NCT00838916 https://clinicaltrials.gov/study/NCT00838916 (accessed on 4 May 2025) NCT00838903 https://clinicaltrials.gov/study/NCT00838903 (accessed on 4 May 2025) NCT01128894 https://clinicaltrials.gov/study/NCT01128894 (accessed on 4 May 2025) NCT00849056 https://clinicaltrials.gov/study/NCT00849056 (accessed on 4 May 2025) NCT00839527 https://clinicaltrials.gov/study/NCT00839527 (accessed on 4 May 2025) | ↓ HbA1c and body weight vs. placebo ↓ body weight and events of severe hypoglycemia vs. insulin lispro Modest reductions in body weight vs. pioglitazone, glimepiride, and insulin glargine |
| Dulaglutide | 0.75 or 1.5 mg q.w sc | T2DM | 2014 | NCT00734474 https://clinicaltrials.gov/study/NCT00734474 (accessed on 4 May 2025) NCT01126580 https://clinicaltrials.gov/study/NCT01126580 (accessed on 4 May 2025) NCT01075282 https://clinicaltrials.gov/study/NCT01075282 (accessed on 4 May 2025) NCT01064687 https://clinicaltrials.gov/study/NCT01064687 (accessed on 4 May 2025) NCT01191268https://clinicaltrials.gov/study/NC01191268 (accessed on 4 May 2025) | ↓ HbA1c vs. metformin ↓ HbA1c and ↓ incidence of total hypoglycemic events vs. both exenatide and placebo ↓ HbA1c, ↓ incidence of total hypoglycemic events, and ↑ body weight reduction vs. insulin glargine |
| Semaglutide | 0.25 mg then 0.5 mg q.w sc | T2DM and obesity | 2017, 2021 | Ozempic: NCT02054897 https://clinicaltrials.gov/study/NCT02054897 (accessed on 6 May 2025) NCT01930188 https://clinicaltrials.gov/study/NCT01930188 (accessed on 6 May 2025) NCT01885208 https://clinicaltrials.gov/study/NCT01885208 (accessed on 6 May 2025) NCT02128932, https://clinicaltrials.gov/study/NCT02128932 (accessed on 6 May 2025) NCT02305381 https://clinicaltrials.gov/study/NCT02305381 (accessed on 6 May 2025) Rybelsus (oral tablet): NCT02827708 https://clinicaltrials.gov/study/NCT02827708 (accessed on 6 May 2025) NCT03021187 https://clinicaltrials.gov/study/NCT03021187 (accessed on 6 May 2025) NCT02906930 https://clinicaltrials.gov/study/NCT02906930 (accessed on 6 May 2025) NCT02863328 https://clinicaltrials.gov/study/NCT02863328 (accessed on 6 May 2025) NCT02607865 https://clinicaltrials.gov/study/NCT02607865, (accessed on 6 May 2025) NCT02863419 https://clinicaltrials.gov/study/NCT02863419 (accessed on 6 May 2025) | (SC) Greater HbA1c reduction and body weight vs. placebo Greater HbA1c and body weight reduction vs. sitagliptin Greater HbA1c and body weight reduction vs. exenatide extended release Greater HbA1c and body weight reduction and ↓ hypoglycemic events vs. insulin glargine (Oral): Greater HbA1c and body weight reduction vs. placebo and in a dose-dependent manner Greater HbA1c and body weight reduction vs. empagliflozin |
| Beinaglutide | 0.06 mg to 0.2 mg t.i.d. | T2DM and obesity | 2016, 2023 | NCT03829891 https://clinicaltrials.gov/study/NCT03829891 (accessed on 6 May 2025) NCT03987308 https://clinicaltrials.gov/study/NCT03987308 (accessed on 6 May 2025) NCT05005741 https://clinicaltrials.gov/study/NCT05005741 (accessed on 6 May 2025) ChiCTR1900023428 https://www.chictr.org.cn/showprojEN.html?proj=38105 (accessed on 6 May 2025) | Greater proportion achieved the glycemic target vs. no treatment |
| Pegloxenatide | 0.2 mg q.w | T2DM | 2019 | NCT02477865 https://clinicaltrials.gov/study/NCT02477865 (accessed on 6 May 2025) NCT02477969 https://clinicaltrials.gov/study/NCT02477969 (accessed on 6 May 2025) NCT01965509 https://clinicaltrials.gov/study/NCT01965509 (accessed on 6 May 2025) ChiCTR1900026514, https://www.chictr.org.cn/showprojEN.html?proj=44112 (accessed on 6 May 2025) ChiCTR2200057800, https://www.chictr.org.cn/showprojEN.html?proj=162400 (accessed on 6 May 2025) | Pegloxenatide added to metformin resulted in greater reductions in HbA1c, FPG, and PPG vs. metformin monotherapy |
| Year/Ref | Study Status | Study Arms | Patients, N | Outcomes |
|---|---|---|---|---|
| Klausen et al. 2022/[61] | Published | Exenatide sc 2 mg/week sc vs. placebo | 127 | Exenatide reduced the number of heavy drinking days and total alcohol intake in a subgroup of obese patients (BMI > 30 kg/m2) |
| Probst et al. 2023/[63] | Published | Dulaglutide sc 1.5 mg/week vs. placebo | 151 | Dulaglutide treatment significantly reduced alcohol intake in individuals treated for smoking cessation in 12 weeks |
| Hendershot et al. 2025/[64] | Published | Semaglutide sc titrated up to 1.0 mg/week vs. placebo | Semaglutide reduced the amount of alcohol consumed during a post-treatment laboratory self-administration task and the concentration of peak alcohol breath Semaglutide reduced the number of drinks per drinking day | |
| NCT05895643 | Recruiting | Semaglutide sc titrated up to 2.4 mg/week vs. placebo | 108 | Primary endpoint: change in heavy drinking days (alcohol consumption > 60/48 gr for men/women in one day) Secondary endpoints: alterations in alcohol consumption, smoking status, plasma concentration of phosphatidyl ethanol, brain gamma-aminobutyric acid (GABA) levels, quality of life, Fibrosis-4 score, alcohol cue reactivity, functional connectivity, and white matter tract integrity at 26 weeks vs. baseline |
| NCT05891587 | Recruiting | Semaglutide sc at a dose of 0.25 mg/week titrated up to 1 mg/week vs. placebo | 80 | Alteration in the number of standard alcoholic drinks consumed per week in a time frame from baseline to 13th week |
| NCT06015893 | Recruiting | Semaglutide sc titrated up to 2.4 mg/week or maximum tolerated dose vs. placebo | 52 | Difference in number of standard alcohol-containing drinks consumed per week from baseline to end of the study Number of severity adverse events during the study |
| NCT05892432 | Recruiting | Semaglutide po 3 mg titrated to 7 mg vs. placebo | 135 | Change in Cue Craving Visual Analog Score on a time frame from baseline and week 6 visit |
| NCT05520775 | Completed | Semaglutide sc 0.25 mg/week titrated up to 1 mg/week | 48 | Change in volume of alcohol consumption during a self-administration procedure from baseline to 8 weeks Change in peak breath alcohol concentration during a self-administration procedure from baseline to 8 weeks |
| Pathophysiological Target | Molecular Mechanism | Therapeutic Outcome |
|---|---|---|
| Hepatic steatosis | ↑ AMPK activation → ↓ de novo lipogenesis ↑ PPARα-mediated β-oxidation | Reduction in intrahepatic lipid accumulation |
| Inflammation | ↓ NF-κB pathway activity ↓ Pro-inflammatory cytokines (e.g., TNF-α, IL-6) | Attenuation of hepatic inflammation |
| Oxidative stress | ↑ Antioxidant enzyme expression ↓ Reactive oxygen species (ROS) production | Protection against hepatocellular damage |
| Fibrosis | ↓ TGF-β1/Smad signaling ↑ PPARγ expression ↓ α-SMA and COL1A1 transcription | Inhibition of hepatic stellate cell activation and fibrosis |
| Mitochondrial dysfunction | ↑ SIRT1 and PGC-1α expression Restoration of mitochondrial membrane potential | Improved mitochondrial integrity and energy metabolism |
| Insulin resistance | ↑ Insulin sensitivity ↓ Glucagon secretion | Improved glucose metabolism and hepatic insulin signaling |
| Appetite and energy intake | Central GLP-1 receptor activation ↑ Satiety signals in hypothalamus | Weight loss; reduced caloric intake and visceral adiposity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysavgis, L.; Mourelatou, N.-G.; Cholongitas, E. Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A Pan-Steatotic Liver Disease Treatment? Biomedicines 2025, 13, 1516. https://doi.org/10.3390/biomedicines13071516
Chrysavgis L, Mourelatou N-G, Cholongitas E. Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A Pan-Steatotic Liver Disease Treatment? Biomedicines. 2025; 13(7):1516. https://doi.org/10.3390/biomedicines13071516
Chicago/Turabian StyleChrysavgis, Lampros, Niki-Gerasimoula Mourelatou, and Evangelos Cholongitas. 2025. "Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A Pan-Steatotic Liver Disease Treatment?" Biomedicines 13, no. 7: 1516. https://doi.org/10.3390/biomedicines13071516
APA StyleChrysavgis, L., Mourelatou, N.-G., & Cholongitas, E. (2025). Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs): A Pan-Steatotic Liver Disease Treatment? Biomedicines, 13(7), 1516. https://doi.org/10.3390/biomedicines13071516






