Optimization, Characterization and Pharmacological Validation of the Endotoxin-Induced Acute Pneumonitis Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
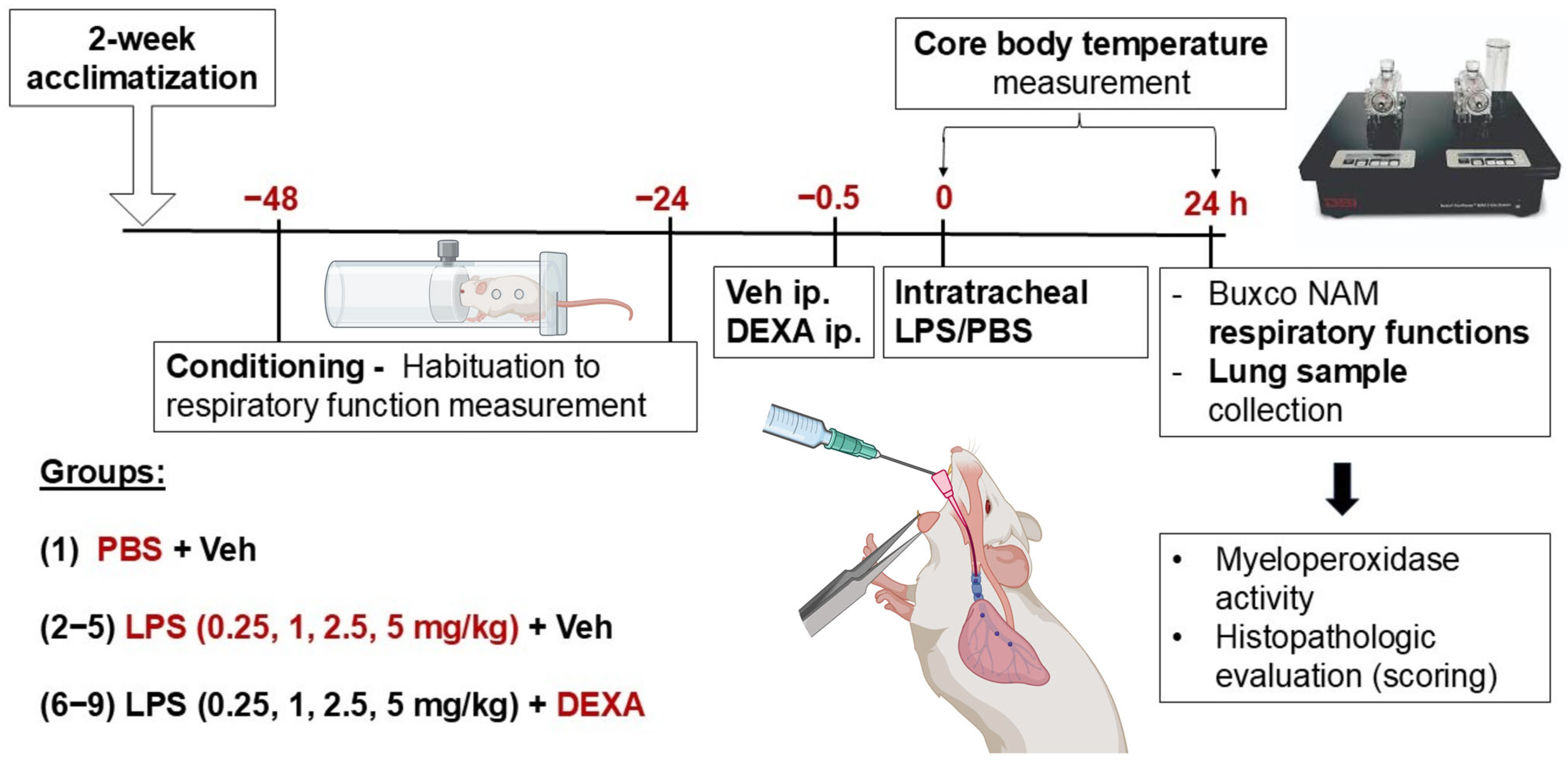
2.3. Experimental Protocol of Endotoxin-Induced Acute Interstitial Pneumonitis Model
2.4. Induction of Acute Pneumonitis by Intratracheal Administration of LPS
2.5. Pulmonary Function Measurement with Buxco Non-Invasive Airway Mechanics Plethysmometry
2.6. Investigation of Core Body Temperature
2.7. Histopathological Assessment of the Lungs
2.8. Determination of MPO Activity from Lung Homogenates
2.9. Statistical Analysis
3. Results
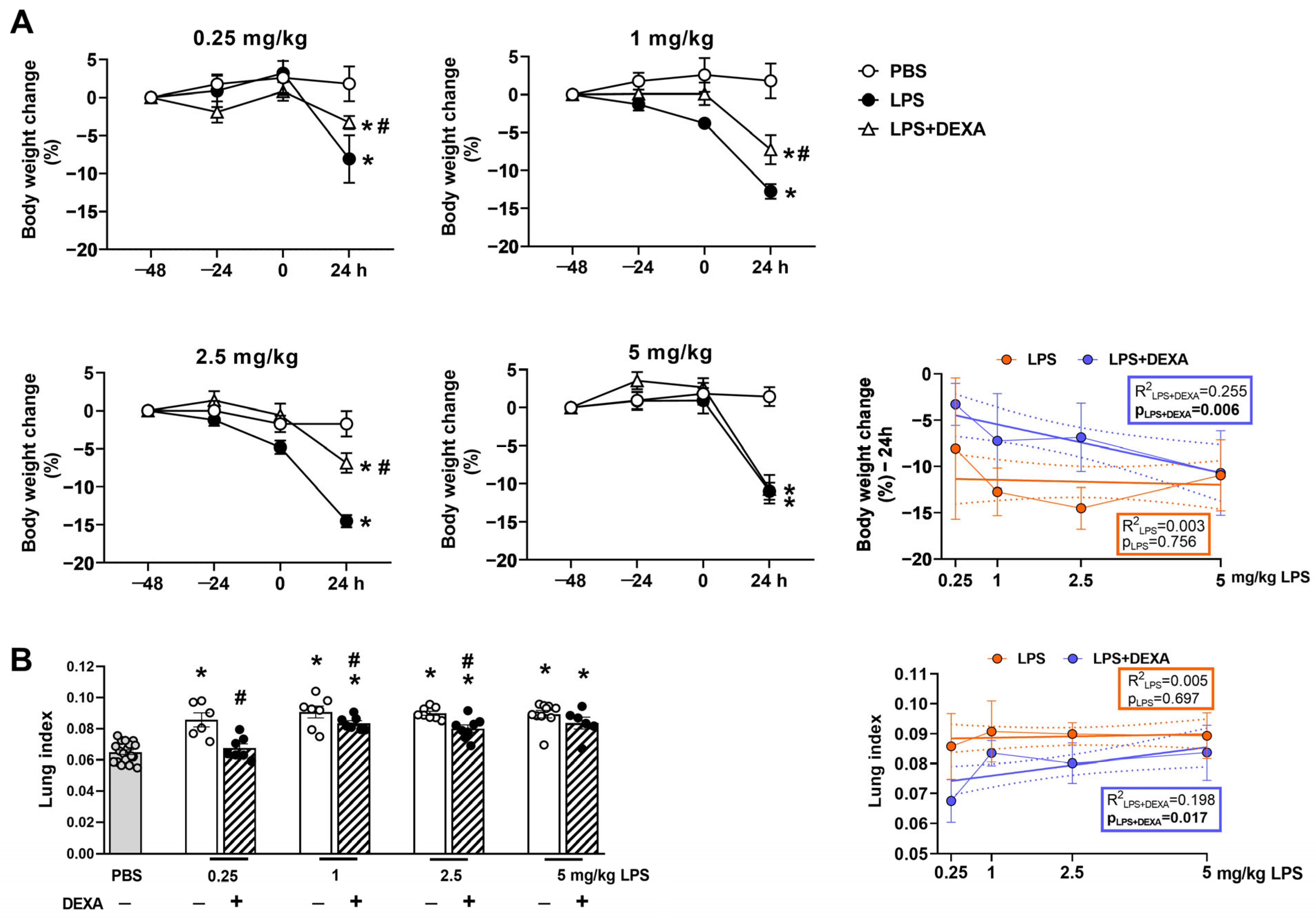
3.1. Dexamethasone Can Diminish LPS-Induced Body Weight Loss and Increased Lung Index Depending on the LPS Dose
3.2. Dexamethasone Counteracts Endotoxin-Induced Respiratory Function Impairment Depending on the LPS Dose
3.3. LPS Induces Hypothermia Dose-Dependently
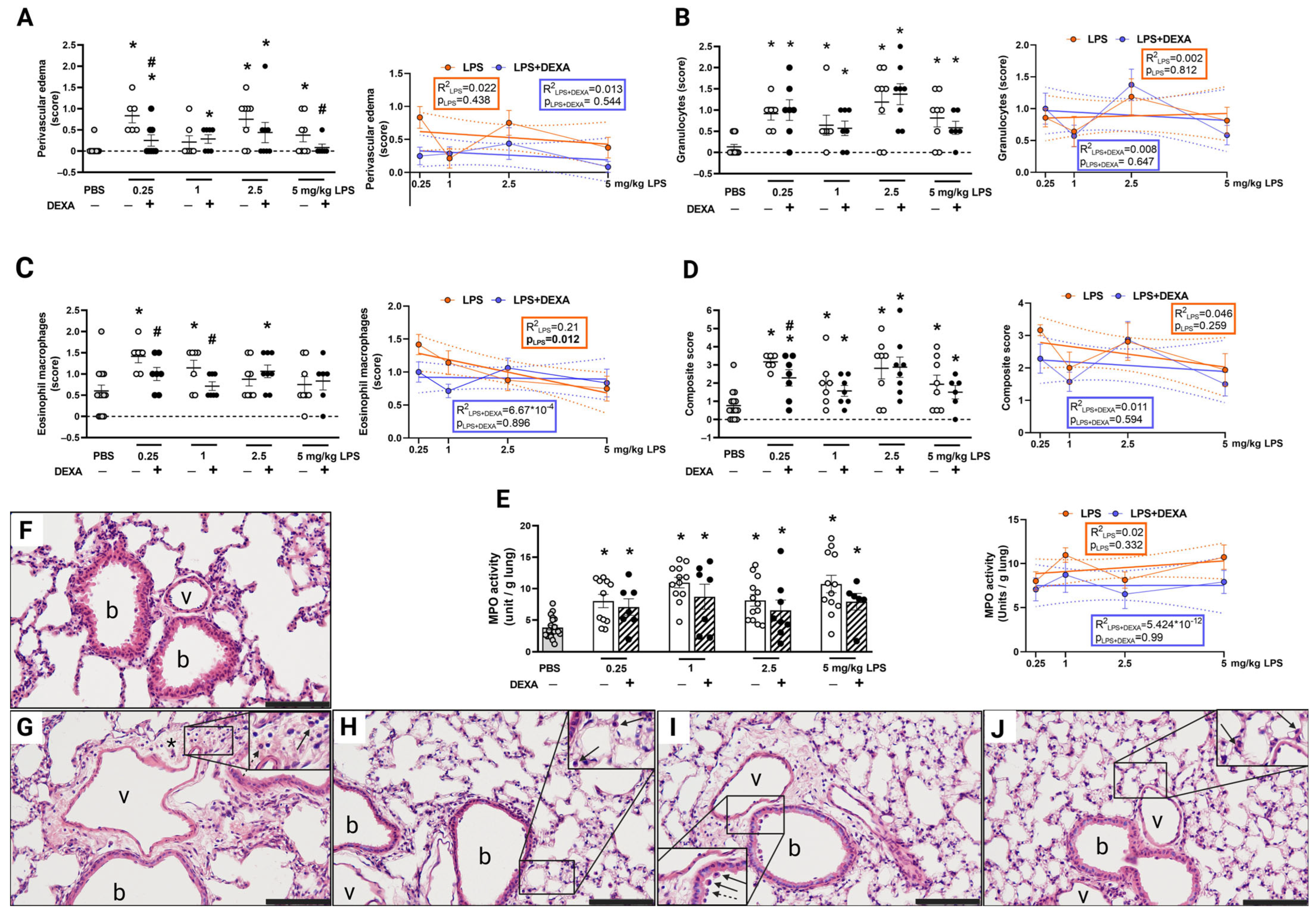
3.4. LPS-Induced Histopathological Alterations Are Not Dose-Dependent
3.5. LPS-Induced MPO Activity Was Not Influenced by Dexamethasone
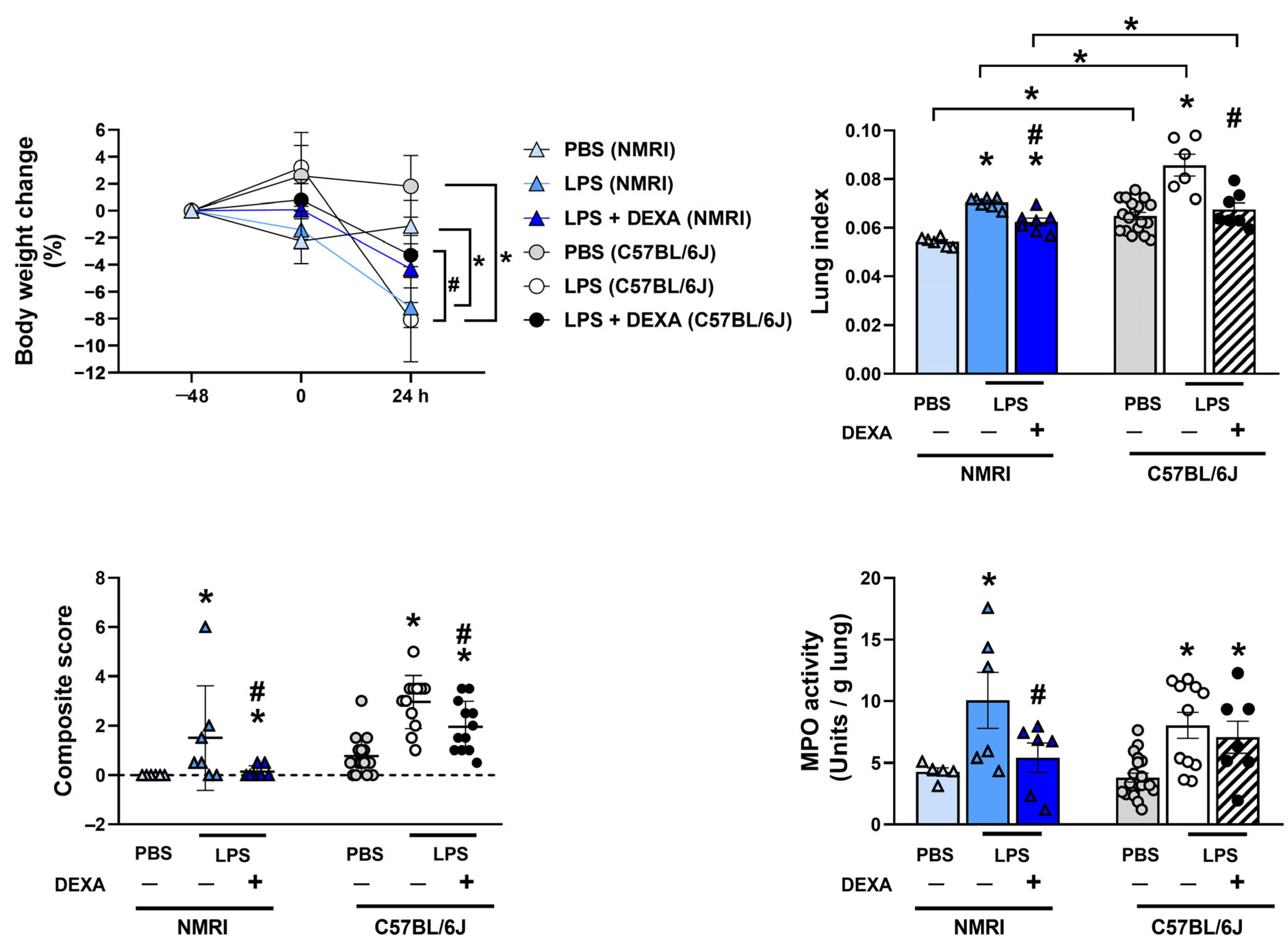
3.6. Dexamethasone Counteracts LPS-Induced Increased Total Lung Weight Along with Composite Score and MPO Activity in NMRI Mice
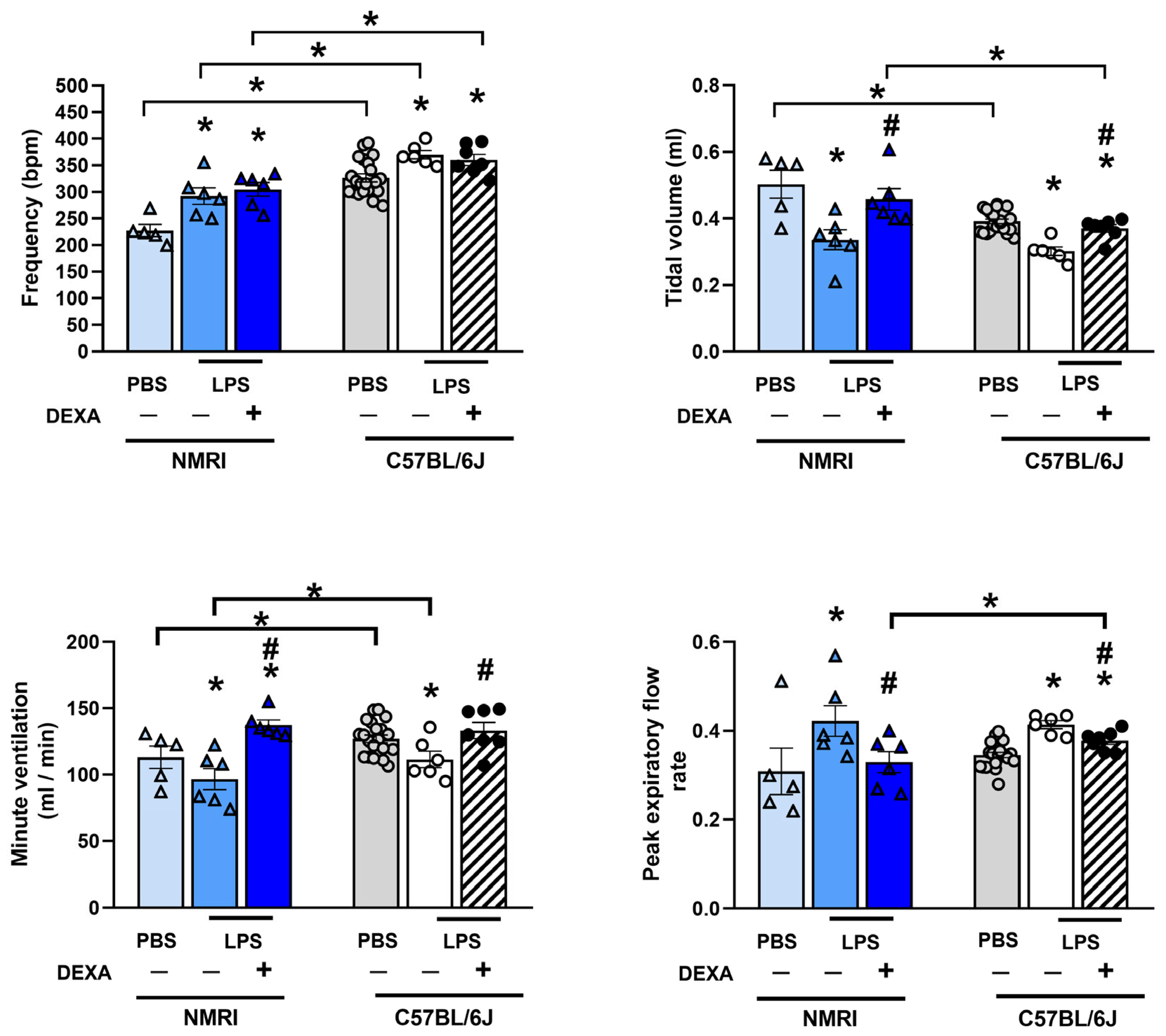
3.7. Dexamethasone Diminishes LPS-Induced Respiratory Function Alterations in NMRI Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | acute lung injury |
| ARDS | acute respiratory distress syndrome |
| BALF | bronchoalveolar lavage fluid |
| COVID-19 | Coronavirus disease 2019 |
| DEXA | dexamethasone |
| HE | hematoxylin-eosin |
| IL | interleukin |
| i.p. | intraperitoneal |
| LPS | lipopolysaccharide |
| MPO | myeloperoxidase |
| NAM | Non-Invasive Airway Mechanics |
| n.r. | not reported |
| n.u | not used |
| PBS | phosphate-buffered saline |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus-2 |
| SEM | standard error of the mean |
| Tab | abdominal temperature |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| Veh | Vehicle |
References
- Mokra, D. Acute Lung Injury—From Pathophysiology to Treatment. Physiol. Res. 2020, 69, S353–S366. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.S.; Capstick, T.; Ahmed, R.; Kow, C.S.; Mazhar, F.; Merchant, H.; Zaidi, S.T.R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert. Rev. Respir. Med. 2020, 14, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef] [PubMed]
- Azagew, A.W.; Beko, Z.W.; Ferede, Y.M.; Mekonnen, H.S.; Abate, H.K.; Mekonnen, C.K. Global prevalence of COVID-19-induced acute respiratory distress syndrome: Systematic review and meta-analysis. Syst. Rev. 2023, 12, 212. [Google Scholar] [CrossRef]
- Long, M.E.; Mallampalli, R.K.; Horowitz, J.C. Pathogenesis of pneumonia and acute lung injury. Clin. Sci. 2022, 136, 747–769. [Google Scholar] [CrossRef]
- Aliska, G.; Nafrialdi, N.; Lie, K.C.; Setiabudy, R.; Putra, A.E.; Widyahening, I.S.; Harahap, A.R. The role of the glucocorticoid receptor and its impact on steroid response in moderate-severe COVID-19 patients. Eur. J. Pharmacol. 2023, 943, 175555. [Google Scholar] [CrossRef]
- Chan, J.F.W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.Y. COVID-19 drug discovery and treatment options. Nat. Rev. Microbiol. 2024, 22, 391–407. [Google Scholar] [CrossRef]
- Akter, F.; Araf, Y.; Hosen, M.J. Corticosteroids for COVID-19, Worth it or not? Mol. Biol. Rep. 2021, 49, 567–576. [Google Scholar] [CrossRef]
- Golomb, B.A.; Han, J.H.; Langsjoen, P.H.; Dinkeloo, E.; Zemljic-Harpf, A.E. Statin Use in Relation to COVID-19 and Other Respiratory Infections: Muscle and Other Considerations. J. Clin. Med. 2023, 12, 4659. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Mak, M.; Tao, Z. Translational medicine for acute lung injury. J. Transl. Med. 2024, 22, 25. [Google Scholar] [CrossRef]
- Domscheit, H.; Hegeman, M.A.; Carvalho, N.; Spieth, P.M. Molecular Dynamics of Lipopolysaccharide-Induced Lung Injury in Rodents. Front. Physiol. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, Y.; Huang, M.; Chen, J.; Zhang, Z.; Li, J.; Yang, R.; Liu, Y.; Cai, S. Fuzhengjiedu formula exerts protective effect against LPS-induced acute lung injury via gut-lung axis. Phytomedicine 2024, 123, 155190. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Li, Q.; Ling, Y.; Zhou, Y.; Chu, K.; Xue, L.; Tao, S. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death Dis. 2022, 13, 530. [Google Scholar] [CrossRef]
- Puntorieri, V.; McCaig, L.A.; Howlett, C.J.; Yao, L.J.; Lewis, J.F.; Yamashita, C.M.; Veldhuizen, R.A.W. Lack of matrix metalloproteinase 3 in mouse models of lung injury ameliorates the pulmonary inflammatory response in female but not in male mice. Exp. Lung Res. 2016, 42, 365–379. [Google Scholar] [CrossRef]
- Speyer, C.L.; Rancilio, N.J.; McClintock, S.D.; Crawford, J.D.; Gao, H.; Vidya Sarma, J.; Ward, P.A. Regulatory effects of estrogen on acute lung inflammation in mice. Am. J. Physiol. Cell Physiol. 2005, 288, 881–890. [Google Scholar] [CrossRef]
- Kovacs-Kasa, A.; Kovacs, L.; Cherian-Shaw, M.; Patel, V.; Meadows, M.L.; Fulton, D.J.; Su, Y.; Verin, A.D. Inhibition of Class IIa HDACs improves endothelial barrier function in endotoxin-induced acute lung injury. J. Cell Physiol. 2021, 236, 2893–2905. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Foley, N.M.; Xu, Y.; Li, Y.P.; Pan, J.; Redmond, H.P.; Wang, J.H. B7H3 ameliorates LPS-induced acute lung injury via attenuation of neutrophil migration and infiltration. Sci. Rep. 2016, 6, 31284. [Google Scholar] [CrossRef]
- Tao, H.; Li, N.; Zhang, Z.; Mu, H.; Meng, C.; Xia, H.; Fu, L.; Xu, Y.; Zhang, S. Erlotinib Protects LPS-Induced Acute Lung Injury in Mice by Inhibiting EGFR/TLR4 Signaling Pathway. Shock 2019, 51, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Card, J.W.; Carey, M.A.; Bradbury, J.A.; Degraff, L.M.; Morgan, D.L.; Moorman, M.P.; Flake, G.P.; Zeldin, D.C. Gender Differences in Murine Airway Responsiveness and Lipopolysaccharide-Induced Inflammation 1. J. Immunol. 2006, 177, 621–630. [Google Scholar] [CrossRef]
- Li, Z.; Pan, H.; Yang, J.; Chen, D.; Wang, Y.; Zhang, H.; Cheng, Y. Xuanfei Baidu formula alleviates impaired mitochondrial dynamics and activated NLRP3 inflammasome by repressing NF-κB and MAPK pathways in LPS-induced ALI and inflammation models. Phytomedicine 2023, 108, 154545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, C.; Yuan, J.; Chen, X.; Li, Q.; Shen, F. Rhein-attenuates LPS-induced acute lung injury via targeting NFATc1/Trem2 axis. Inflamm. Res. 2023, 72, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.R.; Tune, M.K.; Bose, P.G.; McCullough, M.J.; Doerschuk, C.M. Comparison of different methods of initiating lung inflammation and the sex-specific effects on inflammatory parameters. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 324, L199–L210. [Google Scholar] [CrossRef]
- Tsikis, S.T.; Fligor, S.C.; Hirsch, T.I.; Pan, A.; Yu, L.J.; Kishikawa, H.; Joiner, M.M.; Mitchell, P.D.; Puder, M. Lipopolysaccharide-induced murine lung injury results in long-term pulmonary changes and downregulation of angiogenic pathways. Sci. Rep. 2022, 12, 10245. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Xue, Z.; Shao, R.; Li, L.; Zhu, Y.; Zhang, H.; Yang, J. Xuanfei Baidu Decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacol Res 2022, 176, 106083. [Google Scholar] [CrossRef]
- Dhlamini, Q.; Wang, W.; Feng, G.; Chen, A.; Chong, L.; Li, X.; Li, Q.; Wu, J.; Zhou, D.; Wang, J.; et al. FGF1 alleviates LPS-induced acute lung injury via suppression of inflammation and oxidative stress. Mol. Med. 2022, 28, 73. [Google Scholar] [CrossRef]
- Yang, H.H.; Duan, J.X.; Liu, S.K.; Xiong, J.B.; Guan, X.X.; Zhong, W.J.; Sun, C.C.; Zhang, C.Y.; Luo, X.Q.; Zhang, X.Q.; et al. A COX-2/sEH dual inhibitor PTUPB alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting NLRP3 inflammasome activation. Theranostics 2020, 10, 4749–4761. [Google Scholar] [CrossRef]
- Lv, X.; Yao, T.; He, R.; He, Y.; Li, M.; Han, Y.; Zhang, Y.; Long, L.; Jiang, G.; Cheng, X.; et al. Protective Effect of Fluorofenidone Against Acute Lung Injury Through Suppressing the MAPK/NF-κB Pathway. Front. Pharmacol. 2021, 12, 772031. [Google Scholar] [CrossRef]
- Jin, Z.; Shao, Z.; Yang, S.; Guo, A.; Han, Y.; Wu, Y.; Zhao, Y.; Wu, Y.; Shen, J.; Zhang, M.; et al. Airway epithelial cGAS inhibits LPS-induced acute lung injury through CREB signaling. Cell Death Dis. 2023, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, X.; Zhao, X.; Luo, L.; Liu, J.; Dai, S.; Zhang, F.; Wu, R.; Liu, Y.; Peng, C.; et al. Forsythiaside A alleviates acute lung injury by inhibiting inflammation and epithelial barrier damages in lung and colon through PPAR-γ/RXR-α complex. J. Adv. Res. 2024, 60, 183–200. [Google Scholar] [CrossRef]
- Zhou, M.; Meng, L.; He, Q.; Ren, C.; Li, C. Valsartan attenuates LPS-induced ALI by modulating NF-κB and MAPK pathways. Front. Pharmacol. 2024, 15, 1321095. [Google Scholar] [CrossRef] [PubMed]
- Burkard, P.; Schonhart, C.; Vögtle, T.; Köhler, D.; Tang, L.; Johnson, D.; Hemmen, K.; Heinze, K.G.; Zarbock, A.; Hermanns, H.M.; et al. A key role for platelet GPVI in neutrophil recruitment, migration, and NETosis in the early stages of acute lung injury. Blood 2023, 142, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Hsu, S.P.; Hu, M.C.; Lan, Y.T.; Yeh, E.T.H.; Yang, F.M. PEP-sNASP Peptide Alleviates LPS-Induced Acute Lung Injury Through the TLR4/TRAF6 Axis. Front. Med. 2022, 9, 832713. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Peng, H.; Zhang, M.; Wu, X.; Gui, F.; Li, W.; Ai, F.; Yu, B.; Liu, Y. Meteorin-like/Meteorin-β protects LPS-induced acute lung injury by activating SIRT1-P53-SLC7A11 mediated ferroptosis pathway. Mol. Med. 2023, 29, 144. [Google Scholar] [CrossRef]
- Nie, Y.; Liang, J.; Sun, J.; Li, J.; Zhai, X.; Zhao, P. Orexin A alleviates LPS-induced acute lung injury by inhibiting macrophage activation through JNK-mediated autophagy. Int. Immunopharmacol. 2023, 124, 111018. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, M.; Jin, L.; Yang, B.; Bai, B.; Mutsinze, R.N.; Zuo, W.; Chattipakorn, N.; Huh, J.Y.; Liang, G.; et al. Licochalcone A protects against LPS-induced inflammation and acute lung injury by directly binding with myeloid differentiation factor 2 (MD2). Br. J. Pharmacol. 2023, 180, 1114–1131. [Google Scholar] [CrossRef]
- Tesfaigzi, Y.; Rudolph, K.; Fischer, M.J.; Conn, C.A. Bcl-2 mediates sex-specific differences in recovery of mice from LPS-induced signs of sickness independent of IL-6. J. Appl. Physiol. 2001, 91, 2182–2189. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Wu, X.; Ma, Z.; Shi, J.; He, S.; Li, S.; Li, X.; Li, X.; Li, Y.; et al. 5-Methoxytryptophan ameliorates endotoxin-induced acute lung injury in vivo and in vitro by inhibiting NLRP3 inflammasome-mediated pyroptosis through the Nrf2/HO-1 signaling pathway. Inflamm. Res. 2023, 72, 1633–1647. [Google Scholar] [CrossRef]
- Hajna, Z.; Csekő, K.; Kemény, Á.; Kereskai, L.; Kiss, T.; Perkecz, A.; Szitter, I.; Kocsis, B.; Pintér, E.; Helyes, Z. Complex regulatory role of the trpa1 receptor in acute and chronic airway inflammation mouse models. Int. J. Mol. Sci. 2020, 21, 4109. [Google Scholar] [CrossRef]
- Helyes, Z.; Kemény, Á.; Csekő, K.; Szőke, É.; Elekes, K.; Mester, M.; Sándor, K.; Perkecz, A.; Kereskai, L.; Mark, L.; et al. Marijuana smoke induces severe pulmonary hyperresponsiveness, inflammation, and emphysema in a predictive mouse model not via CB1 receptor activation. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Csikós, E.; Csekő, K.; Kemény, Á.; Draskóczi, L.; Kereskai, L.; Kocsis, B.; Böszörményi, A.; Helyes, Z.; Horváth, G. Pinus sylvestris L. and Syzygium aromaticum (L.) Merr. & L. M. Perry Essential Oils Inhibit Endotoxin-Induced Airway Hyperreactivity despite Aggravated Inflammatory Mechanisms in Mice. Molecules 2022, 27, 3868. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Suzuki, S.; Tsubochi, H.; Sugita, M.; Maeda, S.; Kobayashi, S.; Kubo, H.; Kondo, T. Single dexamethasone injection increases alveolar fluid clearance in adult rats. Crit. Care Med. 2003, 31, 1183–1189. [Google Scholar] [CrossRef]
- Barnthaler, T.; Ramachandra, A.B.; Ebanks, S.; Guerrera, N.; Sharma, L.; Dela Cruz, C.S.; Humphrey, J.D.; Manning, E.P. Developmental changes in lung function of mice are independent of sex as a biological variable. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, L627–L637. [Google Scholar] [CrossRef]
- Wahlström, E.; Ollerstam, A.; Sundius, L.; Zhang, H. Use of Lung Weight as Biomarker for Assessment of Lung Toxicity in Rat Inhalation Studies. Toxicol. Pathol. 2013, 41, 902–912. [Google Scholar] [CrossRef]
- Pohorec, V.; Križančić Bombek, L.; Skelin Klemen, M.; Dolenšek, J.; Stožer, A. Glucose-Stimulated Calcium Dynamics in Beta Cells From Male C57BL/6J, C57BL/6N, and NMRI Mice: A Comparison of Activation, Activity, and Deactivation Properties in Tissue Slices. Front. Endocrinol. 2022, 13, 867663. [Google Scholar] [CrossRef]
- Garami, A.; Ibrahim, M.; Gilbraith, K.; Khanna, R.; Pakai, E.; Miko, A.; Pinter, E.; Romanovsky, A.A.; Porreca, F.; Patwardhan, A.M. Transient Receptor Potential Vanilloid 1 Antagonists Prevent Anesthesia-induced Hypothermia and Decrease Postincisional Opioid Dose Requirements in Rodents. Anesthesiology 2017, 127, 813–823. [Google Scholar] [CrossRef]
- Irwin, M.R.; Curay, C.M.; Choi, S.; Kiyatkin, E.A. Basic physiological effects of ketamine-xylazine mixture as a general anesthetic preparation for rodent surgeries. Brain Res. 2023, 1804, 148251. [Google Scholar] [CrossRef]
- Helyes, Z.; Elekes, K.; Sándor, K.; Szitter, I.; Kereskai, L.; Pintér, E.; Kemény, Á.; Szolcsányi, J.; McLaughlin, L.; Vasiliou, S.; et al. Involvement of preprotachykinin A gene-encoded peptides and the neurokinin 1 receptor in endotoxin-induced murine airway inflammation. Neuropeptides 2010, 44, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, E.; Chander, N.; Stewart, D.J.; Jahandideh, F.; Zhang, H.; Kristof, A.S.; Bastarache, J.A.; Schmidt, E.P.; Taljaard, M.; Thebaud, B.; et al. A preclinical systematic review and meta-analysis assessing the effect of biological sex in lipopolysaccharide-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, L661–L671. [Google Scholar] [CrossRef] [PubMed]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzales, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszynski, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef]
- Britt, R.D.; Thompson, M.A.; Sasse, S.; Pabelick, C.M.; Gerber, A.N.; Prakash, X.Y.S. Th1 cytokines TNF-and IFN-promote corticosteroid resistance in developing human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, 71–81. [Google Scholar] [CrossRef]
- Webster, J.C.; Oakley, R.H.; Jewell, C.M.; Cidlowski, J.A. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative isoform: A mechanism for the generation of glucocorticoid resistance. Proc. Natl. Acad. Sci. USA 2001, 12, 6865–6870. [Google Scholar] [CrossRef]


| LPS Dose | LPS Route of Admin | LPS Serotype | Strain | Sex | Endpoint | Anti-Inflammatory Drug Testing | Reference Compound | Reference |
|---|---|---|---|---|---|---|---|---|
| 0.5 mg/kg (10 µg) | intratracheal | n.r. | C57BL/6J | M | 24 h | Panaxydol | n.u. | Li et al., 2021 [13] |
| intranasal | n.r. | BALB/c | M | day 7 | Fuzhengjiedu formula | DEXA (1.5 mg/kg) | Lu et al., 2024 [14] | |
| 1 mg/kg | intratracheal | O111:B4 | C57BL/6J, STAT6flox/flox | n.r. | 24 h | n.u. | n.u. | Yang et al., 2022 [15] |
| O111:B4 | MMP3+/+, MMP3−/− | M, F | 18 h | n.u. | n.u. | Puntorieri et al., 2016 [16] | ||
| O111:B4 | n.r. | M, F | 6 h | n.u. | n.u. | Speyer et al., 2005 [17] | ||
| n.r. | C57BL/6J | n.r. | 16 h | n.u. | n.u. | Kovacs-Kasa et al., 2021 [18] | ||
| intranasal | O55:B5 | BALB/c | M | 24 h | B7H3 | n.u. | Li et al., 2016 [19] | |
| 2 mg/kg | intratracheal | O55:B5 | C57BL/6J | M | 6, 12, 24, 48, 72 h | erlotinib | n.u. | Tao et al., 2019 [20] |
| 2.5 mg/kg (50 µg) | aspiration | O111:B4 | C57BL/6J | M, F | 6 h | n.u. | n.u. | Card et al., 2006 [21] |
| inhalation | O55:B5 | ICR | M | day 14 | Xuanfei Baidu formula | DEXA (0.4 mg/kg) | Li Z et al., 2023 [22] | |
| 3 mg/kg | intratracheal | O111:B4 | C57BL/6J, Trem2−/− | M | 48 h | rhein, NFATc1 inhibitor | n.u. | Li X et al., 2023 [23] |
| O55:B5 | C57BL/6J | M, F | day 3, 7 | n.u. | n.u. | Mock et al., 2023 [24] | ||
| 75 µg | intratracheal | O111:B4 | C57BL/6J | M | 24 h, day 4, 7, 28 | n.u. | n.u. | Tsikis et al., 2022 [25] |
| 4 mg/kg | n.r. | n.r. | C57BL/6J | M | day 7 | Xuanfei Baidu Decoction | DEXA (5 mg/kg) | Wang et al., 2022 [26] |
| 5 mg/kg | intratracheal | O111:B4 | C57BL/6J | M | 12, 24 h | FGF1 | n.u. | Dhlamini et al., 2022 [27] |
| O111:B4 | C57BL/6J | M | 12 h | PTUPB | n.u. | Yang et al., 2020 [28] | ||
| O111:B4 | C57BL/6J | n.r. | 48 h | fluorofenidone | n.u. | Lv et al., 2021 [29] | ||
| O55:B5 | CC10-rtTA/(tetO)7-Cre/cGASflox/flox | n.r. | 24 h | n.u. | n.u. | Jin et al., 2023 [30] | ||
| O55:B5 | ICR | M | 72 h | Forsythiaside A | DEXA (5 mg/kg) | Wang et al., 2024 [31] | ||
| n.r. | C57BL/6J | n.r. | 24 h | valsartan | n.u. | Zhou et al., 2024 [32] | ||
| intranasal | O111:B4 | C57BL/6J, GP6−/− | M | 4 h | JAQ1 | n.u. | Burkard et al., 2023 [33] | |
| n.r. | C57BL/6J | M | 24 h | PEP-sNASP peptide | n.u. | Wu et al., 2022 [34] | ||
| i.p. injection | O111:B4 | C57BL/6J | M | 48 h | n.u. | n.u. | Chen et al., 2023 [35] | |
| n.r. | C57BL/6J | M | 24 h | orexin-A | n.u. | Nie et al., 2023 [36] | ||
| n.r. | n.r. | C57BL/6J, MD2−/−, TLR4−/− | M | 6, 24 h | licochalcone A | n.u. | Zhu et al., 2023 [37] | |
| 180 µg; 3 × 60 µg | intranasal | n.r. | Bcl-2-(1/2) | M, F | day 1, 2, 3, 4 | n.u. | n.u. | Tesfaigzi et al., 2001 [38] |
| 15 mg/kg | Caudal vein | O55:B5 | C57BL/6J, Nrf2−/− | M | 12 h | n.u. | n.u. | Ma et al., 2023 [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, E.; Hohl, K.; Kereskai, L.; Kemény, Á.; Hargitai, D.; Szombati, V.; Perkecz, A.; Pakai, E.; Garami, A.; Zsembery, Á.; et al. Optimization, Characterization and Pharmacological Validation of the Endotoxin-Induced Acute Pneumonitis Mouse Model. Biomedicines 2025, 13, 1498. https://doi.org/10.3390/biomedicines13061498
Ritter E, Hohl K, Kereskai L, Kemény Á, Hargitai D, Szombati V, Perkecz A, Pakai E, Garami A, Zsembery Á, et al. Optimization, Characterization and Pharmacological Validation of the Endotoxin-Induced Acute Pneumonitis Mouse Model. Biomedicines. 2025; 13(6):1498. https://doi.org/10.3390/biomedicines13061498
Chicago/Turabian StyleRitter, Emese, Kitti Hohl, László Kereskai, Ágnes Kemény, Dóra Hargitai, Veronika Szombati, Anikó Perkecz, Eszter Pakai, Andras Garami, Ákos Zsembery, and et al. 2025. "Optimization, Characterization and Pharmacological Validation of the Endotoxin-Induced Acute Pneumonitis Mouse Model" Biomedicines 13, no. 6: 1498. https://doi.org/10.3390/biomedicines13061498
APA StyleRitter, E., Hohl, K., Kereskai, L., Kemény, Á., Hargitai, D., Szombati, V., Perkecz, A., Pakai, E., Garami, A., Zsembery, Á., Helyes, Z., & Csekő, K. (2025). Optimization, Characterization and Pharmacological Validation of the Endotoxin-Induced Acute Pneumonitis Mouse Model. Biomedicines, 13(6), 1498. https://doi.org/10.3390/biomedicines13061498







