Sinusoidal Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Promotes Angiogenesis In Vitro
Abstract
1. Introduction
2. Materials and Methods
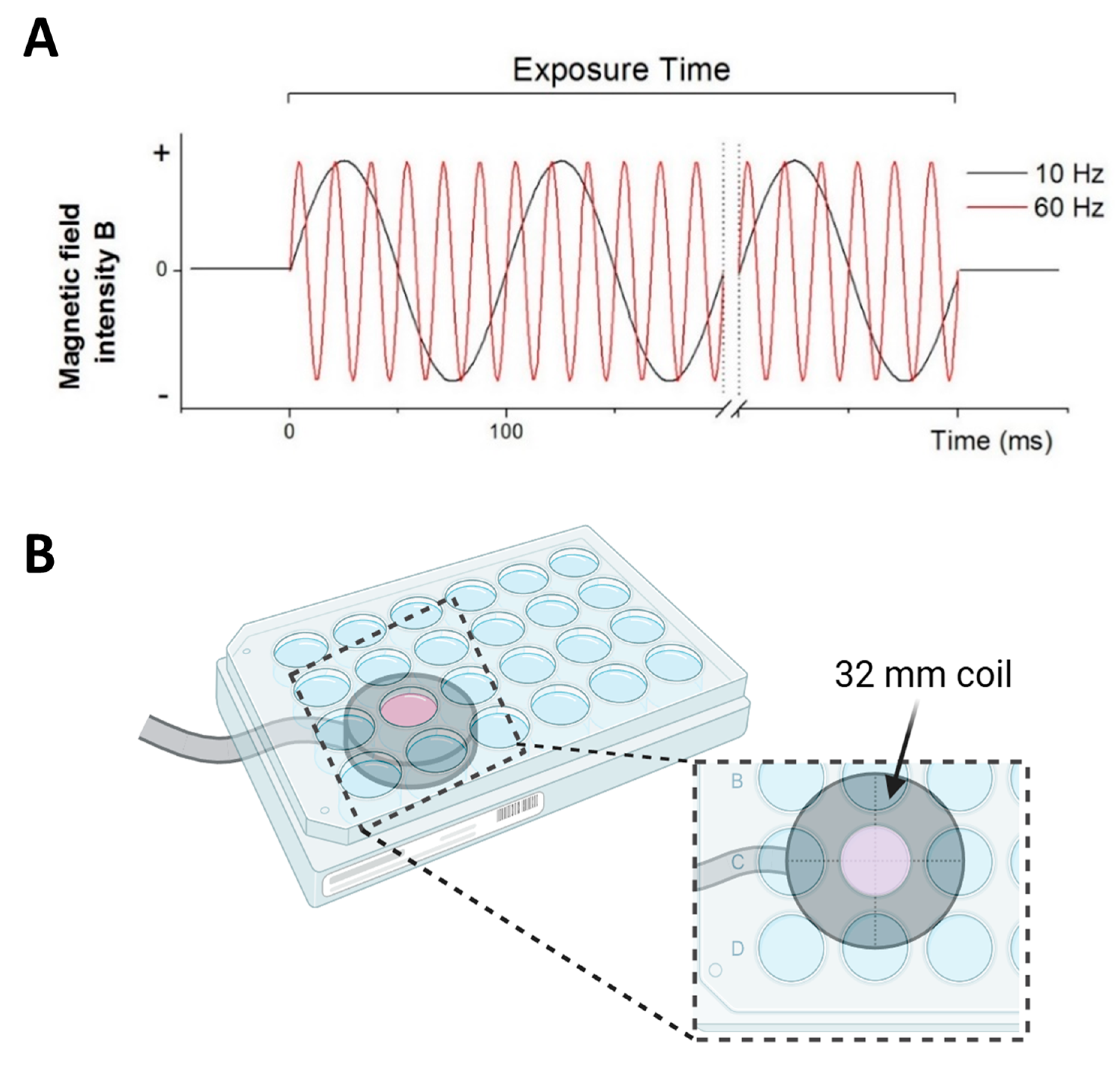
2.1. Specifications of ELF-EMS Exposure System
2.2. In Vitro Angiogenic Assays
2.2.1. Proliferation Assay
2.2.2. Wound Healing Assay
2.2.3. Transwell Migration Assay
2.2.4. Tube Formation Assay
2.3. Quantification of Nitrite Production
2.4. Chorioallantoic Membrane Assay (CAM)
2.5. Statistical Analysis
3. Results
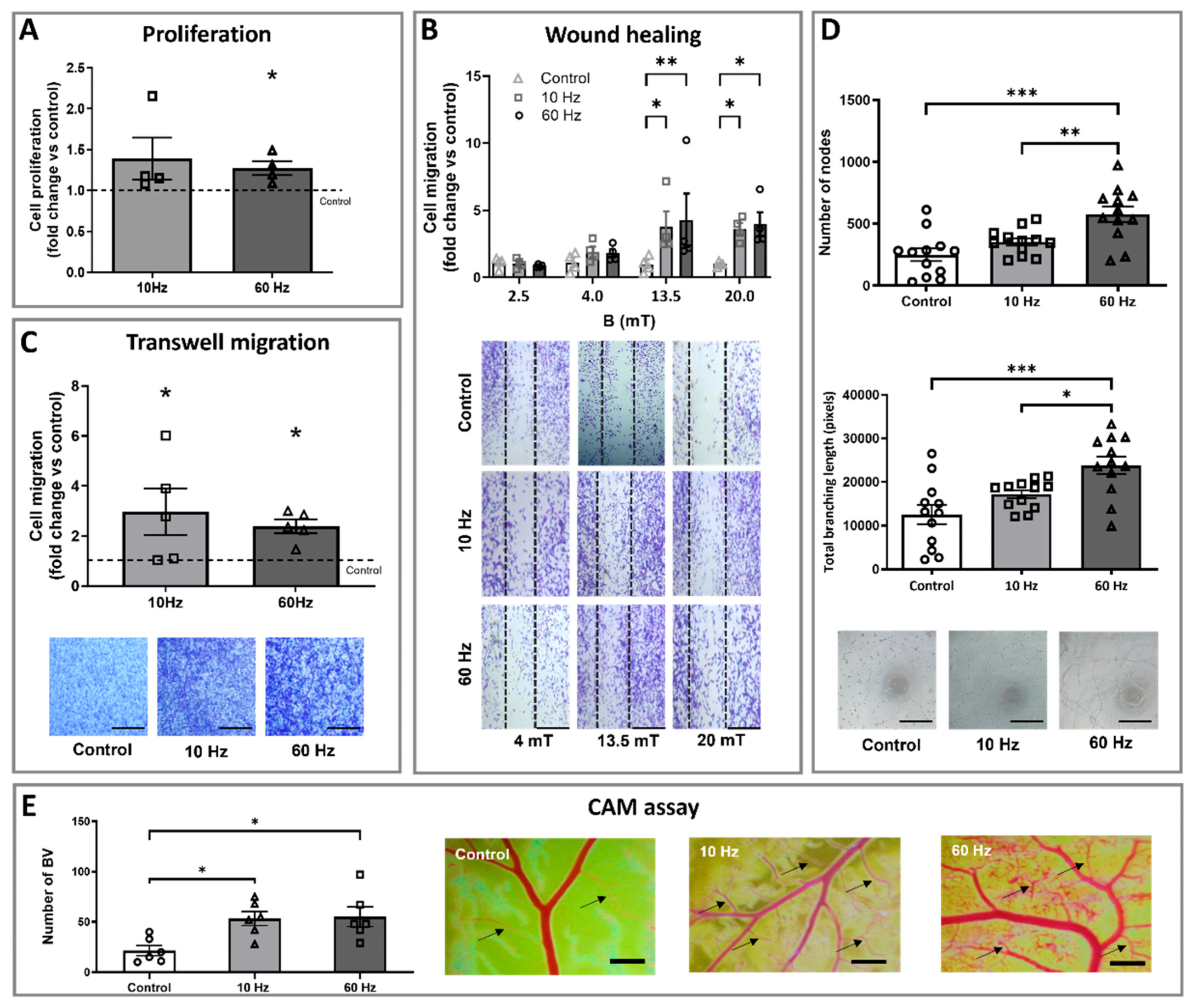
3.1. ELF-EMS Stimulates Proliferation, Migration, and Tube Formation of HMEC-1 Cells
3.2. ELF-EMS Enhances In Ovo Angiogenesis
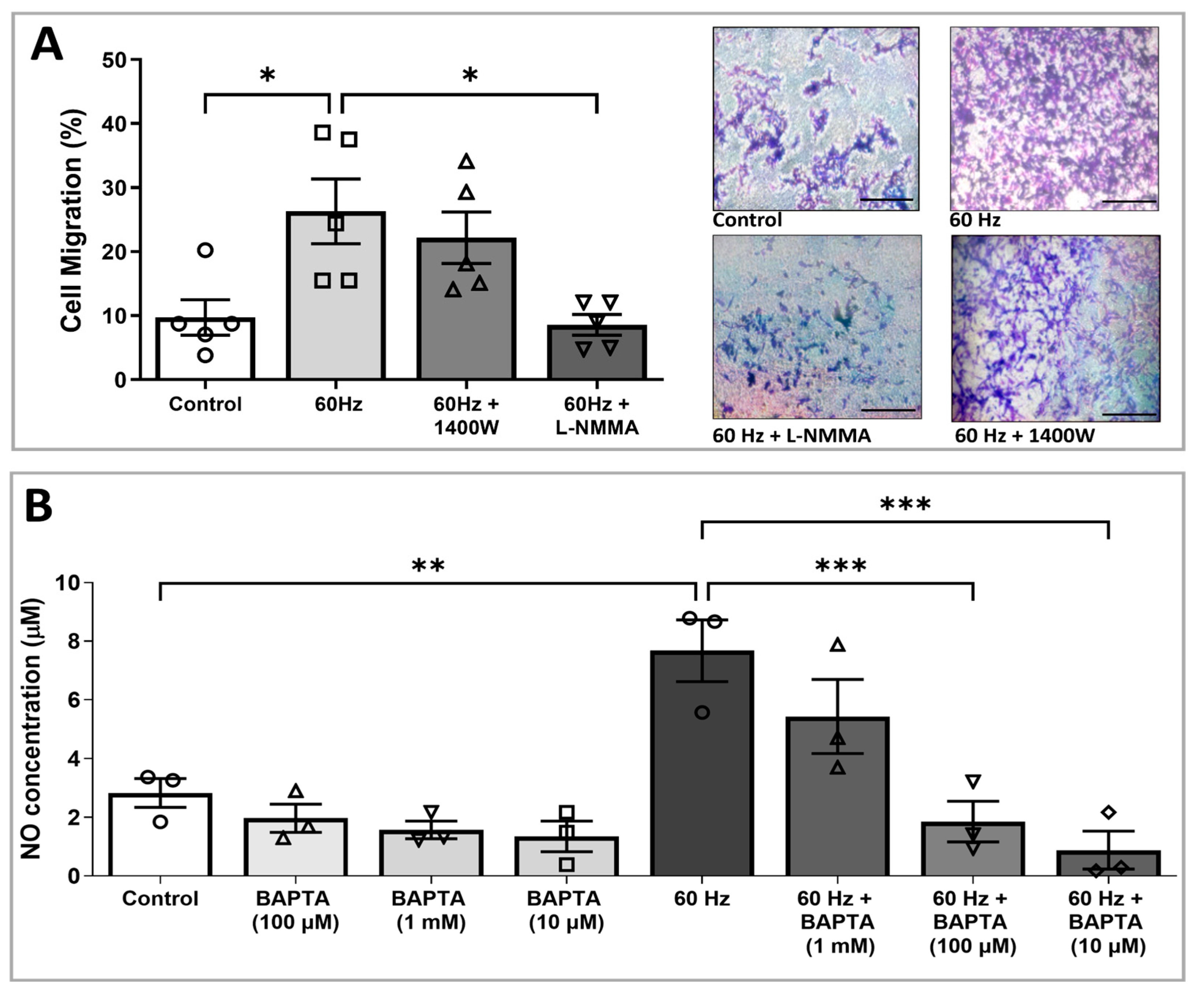
3.3. ELF-EMS-Induced HMEC-1 Migration Is Mediated by NOS
3.4. Increased NO Production Induced by ELF-EMS Is Mediated via Calcium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELF-EMS | extremely low-frequency electromagnetic stimulation |
| HMEC-1 | human microvascular endothelial cells |
| NO | nitric oxide |
| CAM | chorioallantois membrane |
| VEGF | vascular endothelial growth factor |
| ECs | endothelial cells |
| eNOS | endothelial nitric oxide synthase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| FBS | fetal bovine serum |
| iNOS | inducible nitric oxide synthase |
| nNOS | neural nitric oxide synthase |
| L-NMMA | NG-Monomethyl-L-arginine acetate |
| LPS | lipopolysaccharide |
| BAPTA-AM | 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) |
| SEM | standard error of the mean |
| BVs | blood vessels |
| PEMF | pulsed electromagnetic field |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| HUVECs | human umbilical vein endothelial cells |
| L-NAME | LN G-Nitro arginine methyl ester |
| CaM | calmodulin |
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Y.; Gao, S.-J.; Sun, J.; Zhang, L.-Q.; Wu, J.-Y.; Song, F.-H.; Liu, D.-Q.; Zhou, Y.-Q.; Mei, W. Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain. Neural Regen Res. 2023, 18, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, Z.; Miao, C. Angiogenesis after ischemic stroke. Acta Pharmacol. Sin. 2023, 44, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Ergul, A.; Alhusban, A.; Fagan, S.C. Angiogenesis: A harmonized target for recovery after stroke. Stroke 2012, 43, 2270–2274. [Google Scholar] [CrossRef]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-Angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef]
- Rust, R. Insights into the dual role of angiogenesis following stroke. J. Cereb. Blood Flow Metab. 2020, 40, 1167–1171. [Google Scholar] [CrossRef]
- Nagai, N.; Kawao, N.; Okada, K.; Okumoto, K.; Teramura, T.; Ueshima, S.; Umemura, K.; Matsuo, O. Systemic transplantation of embryonic stem cells accelerates brain lesion decrease and angiogenesis. NeuroReport 2010, 21, 575–579. [Google Scholar] [CrossRef]
- Fan, Y.; Shen, F.; Frenzel, T.; Zhu, W.; Ye, J.; Liu, J.; Chen, Y.; Su, H.; Young, W.L.; Yang, G.-Y.Y. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann. Neurol. 2010, 67, 488–497. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Wang, G.; Sun, Q.; Lin, P.; Wang, Q.; Zhang, H.; Wang, Y.; Zhang, T.; Cui, F.; et al. Advances in Engineered Nanoparticles for the Treatment of Ischemic Stroke by Enhancing Angiogenesis. Int. J. Nanomed. 2024, 19, 4377–4409. [Google Scholar] [CrossRef]
- Nakamura, K.; Henry, T.D.; Traverse, J.H.; Latter, D.A.; Mokadam, N.A.; Answini, G.A.; Williams, A.R.; Sun, B.C.; Burke, C.R.; Bakaeen, F.G.; et al. Angiogenic gene therapy for refractory angina: Results of the EXACT phase 2 trial. Circ. Cardiovasc. Interv. 2024, 17, e014054. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast growth factor in diabetic foot ulcer: Progress and therapeutic prospects. Front. Endocrinol. 2021, 14, 744868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, Q.; Jing, C.; Sun, L.; Jiang, L. Angiogenic responses are enhanced by recombinant human erythropoietin in a model of periventricular white matter damage of neonatal rats through EPOR-ERK1 signaling. J. Neuropathol. Exp. Neurol. 2024, 83, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Lyttle, B.D.; Vaughn, A.E.; Bardill, J.R.; Apte, A.; Gallagher, L.T.; Zgheib, C.; Liechty, K.W. Effects of microRNAs on angiogenesis in diabetic wounds. Front. Med. 2023, 10, 1140979. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.J.; Hamblin, M.; Eugene Chen, Y. Angiogenesis-regulating microRNAs and ischemic stroke. Curr. Vasc. Pharmacol. 2015, 13, 62–365. [Google Scholar] [CrossRef]
- Peng, L.; Fu, C.; Wang, L.; Zhang, Q.; Liang, Z.; He, C.; Wei, Q. The Effect of Pulsed Electromagnetic Fields on Angiogenesis. Bioelectromagnetics 2021, 42, 250–258. [Google Scholar] [CrossRef]
- Oladnabi, M.; Bagheri, A.; Kanavi, M.R.; Azadmehr, A.; Kianmehr, A. Extremely low frequency-pulsed electromagnetic fields affect proangiogenic-related gene expression in retinal pigment epithelial cells. Iran. J. Basic Med. Sci. 2019, 22, 128–133. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Font, L.P.; Cardonne, M.M.; Kemps, H.; Meesen, R.; Salmon, O.F.; González, F.G.; Lambrichts, I.; Rigo, J.-M.; Brône, B.; Bronckaers, A. Non-pulsed Sinusoidal Electromagnetic Field Rescues Animals From Severe Ischemic Stroke via NO Activation. Front. Neurosci. 2019, 13, 561. [Google Scholar] [CrossRef]
- Kemps, H.; Dessy, C.; Dumas, L.; Sonveaux, P.; Alders, L.; Van Broeckhoven, J.; Font, L.P.; Lambrichts, S.; Foulquier, S.; Hendrix, S.; et al. Extremely low frequency electromagnetic stimulation reduces ischemic stroke volume by improving cerebral collateral blood flow. J. Cereb. Blood Flow Metab. 2022, 42, 979–996. [Google Scholar] [CrossRef]
- Delle Monache, S.; Alessandro, R.; Iorio, R.; Gualtieri, G.; Colonna, R. Extremely low frequency electromagnetic fields (ELF-EMFs) induce in vitro angiogenesis process in human endothelial cells. Bioelectromagnetics 2008, 29, 640–648. [Google Scholar] [CrossRef]
- Saliev, T.; Mustapova, Z.; Kulsharova, G.; Bulanin, D.; Mikhalovsky, S. Therapeutic potential of electromagnetic fields for tissue engineering and wound healing. Cell Prolif. 2014, 47, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Tabrez, S.; Pesce, M.; Shakil, S.; Kamal, M.A.; Reale, M. Effects of extremely low frequency electromagnetic field (ELF-EMF) on catalase, cytochrome P450 and nitric oxide synthase in erythro-leukemic cells. Life Sci. 2015, 121, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Extremely low frequency electromagnetic field and wound healing: Implication of cytokines as biological mediators. Eur. Cytokine Netw. 2013, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Costantini, E.; Reale, M.; Amerio, P. Wound Repair and Extremely Low Frequency-Electromagnetic Field: Insight from In Vitro Study and Potential Clinical Application. Int. J. Mol. Sci. 2021, 22, 5037. [Google Scholar] [CrossRef]
- Cios, A.; Ciepielak, M.; Lieto, K.; Matak, D.; Lewicki, S.; Palusińska, M.; Stankiewicz, W.; Szymański, Ł. Extremely low-frequency electromagnetic field (ELF-EMF) induced alterations in gene expression and cytokine secretion in clear cell renal carcinoma cells. Med. Pr. Work. Health Saf. 2024, 75, 133–141. [Google Scholar] [CrossRef]
- Wang, M.H.; Chen, K.W.; Ni, D.X.; Fang, H.J.; Jang, L.S.; Chen, C.H. Effect of extremely low frequency electromagnetic field parameters on the proliferation of human breast cancer. Electromagn. Biol. Med. 2021, 40, 384–392. [Google Scholar] [CrossRef]
- Delle Monache, S.; Angelucci, A.; Sanità, P.; Iorio, R.; Bennato, F.; Mancini, F.; Gualtieri, G.; Colonna, R.C. Inhibition of angiogenesis mediated by extremely low-frequency magnetic fields (ELF-MFs). PLoS ONE 2013, 8, e79309. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Fanton, Y.; Struys, T.; Gervois, P.; Politis, C.; Martens, W.; Lambrichts, I. Angiogenic Properties of Human Dental Pulp Stem Cells. PLoS ONE 2013, 8, e71104. [Google Scholar] [CrossRef]
- Kokilakanit, P.; Koontongkaew, S.; Utispan, K. Nitric oxide has diverse effects on head and neck cancer cell proliferation and glycolysis. Biomed. Rep. 2024, 21, 106. [Google Scholar] [CrossRef]
- Olomu, I.N.; Hoang, V.; Madhukar, B.V. Low levels of nicotine and cotinine but not benzo[a]pyrene induce human trophoblast cell proliferation. Reprod. Toxicol. 2024, 125, 108572. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.-L. Wound-Healing Assay. In Cell Migration; Humana Press: Totowa, NJ, USA, 2005; pp. 23–30. [Google Scholar] [CrossRef]
- Huuskes, B.M.; DeBuque, R.J.; Kerr, P.G.; Samuel, C.S.; Ricardo, S.D. The Use of Live Cell Imaging and Automated Image Analysis to Assist With Determining Optimal Parameters for Angiogenic Assay in vitro. Front. Cell Dev. Biol. 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Proust, R.; Ponsen, A.-C.; Rouffiac, V.; Schenowitz, C.; Montespan, F.; Roux, K.S.-L.; De Leeuw, F.; Laplace-Builhé, C.; Mauduit, P.; Carosella, E.D.; et al. Cord blood-endothelial colony forming cells are immunotolerated and participate at post-ischemic angiogenesis in an original dorsal chamber immunocompetent mouse model. Stem Cell Res. Ther. 2020, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Shekatkar, M.; Kheur, S.; Deshpande, S.; Sakhare, S.; Sanap, A.; Kheur, M.; Bhonde, R. Critical appraisal of the chorioallantoic membrane model for studying angiogenesis in preclinical research. Mol. Biol. Rep. 2024, 51, 1026. [Google Scholar] [CrossRef] [PubMed]
- Katsir, G.; Parola, A.H. Enhanced proliferation caused by a low frequency weak magnetic field in chick embryo fibroblasts is suppressed by radical scavengers. Biochem. Biophys. Res. Commun. 1998, 252, 753–756. [Google Scholar] [CrossRef]
- McKay, J.C.; Prato, F.S.; Thomas, A.W. A literature review: The effects of magnetic field exposure on blood flow and blood vessels in the microvasculature. Bioelectromagnetics 2007, 28, 81–98. [Google Scholar] [CrossRef]
- Robertson, J.A.; Thomas, A.W.; Bureau, Y.; Prato, F.S. The influence of extremely low frequency magnetic fields on cytoprotection and repair. Bioelectromagnetics 2007, 28, 16–30. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Guo, Y.; Liu, N.; Jing, D.; Wang, H.; Guo, W. Pulsed magnetic field accelerate proliferation and migration of cardiac microvascular endothelial cells. Bioelectromagnetics 2015, 36, 1–9. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, Z.; Fu, X.; Jiang, Q.; Fei, J. Hydroxytyrosol contributes to cell proliferation and inhibits apoptosis in pulsed electromagnetic fields treated human umbilical vein endothelial cells in vitro. Mol. Med. Rep. 2017, 16, 8826–8832. [Google Scholar] [CrossRef]
- Alonso-Matilla, R.; Provenzano, P.P.; Odde, D.J. Physical principles and mechanisms of cell migration. Biol. Phys. Mech. 2025, 2, 2. [Google Scholar] [CrossRef]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef]
- Migliaccio, G.; Ferraro, R.; Wang, Z.; Cristini, V.; Dogra, P.; Caserta, S. Exploring cell migration mechanisms in cancer: From wound healing assays to cellular automata models. Cancers 2023, 15, 5284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Bai, L.; Uchida, K.; Bai, W.; Wu, B.; Xu, W.; Zhu, H.; Huang, H. Effects of low frequency electromagnetic field on proliferation of human epidermal stem cells: An in vitro study. Bioelectromagnetics 2013, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zuo, H.; Li, Y. Effects of Radiofrequency Electromagnetic Radiation on Neurotransmitters in the Brain. Front. Public Health 2021, 9, 691880. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Paul, S.; Bhattacharya, P.; Patnaik, R. Electromagnetic Field as a Treatment for Cerebral Ischemic Stroke. Front. Mol. Biosci. 2021, 8, 742596. [Google Scholar] [CrossRef]
- Ruggiero, M.; Bottaro, D.P.; Liguri, G.; Gulisano, M.; Peruzzi, B.; Pacini, S. 0.2 T magnetic field inhibits angiogenesis in chick embryo chorioallantoic membrane. Bioelectromagnetics 2004, 25, 390–396. [Google Scholar] [CrossRef]
- Eydgahi, S.M.; Baharara, J.; Balanezhad, S.Z.; Samani, M.A. The synergic effect of glycyrrhizic acid and low frequency electromagnetic field on angiogenesis in chick chorioallantoic membrane. Avicenna J. Phytomed. 2015, 5, 174–181. [Google Scholar] [CrossRef]
- Sadoughi, S.D.; Zafar-Balanjad, S.; Baharara, J.; Nejhad Shahrokhabadi, K.H.; Sepehri Moghadam, H.; Rahbarian, R. The effect of shoots of Allium sativum L., Ferula assa-foetida aqueous extract and low frequency electromagnetic field on angiogenesis in chick chorioallantoic membrane (in vivo). J. Med. Plants 2015, 14, 149–160. [Google Scholar]
- Balanezhad, S.Z.; Parivar, K.; Baharara, J.; Kouchesfeh, H.M.; Ashraf, A. The Effect of Extremely Low Frequency Electromagnetic Field on Angiogenesis. Res. J. Environ. Sci. 2010, 4, 300–304. [Google Scholar] [CrossRef]
- Moya-Gómez, A.; Font, L.P.; Burlacu, A.; Alpizar, Y.A.; Cardonne, M.M.; Brône, B.; Bronckaers, A. Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Improves Neurological Outcome and Reduces Microglial Reactivity in a Rodent Model of Global Transient Stroke. Int. J. Mol. Sci. 2023, 24, 11117. [Google Scholar] [CrossRef]
- Berg, H.; Günther, B.; Hilger, I.; Radeva, M.; Traitcheva, N.; Wollweber, L. Bioelectromagnetic Field Effects on Cancer Cells and Mice Tumors. Electromagn. Biol. Med. 2010, 29, 132–143. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.; Wang, X.; Xu, Y.; Liang, Z.; Gu, X.; He, C. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone 2022, 154, 116211. [Google Scholar] [CrossRef]
- Li, R.L.; Huang, J.J.; Shi, Y.Q.; Hu, A.; Lu, Z.Y.; Weng, L.; Wang, S.Q.; Han, Y.P.; Zhang, L.; Hao, C.N.; et al. Pulsed electromagnetic field improves postnatal neovascularization in response to hindlimb ischemia. Am. J. Transl. Res. 2015, 7, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, W.; Li, X.; Tran, B.H.; Suguro, R.; Guan, R.; Hou, C.; Wang, H.; Zhang, A.; Zhu, Y.; et al. Novel protective effects of pulsed electromagnetic field ischemia/reperfusion injury rats. Biosci. Rep. 2016, 36, e00420. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; del Zoppo, G.J. Angiogenesis in the ischemic core: A potential treatment target? J. Cereb. Blood Flow Metab. 2019, 39, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Bragin, D.E.; Statom, G.L.; Hagberg, S.; Nemoto, E.M. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J. Neurosurg. 2015, 122, 1239–1247. [Google Scholar] [CrossRef]
- Erkens, R.; Suvorava, T.; Kramer, C.M.; Diederich, L.D.; Kelm, M.; Cortese-Krott, M.M. Modulation of Local and Systemic Heterocellular Communication by Mechanical Forces: A Role of Endothelial Nitric Oxide Synthase. Antioxid. Redox Signal. 2017, 26, 917–935. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; House, D.E.; Joines, W.T. Effects of ELF (1–120 Hz) and modulated (50 Hz) RF fields on the efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 1985, 6, 1–11. [Google Scholar] [CrossRef]
- Santoro, N.; Lisi, A.; Pozzi, D.; Pasquali, E.; Serafino, A.; Grimaldi, S. Effect of extremely low frequency (ELF) magnetic field exposure on morphological and biophysical properties of human lymphoid cell line (Raji). Biochim. Biophys. Acta Mol. Cell Res. 1997, 1357, 281–290. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell. Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font, L.P.; Moya-Gomez, A.; Kemps, H.; Lambrichts, I.; Rigo, J.-M.; Brône, B.; Bronckaers, A. Sinusoidal Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Promotes Angiogenesis In Vitro. Biomedicines 2025, 13, 1490. https://doi.org/10.3390/biomedicines13061490
Font LP, Moya-Gomez A, Kemps H, Lambrichts I, Rigo J-M, Brône B, Bronckaers A. Sinusoidal Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Promotes Angiogenesis In Vitro. Biomedicines. 2025; 13(6):1490. https://doi.org/10.3390/biomedicines13061490
Chicago/Turabian StyleFont, Lena Perez, Amanda Moya-Gomez, Hannelore Kemps, Ivo Lambrichts, Jean-Michel Rigo, Bert Brône, and Annelies Bronckaers. 2025. "Sinusoidal Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Promotes Angiogenesis In Vitro" Biomedicines 13, no. 6: 1490. https://doi.org/10.3390/biomedicines13061490
APA StyleFont, L. P., Moya-Gomez, A., Kemps, H., Lambrichts, I., Rigo, J.-M., Brône, B., & Bronckaers, A. (2025). Sinusoidal Extremely Low-Frequency Electromagnetic Stimulation (ELF-EMS) Promotes Angiogenesis In Vitro. Biomedicines, 13(6), 1490. https://doi.org/10.3390/biomedicines13061490






