Effects of SGLT2 Inhibitors on Sleep Apnea Parameters and Cheyne–Stokes Respiration in Patients with Acute Decompensated Heart Failure: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Sleep-Related Respiratory Outcomes
3.3. Cardiac and Functional Parameters
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-Converting Enzyme |
| ADHF | Acute Decompensated Heart Failure |
| AF | Atrial Fibrillation |
| AHI | Apnea–Hypopnea Index |
| ARB | Angiotensin II Receptor Blocker |
| ARNi | Angiotensin Receptor–Neprilysin Inhibitor |
| CSR | Cheyne–Stokes Respiration |
| DPP-4i | Dipeptidyl Peptidase-4 Inhibitor |
| eGFR | Estimated Glomerular Filtration Rate |
| ESS | Epworth Sleepiness Scale |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| KCCQ | Kansas City Cardiomyopathy Questionnaire |
| LAVi | Left Atrial Volume Index |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVEF | Left Ventricular Ejection Fraction |
| MRA | Mineralocorticoid Receptor Antagonist |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association |
| ODI | Oxygen Desaturation Index |
| OSA | Obstructive Sleep Apnea |
| RA | Right Atrium |
| RV | Right Ventricle |
| RVOT | Right Ventricular Outflow Tract |
| RV–PA | Right Ventricular–Pulmonary Arterial |
| SDB | Sleep-Disordered Breathing |
| SD | Standard Deviation |
| SGLT2i | Sodium–Glucose Cotransporter 2 Inhibitor |
| SpO2 | Peripheral Oxygen Saturation |
| SPSS | Statistical Package for the Social Sciences |
| T2DM | Type 2 Diabetes Mellitus |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TAPSE/sPAP | Ratio of TAPSE to Systolic Pulmonary Artery Pressure |
References
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-disordered breathing in patients with symptomatic heart failure: A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Shukla, R.; Zeigler, H.; Wexler, L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J. Am. Coll. Cardiol. 2007, 49, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Yumino, D.; Bradley, T.D. Central sleep apnea and Cheyne-Stokes respiration. Proc. Am. Thorac. Soc. 2008, 5, 226–236. [Google Scholar] [CrossRef]
- White, L.H.; Motwani, S.; Kasai, T.; Yumino, D.; Amirthalingam, V.; Bradley, T.D. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir. Physiol. Neurobiol. 2014, 192, 17–22. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Sleep apnea and heart failure: Part I: Obstructive sleep apnea. Circulation 2003, 107, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Naughton, M.T. Pathophysiology and treatment of Cheyne-Stokes respiration. Thorax 1998, 53, 514–520. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef]
- Menon, T.; Kalra, D.K. Sleep Apnea and Heart Failure-Current State-of-The-Art. Int. J. Mol. Sci. 2024, 25, 5251. [Google Scholar] [CrossRef]
- Perger, E.; Jutant, E.M.; Redolfi, S. Targeting volume overload and overnight rostral fluid shift: A new perspective to treat sleep apnea. Sleep. Med. Rev. 2018, 42, 160–170. [Google Scholar] [CrossRef]
- Floras, J.S.; Bradley, T.D. Sleep apnoea in acute heart failure: Fluid in flux. Eur. Heart J. 2015, 36, 1428–1430. [Google Scholar] [CrossRef]
- Sekizuka, H. Body fluid management as a treatment for obstructive sleep apnea: A new possibility for sodium-glucose cotransporter 2 inhibitors. Hypertens. Res. 2025, 48, 1225–1227. [Google Scholar] [CrossRef]
- Packer, M. Activation and inhibition of sodium–hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 2017, 136, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- DiCaro, M.V.; Lei, K.; Yee, B.; Tak, T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. J. Clin. Med. 2024, 13, 3223. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Chandramouli, C.; Ahooja, V.; Verma, S. SGLT-2 Inhibitors in Heart Failure: Current Management, Unmet Needs, and Therapeutic Prospects. J. Am. Heart Assoc. 2019, 8, e013389. [Google Scholar] [CrossRef]
- Erman, M.K.; Stewart, D.; Einhorn, D.; Gordon, N.; Casal, E. Validation of the ApneaLink™ for the screening of sleep apnea: A novel and simple single-channel recording device. J. Clin. Sleep. Med. 2007, 3, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Porter, C.B.; Bresnahan, D.R.; Spertus, J.A. Development and validation of the Kansas City Cardiomyopathy Questionnaire. J. Am. Coll. Cardiol. 2000, 35, 1245–1255. [Google Scholar] [CrossRef]
- Csipor Fodor, A.; Huțanu, D.; Budin, C.E.; Ianoși, M.B.; Rachiș, D.L.; Sárközi, H.-K.; Vultur, M.A.; Jimborean, G. Central Sleep Apnea in Adults: An Interdisciplinary Approach to Diagnosis and Management—A Narrative Review. J. Clin. Med. 2025, 14, 2369. [Google Scholar] [CrossRef]
- Randerath, W.; Schwarz, E.I. Central sleep apnea: Realignment required. J. Clin. Sleep. Med. 2025, 21, 657–665. [Google Scholar] [CrossRef]
- Chowdhuri, S.; Badr, M.S. Opioid-related sleep-disordered breathing: Correlates and conundrums. Sleep 2024, 47, zsae104. [Google Scholar] [CrossRef]
- Patel, S.R.; Sykes, A.V.; Malhotra, A. Sleep apnoea in congestive heart failure: One step forwards. Lancet Respir. Med. 2024, 12, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Sagris, M.; Patoulias, D.; Koufakis, T.; Theofilis, P.; Klisic, A.; Fragakis, N.; El Tanani, M.; Rizzo, M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines 2024, 12, 2503. [Google Scholar] [CrossRef]
- Manzi, L.; Buongiorno, F.; Narciso, V.; Florimonte, D.; Forzano, I.; Castiello, D.S.; Sperandeo, L.; Paolillo, R.; Verde, N.; Spinelli, A.; et al. Acute Heart Failure and Non-Ischemic Cardiomyopathies: A Comprehensive Review. Diagnostics 2025, 15, 540. [Google Scholar] [CrossRef]
- Kumar, S.; Negi, P.C.; Asotra, S.; Chandel, M.; Kumar, J.; Merwah, R.; Sharma, R.; Kumar, R.; Bhardwaj, V.; Thakur, P.S. Determinants of LVEF recovery in non-ischemic HFrEF: A hospital-based study. Indian Heart J. 2025, 77, 59–62. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Guo, Y.; Shao, H.; Kimmel, S.E.; Bian, J.; Schatz, D.A.; Guo, J. SGLT2 inhibitors and incidence of atrial fibrillation in older adults with type 2 diabetes: A population-based study. Front. Pharmacol. 2024, 15, 1379251. [Google Scholar] [CrossRef]
- Farrero, M.; Bellumkonda, L.; Gomez Otero, I.; Molina, B.D. Sex and heart failure treatment prescription and adherence: Current insights from registry data. Front. Cardiovasc. Med. 2021, 8, 630141. [Google Scholar] [CrossRef]
- Tsigkas, G.; Apostolos, A.; Aznaouridis, K.; Despotopoulos, S.; Chrysohoou, C.; Naka, K.K.; Davlouros, P. Real-world implementation of European Society of Cardiology guidelines for heart failure management. Hellenic J. Cardiol. 2022, 63, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.W.; Makwana, B.; Khadke, S.; Dani, S.S.; Ganatra, S. Reply: Balancing Promise and Evidence: Critical Perspectives on SGLT2 Inhibitors for Cardiotoxicity Prevention. JACC: CardioOncology 2025, 7, 316. [Google Scholar] [CrossRef]
- Xie, L.; Li, S.; Yu, X.; Wei, Q.; Yu, F.; Tong, J. DAHOS Study: Efficacy of dapagliflozin in treating heart failure with reduced ejection fraction and obstructive sleep apnea syndrome—A 3-month, multicenter trial. Eur. J. Clin. Pharmacol. 2024, 80, 771–780. [Google Scholar] [CrossRef]
- Polecka, A.; Olszewska, N.; Danielski, Ł.; Olszewska, E. Association between obstructive sleep apnea and heart failure in adults: A systematic review. J. Clin. Med. 2023, 12, 6139. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; D’Alessandro, D.; Tricarico, L.; Ceci, V.; Mazzeo, P.; Capasso, R.; Ferrara, S.; Barile, M.; Di Nunno, N.; Rossi, L.; et al. Left ventricular reverse remodeling after combined ARNI and SGLT2 therapy in heart failure patients with reduced or mildly reduced ejection fraction. Int. J. Cardiol. Heart Vasc. 2024, 54, 101492. [Google Scholar] [CrossRef] [PubMed]
- Jaffuel, D.; Nogue, E.; Berdague, P.; Galinier, M.; Fournier, P.; Dupuis, M.; Georger, F.; Cadars, M.; Ricci, J.; Plouvier, N.; et al. Sacubitril-valsartan initiation in chronic heart failure patients impacts sleep apnea: The ENTRESTO-SAS study. ESC Heart Fail. 2021, 8, 2513–2526. [Google Scholar] [CrossRef]
- Mantegazza, V.; Volpato, V.; Mapelli, M.; Sassi, V.; Salvioni, E.; Mattavelli, I.; Tamborini, G.; Agostoni, P.; Pepi, M. Cardiac Reverse Remodelling by 2D and 3D Echocardiography in Heart Failure Patients Treated with Sacubitril/Valsartan. Diagnostics 2021, 11, 1845. [Google Scholar] [CrossRef]
- Mariani, M.V.; Lavalle, C.; Palombi, M.; Pierucci, N.; Trivigno, S.; D’AMato, A.; Filomena, D.; Cipollone, P.; Laviola, D.; Piro, A.; et al. SGLT2i reduce arrhythmic events in heart failure patients with cardiac implantable electronic devices. ESC Heart Fail. 2025, 12, 2125–2133. [Google Scholar] [CrossRef]
- Lo Giudice, F.; Escribano-Subias, P.; Tello, K.; Kopec, G.; Ghio, S.; Giannakoulas, G.; D’aLto, M.; Filomena, D.; Manzi, G.; Orlando, A.; et al. Echocardiography of the right heart in pulmonary arterial hypertension: Insights from the ULTRA RIGHT VALUE study. Eur. Heart J. Imaging Methods Pract. 2025, 3, qyae121. [Google Scholar] [CrossRef]
- Karacaglar, E.; Bal, U.; Eroglu, S.; Colak, A.; Bozbas, S.; Muderrisoglu, H. Pulmonary Artery Distensibility is Worsened in Obstructive Sleep Apnea Syndrome. Acta Cardiol. Sin. 2019, 35, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef]
- Spahillari, A.; Cohen, L.P.; Lin, C.; Liu, Y.; Tringale, A.; Sheppard, K.E.; Ko, C.; Khairnar, R.; Williamson, K.M.; Wasfy, J.H.; et al. Efficacy, Safety and Mechanistic Impact of a Heart Failure Guideline-Directed Medical Therapy Clinic. JACC Heart Fail. 2025, 13, 554–568. [Google Scholar] [CrossRef]
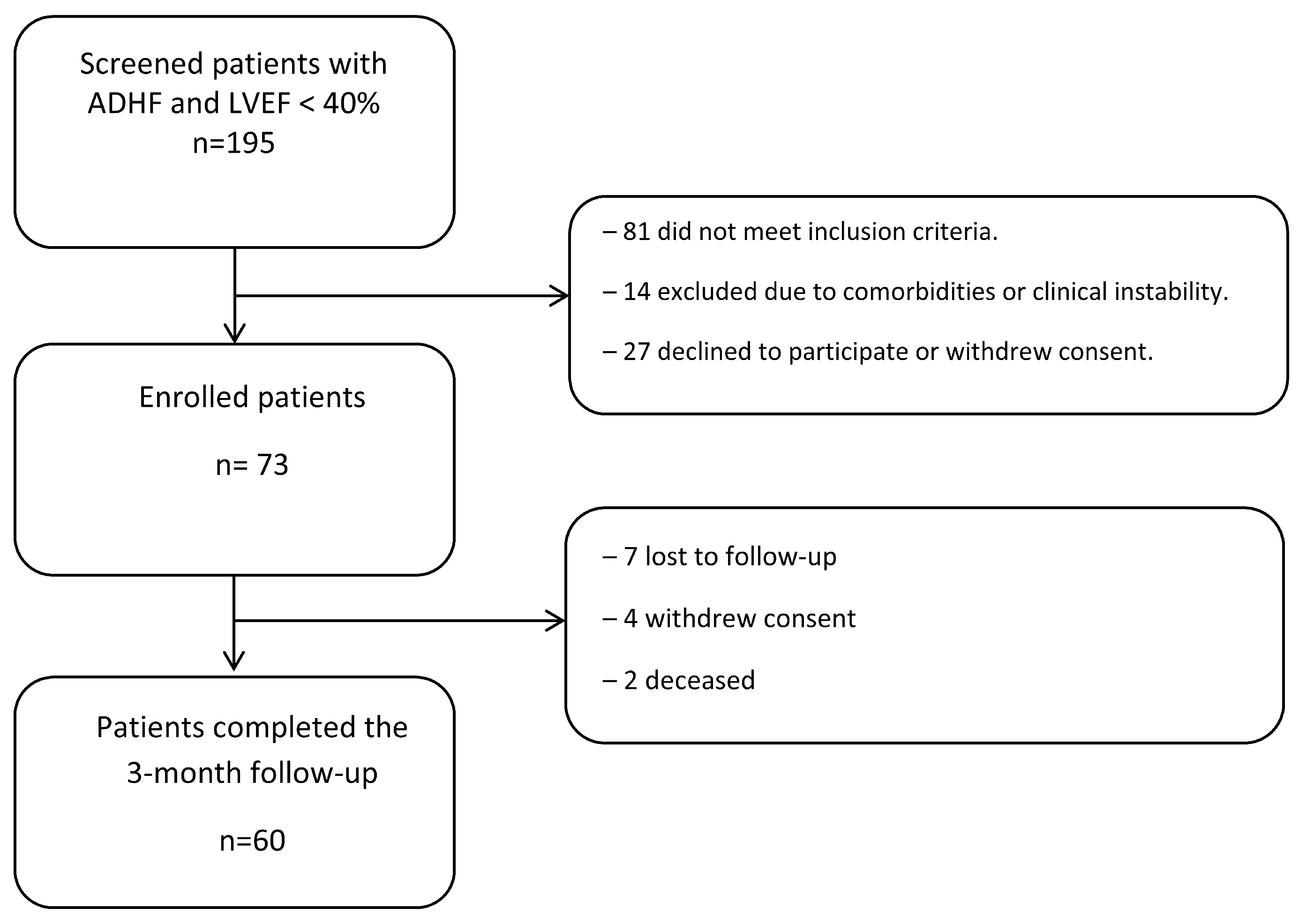
| Characteristic | Value, n (%) |
|---|---|
| Age, years | 69.3 ± 9.4 (45–86) |
| Sex, n (%) | |
| Female | 19 (31.7) |
| Male | 41 (68.3) |
| Non-ischemic etiology, n (%) | 38 (63.3) |
| Atrial fibrillation, n (%) | 43 (71.7) |
| Diabetes mellitus type 2, n (%) | 34 (56.7) |
| Medications, n (%) | |
| Beta-blockers | 54 (90.0) |
| ACEI/ARB | 44 (73.3) |
| ARNi | 33 (55.0) |
| MRA | 26 (43.3) |
| Loop diuretics | 52 (86.7) |
| Antidiabetic therapy among patients with DM2 | |
| Dapagliflozin 5 mg | 10 (16.7) |
| Dapagliflozin 10 mg | 26 (43.3) |
| Empagliflozin 5 mg | 3 (5.0) |
| Empagliflozin 10 mg | 21 (35.0) |
| Metformin | 21 (61.8%) |
| DPP-4 inhibitors | 6 (17.6%) |
| Insulin (basal and/or bolus) | 8 (23.5%) |
| Sulfonylureas | 2 (5.9%) |
| Parameter | Baseline | Follow-Up | Δ (Follow-Up − Baseline) | p-Value |
|---|---|---|---|---|
| ESS | 10.72 ± 1.61 | 8.04 ± 2.27 | −2.68 | <0.001 |
| KCCQ overall score | 62.87 ± 5.73 | 72.03 ± 6.99 | +9.16 | <0.001 |
| CSR index (events/h) | 32.63 ± 11.05 | 27.01 ± 9.61 | −5.63 | <0.001 |
| AHI (events/h) | 21.18 ± 4.82 | 18.11 ± 4.64 | −3.07 | <0.001 |
| ODI (events/h) | 24.35 ± 6.66 | 18.24 ± 5.83 | −6.11 | <0.001 |
| Mean nocturnal SpO2 (%) | 89.98 ± 2.85 | 91.93 ± 2.58 | +1.95 | <0.001 |
| Lowest nocturnal SpO2 (%) | 80.91 ± 5.55 | 81.69 ± 5.26 | +0.78 | 0.206 |
| Parameter | Baseline | Follow-Up | Δ (Follow-Up − Baseline) | p-Value |
|---|---|---|---|---|
| NT-proBNP (pg/mL) | 1780.9 ± 882.8 | 1451.3 ± 923.4 | −329.64 | <0.001 |
| E/e′ | 14.55 ± 2.76 | 13.48 ± 2.65 | −1.08 | <0.001 |
| LVEF (%) | 35.06 ± 4.65 | 35.16 ± 4.85 | +0.10 | 0.490 |
| LVEDV (mL) | 191.76 ± 19.20 | 189.78 ± 17.97 | −1.98 | 0.094 |
| LAVi (mL/m2) | 45.58 ± 4.20 | 45.47 ± 4.24 | −0.11 | 0.239 |
| eGFR (mL/min/1.73 m2) | 61.87 ± 9.42 | 62.37 ± 9.56 | +0.50 | 0.210 |
| Serum creatinine, µmol/L | 108 ± 9.1 | 103 ± 8.2 | −5 | 0.081 |
| BMI, kg/m2 | 29.2 ± 2.6 | 27.8 ± 2.5 | −1.4 | 0.011 |
| RVOT diameter (mm) | 39.44 ± 4.18 | 38.53 ± 3.91 | −0.92 | 0.001 |
| RA area (cm2) | 21.93 ± 3.15 | 21.99 ± 3.04 | +0.06 | 0.728 |
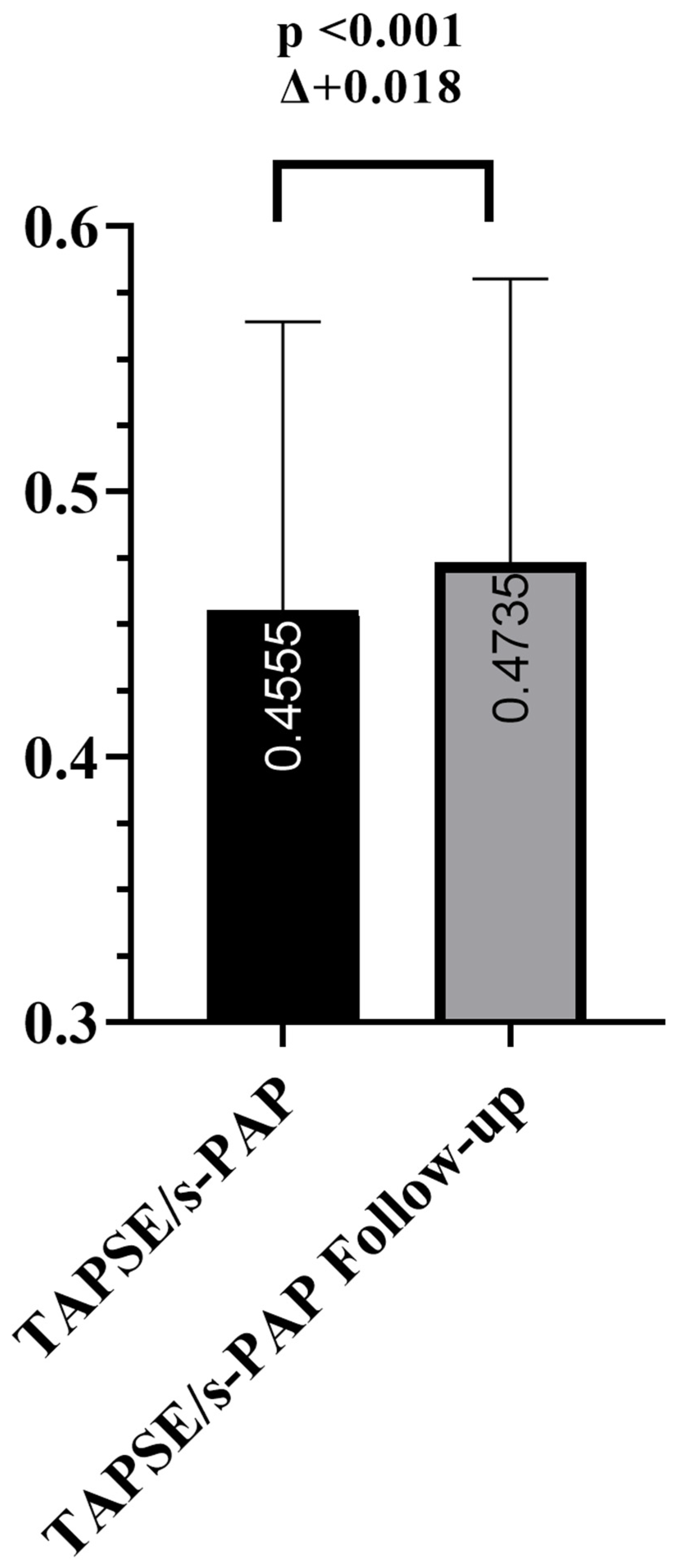
| TAPSE/s-PAP (mm/mmHg) | 0.455 ± 0.108 | 0.474 ± 0.107 | +0.018 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaydzhiev, P.; Velikova, T.; Davidkova, Y.; Voynova, G.; Borizanova, A.; Spasova, N.; Georgieva, N.; Ilieva, R.; Kinova, E.; Goudev, A. Effects of SGLT2 Inhibitors on Sleep Apnea Parameters and Cheyne–Stokes Respiration in Patients with Acute Decompensated Heart Failure: A Prospective Cohort Study. Biomedicines 2025, 13, 1474. https://doi.org/10.3390/biomedicines13061474
Kalaydzhiev P, Velikova T, Davidkova Y, Voynova G, Borizanova A, Spasova N, Georgieva N, Ilieva R, Kinova E, Goudev A. Effects of SGLT2 Inhibitors on Sleep Apnea Parameters and Cheyne–Stokes Respiration in Patients with Acute Decompensated Heart Failure: A Prospective Cohort Study. Biomedicines. 2025; 13(6):1474. https://doi.org/10.3390/biomedicines13061474
Chicago/Turabian StyleKalaydzhiev, Petar, Tsvetelina Velikova, Yanitsa Davidkova, Gergana Voynova, Angelina Borizanova, Natalia Spasova, Neli Georgieva, Radostina Ilieva, Elena Kinova, and Assen Goudev. 2025. "Effects of SGLT2 Inhibitors on Sleep Apnea Parameters and Cheyne–Stokes Respiration in Patients with Acute Decompensated Heart Failure: A Prospective Cohort Study" Biomedicines 13, no. 6: 1474. https://doi.org/10.3390/biomedicines13061474
APA StyleKalaydzhiev, P., Velikova, T., Davidkova, Y., Voynova, G., Borizanova, A., Spasova, N., Georgieva, N., Ilieva, R., Kinova, E., & Goudev, A. (2025). Effects of SGLT2 Inhibitors on Sleep Apnea Parameters and Cheyne–Stokes Respiration in Patients with Acute Decompensated Heart Failure: A Prospective Cohort Study. Biomedicines, 13(6), 1474. https://doi.org/10.3390/biomedicines13061474








