Neutrophil Spatiotemporal Regulatory Networks: Dual Roles in Tumor Growth Regulation and Metastasis
Abstract
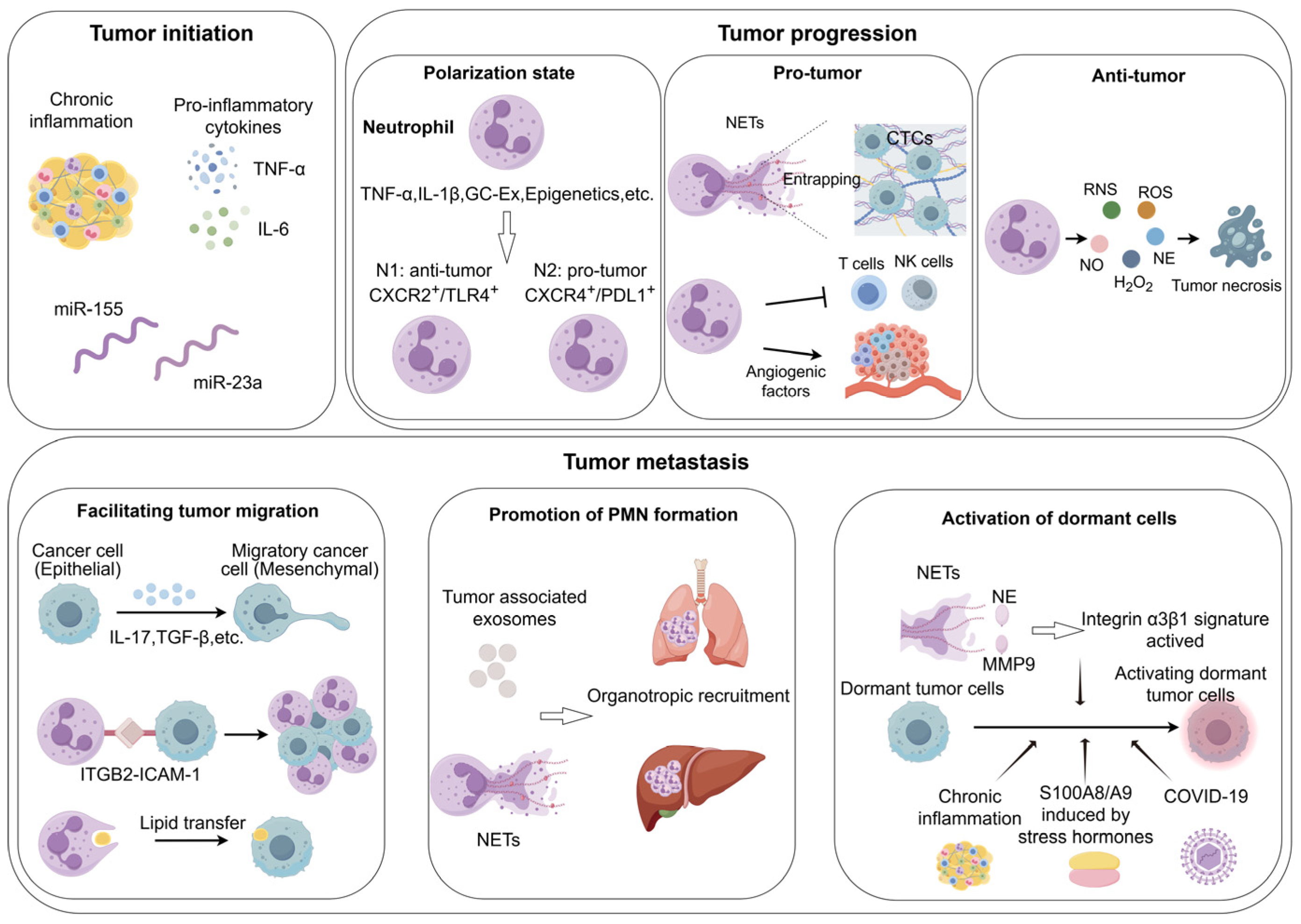
1. Introduction
2. Neutrophil Biology: Development, Homeostasis, and Circulation
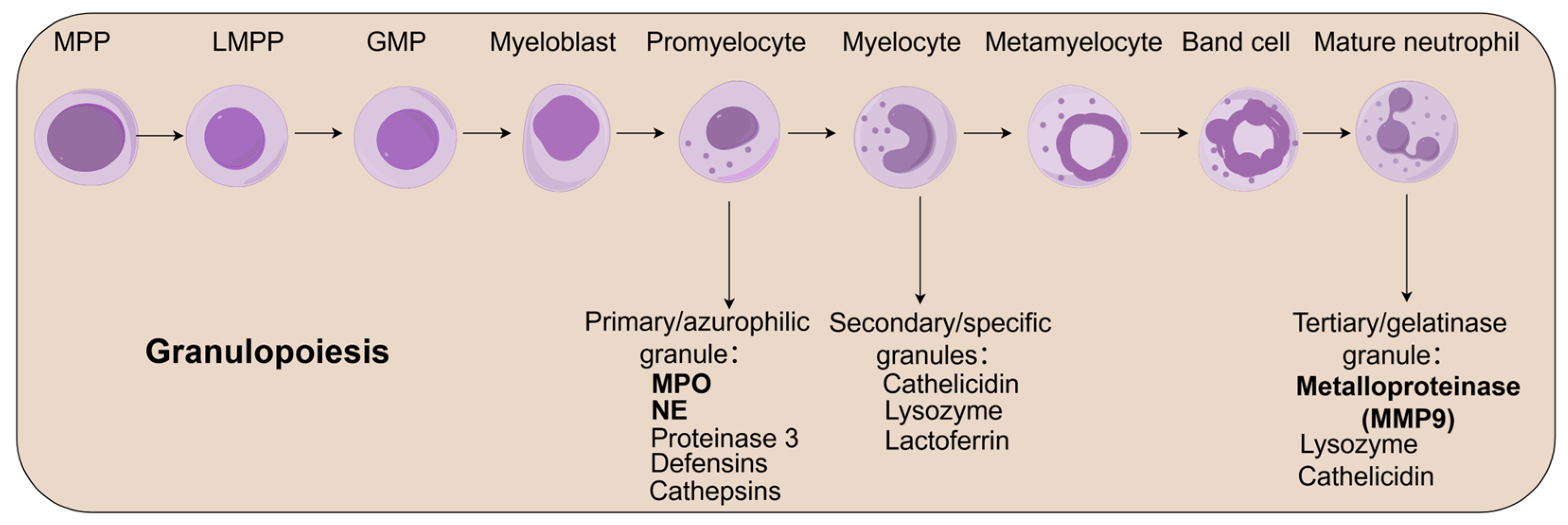
2.1. Neutrophil Development and Functional Maturation
2.2. Bone Marrow Reservoir and Regulatory Functions
2.3. Circulatory Dynamics and Homeostatic Regulation
3. Neutrophils as Key Mediators of Inflammation-to-Carcinogenesis
4. The Behavior of Neutrophils in the Tumor Progression and Tumor Metastasis
4.1. The Dualistic Behavior of Neutrophils in the Tumor Progression
4.1.1. Pro-Tumorigenic Roles of Neutrophils
Pro-Angiogenesis
NETs Formation
Immunosuppressive Microenvironment Remodeling
4.1.2. Anti-Tumorigenic Roles of Neutrophils
Oxidative Stress-Mediated Tumor Cell Killing
Protease-Dependent Selective Cytotoxicity
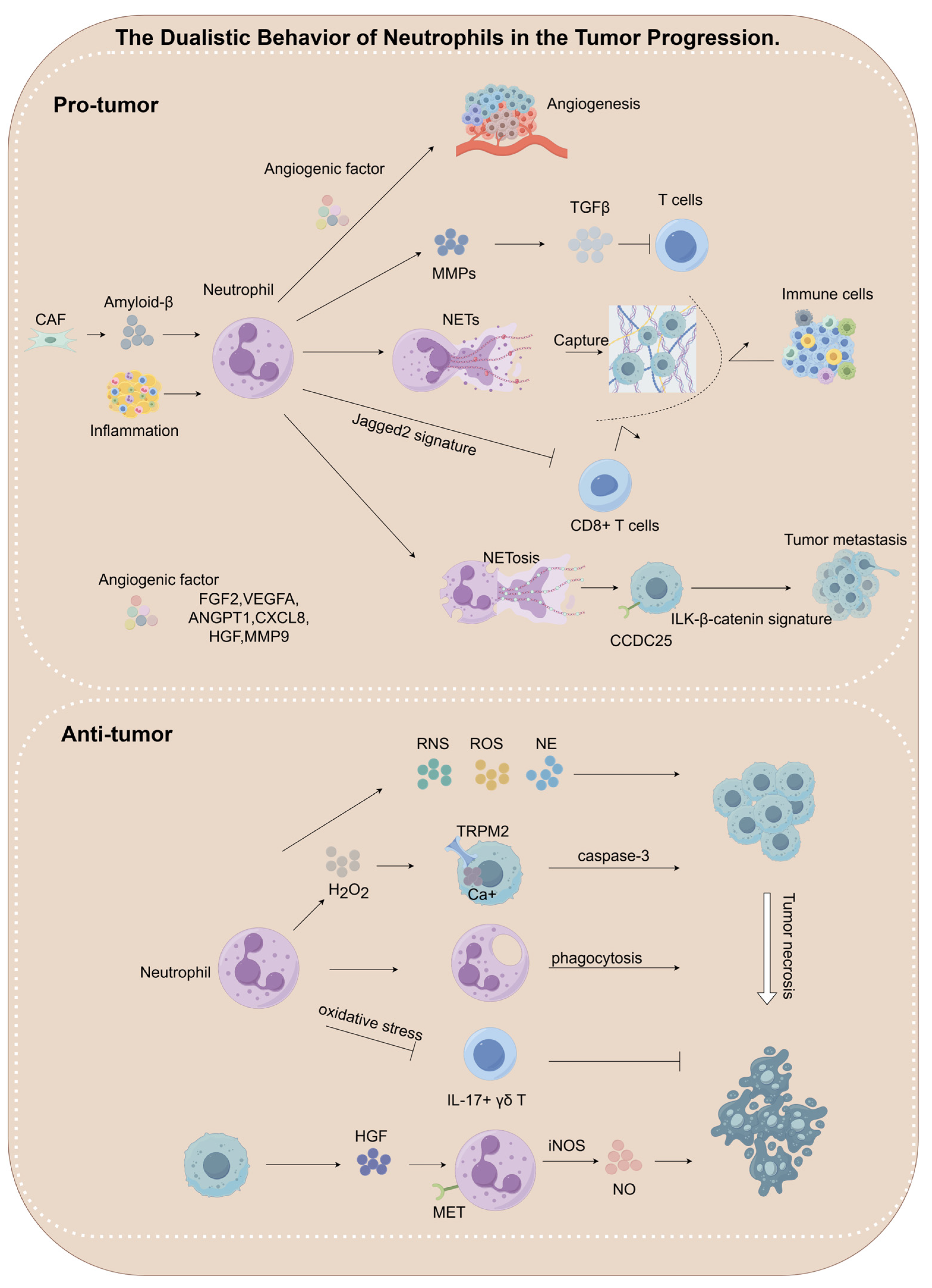
| Characteristics | Mechanisms | References |
|---|---|---|
| Anti-tumor | Neutrophils suppress the proliferation of IL-17+ γδ T cells by secreting ROS, thereby exerting anti-tumor effects in melanoma and hepatocellular carcinoma. | [138] |
| Neutrophils acquire anti-tumor phenotypes in breast cancer under the influence of ACE inhibitors and AGTR1 antagonists, thereby suppressing tumor growth. | [140] | |
| Neutrophils undergo N1-dominant reprogramming upon TGF-β blockade, resulting in effective tumor suppression across various cancers including breast, bile duct, colon, lung, and melanoma. | [141,142,143,144,145,146,147,148] | |
| Neutrophils secrete H2O2, which binds to the TRPM2 receptor on tumor cells, inducing Ca²+ influx and promoting tumor cell death through activation of the caspase-3 apoptosis signaling pathway in breast cancer. | [135,136] | |
| HGF/MET-dependent nitric oxide release by neutrophils promotes cancer cell killing in many tumors, including fibrosarcoma, colon cancer, lung cancer, melanoma, and hepatocellular carcinoma. | [137] | |
| Neutrophils inhibit metastatic seeding in the lungs by generating H2O2 in breast cancer, lung cancer, melanoma. | [30] | |
| Neutrophils release catalytically active NE, which hydrolyzes the CD95 death domain to selectively eliminate cancer cells in pan-cancers. | [139] | |
| Pro-tumor | Neutrophils suppress tumor-infiltrating T cells in colon cancer via MMP9-mediated activation of TGFβ in colon cancer. | [127] |
| Neutrophils contribute to skin carcinogenesis by releasing MMP9. | [118] | |
| CAFs secrete Amyloid β, which enhances the formation of NETs, thereby promoting tumor progression in melanoma, pancreatic cancer. | [121] | |
| Neutrophils promote angiogenesis through FGF2 secretion. | [107] | |
| Neutrophils promote angiogenesis through MMP9 secretion. | [36,108,117] | |
| IFN-β inhibits the production of VEGF and matrix MMP9 by neutrophils, consequently suppressing angiogenesis in melanoma. | [111] | |
| Neutrophils promote angiogenesis by releasing VEGF, HGF, and Angiopoietin-1. | [112,113,114,115,116] | |
| NETs promote tumor cell metastasis in esophageal, gastric, colon and lung cancer. | [120] | |
| Neutrophil-derived NETs engage the CCDC25 receptor on tumor cells, triggering ILK-β-parvin signaling and promoting metastasis in breast and colon cancer. | [122] | |
| NETs promote tumor cell metastasis in breast cancer. | [123] | |
| Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1 in melanoma. | [125] |
4.2. The Role of Neutrophils in Tumor Metastasis
4.2.1. Facilitating Tumor Migration
Mechanisms of EMT Regulation
Adhesive Support and Metastatic Niche Formation
Metabolic Reprogramming and Energy Supply Networks
4.2.2. Promotion of Pre-Metastatic Niche Formation
Theoretical Evolution of Pre-Metastatic Niches
Neutrophils as Central Drivers of PMN Formation
Exosome-Mediated Organotropic Programming
Regulatory Networks of NETs
4.2.3. Activation of Dormant Tumor Cells
Biological Features of Tumor Cell Dormancy
NET-Mediated Reactivation of Dormant Cells
The Regulatory Role of Secreted Protein
Stress Hormone-Driven Activation
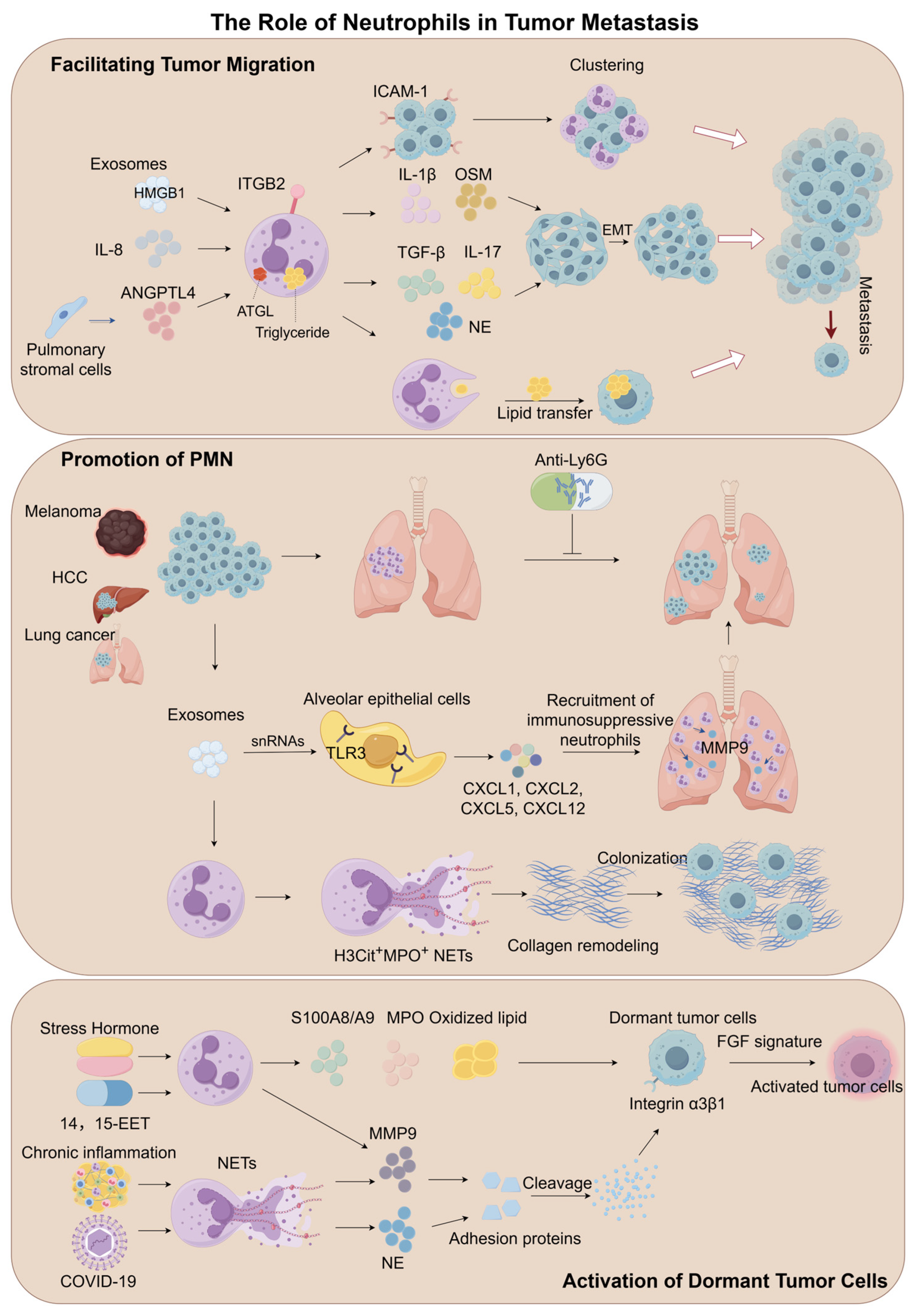
| Characteristics | Mechanisms | References |
|---|---|---|
| Facilitating tumor migration | TANs produce IL-17a, which promotes EMT of GC cells through JAK2/STAT3 signaling in gastric cancer. | [149] |
| Neutrophils secrete NE, which cleaves E-cadherin on tumor cell surfaces while inducing nuclear translocation of β-catenin and Zeb1, promoting tumor cell EMT in pancreatic ductal adenocarcinoma. | [150] | |
| GC-Ex activates neutrophils through the HMGB1/TLR4/NF-κB signaling pathway, thereby promoting tumor metastasis in gastric cancer. | [152] | |
| Clinical cohort analysis shows that the proportion of circulating tumor cell-neutrophil clusters in the peripheral blood of breast cancer patients is positively correlated with metastasis risk in breast cancer. | [153] | |
| ICAM-1 on melanoma cells and β2 integrin on neutrophils interacted, promoting anchoring to vascular endothelium in melanoma. | [154] | |
| The adhesion of lipopolysaccharide-activated neutrophils to cancer cells was mediated by neutrophil Mac-1/ICAM-1 in lung cancer. | [155] | |
| Upon contact with MC cells, neutrophils experience ATGL suppression, leading to intracellular lipid accumulation, which is transferred to tumor cells via the macropinocytosis-lysosome pathway, promoting tumor metastasis in breast cancer. | [156] | |
| Neutrophils accumulate in the pre-metastatic lung microenvironment and promote tumor cell colonization by secreting leukotrienes in breast cancer. | [160] | |
| Promotion of pre-metastatic niche formation | NET formation in rendering the PMN conducive for implantation of ovarian cancer cells, while PAD4 plays a critical role in NETs formation in ovarian cancer. | [124] |
| Chronic nicotine exposure induces neutrophil recruitment in the lung, where neutrophils release LCN2, promoting MET in tumor cells, thereby enhancing their colonization and metastatic potential in breast cancer. | [161] | |
| Lung epithelial cells are critical for initiating neutrophil recruitment and lung metastatic niche formation by sensing tumor exosomal RNAs via TLR3 in melanoma. | [164] | |
| Exosomes from highly metastatic melanoma increased the metastatic behavior of primary tumors by permanently “educating” bone marrow progenitors via the MET receptor in melanoma. | [162] | |
| Neutrophils operate to facilitate extravasation of tumor cells through the secretion of IL1β and matrix metalloproteinases in breast cancer and melanoma. | [165] | |
| Neutrophils promote breast cancer lung metastasis through the SIRT1-Naged-NETs axis in breast cancer. | [166] | |
| LMSCs promote neutrophil recruitment and NETs formation by secreting C3, thereby facilitating cancer cell metastasis to the lung in breast cancer. | [167] | |
| Dormancy activation | NE and MMP9 in NETs promote ECM remodeling, activating the integrin α3β1-FAK/ERK/MLCK/YAP signaling pathway to enhance dormant tumor cell proliferation in breast cancer. | [126] |
| 14,15-EET induces G-CSF/IL-6 production in vivo, enhancing STAT3 activation in neutrophils to promote MMP-9 expression and suppress TRAIL expression, with neutrophil-derived MMP-9 being essential for inducing angiogenesis in dormant micrometastases in melanoma. | [172] | |
| Stress hormones trigger release of S100A8/A9 proteins from neutrophils, which activate MPO, leading to oxidized lipid accumulation. These lipids, upon release, upregulate the fibroblast growth factor pathway in tumor cells, promoting their exit from dormancy and formation of new tumor lesions in lung cancer. | [173] |
5. Single-Cell and Spatial Omics Uncover Neutrophil Heterogeneity and Therapeutic Targets
6. Targeting Neutrophils in Cancer Therapy: Strategies and Approaches
6.1. Strategies to Inhibit TANs Recruitment
6.2. Regulation of Neutrophil-Derived Cytokine Release
6.3. Bispecific Antibodies Enhance Neutrophil-Mediated Antitumor Activity
6.4. Innovative Carriers for Tumor-Targeted Nanodrug Delivery
6.5. Neutrophil-Based Combination Therapy
6.5.1. Combination with Immune Checkpoint Inhibitors
6.5.2. Combination with Chemotherapy
6.5.3. Other Combination Strategies with Neutrophil Modulators
6.6. Neutrophil-Lymphocyte Ratio (NLR): A Critical Biomarker for Cancer Prognosis Evaluation
| Tumor Type | Mechanisms | References |
|---|---|---|
| Breast cancer | Promote tumor cell proliferation, intravascular infiltration, and distant metastasis, PMN formation, and awakening of dormant cancer cells. | [215,216,217,218,219] |
| Neuroblastoma | Play a key role in tumor cell proliferation, metastasis, and immune escape. | [220] |
| Oropharyngeal squamous cell carcinoma | In the initial stages of tumor formation, it may suppress tumorigenesis by clearing bacteria; after the tumor is established, it promotes tumor progression by releasing cytokines and chemokines. | [221] |
| Gastric cancer | Promote tumor progression (e.g., IL-8-induced NETs); facilitate EMT and metastasis through the PAI-1/TGF-β axis. | [222,223,224] |
| Colorectal cancer | Promote tumor progression and liver metastasis. | [225,226,227,228,229] |
| Esophageal squamous cell carcinoma | Promote tumor cell proliferation, migration, invasion, and angiogenesis; CCDC25 is associated with poor prognosis. | [230,231] |
| Osteosarcoma | High levels of NETs formation in diagnostic biopsies are associated with poor response to neoadjuvant chemotherapy and worse overall survival; NETs levels are higher in metastatic sites than in primary lesions. | [232,233] |
| Ovarian cancer | Promote the formation of the pre-metastatic niche in the peritoneum. | [234] |
| Pancreatic adenocarcinoma | Melatonin has been found to enhance anti-tumor immunity by regulating TANs infiltration and NETosis. | [208] |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 14,15-EET | 14,15-epoxyeicosatrienoic acid |
| ACEIs | angiotensin-converting enzyme inhibitors |
| AGTR1 | angiotensin II receptor type 1 |
| ANGPT1 | Angiopoietin 1 |
| ANGPTL4 | angiopoietin-like 4 |
| CAFs | cancer-associated fibroblasts |
| CTCs | circulating tumor cells |
| EMT | epithelial-mesenchymal transition |
| EP4 | prostaglandin E Receptor 4 |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| GC-Ex | gastric cancer cell-derived exosomes |
| GMP | granulocyte-monocyte progenitor |
| HDC+ | decarboxylase-positive |
| HSC | hematopoietic stem cell |
| ICAM-1 | intercellular adhesion molecule 1 |
| ITGB2 | integrin β2 |
| LMPPs | lymphoid-primed multipotent progenitors |
| MMP9 | matrix metalloproteinase 9 |
| MMPs | matrix metalloproteinases |
| MPO | myeloperoxidase |
| mtDNA | mitochondrial DNA |
| NE | neutrophil elastase |
| NETs | neutrophil extracellular traps |
| NO | nitric oxide |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OSM | Oncostatin M |
| PAMPs | pathogen-associated molecular patterns |
| PGE2 | prostaglandin E2 |
| PMN | pre-metastatic niche |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| S100A8/A9 | s100 calcium-binding protein A8/A9 |
| TAMs | tumor-associated macrophages |
| TANs | tumor-associated neutrophils |
| TDEs | Tumor-derived exosomes |
| THPO | Thrombopoietin |
| TLR | Toll-like receptor |
| TME | tumor microenvironment |
| VCAM1 | Vascular cell adhesion molecule 1 |
| VLA-4 | Very late antigen 4 |
References
- Coffelt, S.B.; Wellenstein, M.D.; De Visser, K.E. Neutrophils in Cancer: Neutral No More. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Ng, L.G. Granulopoiesis and Neutrophil Homeostasis: A Metabolic, Daily Balancing Act. Trends Immunol. 2019, 40, 598–612. [Google Scholar] [CrossRef]
- Thanabalasuriar, A.; Scott, B.N.V.; Peiseler, M.; Willson, M.E.; Zeng, Z.; Warrener, P.; Keller, A.E.; Surewaard, B.G.J.; Dozier, E.A.; Korhonen, J.T.; et al. Neutrophil Extracellular Traps Confine Pseudomonas Aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 2019, 25, 526–536.e4. [Google Scholar] [CrossRef]
- Liu, X.; Shin, S. Listening In: Plasmacytoid DC, Monocyte-Derived DC, and Neutrophil Crosstalk in Antifungal Defense. Cell Host Microbe 2020, 28, 9–11. [Google Scholar] [CrossRef]
- Iversen, M.B.; Reinert, L.S.; Thomsen, M.K.; Bagdonaite, I.; Nandakumar, R.; Cheshenko, N.; Prabakaran, T.; Vakhrushev, S.Y.; Krzyzowska, M.; Kratholm, S.K.; et al. An Innate Antiviral Pathway Acting before Interferons at Epithelial Surfaces. Nat. Immunol. 2016, 17, 150–158. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in Modulating Acute and Chronic Inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal Neutrophil Polarization Following Myocardial Infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef]
- Mistry, P.; Nakabo, S.; O’Neil, L.; Goel, R.R.; Jiang, K.; Carmona-Rivera, C.; Gupta, S.; Chan, D.W.; Carlucci, P.M.; Wang, X.; et al. Transcriptomic, Epigenetic, and Functional Analyses Implicate Neutrophil Diversity in the Pathogenesis of Systemic Lupus Erythematosus. Proc. Natl. Acad. Sci. USA 2019, 116, 25222–25228. [Google Scholar] [CrossRef]
- Kuley, R.; Duvvuri, B.; Hasnain, S.; Dow, E.R.; Koch, A.E.; Higgs, R.E.; Krishnan, V.; Lood, C. Neutrophil Activation Markers and Rheumatoid Arthritis Treatment Response to the JAK1/2 Inhibitor Baricitinib. Arthritis Rheumatol. 2025, 77, 395–404. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Qu, M.; Li, W.; Wu, D.; Cata, J.P.; Miao, C. Neutrophil, Neutrophil Extracellular Traps and Endothelial Cell Dysfunction in Sepsis. Clin. Transl. Med. 2023, 13, e1170. [Google Scholar] [CrossRef] [PubMed]
- Herster, F.; Bittner, Z.; Archer, N.K.; Dickhöfer, S.; Eisel, D.; Eigenbrod, T.; Knorpp, T.; Schneiderhan-Marra, N.; Löffler, M.W.; Kalbacher, H.; et al. Neutrophil Extracellular Trap-Associated RNA and LL37 Enable Self-Amplifying Inflammation in Psoriasis. Nat. Commun. 2020, 11, 105. [Google Scholar] [CrossRef]
- Márquez-Coello, M.; Ruiz-Sánchez, C.; Martín-Aspas, A.; Fernández Gutiérrez Del Álamo, C.; Illanes-Álvarez, F.; Cuesta-Sancho, S.; Girón-González, J.-A. Neutrophil Expression of T and B Immunomodulatory Molecules in HIV Infection. Front. Immunol. 2021, 12, 670966. [Google Scholar] [CrossRef]
- Jia, M.; Fu, H.; Jiang, X.; Wang, L.; Xu, J.; Barnes, P.J.; Adcock, I.M.; Liu, Y.; He, S.; Zhang, F.; et al. DEL-1, as an Anti-neutrophil Transepithelial Migration Molecule, Inhibits Airway Neutrophilic Inflammation in Asthma. Allergy 2024, 79, 1180–1194. [Google Scholar] [CrossRef]
- Nakazawa, D.; Masuda, S.; Tomaru, U.; Ishizu, A. Pathogenesis and Therapeutic Interventions for ANCA-Associated Vasculitis. Nat. Rev. Rheumatol. 2019, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Ren, B.; Li, C.; Li, Q.; Kan, S.; Wang, X.; Bai, W.; Wu, C.; Kassegne, K.; Yan, H.; et al. PRL2 Regulates Neutrophil Extracellular Trap Formation Which Contributes to Severe Malaria and Acute Lung Injury. Nat. Commun. 2024, 15, 881. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and Adaptive Immune Cells in the Tumor Microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Treffers, L.W.; Hiemstra, I.H.; Kuijpers, T.W.; Van Den Berg, T.K.; Matlung, H.L. Neutrophils in Cancer. Immunol. Rev. 2016, 273, 312–328. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-Associated Neutrophils as a New Prognostic Factor in Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef]
- Carus, A.; Ladekarl, M.; Hager, H.; Nedergaard, B.S.; Donskov, F. Tumour-Associated CD66b+ Neutrophil Count Is an Independent Prognostic Factor for Recurrence in Localised Cervical Cancer. Br. J. Cancer 2013, 108, 2116–2122. [Google Scholar] [CrossRef]
- Rao, H.-L.; Chen, J.-W.; Li, M.; Xiao, Y.-B.; Fu, J.; Zeng, Y.-X.; Cai, M.-Y.; Xie, D. Increased Intratumoral Neutrophil in Colorectal Carcinomas Correlates Closely with Malignant Phenotype and Predicts Patients’ Adverse Prognosis. PLoS ONE 2012, 7, e30806. [Google Scholar] [CrossRef]
- Kargl, J.; Busch, S.E.; Yang, G.H.Y.; Kim, K.-H.; Hanke, M.L.; Metz, H.E.; Hubbard, J.J.; Lee, S.M.; Madtes, D.K.; McIntosh, M.W.; et al. Neutrophils Dominate the Immune Cell Composition in Non-Small Cell Lung Cancer. Nat. Commun. 2017, 8, 14381. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.B.G.; Coffelt, S.B.; Carlin, L.M. Neutrophil Maturity in Cancer. Front. Immunol. 2019, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.; Galbraith, M.A.; Ellber, R. Selective Reduction of Human Tumor Cell Populations by Human Granulocytesin Vitro. Cancer Res. 1978, 38, 4534–4539. [Google Scholar] [PubMed]
- Gerrard, T.L.; Kaplan, A.M. Human Neutrophil-Mediated Cytotoxicity to Tumor Cells. J. Natl. Cancer Inst. 1981, 66, 483–488. [Google Scholar] [PubMed]
- Cameron, D.J. A Comparison of the Cytotoxic Potential in Polymorphonuclear Leukocytes Obtained from Normal Donors and Cancer Patients. Clin. Immunol. Immunopathol. 1983, 28, 115–124. [Google Scholar] [CrossRef]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor Entrained Neutrophils Inhibit Seeding in the Premetastatic Lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Mantovani, A.; Marone, G. Roles of Neutrophils in Cancer Growth and Progression. J. Leukoc. Biol. 2018, 103, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Neutrophils as Active Regulators of the Immune System in the Tumor Microenvironment. J. Leukoc. Biol. 2017, 102, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Donskov, F. Immunomonitoring and Prognostic Relevance of Neutrophils in Clinical Trials. Semin. Cancer Biol. 2013, 23, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Bianchi, P.; Grizzi, F.; Di Caro, G.; Basso, G.; Ponzetta, A.; Bonavita, E.; Barbagallo, M.; Tartari, S.; Polentarutti, N.; et al. Occurrence and Significance of Tumor-associated Neutrophils in Patients with Colorectal Cancer. Int. J. Cancer 2016, 139, 446–456. [Google Scholar] [CrossRef]
- Jensen, H.K.; Donskov, F.; Marcussen, N.; Nordsmark, M.; Lundbeck, F.; Von Der Maase, H. Presence of Intratumoral Neutrophils Is an Independent Prognostic Factor in Localized Renal Cell Carcinoma. JCO 2009, 27, 4709–4717. [Google Scholar] [CrossRef]
- Kuang, D.-M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.-Y.; Zheng, L. Peritumoral Neutrophils Link Inflammatory Response to Disease Progression by Fostering Angiogenesis in Hepatocellular Carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef]
- Trellakis, S.; Bruderek, K.; Dumitru, C.A.; Gholaman, H.; Gu, X.; Bankfalvi, A.; Scherag, A.; Hütte, J.; Dominas, N.; Lehnerdt, G.F.; et al. Polymorphonuclear Granulocytes in Human Head and Neck Cancer: Enhanced Inflammatory Activity, Modulation by Cancer Cells and Expansion in Advanced Disease. Int. J. Cancer 2011, 129, 2183–2193. [Google Scholar] [CrossRef]
- Wislez, M.; Rabbe, N.; Marchal, J.; Milleron, B.; Crestani, B.; Mayaud, C.; Antoine, M.; Soler, P.; Cadranel, J. Hepatocyte Growth Factor Production by Neutrophils Infiltrating Bronchioloalveolar Subtype Pulmonary Adenocarcinoma: Role in Tumor Progression and Death. Cancer Res. 2003, 63, 1405–1412. [Google Scholar] [PubMed]
- Mollinedo, F.; Borregaard, N.; Boxer, L.A. Novel Trends in Neutrophil Structure, Function and Development. Immunol. Today 1999, 20, 535–537. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Bjerregaard, M.D.; Jurlander, J.; Klausen, P.; Borregaard, N.; Cowland, J.B. The in Vivo Profile of Transcription Factors during Neutrophil Differentiation in Human Bone Marrow. Blood 2003, 101, 4322–4332. [Google Scholar] [CrossRef] [PubMed]
- Görgens, A.; Radtke, S.; Möllmann, M.; Cross, M.; Dürig, J.; Horn, P.A.; Giebel, B. Revision of the Human Hematopoietic Tree: Granulocyte Subtypes Derive from Distinct Hematopoietic Lineages. Cell Rep. 2013, 3, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D. Transcriptional Control of Granulocyte and Monocyte Development. Oncogene 2007, 26, 6816–6828. [Google Scholar] [CrossRef]
- Fiedler, K.; Brunner, C. The Role of Transcription Factors in the Guidance of Granulopoiesis. Am. J. Blood Res. 2012, 2, 57–65. [Google Scholar] [PubMed]
- Rosenbauer, F.; Tenen, D.G. Transcription Factors in Myeloid Development: Balancing Differentiation with Transformation. Nat. Rev. Immunol. 2007, 7, 105–117. [Google Scholar] [CrossRef]
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune Suppression by Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells: Similarities and Differences. Cell. Mol. Life Sci. 2013, 70, 3813–3827. [Google Scholar] [CrossRef]
- Manz, M.G.; Boettcher, S. Emergency Granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Häger, M.; Cowland, J.B.; Borregaard, N. Neutrophil Granules in Health and Disease. J. Intern. Med. 2010, 268, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Sørensen, O.E.; Theilgaard-Mönch, K. Neutrophil Granules: A Library of Innate Immunity Proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef]
- Dancey, J.T.; Deubelbeiss, K.A.; Harker, L.A.; Finch, C.A. Neutrophil Kinetics in Man. J. Clin. Invest. 1976, 58, 705–715. [Google Scholar] [CrossRef]
- Saverymuttu, S.H.; Peters, A.M.; Keshavarzian, A.; Reavy, H.J.; Lavender, J.P. The Kinetics of111 Indium Distribution Following Injection of111 Indium Labelled Autologous Granulocytes in Man. Br. J. Haematol. 1985, 61, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hodgson, G.; Katz, M.; Dunn, A.R. Evaluation of Role of G-CSF in the Production, Survival, and Release of Neutrophils from Bone Marrow into Circulation. Blood 2002, 100, 854–861. [Google Scholar] [CrossRef]
- Santos-Beneit, A.M.; Mollinedo, F. Expression of Genes Involved in Initiation, Regulation, and Execution of Apoptosis in Human Neutrophils and during Neutrophil Differentiation of HL-60 Cells. J. Leukoc. Biol. 2000, 67, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Craddock, C.G.; Perry, S.; Ventzke, L.E.; Lawrence, J.S.; Baker, M.H.; Paul, G. Evaluation of Marrow Granulocytic Reserves in Normal and Disease States. Blood 1960, 15, 840–855. [Google Scholar] [CrossRef]
- Donohue, D.M.; Reiff, R.H.; Hanson, M.L.; Betson, Y.; Finch, C.A. Quantitative Measurement of the Erythrocytic and Granulocytic Cells of the Marrow and Blood1. J. Clin. Invest. 1958, 37, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Weinstein, I.M.; Craddock, C.G.; Lawrence, J.S.; Paul, G.; Baker, M.H.; Green, J.; Cianko, G. The Combined Use of Typhoid Vaccine and P32 Labeling to Assess Myelopoiesis. Blood 1957, 12, 549–558. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic Modulation of the Hematopoietic Niche through Neutrophil Clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Martin, C.; Burdon, P.C.E.; Bridger, G.; Gutierrez-Ramos, J.-C.; Williams, T.J.; Rankin, S.M. Chemokines Acting via CXCR2 and CXCR4 Control the Release of Neutrophils from the Bone Marrow and Their Return Following Senescence. Immunity 2003, 19, 583–593. [Google Scholar] [CrossRef]
- Bowers, E.; Slaughter, A.; Frenette, P.S.; Kuick, R.; Pello, O.M.; Lucas, D. Granulocyte-Derived TNFα Promotes Vascular and Hematopoietic Regeneration in the Bone Marrow. Nat. Med. 2018, 24, 95–102. [Google Scholar] [CrossRef]
- Kawano, Y.; Fukui, C.; Shinohara, M.; Wakahashi, K.; Ishii, S.; Suzuki, T.; Sato, M.; Asada, N.; Kawano, H.; Minagawa, K.; et al. G-CSF-Induced Sympathetic Tone Provokes Fever and Primes Antimobilizing Functions of Neutrophils via PGE2. Blood 2017, 129, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Deng, H.; Churchill, M.J.; Luchsinger, L.L.; Du, X.; Chu, T.H.; Friedman, R.A.; Middelhoff, M.; Ding, H.; Tailor, Y.H.; et al. Bone Marrow Myeloid Cells Regulate Myeloid-Biased Hematopoietic Stem Cells via a Histamine-Dependent Feedback Loop. Cell Stem Cell 2017, 21, 747–760.e7. [Google Scholar] [CrossRef]
- Ma, Q.; Jones, D.; Springer, T.A. The Chemokine Receptor CXCR4 Is Required for the Retention of B Lineage and Granulocytic Precursors within the Bone Marrow Microenvironment. Immunity 1999, 10, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Suratt, B.T.; Petty, J.M.; Young, S.K.; Malcolm, K.C.; Lieber, J.G.; Nick, J.A.; Gonzalo, J.-A.; Henson, P.M.; Worthen, G.S. Role of the CXCR4/SDF-1 Chemokine Axis in Circulating Neutrophil Homeostasis. Blood 2004, 104, 565–571. [Google Scholar] [CrossRef]
- Eash, K.J.; Means, J.M.; White, D.W.; Link, D.C. CXCR4 Is a Key Regulator of Neutrophil Release from the Bone Marrow under Basal and Stress Granulopoiesis Conditions. Blood 2009, 113, 4711–4719. [Google Scholar] [CrossRef]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 Antagonistically Regulate Neutrophil Trafficking from Murine Bone Marrow. J. Clin. Invest. 2010, 120, 2423–2431. [Google Scholar] [CrossRef]
- Köhler, A.; De Filippo, K.; Hasenberg, M.; Van Den Brandt, C.; Nye, E.; Hosking, M.P.; Lane, T.E.; Männ, L.; Ransohoff, R.M.; Hauser, A.E.; et al. G-CSF–Mediated Thrombopoietin Release Triggers Neutrophil Motility and Mobilization from Bone Marrow via Induction of Cxcr2 Ligands. Blood 2011, 117, 4349–4357. [Google Scholar] [CrossRef]
- Levesque, J.-P.; Liu, F.; Simmons, P.J.; Betsuyaku, T.; Senior, R.M.; Pham, C.; Link, D.C. Characterization of Hematopoietic Progenitor Mobilization in Protease-Deficient Mice. Blood 2004, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Burdon, P.C.E.; Martin, C.; Rankin, S.M. The CXC Chemokine MIP-2 Stimulates Neutrophil Mobilization from the Rat Bone Marrow in a CD49d-Dependent Manner. Blood 2005, 105, 2543–2548. [Google Scholar] [CrossRef]
- Petty, J.M.; Lenox, C.C.; Weiss, D.J.; Poynter, M.E.; Suratt, B.T. Crosstalk between CXCR4/Stromal Derived Factor-1 and VLA-4/VCAM-1 Pathways Regulates Neutrophil Retention in the Bone Marrow. J. Immunol. 2009, 182, 604–612. [Google Scholar] [CrossRef]
- Kim, H.K.; De La Luz Sierra, M.; Williams, C.K.; Gulino, A.V.; Tosato, G. G-CSF down-Regulation of CXCR4 Expression Identified as a Mechanism for Mobilization of Myeloid Cells. Blood 2006, 108, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Szyper-Kravitz, M.; Nagler, A.; Lahav, M.; Peled, A.; Habler, L.; Ponomaryov, T.; Taichman, R.S.; Arenzana-Seisdedos, F.; Fujii, N.; et al. G-CSF Induces Stem Cell Mobilization by Decreasing Bone Marrow SDF-1 and up-Regulating CXCR4. Nat. Immunol. 2002, 3, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Semerad, C.L.; Christopher, M.J.; Liu, F.; Short, B.; Simmons, P.J.; Winkler, I.; Levesque, J.-P.; Chappel, J.; Ross, F.P.; Link, D.C. G-CSF Potently Inhibits Osteoblast Activity and CXCL12 mRNA Expression in the Bone Marrow. Blood 2005, 106, 3020–3027. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Nicolás-Ávila, J.A.; Li, J.L.; García-Silva, S.; Balachander, A.; Rubio-Ponce, A.; Weiss, L.A.; Adrover, J.M.; Burrows, K.; A-González, N.; et al. Neutrophils Instruct Homeostatic and Pathological States in Naive Tissues. J. Exp. Med. 2018, 215, 2778–2795. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.-E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic Nerves Govern Circadian Leukocyte Recruitment to Tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef]
- Adrover, J.M.; Nicolás-Ávila, J.A.; Hidalgo, A. Aging: A Temporal Dimension for Neutrophils. Trends Immunol. 2016, 37, 334–345. [Google Scholar] [CrossRef]
- Semerad, C.L.; Liu, F.; Gregory, A.D.; Stumpf, K.; Link, D.C. G-CSF Is an Essential Regulator of Neutrophil Trafficking from the Bone Marrow to the Blood. Immunity 2002, 17, 413–423. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-Producing Cells: The Sentinels of the Immune System. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 Immune Axis: From Mechanisms to Therapeutic Testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Schwarzenberger, P.; Huang, W.; Ye, P.; Oliver, P.; Manuel, M.; Zhang, Z.; Bagby, G.; Nelson, S.; Kolls, J.K. Requirement of Endogenous Stem Cell Factor and Granulocyte-Colony-Stimulating Factor for IL-17-Mediated Granulopoiesis. J. Immunol. 2000, 164, 4783–4789. [Google Scholar] [CrossRef]
- Forlow, S.B.; Schurr, J.R.; Kolls, J.K.; Bagby, G.J.; Schwarzenberger, P.O.; Ley, K. Increased Granulopoiesis through Interleukin-17 and Granulocyte Colony-Stimulating Factor in Leukocyte Adhesion Molecule–Deficient Mice. Blood 2001, 98, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.A.; Huo, Y.; Burcin, T.L.; Morris, M.A.; Olson, T.S.; Ley, K. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 2005, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Gordy, C.; Pua, H.; Sempowski, G.D.; He, Y.-W. Regulation of Steady-State Neutrophil Homeostasis by Macrophages. Blood 2011, 117, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Tittel, A.P.; Heuser, C.; Ohliger, C.; Llanto, C.; Yona, S.; Hämmerling, G.J.; Engel, D.R.; Garbi, N.; Kurts, C. Functionally Relevant Neutrophilia in CD11c Diphtheria Toxin Receptor Transgenic Mice. Nat. Methods 2012, 9, 385–390. [Google Scholar] [CrossRef]
- Jiao, J.; Dragomir, A.-C.; Kocabayoglu, P.; Rahman, A.H.; Chow, A.; Hashimoto, D.; Leboeuf, M.; Kraus, T.; Moran, T.; Carrasco-Avino, G.; et al. Central Role of Conventional Dendritic Cells in Regulation of Bone Marrow Release and Survival of Neutrophils. J. Immunol. 2014, 192, 3374–3382. [Google Scholar] [CrossRef]
- Mei, J.; Liu, Y.; Dai, N.; Hoffmann, C.; Hudock, K.M.; Zhang, P.; Guttentag, S.H.; Kolls, J.K.; Oliver, P.M.; Bushman, F.D.; et al. Cxcr2 and Cxcl5 Regulate the IL-17/G-CSF Axis and Neutrophil Homeostasis in Mice. J. Clin. Invest. 2012, 122, 974–986. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The Microbiota Regulates Neutrophil Homeostasis and Host Resistance to Escherichia Coli K1 Sepsis in Neonatal Mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef]
- Ueda, Y.; Cain, D.W.; Kuraoka, M.; Kondo, M.; Kelsoe, G. IL-1R Type I-Dependent Hemopoietic Stem Cell Proliferation Is Necessary for Inflammatory Granulopoiesis and Reactive Neutrophilia. J. Immunol. 2009, 182, 6477–6484. [Google Scholar] [CrossRef]
- Mankan, A.K.; Canli, O.; Schwitalla, S.; Ziegler, P.; Tschopp, J.; Korn, T.; Greten, F.R. TNF-α–Dependent Loss of IKKβ-Deficient Myeloid Progenitors Triggers a Cytokine Loop Culminating in Granulocytosis. Proc. Natl. Acad. Sci. USA 2011, 108, 6567–6572. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From Inflammation to Cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A. The Inflammation—Cancer Connection. FEBS J. 2018, 285, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory Networks Underlying Colorectal Cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-Induced Cancer: Crosstalk between Tumours, Immune Cells and Microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory Bowel Disease and Cancer: The Role of Inflammation, Immunosuppression, and Cancer Treatment. WJG 2016, 22, 4794. [Google Scholar] [CrossRef]
- Tu, T.; Bühler, S.; Bartenschlager, R. Chronic Viral Hepatitis and Its Association with Liver Cancer. Biol. Chem. 2017, 398, 817–837. [Google Scholar] [CrossRef]
- Naumann, M.; Sokolova, O.; Tegtmeyer, N.; Backert, S. Helicobacter Pylori: A Paradigm Pathogen for Subverting Host Cell Signal Transmission. Trends Microbiol. 2017, 25, 316–328. [Google Scholar] [CrossRef]
- Pastille, E.; Frede, A.; McSorley, H.J.; Gräb, J.; Adamczyk, A.; Kollenda, S.; Hansen, W.; Epple, M.; Buer, J.; Maizels, R.M.; et al. Intestinal Helminth Infection Drives Carcinogenesis in Colitis-Associated Colon Cancer. PLoS Pathog. 2017, 13, e1006649. [Google Scholar] [CrossRef]
- Casper, C.; Fitzmaurice, C. Infection-Related Cancers: Prioritising an Important and Eliminable Contributor to the Global Cancer Burden. Lancet Glob. Health 2016, 4, e580–e581. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-Induced Genomic Instability Impedes Resolution of Inflammation and Wound Healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef]
- Bui, T.M.; Butin-Israeli, V.; Wiesolek, H.L.; Zhou, M.; Rehring, J.F.; Wiesmüller, L.; Wu, J.D.; Yang, G.-Y.; Hanauer, S.B.; Sebag, J.A.; et al. Neutrophils Alter DNA Repair Landscape to Impact Survival and Shape Distinct Therapeutic Phenotypes of Colorectal Cancer. Gastroenterology 2021, 161, 225–238.e15. [Google Scholar] [CrossRef]
- Roessner, A.; Kuester, D.; Malfertheiner, P.; Schneider-Stock, R. Oxidative Stress in Ulcerative Colitis-Associated Carcinogenesis. Pathol.-Res. Pract. 2008, 204, 511–524. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Chen, Z.; Wei, F.; Zhou, Q.; Li, P.; Gu, Q. Reactive Oxygen Species and Gastric Carcinogenesis: The Complex Interaction between Helicobacter Pylori and Host. Helicobacter 2023, 28, e13024. [Google Scholar] [CrossRef]
- Poto, R.; Cristinziano, L.; Modestino, L.; De Paulis, A.; Marone, G.; Loffredo, S.; Galdiero, M.R.; Varricchi, G. Neutrophil Extracellular Traps, Angiogenesis and Cancer. Biomedicines 2022, 10, 431. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Raftopoulou, S.; Valadez-Cosmes, P.; Mihalic, Z.N.; Schicho, R.; Kargl, J. Tumor-Mediated Neutrophil Polarization and Therapeutic Implications. IJMS 2022, 23, 3218. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.N.; Lim, S.Y.; Yuzhalin, A.E.; Jones, K.; Markelc, B.; Kim, K.J.; Buzzelli, J.N.; Fokas, E.; Cao, Y.; Smart, S.; et al. Neutrophils Promote Hepatic Metastasis Growth through Fibroblast Growth Factor 2–Dependent Angiogenesis in Mice. Hepatology 2017, 65, 1920–1935. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-Infiltrating Neutrophils Constitute the Major In Vivo Source of Angiogenesis-Inducing MMP-9 in the Tumor Microenvironment. Neoplasia 2014, 16, 771–788. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-Derived Cytokines Involved in Physiological and Pathological Angiogenesis. In Chemical Immunology and Allergy; Marone, G., Granata, F., Eds.; Karger Publishers: Basel, Switzerland, 2014; Volume 99, pp. 123–137. ISBN 978-3-318-02480-7. [Google Scholar]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatella, M.A.; et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front. Immunol. 2017, 8, 443. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils Responsive to Endogenous IFN-β Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J. Clin. Invest. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Webb, N.J.A.; Myers, C.R.; Watson, C.J.; Bottomley, M.J.; Brenchley, P.E.C. Activated human neutrophils express vascular endothelial growth factor (VEGF). Cytokine 1998, 10, 254–257. [Google Scholar] [CrossRef]
- Grenier, A.; Chollet-Martin, S.; Crestani, B.; Delarche, C.; El Benna, J.; Boutten, A.; Andrieu, V.; Durand, G.; Gougerot-Pocidalo, M.-A.; Aubier, M.; et al. Presence of a Mobilizable Intracellular Pool of Hepatocyte Growth Factor in Human Polymorphonuclear Neutrophils. Blood 2002, 99, 2997–3004. [Google Scholar] [CrossRef]
- Scapini, P.; Morini, M.; Tecchio, C.; Minghelli, S.; Di Carlo, E.; Tanghetti, E.; Albini, A.; Lowell, C.; Berton, G.; Noonan, D.M.; et al. CXCL1/Macrophage Inflammatory Protein-2-Induced Angiogenesis In Vivo Is Mediated by Neutrophil-Derived Vascular Endothelial Growth Factor-A. J. Immunol. 2004, 172, 5034–5040. [Google Scholar] [CrossRef]
- Gaudry, M.; Brégerie, O.; Andrieu, V.; El Benna, J.; Pocidalo, M.-A.; Hakim, J. Intracellular Pool of Vascular Endothelial Growth Factor in Human Neutrophils. Blood 1997, 90, 4153–4161. [Google Scholar] [CrossRef]
- Neagoe, P.-E.; Brkovic, A.; Hajjar, F.; Sirois, M.G. Expression and Release of Angiopoietin-1 from Human Neutrophils: Intracellular Mechanisms. Growth Factors 2009, 27, 335–344. [Google Scholar] [CrossRef]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human Neutrophils Uniquely Release TIMP-Free MMP-9 to Provide a Potent Catalytic Stimulator of Angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 Supplied by Bone Marrow–Derived Cells Contributes to Skin Carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, L.; Peng, Y.; Tao, M.; Li, L.; Xiu, D.; Yuan, C.; Ma, Z.; Jiang, B. Neutrophils Assist the Metastasis of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma: A New Hypothesis and a New Predictor for Distant Metastasis. Medicine 2016, 95, e4932. [Google Scholar] [CrossRef]
- Rayes, R.F.; Mouhanna, J.G.; Nicolau, I.; Bourdeau, F.; Giannias, B.; Rousseau, S.; Quail, D.; Walsh, L.; Sangwan, V.; Bertos, N.; et al. Primary Tumors Induce Neutrophil Extracellular Traps with Targetable Metastasis-Promoting Effects. JCI Insight 2019, 4, e128008. [Google Scholar] [CrossRef]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-Driven and Amyloid β-Dependent Induction of Neutrophil Extracellular Traps Modulates Tumor Growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils Facilitate Ovarian Cancer Premetastatic Niche Formation in the Omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- El Rayes, T.; Catena, R.; Lee, S.; Stawowczyk, M.; Joshi, N.; Fischbach, C.; Powell, C.A.; Dannenberg, A.J.; Altorki, N.K.; Gao, D.; et al. Lung Inflammation Promotes Metastasis through Neutrophil Protease-Mediated Degradation of Tsp-1. Proc. Natl. Acad. Sci. USA 2015, 112, 16000–16005. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil Extracellular Traps Produced during Inflammation Awaken Dormant Cancer Cells in Mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef]
- Germann, M.; Zangger, N.; Sauvain, M.; Sempoux, C.; Bowler, A.D.; Wirapati, P.; Kandalaft, L.E.; Delorenzi, M.; Tejpar, S.; Coukos, G.; et al. Neutrophils Suppress Tumor-infiltrating T Cells in Colon Cancer via Matrix Metalloproteinase-mediated Activation of TGF β. EMBO Mol. Med. 2020, 12, e10681. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, G.; Wang, Y.; He, M.; Xu, Q.; Lu, J.; Liu, H.; Xu, C. Tumour-Associated Neutrophils Orchestrate Intratumoural IL-8-Driven Immune Evasion through Jagged2 Activation in Ovarian Cancer. Br. J. Cancer 2020, 123, 1404–1416. [Google Scholar] [CrossRef]
- Domnich, M.; Pylaeva, E.; Siakaeva, E.; Kabankova, N.; Bedzinska, A.; Sojka, D.; Zebrowska, A.; Gawin, M.; Soldierer, M.; Rist, M.; et al. Serpins A1/A3 within Tumor-Derived Extracellular Vesicles Support pro-Tumoral Bias of Neutrophils in Cancer. bioRxiv 2025. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-Producing Γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Apte, R.N.; Segal, S.; Dinarello, C.A.; Song, X.; Krelin, Y.; Dvorkin, T. Bearing Tumors of IL-1β-Secreting Cells Mediate Suppression of T Cells in Mice CD11b+/Gr-1+ Immature Myeloid Cells. J. Immunol. 2005, 175, 8200–8208. [Google Scholar] [CrossRef]
- Bunt, S.K.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Inflammation Induces Myeloid-Derived Suppressor Cells That Facilitate Tumor Progression. J. Immunol. 2006, 176, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.G.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C.A.J. IL-1β Regulates a Novel Myeloid-derived Suppressor Cell Subset That Impairs NK Cell Development and Function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Bhagat, G.; Cui, G.; Takaishi, S.; Kurt-Jones, E.A.; Rickman, B.; Betz, K.S.; Penz-Oesterreicher, M.; Bjorkdahl, O.; Fox, J.G.; et al. Overexpression of Interleukin-1β Induces Gastric Inflammation and Cancer and Mobilizes Myeloid-Derived Suppressor Cells in Mice. Cancer Cell 2008, 14, 408–419. [Google Scholar] [CrossRef]
- Gershkovitz, M.; Caspi, Y.; Fainsod-Levi, T.; Katz, B.; Michaeli, J.; Khawaled, S.; Lev, S.; Polyansky, L.; Shaul, M.E.; Sionov, R.V.; et al. TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells. Cancer Res. 2018, 78, 2680–2690. [Google Scholar] [CrossRef]
- Buckley, C.D.; Ross, E.A.; McGettrick, H.M.; Osborne, C.E.; Haworth, O.; Schmutz, C.; Stone, P.C.W.; Salmon, M.; Matharu, N.M.; Vohra, R.K.; et al. Identification of a Phenotypically and Functionally Distinct Population of Long-Lived Neutrophils in a Model of Reverse Endothelial Migration. J. Leukoc. Biol. 2005, 79, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Finisguerra, V.; Di Conza, G.; Di Matteo, M.; Serneels, J.; Costa, S.; Thompson, A.A.R.; Wauters, E.; Walmsley, S.; Prenen, H.; Granot, Z.; et al. MET Is Required for the Recruitment of Anti-Tumoural Neutrophils. Nature 2015, 522, 349–353. [Google Scholar] [CrossRef]
- Mensurado, S.; Rei, M.; Lança, T.; Ioannou, M.; Gonçalves-Sousa, N.; Kubo, H.; Malissen, M.; Papayannopoulos, V.; Serre, K.; Silva-Santos, B. Tumor-Associated Neutrophils Suppress pro-Tumoral IL-17+ Γδ T Cells through Induction of Oxidative Stress. PLoS Biol. 2018, 16, e2004990. [Google Scholar] [CrossRef]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffman, A.; Chang, Y.-F.; Blank, A.; Reardon, C.A.; et al. Neutrophil Elastase Selectively Kills Cancer Cells and Attenuates Tumorigenesis. Cell 2021, 184, 3163–3177.e21. [Google Scholar] [CrossRef]
- Shrestha, S.; Noh, J.M.; Kim, S.-Y.; Ham, H.-Y.; Kim, Y.-J.; Yun, Y.-J.; Kim, M.-J.; Kwon, M.-S.; Song, D.-K.; Hong, C.-W. Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Antagonist Attenuate Tumor Growth via Polarization of Neutrophils toward an Antitumor Phenotype. OncoImmunology 2016, 5, e1067744. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Schernberg, A.; Blanchard, P.; Chargari, C.; Deutsch, E. Neutrophils, a Candidate Biomarker and Target for Radiation Therapy? Acta Oncol. 2017, 56, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Bodogai, M.; Moritoh, K.; Lee-Chang, C.; Hollander, C.M.; Sherman-Baust, C.A.; Wersto, R.P.; Araki, Y.; Miyoshi, I.; Yang, L.; Trinchieri, G.; et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015, 75, 3456–3465. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Gara, S.K.; Achyut, B.R.; Li, Z.; Yan, H.H.; Day, C.-P.; Weiss, J.M.; Trinchieri, G.; Morris, J.C.; Yang, L. TGF-β Signaling in Myeloid Cells Is Required for Tumor Metastasis. Cancer Discov. 2013, 3, 936–951. [Google Scholar] [CrossRef]
- Jackstadt, R.; Van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e7. [Google Scholar] [CrossRef]
- Yoo, C.; Javle, M.M.; Verdaguer Mata, H.; De Braud, F.; Trojan, J.; Raoul, J.-L.; Kim, J.W.; Ueno, M.; Lee, C.; Hijioka, S.; et al. Phase 2 Trial of Bintrafusp Alfa as Second-Line Therapy for Patients with Locally Advanced/Metastatic Biliary Tract Cancers. Hepatology 2023, 78, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Gunderson, A.J.; Gilchrist, M.; Whiteford, M.; Kiely, M.X.; Hayman, A.; O’Brien, D.; Ahmad, R.; Manchio, J.V.; Fox, N.; et al. Galunisertib plus Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 1189–1200. [Google Scholar] [CrossRef]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key Role for Neutrophils in Radiation-Induced Antitumor Immune Responses: Potentiation with G-CSF. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-Associated Neutrophils Induce EMT by IL-17a to Promote Migration and Invasion in Gastric Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 6. [Google Scholar] [CrossRef]
- Große-Steffen, T.; Giese, T.; Giese, N.; Longerich, T.; Schirmacher, P.; Hänsch, G.M.; Gaida, M.M. Epithelial-to-Mesenchymal Transition in Pancreatic Ductal Adenocarcinoma and Pancreatic Tumor Cell Lines: The Role of Neutrophils and Neutrophil-Derived Elastase. Clin. Dev. Immunol. 2012, 2012, 720768. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-Derived Exosomes Induce N2 Polarization of Neutrophils to Promote Gastric Cancer Cell Migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently Entrapped Circulating Tumor Cells Interact with Neutrophils to Facilitate Lung Metastasis Development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef]
- Spicer, J.D.; McDonald, B.; Cools-Lartigue, J.J.; Chow, S.C.; Giannias, B.; Kubes, P.; Ferri, L.E. Neutrophils Promote Liver Metastasis via Mac-1–Mediated Interactions with Circulating Tumor Cells. Cancer Res. 2012, 72, 3919–3927. [Google Scholar] [CrossRef]
- Li, P.; Lu, M.; Shi, J.; Gong, Z.; Hua, L.; Li, Q.; Lim, B.; Zhang, X.H.-F.; Chen, X.; Li, S.; et al. Lung Mesenchymal Cells Elicit Lipid Storage in Neutrophils That Fuel Breast Cancer Lung Metastasis. Nat. Immunol. 2020, 21, 1444–1455. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The Seed and Soil Hypothesis Revisited—The Role of Tumor-stroma Interactions in Metastasis to Different Organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Psaila, B.; Lyden, D. Bone Marrow Cells in the ‘Pre-Metastatic Niche’: Within Bone and Beyond. Cancer Metastasis Rev. 2007, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Malanchi, I. Neutrophils Support Lung Colonization of Metastasis-Initiating Breast Cancer Cells. Nature 2015, 528, 413–417. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, S.; Wu, K.; Wu, S.-Y.; Xing, F.; Liu, Y.; Zhao, D.; Deshpande, R.P.; D’Agostino, R.B.; Watabe, K. Nicotine Promotes Breast Cancer Metastasis by Stimulating N2 Neutrophils and Generating Pre-Metastatic Niche in Lung. Nat. Commun. 2021, 12, 474. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor Exosomal RNAs Promote Lung Pre-Metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell–Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Li, L.; Zhang, Z.; Jin, X.; Wu, P.; Sun, S.; Pan, J.; Su, K.; Jia, F.; et al. Aged Neutrophils Form Mitochondria-Dependent Vital NETs to Promote Breast Cancer Lung Metastasis. J. Immunother. Cancer 2021, 9, e002875. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Jia, S.; Zhu, M.; Cao, L.; Tao, M.; Jiang, J.; Zhan, S.; Chen, Y.; Gao, P.-J.; et al. Lung Mesenchymal Stromal Cells Influenced by Th2 Cytokines Mobilize Neutrophils and Facilitate Metastasis by Producing Complement C3. Nat. Commun. 2021, 12, 6202. [Google Scholar] [CrossRef] [PubMed]
- Cackowski, F.C.; Heath, E.I. Prostate Cancer Dormancy and Recurrence. Cancer Lett. 2022, 524, 103–108. [Google Scholar] [CrossRef]
- Park, S.-Y.; Nam, J.-S. The Force Awakens: Metastatic Dormant Cancer Cells. Exp. Mol. Med. 2020, 52, 569–581. [Google Scholar] [CrossRef]
- Francescangeli, F.; De Angelis, M.L.; Zeuner, A. COVID-19: A Potential Driver of Immune-Mediated Breast Cancer Recurrence? Breast Cancer Res. 2020, 22, 117. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Luo, J.; Feng, X.-X.; Luo, C.; Wang, Y.; Li, D.; Shu, Y.; Wang, S.-S.; Qin, J.; Li, Y.-C.; Zou, J.-M.; et al. 14,15-EET Induces the Infiltration and Tumor-Promoting Function of Neutrophils to Trigger the Growth of Minimal Dormant Metastases. Oncotarget 2016, 7, 43324–43336. [Google Scholar] [CrossRef] [PubMed]
- Perego, M.; Tyurin, V.A.; Tyurina, Y.Y.; Yellets, J.; Nacarelli, T.; Lin, C.; Nefedova, Y.; Kossenkov, A.; Liu, Q.; Sreedhar, S.; et al. Reactivation of Dormant Tumor Cells by Modified Lipids Derived from Stress-Activated Neutrophils. Sci. Transl. Med. 2020, 12, eabb5817. [Google Scholar] [CrossRef] [PubMed]
- Hirz, T.; Mei, S.; Sarkar, H.; Kfoury, Y.; Wu, S.; Verhoeven, B.M.; Subtelny, A.O.; Zlatev, D.V.; Wszolek, M.W.; Salari, K.; et al. Dissecting the Immune Suppressive Human Prostate Tumor Microenvironment via Integrated Single-Cell and Spatial Transcriptomic Analyses. Nat. Commun. 2023, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- De Zuani, M.; Xue, H.; Park, J.S.; Dentro, S.C.; Seferbekova, Z.; Tessier, J.; Curras-Alonso, S.; Hadjipanayis, A.; Athanasiadis, E.I.; Gerstung, M.; et al. Single-Cell and Spatial Transcriptomics Analysis of Non-Small Cell Lung Cancer. Nat. Commun. 2024, 15, 4388. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Dai, Y.; Tang, X.; Yin, T.; Wang, C.; Wang, T.; Dong, L.; Shi, M.; Qin, J.; et al. Single-Cell RNA-Seq Analysis Reveals BHLHE40-Driven pro-Tumour Neutrophils with Hyperactivated Glycolysis in Pancreatic Tumour Microenvironment. Gut 2023, 72, 958–971. [Google Scholar] [CrossRef]
- Hwang, W.L.; Jagadeesh, K.A.; Guo, J.A.; Hoffman, H.I.; Yadollahpour, P.; Reeves, J.W.; Mohan, R.; Drokhlyansky, E.; Van Wittenberghe, N.; Ashenberg, O.; et al. Single-Nucleus and Spatial Transcriptome Profiling of Pancreatic Cancer Identifies Multicellular Dynamics Associated with Neoadjuvant Treatment. Nat. Genet. 2022, 54, 1178–1191. [Google Scholar] [CrossRef]
- Surendran, V.; Rutledge, D.; Colmon, R.; Chandrasekaran, A. A Novel Tumor-Immune Microenvironment (TIME)-on-Chip Mimics Three Dimensional Neutrophil-Tumor Dynamics and Neutrophil Extracellular Traps (NETs)-Mediated Collective Tumor Invasion. Biofabrication 2021, 13, 035029. [Google Scholar] [CrossRef]
- Yeo, A.T.; Rawal, S.; Delcuze, B.; Christofides, A.; Atayde, A.; Strauss, L.; Balaj, L.; Rogers, V.A.; Uhlmann, E.J.; Varma, H.; et al. Single-Cell RNA Sequencing Reveals Evolution of Immune Landscape during Glioblastoma Progression. Nat. Immunol. 2022, 23, 971–984. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, L.; Cai, Q.; Xi, W.; Yuan, F.; Zhang, H.; Yan, C.; Huang, L.; Zhu, Z.; Zhang, J. Circulating Neutrophils Activated by Cancer Cells and M2 Macrophages Promote Gastric Cancer Progression during PD-1 Antibody-Based Immunotherapy. Front. Mol. Biosci. 2023, 10, 1081762. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Yang, X.; Nan, F.; Zhang, T.; Ji, S.; Rao, D.; Feng, H.; Gao, K.; Gu, X.; et al. Neutrophil Profiling Illuminates Anti-Tumor Antigen-Presenting Potency. Cell 2024, 187, 1422–1439.e24. [Google Scholar] [CrossRef]
- Nieto, P.; Elosua-Bayes, M.; Trincado, J.L.; Marchese, D.; Massoni-Badosa, R.; Salvany, M.; Henriques, A.; Nieto, J.; Aguilar-Fernández, S.; Mereu, E.; et al. A Single-Cell Tumor Immune Atlas for Precision Oncology. Genome Res. 2021, 31, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, C.; Han, M.; Tian, C.; Song, F.; Feng, S.; Xu, M.; Zhao, Z.; Zhou, H.; Su, W.; et al. CXCL2: A Key Player in the Tumor Microenvironment and Inflammatory Diseases. Cancer Cell Int. 2025, 25, 133. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sharp, A.; Gurel, B.; Crespo, M.; Figueiredo, I.; Jain, S.; Vogl, U.; Rekowski, J.; Rouhifard, M.; Gallagher, L.; et al. Targeting Myeloid Chemotaxis to Reverse Prostate Cancer Therapy Resistance. Nature 2023, 623, 1053–1061. [Google Scholar] [CrossRef]
- Yaeger, M.J.; Ngatikaura, T.; Zecchino, N.; Dunigan-Russell, K.; Lovins, H.B.; Schott, E.; Hutton, G.; Saunders, B.; Lin, Y.; Zhang, J. (Jim); et al. ALX/FPR2 Contributes to Serum Amyloid A-Induced Lung Neutrophil Recruitment Following Acute Ozone Exposure. FASEB J. 2025, 39, e70555. [Google Scholar] [CrossRef]
- Wang, J.; Man, K.; Ng, K.T.-P. Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications. IJMS 2025, 26, 4662. [Google Scholar] [CrossRef]
- Simoncello, F.; Piperno, G.M.; Caronni, N.; Amadio, R.; Cappelletto, A.; Canarutto, G.; Piazza, S.; Bicciato, S.; Benvenuti, F. CXCL5-Mediated Accumulation of Mature Neutrophils in Lung Cancer Tissues Impairs the Differentiation Program of Anticancer CD8 T Cells and Limits the Efficacy of Checkpoint Inhibitors. OncoImmunology 2022, 11, 2059876. [Google Scholar] [CrossRef]
- Chen, M.; Qi, Z.; Meng, X.; Wang, S.; Zheng, X.; Hu, M.; Liu, X.; Song, Y.; Deng, Y. Blockade of Neutrophil Recruitment to Tumor Sites Based on Sialic Acid-Modified Nanoplatforms Enhances the Efficacy of Checkpoint Blockade Immunotherapy. Asian J. Pharm. Sci. 2023, 18, 100784. [Google Scholar] [CrossRef]
- Kato, T.; Fukushima, H.; Furusawa, A.; Okada, R.; Wakiyama, H.; Furumoto, H.; Okuyama, S.; Takao, S.; Choyke, P.L.; Kobayashi, H. Selective Depletion of Polymorphonuclear Myeloid Derived Suppressor Cells in Tumor Beds with near Infrared Photoimmunotherapy Enhances Host Immune Response. OncoImmunology 2022, 11, 2152248. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhai, J.; Yang, Z.; Peng, J.; Wang, C.; Liu, X.; Li, Y.; Yao, J.; Chen, F.; Li, H.; et al. Myeloid-Derived Suppressor Cells in Cancer: Mechanistic Insights and Targeted Therapeutic Innovations. MedComm 2025, 6, e70231. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zhai, E.; Zhao, R.; Qian, Y.; Huang, Z.; Liu, Y.; Zhao, Z.; Xu, X.; Liu, J.; et al. Intratumoral Fusobacterium Nucleatum Recruits Tumor-Associated Neutrophils to Promote Gastric Cancer Progression and Immune Evasion. Cancer Res. 2025, 85, 1819–1841. [Google Scholar] [CrossRef]
- Kasama, T.; Miwa, Y.; Isozaki, T.; Odai, T.; Adachi, M.; Kunkel, S. Neutrophil-Derived Cytokines: Potential Therapeutic Targets in Inflammation. CDTIA 2005, 4, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hein, L.E.; SenGupta, S.; Gunasekaran, G.; Johnson, C.N.; Parent, C.A. TGF-Β1 Activates Neutrophil Signaling and Gene Expression but Not Migration. PLoS ONE 2023, 18, e0290886. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, N.; Gruijs, M.; Temming, A.R.; Heineke, M.H.; Gout, D.Y.; Hellingman, T.; Tuk, C.W.; Winter, P.J.; Lissenberg-Thunnissen, S.; Bentlage, A.E.H.; et al. Augmented Antibody-Based Anticancer Therapeutics Boost Neutrophil Cytotoxicity. J. Clin. Investig. 2021, 131, e134680. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Hu, Q. Convergence of Nanomedicine and Neutrophils for Drug Delivery. Bioact. Mater. 2024, 35, 150–166. [Google Scholar] [CrossRef]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, 1706245. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-Mediated Anticancer Drug Delivery for Suppression of Postoperative Malignant Glioma Recurrence. Nat. Nanotech 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Gryka-Marton, M.; Grabowska, A.D.; Szukiewicz, D. Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction. IJMS 2025, 26, 3532. [Google Scholar] [CrossRef]
- Shimizu, F.; Nakamori, M. Blood–Brain Barrier Disruption in Neuroimmunological Disease. IJMS 2024, 25, 10625. [Google Scholar] [CrossRef]
- Chu, Y.; Luo, Y.; Su, B.; Li, C.; Guo, Q.; Zhang, Y.; Liu, P.; Chen, H.; Zhao, Z.; Zhou, Z.; et al. A Neutrophil-Biomimic Platform for Eradicating Metastatic Breast Cancer Stem-like Cells by Redox Microenvironment Modulation and Hypoxia-Triggered Differentiation Therapy. Acta Pharm. Sin. B 2023, 13, 298–314. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. IJMS 2022, 23, 4153. [Google Scholar] [CrossRef]
- Wang, J.; Tang, W.; Yang, M.; Yin, Y.; Li, H.; Hu, F.; Tang, L.; Ma, X.; Zhang, Y.; Wang, Y. Inflammatory Tumor Microenvironment Responsive Neutrophil Exosomes-Based Drug Delivery System for Targeted Glioma Therapy. Biomaterials 2021, 273, 120784. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Li, J.; Cai, H.; Liu, K.; Duan, D.; Zhang, W.; Han, G.; Zhao, Y. Controlled Inflammation Drives Neutrophil-Mediated Precision Drug Delivery in Heterogeneous Tumors. Adv. Sci. 2025, 12, 2411307. [Google Scholar] [CrossRef]
- Wang, P.-F.; Zhang, Y.-X.; Su, J.; Yao, K.; Li, S.-W.; Huang, G.-R.; Yan, C.-X. Neutrophil Depletion Enhances the Therapeutic Effect of PD-1 Antibody on Glioma. Aging 2020, 12, 15290–15301. [Google Scholar] [CrossRef]
- Ager, A. Cancer Immunotherapy: T Cells and Neutrophils Working Together to Attack Cancers. Cell 2023, 186, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- De Castro Silva, I.; Bianchi, A.; Deshpande, N.U.; Sharma, P.; Mehra, S.; Garrido, V.T.; Saigh, S.J.; England, J.; Hosein, P.J.; Kwon, D.; et al. Neutrophil-Mediated Fibroblast-Tumor Cell Il-6/Stat-3 Signaling Underlies the Association between Neutrophil-to-Lymphocyte Ratio Dynamics and Chemotherapy Response in Localized Pancreatic Cancer: A Hybrid Clinical-Preclinical Study. eLife 2022, 11, e78921. [Google Scholar] [CrossRef]
- De Los Reyes, A.A.; Kim, Y. Optimal Regulation of Tumour-Associated Neutrophils in Cancer Progression. R. Soc. Open Sci. 2022, 9, 210705. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Tan, H.; Lu, Y.; Zhang, C.; Cheng, C.; Wu, J.; Wang, N.; Feng, Y. Pancreatic Melatonin Enhances Anti-Tumor Immunity in Pancreatic Adenocarcinoma through Regulating Tumor-Associated Neutrophils Infiltration and NETosis. Acta Pharm. Sin. B 2023, 13, 1554–1567. [Google Scholar] [CrossRef]
- Mangrolia, U.; Osborne, J.W. Probiotics in Counteracting the Role of Neutrophils in Cancer Metastasis. Vaccines 2021, 9, 1306. [Google Scholar] [CrossRef]
- Garcia-Flores, L.A.; Dawid De Vera, M.T.; Pilo, J.; Rego, A.; Gomez-Casado, G.; Arranz-Salas, I.; Hierro Martín, I.; Alcaide, J.; Torres, E.; Ortega-Gomez, A.; et al. Increased Neutrophil Counts Are Associated with Poor Overall Survival in Patients with Colorectal Cancer: A Five-Year Retrospective Analysis. Front. Immunol. 2024, 15, 1415804. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, T.; Yang, Z.; Yin, L.; Zhao, C.; Feng, L.; Lin, S.; Liu, B.; Cheng, S.; Zhang, K. Telomerase-Positive Circulating Tumor Cells Are Associated with Poor Prognosis via a Neutrophil-Mediated Inflammatory Immune Environment in Glioma. BMC Med. 2021, 19, 277. [Google Scholar] [CrossRef]
- Anderson, R.; Blidner, A.G.; Rapoport, B.L. Frontiers in Pharmacology: Review Manuscript Targeting of the Neutrophil as an Adjunctive Strategy in Non-Small Cell Lung Cancer. Front. Pharmacol. 2021, 12, 676399. [Google Scholar] [CrossRef]
- Kast, R.E. High Neutrophil-to-Lymphocyte Ratio Facilitates Cancer Growth—Currently Marketed Drugs Tadalafil, Isotretinoin, Colchicine, and Omega-3 to Reduce It: The TICO Regimen. Cancers 2022, 14, 4965. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, J.; Zhang, G.; Yu, T.; Piao, H. Approaches for Neutrophil Imaging: An Important Step in Personalized Medicine. Bioengineered 2022, 13, 14844–14855. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Tao, Y.; Han, Y.; He, Y.; Fu, Y.; Yang, H.; Chen, Y.; Shi, Y. Quercetin Alleviates Breast Cancer-Related Depression by Inhibiting Neutrophil Extracellular Traps via Inhibition of Sphingosine 1-Phosphate/Sphingosine 1-Phosphate Receptor Axis. Phytother. Res. 2025, ptr.8513. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.; Liao, J.; Wu, M.; Lu, X.; Li, J.; Zeng, Y. BCL2A1- and G0S2-driven Neutrophil Extracellular Traps: A Protective Mechanism Linking Preeclampsia to Reduced Breast Cancer Risk. Oncol. Rep. 2025, 53, 64. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Guo, J.; Kang, K.; Wang, S.; Huang, M. Advances in Targeting Neutrophil Extracellular Traps as a PromisingApproach for Breast Cancer Treatment. CCHTS 2025, 28. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, W.; Du, J.; Teng, L.; Yu, T.; Xu, P.; Liu, J.; Yang, R.; Dong, Y.; Wang, H.; et al. C-FOS Promotes the Formation of Neutrophil Extracellular Traps and the Recruitment of Neutrophils in Lung Metastasis of Triple-Negative Breast Cancer. J. Exp. Clin. Cancer Res. 2025, 44, 108. [Google Scholar] [CrossRef]
- Huang, Z.; Mo, C.; Li, L.; Hou, Q.; Pan, Y.; Zhu, G.; Qiu, F.; Zou, Q.; Yang, J. Identification of Novel Neutrophil-Extracellular-Traps-Related Genes as Biomarkers for Breast Cancer Prognosis and Immunotherapy. Transl. Cancer Res. 2025, 14, 1737–1752. [Google Scholar] [CrossRef]
- Aierken, Y.; Tan, K.; Liu, T.; Lv, Z. Prognosis and Immune Infiltration Prediction in Neuroblastoma Based on Neutrophil Extracellular Traps-Related Gene Signature. Sci. Rep. 2025, 15, 5343. [Google Scholar] [CrossRef]
- Shen, J.; Lin, H.; Mo, K.; Liang, Z.; Zhang, Y.; Quan, H.; Wang, X.; Zhang, C.; Chen, C. Bidirectional Roles of Neutrophil Extracellular Traps in Oral Microbiota Carcinogenesis: A Systematic Review. Transl. Oncol. 2025, 56, 102361. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ding, W.; Feng, C.; Wang, Y.; Wei, X.; Qu, Z.; Wang, H.; Liu, X.; Wang, H.; et al. Neutrophil Extracellular Traps Induced by Interleukin 8 via CXCR1/2 Promote the Progression of Gastric Carcinoma through Transcription Factor IIB-Related Factor 1 and Cyclin. Genes Dis. 2024, 11, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, X.; Yang, S.; Yang, H.; Zhang, A.; Li, N.; Zou, X. Molecular Mechanisms of Neutrophil Extracellular Traps in Promoting Gastric Cancer Epithelial–Mesenchymal Transition Through SERPINE-1 Expression. J. Biochem. Amp; Mol. Tox 2025, 39, e70157. [Google Scholar] [CrossRef]
- Qian, Y.-Y.; Xu, M.; Huang, X.-K.; Zhu, B. Bioinformatic Analysis Indicated That LINC01150 Might Be a Novel Neutrophil Extracellular Traps-Related Biomarker of Gastric Cancer. Sci. Rep. 2025, 15, 7875. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Ma, Y.; Wang, B.; Wang, Y.; Yuan, J.; Zhang, F.; Zhao, X.; Chen, K.; Zhang, X.; et al. Neutrophil Extracellular Traps Impede Cancer Metastatic Seeding via Protease-Activated Receptor 2-Mediated Downregulation of Phagocytic Checkpoint CD24. J. Immunother. Cancer 2025, 13, e010813. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, J.; Meng, Q.; Zuo, X.; Sun, B.; Liu, Y.; Guo, Y.; Wang, M.; Yan, X.; Zhang, J.; et al. Neutrophil Extracellular Traps Promote a Liver Metastatic Phenotype in Human Colorectal Cancer Cells through Epithelial—Mesenchymal Transition. Preprint 2025. [Google Scholar] [CrossRef]
- Zhou, M.; Guan, B.; Liu, Y.; Gu, Q.; Chen, W.; Xie, B.; Zhou, M.; Xiang, J.; Zhao, S.; Zhao, Q.; et al. Fibrinogen-like 2 in Tumor-Associated Macrophage-Derived Extracellular Vesicles Shapes an Immunosuppressive Microenvironment in Colorectal Liver Metastases by Promoting Tumor Stemness and Neutrophil Extracellular Traps Formation. Cancer Lett. 2025, 618, 217642. [Google Scholar] [CrossRef]
- Wang, X.; He, S.; Gong, X.; Lei, S.; Zhang, Q.; Xiong, J.; Liu, Y. Neutrophils in Colorectal Cancer: Mechanisms, Prognostic Value, and Therapeutic Implications. Front. Immunol. 2025, 16, 1538635. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Xiang, L.; You, Y.; Duan, Y.; Zhao, Y.; Li, S.; Wu, R.; Zhang, J.; Zhou, L.; et al. Fusobacterium Nucleatum-Triggered Neutrophil Extracellular Traps Facilitate Colorectal Carcinoma Progression. J. Exp. Clin. Cancer Res. 2023, 42, 236. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsujimoto, H.; Watanabe, T.; Ishibashi, Y.; Fujishima, S.; Itazaki, Y.; Kariya, R.; Uehata, N.; Shinada, H.; Mochizuki, S.; et al. Clinical Significance of Coiled-Coil Domain-Containing Protein 25 Expression in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2025, 32, 3839–3850. [Google Scholar] [CrossRef]
- Hong, Z.; Liu, Q.; Zhang, Y.; Shi, X.; Jing, D.; Cheng, T.; Liu, H.; Piao, H.; Gou, Y. The Tumor-Promoting Role of Neutrophil Extracellular Traps in Esophageal Squamous Cell Carcinoma and Their Interaction with the Gut Microbiota. Preprint 2025. [Google Scholar] [CrossRef]
- Baron, S.; Binenbaum, Y.; Maman, R.; Fidel, V.; Shusterman, A.; Vaisman, D.; Sher, O.; Manisterski, M.; Shukrun, R.; Rössig, C.; et al. Neutrophil Extracellular Traps Are Associated with Poor Response to Neoadjuvant Therapy and Poor Survival in Pediatric Osteosarcoma. Front. Oncol. 2025, 15, 1472716. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Huang, R.; Shi, J.; Xu, R.; Wei, D. NETs-Related Genes Predict Prognosis and Are Correlated with the Immune Microenvironment in Osteosarcoma. Front. Oncol. 2025, 15, 1551074. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, S.Y.; Akasaka, H.; Weigert, M.; Lengyel, E.; Naora, H. Neutrophil Extracellular Traps Promote Pre-Metastatic Niche Formation in the Omentum by Expanding Innate-like B Cells That Express IL-10. Cancer Cell 2025, 43, 69–85.e11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Fan, F.; Zhang, B.; Yuan, C.; Liang, H. Neutrophil Spatiotemporal Regulatory Networks: Dual Roles in Tumor Growth Regulation and Metastasis. Biomedicines 2025, 13, 1473. https://doi.org/10.3390/biomedicines13061473
Li P, Fan F, Zhang B, Yuan C, Liang H. Neutrophil Spatiotemporal Regulatory Networks: Dual Roles in Tumor Growth Regulation and Metastasis. Biomedicines. 2025; 13(6):1473. https://doi.org/10.3390/biomedicines13061473
Chicago/Turabian StyleLi, Pengcheng, Feimu Fan, Bixiang Zhang, Chaoyi Yuan, and Huifang Liang. 2025. "Neutrophil Spatiotemporal Regulatory Networks: Dual Roles in Tumor Growth Regulation and Metastasis" Biomedicines 13, no. 6: 1473. https://doi.org/10.3390/biomedicines13061473
APA StyleLi, P., Fan, F., Zhang, B., Yuan, C., & Liang, H. (2025). Neutrophil Spatiotemporal Regulatory Networks: Dual Roles in Tumor Growth Regulation and Metastasis. Biomedicines, 13(6), 1473. https://doi.org/10.3390/biomedicines13061473






