Drug-Coated Balloon PCI in Different Plaque Morphologies: A Narrative Review
Abstract
1. Introduction
2. Evolution of PCI and the Rise of Drug-Coated Balloons
3. The Advantages of Drug-Coated Balloons over Stents
4. Drug Carriers for Paclitaxel and Sirolimus DCBs
5. DCB PCI for De Novo Lesions
6. Paclitaxel vs. Sirolimus: Differences and Clinical Applications
7. When to Choose Paclitaxel vs. Sirolimus Drug-Coated Balloon?
8. Antithrombotic Therapy in DCB-Based PCI
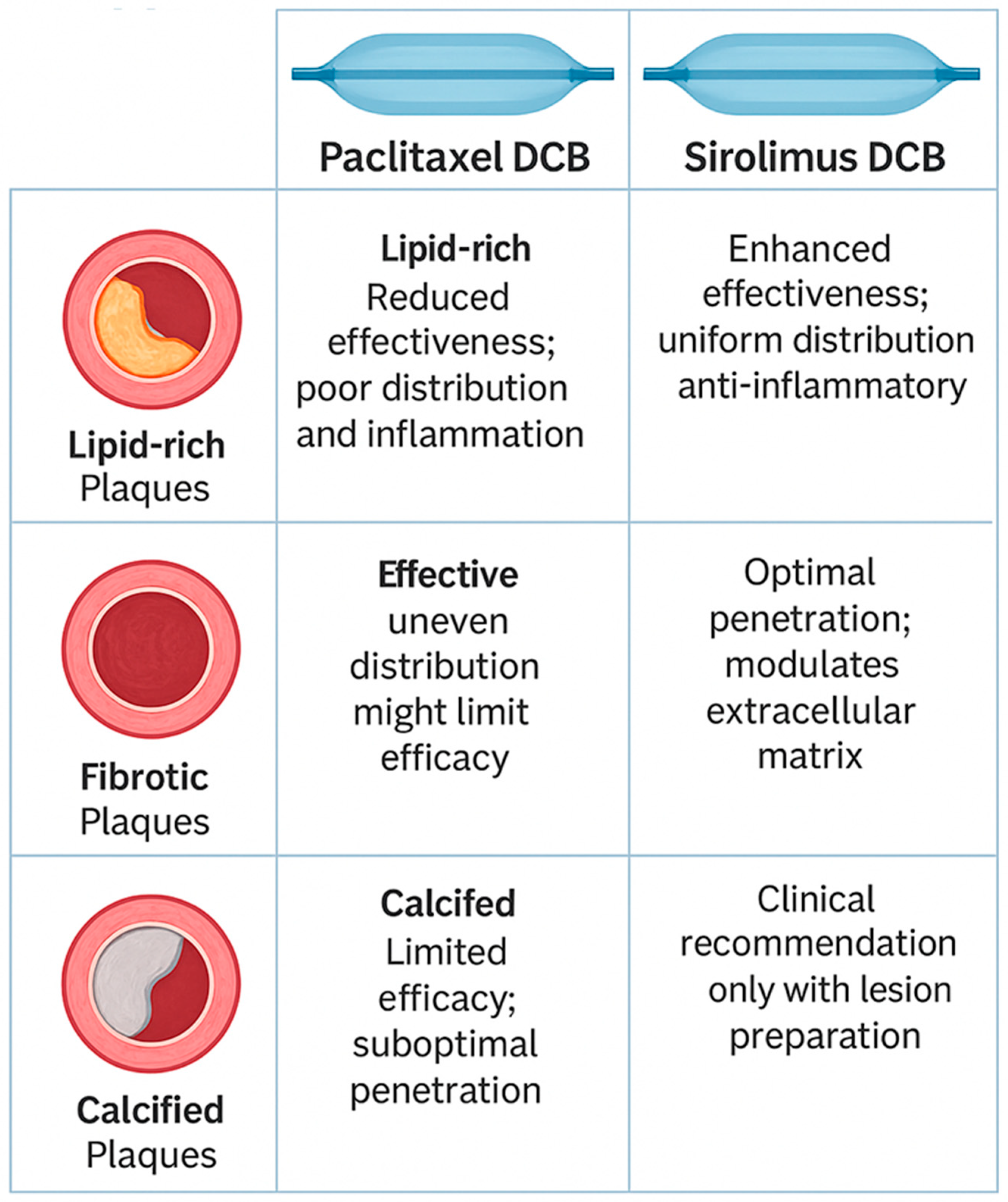
9. Paclitaxel and Sirolimus in Different Plaque Morphologies
9.1. Lipid-Rich Plaques
9.2. Fibrotic Plaques
9.3. Calcified Plaques
10. Clinical Scenarios Where DCB Use May Be Avoided
11. Clinical Implications and Decision-Making Framework
12. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMS | Bare Metal Stent |
| CAD | Coronary Artery Disease |
| DAPT | Dual Antiplatelet Therapy |
| DCB | Drug-Coated Balloon |
| DES | Drug-Eluting Stent |
| ISR | In-Stent Restenosis |
| IVUS | Intravascular Ultrasound |
| LCBI | Lipid Core Burden Index |
| LLL | Late Lumen Loss |
| MACE | Major Adverse Cardiac Events |
| OCT | Optical Coherence Tomography |
| PCB | Paclitaxel-Coated Balloon |
| PCI | Percutaneous Coronary Intervention |
| SCB | Sirolimus-Coated Balloon |
| TLF | Target Lesion Failure |
| TLR | Target Lesion Revascularization |
References
- Payne, M.M. Charles Theodore Dotter: The Father of Intervention. Tex. Heart Inst. J. 2001, 28, 28–38. [Google Scholar]
- Barton, M.; Grüntzig, J.; Husmann, M.; Rösch, J. Balloon Angioplasty—The Legacy of Andreas Grüntzig, M.D. (1939–1985). Front. Cardiovasc. Med. 2014, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Tomberli, B.; Mattesini, A.; Baldereschi, G.J.; Di Mario, C. A Brief History of Coronary Artery Stents. Rev. Esp. Cardiol. (Engl. Ed.) 2018, 71, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Dhall, A. A Comparative Study of Restenosis Rates in Bare Metal and Drug-Eluting Stents. Int. J. Angiol. 2010, 19, e66–e72. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Moses, J.W.; Ellis, S.G.; Schofer, J.; Dawkins, K.D.; Morice, M.-C.; Colombo, A.; Schampaert, E.; Grube, E.; Kirtane, A.J.; et al. Safety and Efficacy of Sirolimus- and Paclitaxel-Eluting Coronary Stents. N. Engl. J. Med. 2007, 356, 998–1008. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Claessen, B.E.; Caixeta, A.; Henriques, J.P.; Piek, J.J. Current Status of the Xience V® Everolimus-Eluting Coronary Stent System. Expert Rev. Cardiovasc. Ther. 2010, 8, 1363–1374. [Google Scholar] [CrossRef]
- Leon, M.B.; Mauri, L.; Popma, J.J.; Cutlip, D.E.; Nikolsky, E.; O’Shaughnessy, C.; Overlie, P.A.; McLaurin, B.T.; Solomon, S.L.; Douglas, J.S.; et al. A Randomized Comparison of the Endeavor Zotarolimus-Eluting Stent Versus the TAXUS Paclitaxel-Eluting Stent in De Novo Native Coronary Lesions: 12-Month Outcomes From the ENDEAVOR IV Trial. J. Am. Coll. Cardiol. 2010, 55, 543–554. [Google Scholar] [CrossRef]
- Whitbeck, M.G.; Applegate, R.J. Second Generation Drug-Eluting Stents: A Review of the Everolimus-Eluting Platform. Clin. Med. Insights Cardiol. 2013, 7, CMC.S11516. [Google Scholar] [CrossRef]
- Rheude, T.; Koch, T.; Joner, M.; Lenz, T.; Xhepa, E.; Wiebe, J.; Coughlan, J.J.; Aytekin, A.; Cassese, S.; Laugwitz, K.-L.; et al. Ten-Year Clinical Outcomes of Drug-Eluting Stents with Different Polymer Coating Strategies by Degree of Coronary Calcification: A Pooled Analysis of the ISAR-TEST 4 and 5 Randomised Trials. Available online: https://eurointervention.pcronline.com/article/ten-year-clinical-outcomes-of-drug-eluting-stents-with-different-polymer-coating-strategies-by-degree-of-coronary-calcification-a-pooled-analysis-of-the-isar-test-4-and-5-randomised-trials (accessed on 13 March 2025).
- EI-Hayek, G.; Bangalore, S.; Casso, D.A.; Devireddy, C.; Jaber, W.; Kumar, G.; Mavromatis, K.; Tamis-Holland, J.; Samady, H. Meta-Analysis of Randomized Clinical Trials Comparing Biodegradable Polymer Drug-Eluting Stent to Second-Generation Durable Polymer Drug-Eluting Stents. JACC Cardiovasc. Interv. 2017, 10, 462–473. [Google Scholar] [CrossRef]
- Palmerini, T.; Biondi-Zoccai, G.; Della Riva, D.; Mariani, A.; Sabaté, M.; Valgimigli, M.; Frati, G.; Kedhi, E.; Smits, P.C.; Kaiser, C.; et al. Clinical Outcomes With Drug-Eluting and Bare-Metal Stents in Patients With ST-Segment Elevation Myocardial Infarction: Evidence From a Comprehensive Network Meta-Analysis. J. Am. Coll. Cardiol. 2013, 62, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Marengo, G.; Bruno, F.; Scudeler, L.; Savoca, F.; Zugna, D.; Isaevska, E.; Pilgrim, T.; Jensen, L.O.; Filippo, O.D.; Richiardi, L.; et al. Comparison Among Ultra-Thin Coronary Stents: A Network Meta-Analysis. Am. J. Cardiol. 2024, 216, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Howard, J.P.; Naqvi, A.; Ben-Yehuda, O.; Redfors, B.; Prasad, M.; Shahim, B.; Leon, M.B.; Bangalore, S.; Stone, G.W.; et al. Long-Term Follow-up after Ultrathin vs. Conventional 2nd-Generation Drug-Eluting Stents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. Heart J. 2021, 42, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F. Long-Term Outcomes Following Ultrathin vs Thin-Strut Drug-Eluting Stents for Percutaneous Coronary Intervention: An Updated Systematic Review and Meta-Analysis of Randomized Control Trials. Am. J. Cardiovasc. Dis. 2024, 14, 267–280. [Google Scholar] [CrossRef]
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; Böhm, M.; Speck, U. Treatment of Coronary In-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter. N. Engl. J. Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef]
- Unverdorben, M.; Vallbracht, C.; Cremers, B.; Heuer, H.; Hengstenberg, C.; Maikowski, C.; Werner, G.; Antoni, D.; Kleber, F.-X.; Bocksch, W.; et al. Paclitaxel-Coated Balloon Catheter versus Paclitaxel-Coated Stent for the Treatment of Coronary in-Stent Restenosis: The Three-Year Results of the PEPCAD II ISR Study. Available online: https://eurointervention.pcronline.com/article/paclitaxel-coated-balloon-catheter-versus-paclitaxel-coated-stent-for-the-treatment-of-coronary-in-stent-restenosis-the-three-year-results-of-the-pepcad-ii-isr-study (accessed on 13 March 2025).
- Her, A.-Y.; Shin, E.-S.; Bang, L.H.; Nuruddin, A.A.; Tang, Q.; Hsieh, I.-C.; Hsu, J.-C.; Kiam, O.T.; Qiu, C.; Qian, J.; et al. Drug-Coated Balloon Treatment in Coronary Artery Disease: Recommendations from an Asia-Pacific Consensus Group. Cardiol. J. 2021, 28, 136–149. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-Coated Balloons for Small Coronary Artery Disease (BASKET-SMALL 2): An Open-Label Randomised Non-Inferiority Trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Cortese, B.; Di, P.G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef]
- Alfonso, F.; Scheller, B. State of the Art: Balloon Catheter Technologies—Drug-Coated Balloon. Available online: https://eurointervention.pcronline.com/article/state-of-the-art-balloon-catheter-technologies-drug-coated-balloon (accessed on 14 March 2025).
- Woehrle, J.; Motz, W.; Moebius-Winkler, S.; Leschke, M.; Opitz, C.; Ahmed, W.; Barragan, P.; Simon, J.-P.; Cassel, G.; Elbal, L.; et al. Sequent Please Worl Wide Registry: Results of Paclitaxel Coated Balloon Angioplasty for Treatment of de-Novo Coronary Artery Disease. JACC 2012, 59, E57. [Google Scholar] [CrossRef]
- Assadi-Schmidt, A.; Mohring, A.; Liebsch, E.; Dannenberg, L.; Achilles, A.; Pöhl, M.; Afzal, S.; Veulemans, V.; Horn, P.; Sansone, R.; et al. SeQuent Please vs. Pantera Lux Drug Coated Balloon Angioplasty in Real Life: Results from the Düsseldorf DCB Registry. Int. J. Cardiol. 2017, 231, 68–72. [Google Scholar] [CrossRef]
- von Koch, S.; Frank, E.; Gabrysch, K.; Erlinge, D. 100.04 Real-World Usage of Prevail Paclitaxel-Coated Balloon Compared With Other Contemporary Drug-Coated Balloons: Two-Year Analysis From SCAAR in Over 6000 Patients. JACC Cardiovasc. Interv. 2025, 18, S17–S18. [Google Scholar] [CrossRef]
- Cortese, B.; Malakouti, S.; Khater, J.; Munjal, A. Magic Touch Sirolimus-Coated Balloon: Animal and Clinical Evidence of a Coronary Sirolimus Drug-Coated Balloon. Future Cardiol. 2024, 20, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.; Krackhardt, F.; Bogaerts, K.; Urban, P.; Meis, S.; Morice, M.-C.; Eccleshall, S. Comparing a Strategy of Sirolimus-Eluting Balloon Treatment to Drug-Eluting Stent Implantation in de Novo Coronary Lesions in All-Comers: Design and Rationale of the SELUTION DeNovo Trial. Am. Heart J. 2023, 258, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yerasi, C.; Case, B.C.; Forrestal, B.J.; Torguson, R.; Weintraub, W.S.; Garcia-Garcia, H.M.; Waksman, R. Drug-Coated Balloon for De Novo Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1061–1073. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Ji, F.; Zhang, W.; Yang, C.; Xu, F.; Wang, F. A Non-Inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon Versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc. Drugs Ther. 2022, 36, 655–664. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Gad, M.M.; Elgendy, A.Y.; Mahmoud, A.; Mahmoud, A.N.; Cuesta, J.; Rivero, F.; Alfonso, F. Clinical and Angiographic Outcomes With Drug-Coated Balloons for De Novo Coronary Lesions: A Meta-Analysis of Randomized Clinical Trials. J. Am. Heart Assoc. 2020, 9, e016224. [Google Scholar] [CrossRef]
- Jain, H.; Odat, R.; Pervez, N.; Goyal, A.; Jain, J.; Patel, N.; Yadav, A.; Shah, J.; Jha, J.; Passey, S. Abstract 4144620: Outcomes with Drug-Coated Balloon Versus Drug-Eluting Stents for De-Novo Coronary Artery Disease: A Meta-Analysis. Circulation 2024, 150, A4144620. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Y.; Zhu, X.; Miao, L.; Liang, X.; Duan, J.; Li, H.; Tian, X.; Pang, L.; Wei, Y.; et al. Significant Difference between Sirolimus and Paclitaxel Nanoparticles in Anti-Proliferation Effect in Normoxia and Hypoxia: The Basis of Better Selection of Atherosclerosis Treatment. Bioact. Mater. 2021, 6, 880–889. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; De Gregorio, M.; Benatti, G.; Donelli, D.; Vignali, L.; Solinas, E.; Tadonio, I.; Denegri, A.; Covani, M.; Dallaglio, G.; et al. Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives. Future Pharmacol. 2024, 4, 775–787. [Google Scholar] [CrossRef]
- Oliva, A.; Castiello, D.S.; Franzone, A.; Condorelli, G.; Colombo, A.; Esposito, G.; Stefanini, G.G.; Piccolo, R. P2Y12 Inhibitors Monotherapy in Patients Undergoing Complex vs Non-Complex Percutaneous Coronary Intervention: A Meta-Analysis of Randomized Trials. Am. Heart J. 2023, 255, 71–81. [Google Scholar] [CrossRef]
- Castiello, D.S.; Buongiorno, F.; Manzi, L.; Narciso, V.; Forzano, I.; Florimonte, D.; Sperandeo, L.; Canonico, M.E.; Avvedimento, M.; Paolillo, R.; et al. Procedural and Antithrombotic Therapy Optimization in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, A.; Küçük, I.T.; Garcia-Garcia, H.M.; Fuentes, F.H.; Kahsay, Y.; Delewi, R.; Beijk, M.A.; den Hartog, A.W.; Grundeken, M.J.; Vis, M.M.; et al. Paclitaxel-Coated Balloons for Vulnerable Lipid-Rich Plaques. Available online: https://eurointervention.pcronline.com/article/paclitaxel-coated-balloons-for-vulnerable-lipid-rich-plaques (accessed on 14 March 2025).
- Zhao, L.; Ding, T.; Cyrus, T.; Cheng, Y.; Tian, H.; Ma, M.; Falotico, R.; Praticò, D. Low-Dose Oral Sirolimus Reduces Atherogenesis, Vascular Inflammation and Modulates Plaque Composition in Mice Lacking the LDL Receptor. Br. J. Pharmacol. 2009, 156, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Drzewiecki, J.; Banning, A.; Grube, E.; Hauptmann, K.; Silber, S.; Dudek, D.; Fort, S.; Schiele, F.; Zmudka, K.; et al. Randomized Study to Assess the Effectiveness of Slow- and Moderate-Release Polymer-Based Paclitaxel-Eluting Stents for Coronary Artery Lesions. Circulation 2003, 108, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular Effects of Immunosuppression Agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef]
- Scheller, B.; Mangner, N.; Abdul Kader, M.A.S.K.; Wan Ahmad, W.A.; Jeger, R.; Wöhrle, J.; Ong, T.K.; Liew, H.B.; Gori, T.; Mahfoud, F.; et al. Combined Analysis of Two Parallel Randomized Trials of Sirolimus-Coated and Paclitaxel-Coated Balloons in Coronary In-Stent Restenosis Lesions. Circ. Cardiovasc. Interv. 2022, 15, e012305. [Google Scholar] [CrossRef]
- Scheller, B.; Mangner, N.; Jeger, R.V.; Afan, S.; Mahfoud, F.; Woitek, F.J.; Fahrni, G.; Schwenke, C.; Schnorr, B.; Kleber, F. A Randomised Trial of Sirolimus- versus Paclitaxel-Coated Balloons for de Novo Coronary Lesions. EuroIntervention 2024, 20, e1322–e1329. [Google Scholar] [PubMed]
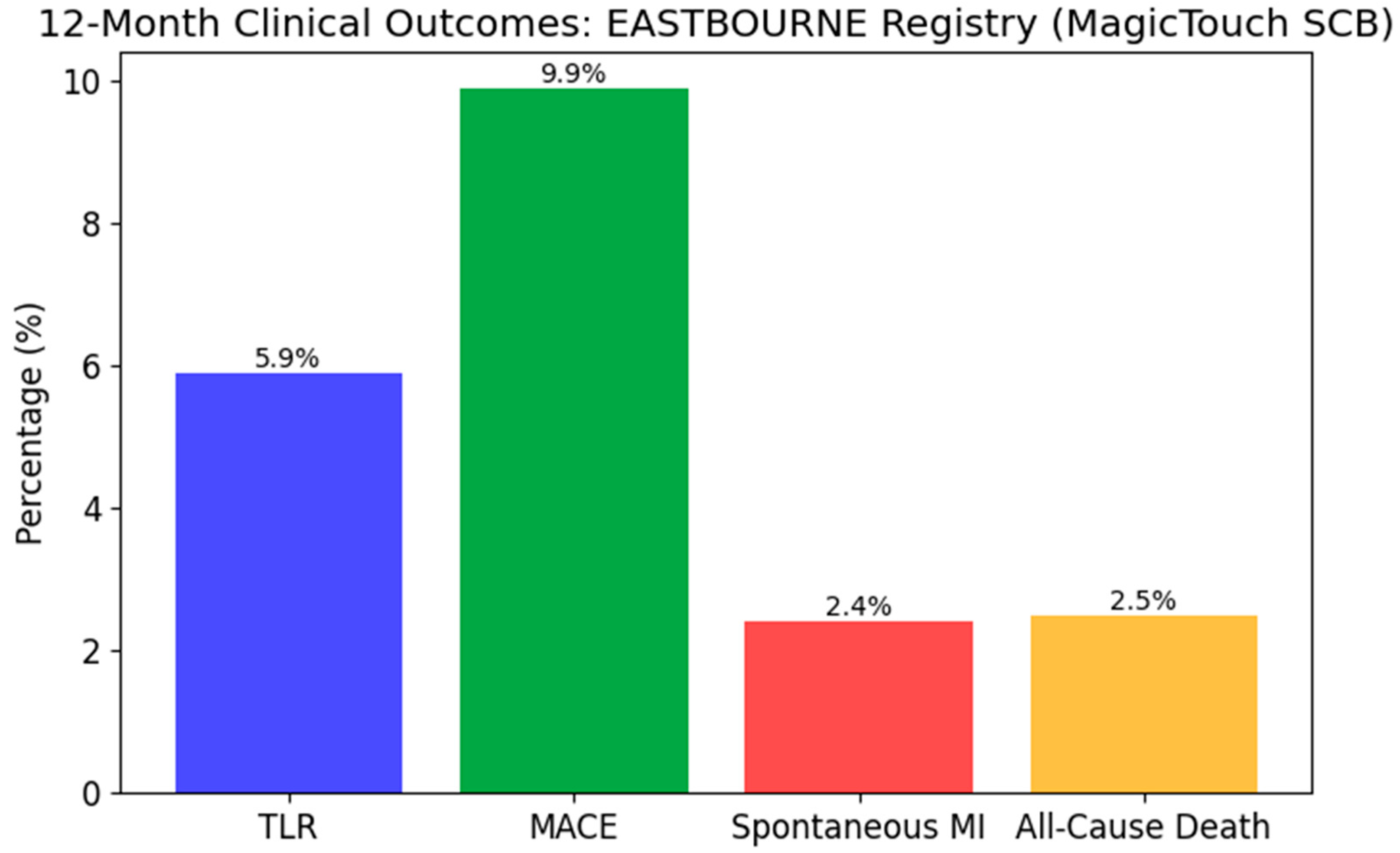
- Cortese, B.; Testa, L.; Heang, T.M.; Ielasi, A.; Bossi, I.; Latini, R.A.; Lee, C.Y.; Perez, I.S.; Milazzo, D.; Caiazzo, G.; et al. Sirolimus-Coated Balloon in an All-Comer Population of Coronary Artery Disease Patients: The EASTBOURNE Prospective Registry. JACC Cardiovasc. Interv. 2023, 16, 1794–1803. [Google Scholar] [CrossRef]
- Jensen, C.J.; Richardt, G.; Tölg, R.; Erglis, A.; Skurk, C.; Jung, W.; Neumann, F.J.; Stangl, K.; Brachmann, J.; Fischer, D.; et al. Angiographic and Clinical Performance of a Paclitaxel-Coated Balloon Compared to a Second-Generation Sirolimus-Eluting Stent in Patients with in-Stent Restenosis: The BIOLUX Randomised Controlled Trial. EuroIntervention 2018, 14, 1096–1103. [Google Scholar] [CrossRef]



| Carrier | Drug Type | Industry Example | Solubility | Inflation Time for Drug Transfer | Mechanism | Clinical Impact |
|---|---|---|---|---|---|---|
| Iopromide | Paclitaxel | SeQuent Please DCB [22] | Hydrophilic | 30–60 s | Enhances rapid drug transfer but may cause distal embolization | Effective in restenosis reduction but has potential embolization risk |
| BTHC (Butyryl-trihexyl-citrate) | Paclitaxel | Pantera Lux DCB [23] | Lipophilic | 45–60 s | Increases retention in tissues, reducing drug washout | Provides prolonged drug effect but may delay healing |
| Urea | Paclitaxel | Prevail DCB [24] | Hydrophilic | 30 s | Improves drug diffusion but allows faster washout | Rapid transfer but shorter tissue retention |
| Shellac | Sirolimus | Magic Touch DCB [25] | Hydrophobic | 60 s | Forms a controlled-release layer for sustained retention | Reduces inflammation and enhances endothelial recovery |
| Urea | Sirolimus | Selution SLR DCB [26] | Hydrophilic | 30 s | Allows rapid drug diffusion but may lead to shorter retention | Effective but may have shorter therapeutic duration |
| BTHC (Butyryl-trihexyl-citrate) | Sirolimus | Virtue DCB [27] | Lipophilic | 45–60 s | Enhances adhesion and retention, ensuring extended release | Prolonged therapeutic action, useful for complex lesions |
| Phospholipid-based | Sirolimus | Experimental SCBs 27] | Amphiphilic | Variable | Mimics cell membranes to improve sirolimus absorption | Sustained drug release with improved vessel healing |
| Study Name | Study Design | Number of Patients | Lesion Type | Intervention Arms | Main Findings | Follow-Up |
|---|---|---|---|---|---|---|
| PEPCAD II | RCT | 131 | ISR | DCB vs. DES | DCB non-inferior to paclitaxel-DES in ISR | 12 months |
| BIOLUX RCT | RCT | 302 | ISR | DCB vs. DES | DCB showed comparable efficacy to DES | 12 months |
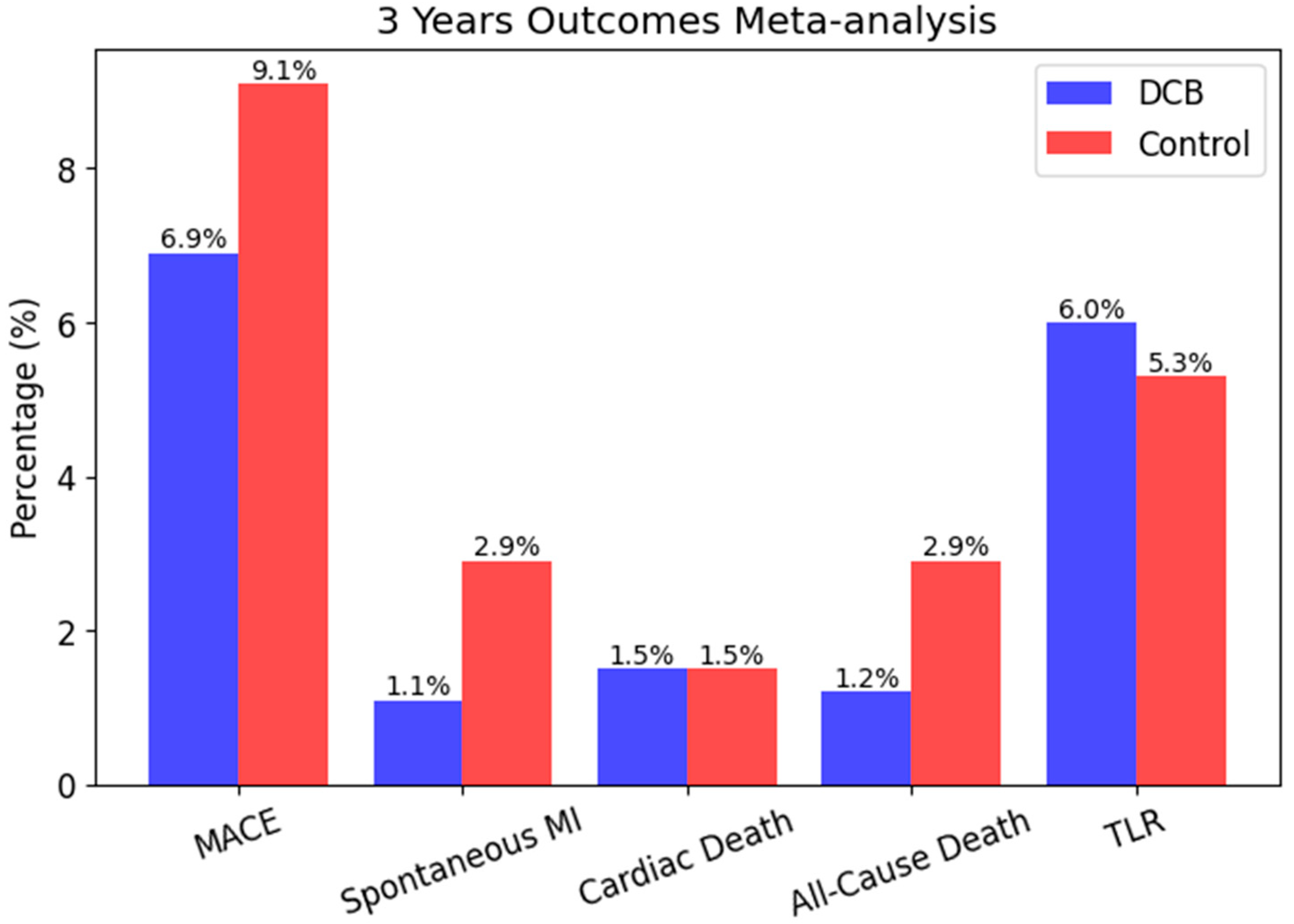
| EASTBOURNE | Prospective Registry | 2111 | All-comers | SCB | SCB showed safety and efficacy in large-scale use | 12 months |
| BASKET-SMALL 2 | RCT | 758 | Small vessels | DCB vs. DES | DCB non-inferior to DES in small vessels | 12 months |
| FIRE | RCT | 1500 | Complex PCI in elderly | DCB vs. DES | DCB associated with lower bleeding, non-inferior ischemic outcomes | 12 months |
| RCT | RCT | 232 | Small vessel CAD | PCB vs. DES | PCB DCB non-inferior to everolimus DES for late lumen loss | 6 months |
| Aspect | Paclitaxel | Sirolimus |
|---|---|---|
| Mechanism of Action | Non-uniform (crystalline, lipophilic), potential for distal embolization | Cytostatic and immunosuppressive; inhibits mTOR pathway and cell-cycle transition (G1/S), reducing proliferation and inflammation |
| Distribution in Vessel Wall | Non-uniform distribution, potential embolization | Homogeneous distribution, lower embolization risk |
| Stable Fibrocalcific Plaque | Preferred; uneven distribution might limit efficacy | Effective; uniform penetration, inflammatory control |
| Lipid-rich Plaque | Reduced effectiveness due to poor distribution and inflammation | Enhanced effectiveness; uniform distribution, anti-inflammatory properties |
| Vulnerable Plaque (thin-cap fibroatheroma) | Limited efficacy; increased inflammation risk | Superior; stabilizes plaque due to anti-inflammatory effects |
| Plaque with Thrombus | Limited; risk of inflammation and embolization | Preferred; anti-inflammatory and stabilizing properties |
| Clinical Recommendations | Stable lesions, restenosis, fibrocalcific plaques | Unstable, inflammatory, lipid-rich, thrombotic lesions |
| Combined Analysis (2022) | Randomized Trial (2024) | EASTBOURNE Registry (2023) | BIOLUX Trial (2024) | |
|---|---|---|---|---|
| Population | 101 patients with DES-ISR | 70 patients with de novo lesions | 2123 patients (2440 lesions) with CAD | 229 patients with ISR |
| Inclusion Criteria | DES in-stent restenosis | De novo coronary lesions suitable for PCI | All-comer CAD, including small vessels and ISR | ISR in BMS or DES |
| Exclusion Criteria | Not explicitly stated | Not explicitly stated | Not explicitly stated | Not explicitly stated |
| Intravascular Imaging | Not specified | OCT-guided balloon sizing | Not specified | Not specified |
| Primary Objectives | In-segment late lumen loss (6 months) | Net lumen diameter gain at 6 months | Target lesion revascularization (12 months) | In-stent late lumen loss (6 months) |
| Secondary Objectives | MACE, TLR, stent thrombosis (12 months) | MACE, TLR (12 months) | MACE, spontaneous MI, cardiac/all-cause death | Target lesion failure (18 months) |
| Key Conclusions | SCB and PCB show similar angiographic and clinical outcomes | SCB non-inferior to PCB in de novo lesions | SCB safe and effective with low TLR and MACE rates | Paclitaxel DCB comparable to sirolimus DES in ISR lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherasie, F.-A.; Ciomag, R.; Gherasie, L.-M. Drug-Coated Balloon PCI in Different Plaque Morphologies: A Narrative Review. Biomedicines 2025, 13, 1472. https://doi.org/10.3390/biomedicines13061472
Gherasie F-A, Ciomag R, Gherasie L-M. Drug-Coated Balloon PCI in Different Plaque Morphologies: A Narrative Review. Biomedicines. 2025; 13(6):1472. https://doi.org/10.3390/biomedicines13061472
Chicago/Turabian StyleGherasie, Flavius-Alexandru, Raluca Ciomag (Ianula), and Luana-Maria Gherasie. 2025. "Drug-Coated Balloon PCI in Different Plaque Morphologies: A Narrative Review" Biomedicines 13, no. 6: 1472. https://doi.org/10.3390/biomedicines13061472
APA StyleGherasie, F.-A., Ciomag, R., & Gherasie, L.-M. (2025). Drug-Coated Balloon PCI in Different Plaque Morphologies: A Narrative Review. Biomedicines, 13(6), 1472. https://doi.org/10.3390/biomedicines13061472







