Polymer-Free Versus Biodegradable Polymer Drug-Eluting Stents in Coronary Artery Disease: Updated Systematic Review and Meta-Analysis of Clinical, Angiographic, and OCT Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Design and Reporting Guidelines
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Collection
2.6. Data Items
2.7. Risk of Bias Assessment
2.8. Data Synthesis and Statistical Analysis
2.9. Reporting Bias
2.10. Additional Analyses
2.11. Certainty of Evidence
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. 12-Month Clinical Outcomes
3.4. 24-Month Clinical Outcomes
3.5. 12-Month Device-Oriented Clinical Outcomes
3.6. Angiographic and OCT Outcomes
3.7. Additional Analyses
3.8. Quality Assessment and Publication Bias
3.9. Certainty of Evidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndromes |
| BP-DES | biodegradable polymer drug-eluting stent |
| CAD | coronary artery disease |
| CCS | chronic coronary syndromes |
| DAPT | dual antiplatelet therapy |
| DES | drug-eluting stent |
| LLL | late lumen loss |
| MD | mean difference |
| MI | myocardial infarction |
| MLD | mean lumen diameter |
| OCT | optical coherence tomography |
| PCI | percutaneous coronary intervention |
| PF-DES | polymer-free drug-eluting stent |
| RCT | randomized controlled trial |
| RR | risk ratio |
| STEMI | ST-elevated myocardial infarction |
| TLR | target lesion revascularization |
| TVR | target vessel revascularization |
References
- Piccolo, R.; Bonaa, K.H.; Efthimiou, O.; Varenne, O.; Baldo, A.; Urban, P.; Kaiser, C.; Remkes, W.; Räber, L.; de Belder, A.; et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: A systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2019, 393, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of Drug-Eluting Stents in Humans. J. Am. Coll. Cardiol. 2006, 48, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hausleiter, J.; Kastrati, A.; Wessely, R.; Dibra, A.; Mehilli, J.; Schratzenstaller, T.; Graf, I.; Renke-Gluszko, M.; Behnisch, B.; Dirschinger, J.; et al. Prevention of restenosis by a novel drug-eluting stent system with a dose-adjustable, polymer-free, on-site stent coating. Eur. Heart J. 2005, 26, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- John, M.C.; Wessely, R.; Kastrati, A.; Schömig, A.; Uchihashi, M.; Crimins, J.; Lajoie, S.; Kolodgie, F.D.; Gold, H.K.; Virmani, R.; et al. Differential healing responses in polymer- and nonpolymer-based sirolimus-eluting stents. JACC Cardiovasc. Interv. 2008, 1, 535–544. [Google Scholar] [CrossRef]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef]
- Briguori, C.; Sarais, C.; Pagnotta, P.; Liistro, F.; Montorfano, M.; Chieffo, A.; Sgura, F.; Corvaja, N.; Albiero, R.; Stankovic, G.; et al. In-stent restenosis in small coronary arteries. J. Am. Coll. Cardiol. 2002, 40, 403–409. [Google Scholar] [CrossRef]
- Kastrati, A.; Mehilli, J.; Dirschinger, J.; Dotzer, F.; Schuhlen, H.; Neumann, F.-J.; Fleckenstein, M.; Pfafferott, C.; Seyfarth, M.; Schomig, A. Intracoronary Stenting and Angiographic Results. Circulation 2001, 103, 2816–2821. [Google Scholar] [CrossRef]
- Nogic, J.; Baey, Y.; Nerlekar, N.; Ha, F.J.; Cameron, J.D.; Nasis, A.; West, N.E.; Brown, A.J. Polymer-free versus permanent polymer-coated drug eluting stents for the treatment of coronary artery disease: A meta-analysis of randomized trials. J. Interv. Cardiol. 2018, 31, 608–616. [Google Scholar] [CrossRef]
- Nogic, J.; Thein, P.; Mirzaee, S.; Comella, A.; Soon, K.; Cameron, J.D.; West, N.E.; Brown, A.J. Biodegradable-Polymer Versus Polymer-Free Drug-Eluting Stents for the Treatment of Coronary Artery Disease. Cardiovasc. Revasc. Med. 2019, 20, 865–870. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.N.; Maeng, M.; Antonsen, L.; Maehara, A.; Jakobsen, L.; Ellert, J.; Terkelsen, C.J.; Ahlehoff, O.; Thim, T.; Fallesen, C.O.; et al. Early vascular healing after implantation of the polymer-free biolimus-eluting stent or the ultrathin strut biodegradable polymer sirolimus-eluting stent in patients with ST-segment elevation myocardial infarction. Coron. Artery Dis. 2022, 33, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Mehilli, J.; Byrne, R.A.; Wieczorek, A.; Iijima, R.; Schulz, S.; Bruskina, O.; Pache, J.; Wessely, R.; Schömig, A.; Kastrati, A.; et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur. Heart J. 2008, 29, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Kufner, S.; Tiroch, K.; Massberg, S.; Laugwitz, K.-L.; Birkmeier, A.; Schulz, S.; Mehilli, J.; ISAR-TEST-3 Investigators. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart 2009, 95, 1489–1494. [Google Scholar] [CrossRef]
- Jensen, L.O.; Maeng, M.; Raungaard, B.; Kahlert, J.; Ellert, J.; Jakobsen, L.; Villadsen, A.B.; Veien, K.T.; Kristensen, S.D.; Ahlehoff, O.; et al. Randomized Comparison of the Polymer-Free Biolimus-Coated BioFreedom Stent With the Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Orsiro Stent in an All-Comers Population Treated With Percutaneous Coronary Intervention. Circulation 2020, 141, 2052–2063. [Google Scholar] [CrossRef]
- Ellert-Gregersen, J.; Jensen, L.J.; Jakobsen, L.; Freeman, P.M.; Eftekhari, A.; Maeng, M.; Raungaard, B.; Engstroem, T.; Kahlert, J.; Hansen, H.H.; et al. Polymer-free biolimus-coated stents versus ultrathin-strut biodegradable polymer sirolimus-eluting stents: Two-year outcomes of the randomised SORT OUT IX trial. EuroIntervention 2022, 18, e124–e131. [Google Scholar] [CrossRef]
- Gómez-Lara, J.; Oyarzabal, L.; Brugaletta, S.; Salvatella, N.; Romaguera, R.; Roura, G.; Fuentes, L.; Fuentes, P.P.; Ortega-Paz, L.; Ferreiro, J.L. Coronary endothelial and microvascular function distal to polymer-free and endothelial cell-capturing drug-eluting stents. The randomized FUNCOMBO trial. Rev. Esp. Cardiol. 2021, 74, 1013–1022. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, J.-S.; Hong, S.J.; Lim, D.-S.; Lee, S.-Y.; Yun, K.H.; Park, J.-K.; Kang, W.C.; Kim, Y.H.; Yoon, H.-J.; et al. 1-Month Dual-Antiplatelet Therapy Followed by Aspirin Monotherapy After Polymer-Free Drug-Coated Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 1801–1811. [Google Scholar] [CrossRef]
- Otaegui Irurueta, I.; González Sucarrats, S.; Barrón Molina, J.L.; Pérez de Prado, A.; Massotti, M.; Carmona Ramírez, M.Á.; Martí, G.; Bellera, N.; Serra, B.; Serra, V. Can an ultrathin strut stent design and a polymer free, proendothelializing probucol matrix coating improve early strut healing? The FRIENDLY-OCT trial. An intra-patient randomized study with OCT, evaluating early strut coverage of a novel probucol coated polymer-free and ultra-thin strut sirolimus-eluting stent compared to a biodegradable polymer sirolimus-eluting stent. Int. J. Cardiol. 2022, 360, 13–20. [Google Scholar]
- Piccolo, R.; Calabrò, P.; Carrara, G.; Varricchio, A.; Baldi, C.; Napolitano, G.; De Simone, C.; Mauro, C.; Stabile, E.; Caiazzo, G.; et al. Polymer-free versus biodegradable-polymer drug-eluting stent in patients undergoing percutaneous coronary intervention: An assessor-blind, non-inferiority, randomised controlled trial. EuroIntervention 2025, 21, 58–72. [Google Scholar] [CrossRef]
- Tao, L.; Li, Z.; Yin, Z.; Lin, W.; Liu, Y.; Li, H.; Yu, B.; Li, W.; Xu, B.; RECOVERY Trial Investigators. Nine-month angiographic and 2-year clinical outcomes of the RECOVERY trial: A randomized study of the biodegradable polymer sirolimus-eluting COMBO dual-therapy stent versus a polymer-free sirolimus-eluting stent in Chinese patients. Catheter. Cardiovasc. Interv. 2021, 97, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Gopinath, K.; Koshy, G.; Gupta, P.N.; Velappan, P. Open-labeled randomized controlled trial to evaluate the 1-year clinical outcomes of polymer-free sirolimus-eluting coronary stents as compared with biodegradable polymer-based sirolimus-eluting coronary stents. Indian Heart J. 2018, 70, S323–S328. [Google Scholar] [CrossRef] [PubMed]

| Study | Design | Country | N. Patients PF/BP | N. Lesions Treated PF/BP | PF-DES, Name | PF-DES, Platform Material | PF-DES, Strut Thickness (mcm) | PF-DES, Drug Eluted | BP-DES, Name | BP-DES, Platform Material | BP-DES, Strut Thickness (mcm) | BP-DES, Drug Eluted | BP-DES, Polymer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISAR-TEST 3 [14,15] | RCT | Germany | 201/202 | 231/232 | NA | 316L stainless steel | 140 | Rapamycine | NA | 316L stainless steel | 87 | Sirolimus | PLA |
| SORT-OUT IX [16,17] | RCT | Denmark | 1572/1579 | 1966/1985 | BioFreedom | 316L stainless steel | 114–119 | Biolimus A9 | Orsiro | CoCr | 60–80 | Sirolimus | PLLA |
| Hansen 2022 [13] | RCT | Denmark | 38/42 | 38/42 | BioFreedom | 316L stainless steel | 120 | Biolimus A9 | Orsiro | CoCr | 60–80 | Sirolimus | PLLA |
| Gomez 2021 [18] | RCT | Spain | 30/30 | 29/31 | BioFreedom | 316L stainless steel | 120 | Biolimus A9 | Combo | CoCr | 100 | Sirolimus | PLLA |
| Hong 2021 [19] | RCT | South Korea | 1507/1513 | 1507/1513 | BioFreedom | 316L stainless steel | 120 | Biolimus A9 | Ultimaster | CoCr | 80 | Sirolimus | PLLA |
| Otaegui 2022 [20] | RCT | Spain | 70/70 | 70/70 | Coroflex ISAR | CoCr | 60 | Probucol | Ultimaster | CoCr | 80 | Sirolimus | PLLA |
| Piccolo 2025 [21] | RCT | Italy | 1051/1056 | 1513/1529 | Cre8 EVO | CoCr | 70–80 | Amphilimus | SYNERGY | PtCr | 74–81 | Everolimus | PLGA |
| Tao 2021 [22] | RCT | China | 216/216 | 249/245 | Nano | CoCr | 90–100 | Sirolimus | Combo | CoCr | 100 | Sirolimus | PLLA |
| Viswanathan 2018 [23] | RCT | India | 91/113 | 91/113 | NA | 316L stainless steel | 87 | Sirolimus | NA | 316L stainless steel | 87 | Sirolimus | PDLLA |
| Study | Clinical Setting | Age †, y PF/BP | BMI †, PF/BP | Male, % PF/BP | DM, % PF/BP | HTN, % PF/BP | Smokers, % PF/BP | Dyslipidemia, % PF/BP | Prior MI, % PF/BP | Prior CABG, % PF/BP | Prior PCI, % PF/BP | LVEF †, % PF/BP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISAR-TEST 3 [14,15] | Non-emergent PCI 1 | 66.8/66.5 | 27.2/27.4 | 78.1/78.2 | 27.2/28.7 | 67.2/71.8 | 17.8/16.3 | 71.1/71.3 | 32.9/32.2 | 13.4/10.4 | NA | 53.5/53.8 |
| SORT-OUT IX [16,17] | All comers | 66.4/66.1 | 27.8/27.6 | 77.5/77.3 | 19.3/19.2 | 59.0/56.0 | 29.8/29.3 | 55.0/51.5 | 14.7/15.2 | 8.4/7.0 | 20.9/20.9 | NA |
| Hansen 2022 [13] | STEMI | 61.3/62.6 | 27.3/27.1 | 81.6/83.3 | 5.3/11.9 | 34.2/40.5 | 45.2/39.5 | 18.4/14.2 | 7.9/2.4 | 0/0 | 7.9/4.8 | NA |
| Gomez 2021 [18] | STEMI | 57.1/57.2 | 28.1/27.4 | 93.1/77.4 | 20.7/6.5 | 48.3/32.3 | 69.0/67.7 | 58.6/51.6 | NA | NA | 0/3.2 | 52.0/52.4 |
| Hong 2021 [19] | Non-emergent PCI 2 | 67.0/67.0 | 24.7/24.7 | 69/69 | 37.0/38.0 | 67.0/66.0 | 17.0/16.0 | 81.0/82.0 | 4.0/4.0 | 1.0/2.0 | 16/18.1 | 63.0/63.0 |
| Otaegui 2022 [20] § | Non-emergent PCI 3 | 67.0/67.0 | NA | 78.6 | 30 | 75.7 | 55.7 | 61.4 | 17.1 | 4.3 | 21.4 | NA |
| Piccolo 2025 [21] | All comers | 63.8/64.0 | 28.0/28.2 | 77.2/78.7 | 30.6/31.8 | 73.9/75.7 | 49.9/49.8 | 62.4/62.5 | 20.5/19.3 | 3.4/4.2 | 21.3/21.8 | 49.2/49.1 |
| Tao 2021 [22] | All comers | 59.3/58.3 | NA | 63.4/68.1 | 21.3/19.9 | 60.2/53.7 | 43.1/44.9 | 17.1/12.5 | 13.0/13.4 | 0.5/0.5 | 9.3/9.7 | 60.2/60.0 |
| Viswanathan 2018 [23] | Elective PCI | 55.8/56.9 | NA | 87.9/73.4 | 49.4/44.2 | 49.2/51.3 | NA | NA | NA | NA | NA | 57.3/58.7 |
| Study | TL:LAD, %, PF/BP | TL:LCX, %, PF/BP | TL:RCA, %, PF/BP | TL:LM, %, PF/BP | Stent Diameter †, mm, PF/BP | Stent Length †, mm, PF/BP | Diameter Stenosis †, %, PF/BP | MLD, pre, mm, PF/BP | Predilatation, %, PF/BP | Postdilatation, %, PF/BP |
|---|---|---|---|---|---|---|---|---|---|---|
| ISAR-TEST 3 [14,15] | 39.8/46.0 | 29.5/22.2 | 30.7/31.8 | NA | 2.75/2.74 | 14.3/13.9 | 58.8/61.5 | 1.13/1.06 | NA | NA |
| SORT-OUT IX [16,17] | 43.0/43.0 | 23.0/22.4 | 30.0/31.3 | 2.5/2.9 | NA | 30.6/31.1 | NA | NA | 91.8/90.2 | NA |
| Hansen 2022 [13] | 42.9/31.6 | 11.9/13.2 | 45.2/55.3 | NA | NA | 25.0/25.6 | NA | NA | NA | NA |
| Gomez 2021 [18] | 44.8/48.4 | 20.7/16.1 | 34.5/35.5 | NA | 3.08/3.04 | 20.1/18.3 | NA | 2.70/2.69 | 20.6/22.5 | 3.44/12.9 |
| Hong 2021 [19] | 56.0/55.0 | 19.0/18.1 | 25.0/26.7 | NA | 3.1/3.1 | 20.3/20.5 | 71.4/71.4 | 0.8/0.8 | NA | NA |
| Otaegui 2022 § [20] | 34.3/34.3 | 35.7/31.4 | 30.0/34.3 | NA | 2.9/3.0 | 19.9/19.4 | 71.4/66.3 | 0.88/1.02 | 71.4/70 | 38.6/47.1 |
| Piccolo 2025 [21] | 46.3/44.6 | 22.9/23.3 | 29.5/29.6 | 1.3/2.4 | 3.0/3.0 | 29.1/29.1 | NA | NA | 62.6/60.9 | 61.9/57.2 |
| Tao 2021 [22] | 47.0/51.8 | 24.1/19.2 | 28.9/29.0 | NA | 3.17/3.17 | 24.9/24.1 | 68.4/67.7 | 0.92/0.94 | 91.6/91.8 | 72.3/74.3 |
| Viswanathan 2018 [23] | NA | NA | NA | NA | 3.18/2.97 | 28.2/29.6 | NA | NA | 68.9/67.9 | 26.0/38.3 |
| Study | DAPT Duration PF-DES | DAPT Duration BP-DES | ASA (%) | Clopidogrel (%) | Prasugrel (%) | Ticagrelor (%) | Notes |
|---|---|---|---|---|---|---|---|
| ISAR-TEST 3 [14,15] | 12 months | 12 months | All patients | All patients | Not used | Not used | Clopidogrel 600 mg pre-PCI, then 75 mg/day × 12 m |
| SORT-OUT IX [16,17] | 12 months | 12 months | All patients | Standard per protocol | Allowed | Allowed | DAPT per guideline, no stratified data |
| Hansen 2022 [13] | 1 month | 12 months | All patients | All patients | Not reported | Not reported | DAPT: ASA + Clopidogrel for 1 month (PF), 12 months (BP) |
| Hong 2021 [19] | 1 month | 6–12 months | All patients | 95.3 | 0.4 | 4.3 | Data from Table 2 of publication |
| Otaegui 2022 [20] | 3 months | 3 months | All patients | All patients | Not used | Not used | 3 months DAPT confirmed in Methods Section. |
| Piccolo 2025 [21] | 3–24 months | 3–24 months | All patients | CCS | ACS | ACS | Individualized by DAPT score in PARTHENOPE trial |
| Viswanathan 2018 [23] | 12 months | 12 months | All patients | All patients | Not used | Not used | Standard DAPT for 12 months |
| Gomez 2021 [18] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Tao 2021 [22] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | PF-DES | BP-DES | Relative (95% CI) | Absolute (95% CI) | ||
| Myocardial Infarction (follow-up: mean 12 months; assessed with: n) | ||||||||||||
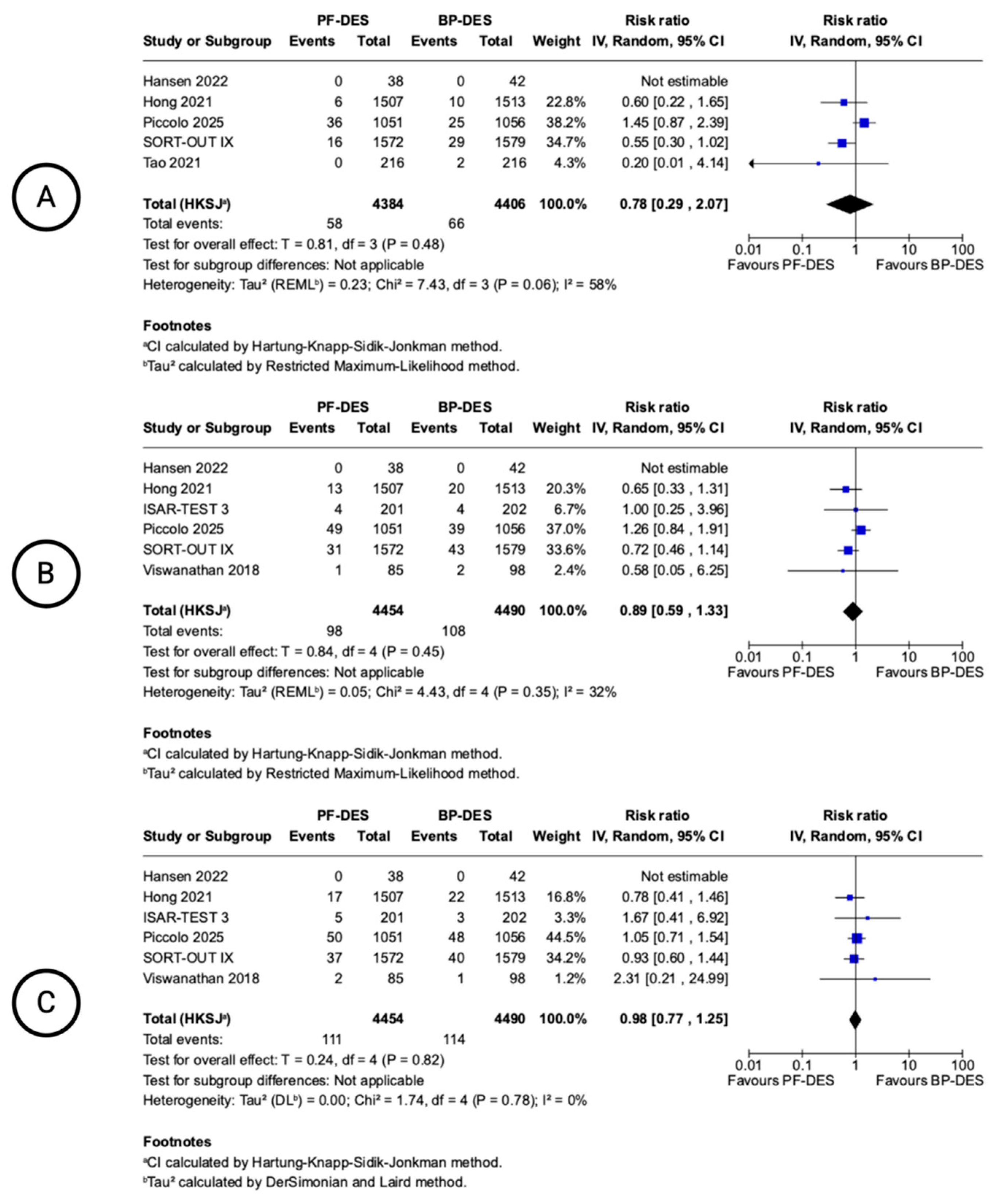
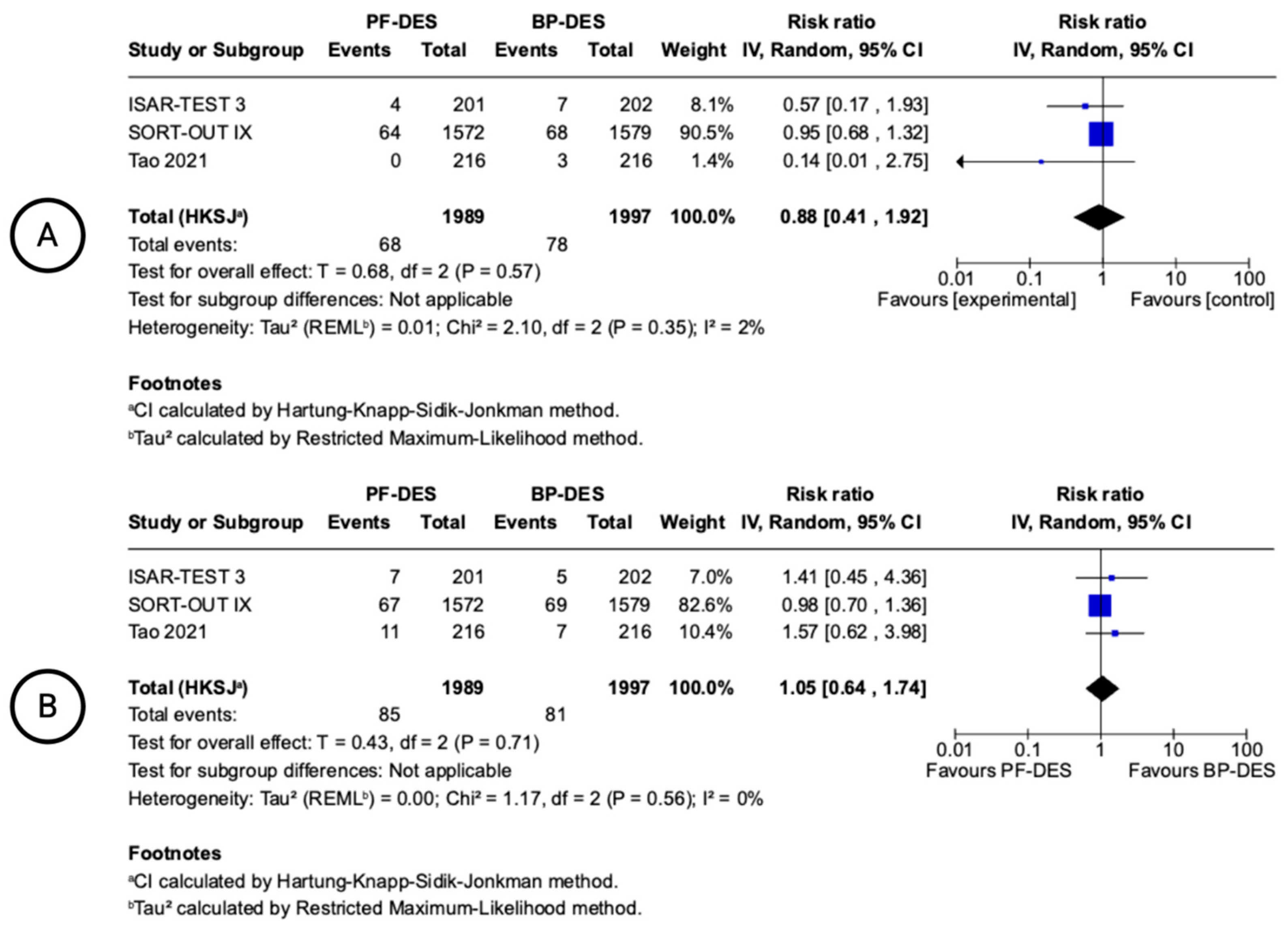
| 6 | randomised trials | not serious | not serious a | not serious b | not serious c | none | 111/4454 (2.5%) | 114/4490 (2.5%) | RR 0.98 (0.77 to 1.25) | 1 fewer per 1.000 (from 6 fewer to 6 more) | ⨁⨁⨁⨁ High a,b,c | CRITICAL |
| All-cause death (follow-up: mean 12 months; assessed with: n) | ||||||||||||
| 6 | randomised trials | not serious | Serious d | not serious e | Serious f | none | 98/4454 (2.2%) | 108/4490 (2.4%) | RR 0.89 (0.59 to 1.33) | 3 fewer per 1.000 (from 10 fewer to 8 more) | ⨁⨁◯◯ Low d,e,f | CRITICAL |
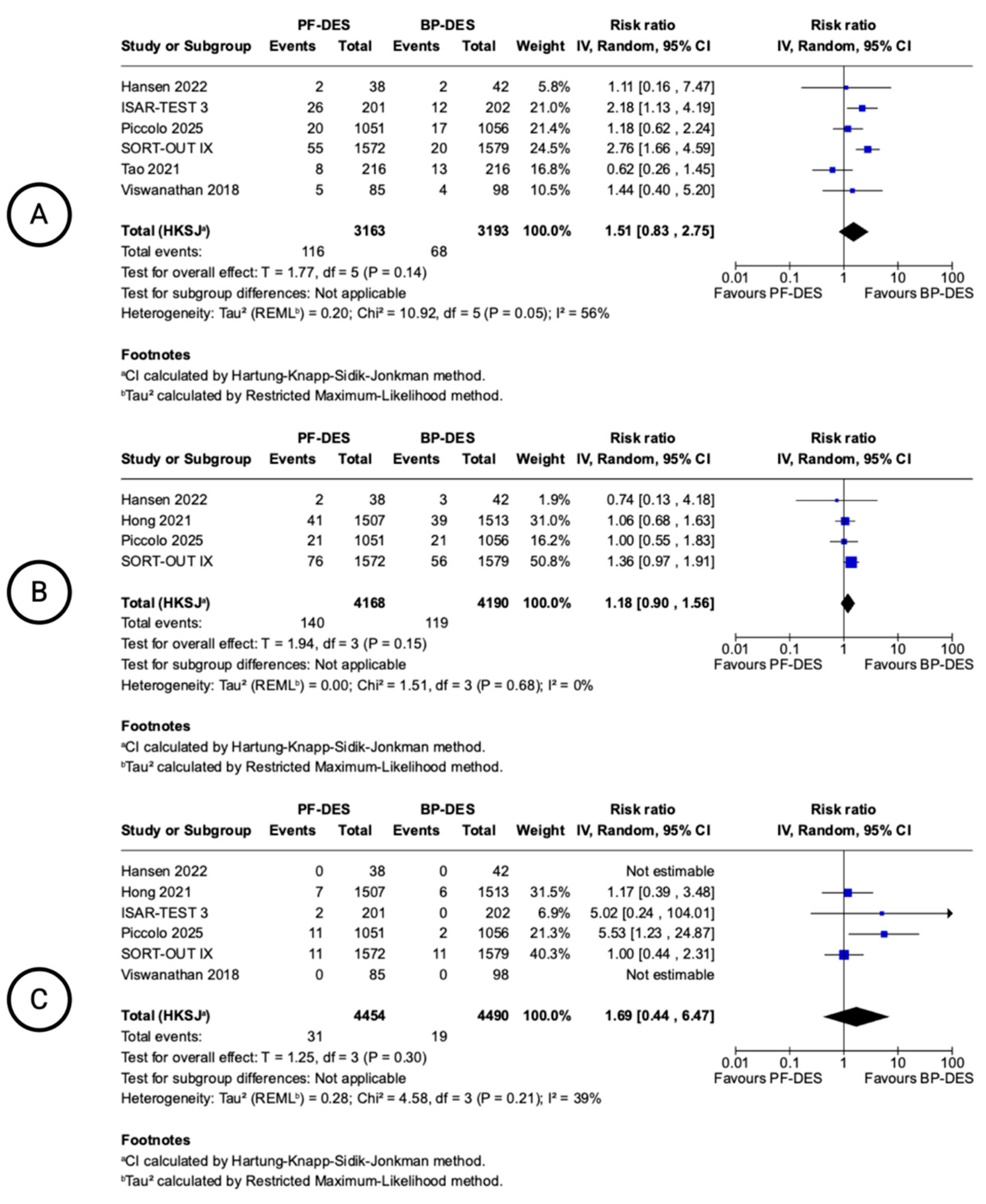
| Target Lesion Revascularization (follow-up: mean 12 months; assessed with: n) | ||||||||||||
| 6 | randomised trials | not serious | not serious | not serious g | Serious h | none | 116/3163 (3.7%) | 68/3193 (2.1%) | RR 1.51 (0.83 to 2.75) | 11 more per 1.000 (from 4 fewer to 37 more) | ⨁⨁⨁◯ Moderate g,h | CRITICAL |
| Stent thrombosis (follow-up: mean 12 months; assessed with: n) | ||||||||||||
| 6 | randomised trials | not serious | not serious a | not serious g | Serious i | none | 31/4454 (0.7%) | 19/4490 (0.4%) | RR 1.69 (0.44 to 6.47) | 3 more per 1.000 (from 2 fewer to 23 more) | ⨁⨁⨁◯ Moderate a,g,i | CRITICAL |
| Cardiac death (follow-up: mean 12 months; assessed with: n) | ||||||||||||
| 5 | randomised trials | serious d | not serious g | not serious f | serious f | none | 58/4384 (1.3%) | 66/4406 (1.5%) | RR 0.78 (0.29 to 2.07) | 3 fewer per 1.000 (from 11 fewer to 16 more) | ⨁⨁◯◯ Low d,f,g | CRITICAL |
| Target Vessel revascularization (follow-up: mean 12 months; assessed with: n) | ||||||||||||
| 4 | randomised trials | not serious | serious j | not serious g | not serious k | none | 140/4168 (3.4%) | 119/4190 (2.8%) | RR 1.18 (0.90 to 1.56) | 5 more per 1.000 (from 3 fewer to 16 more) | ⨁⨁⨁◯ Moderate g,j,k | CRITICAL |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | PF-DES | BP-DES | Relative (95% CI) | Absolute (95% CI) | ||
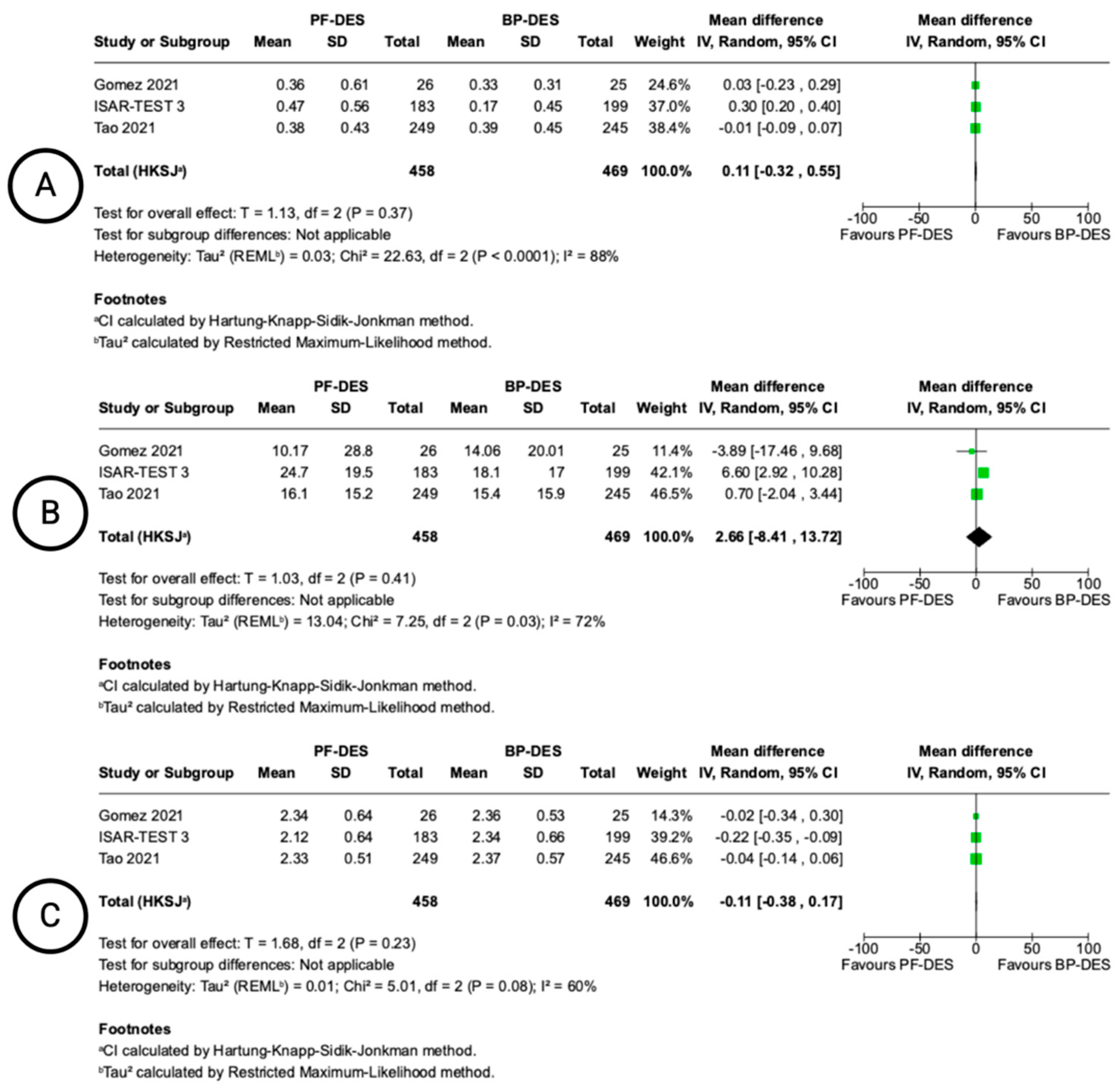
| Late Lumen Loss (follow-up: range 6 months to 9 months; assessed with: QCA) | ||||||||||||
| 3 | randomised trials | not serious | very serious a | Serious b | Serious c | none | 458 | 469 | - | MD 0.11 mm higher (0.32 lower to 0.55 higher) | ⨁◯◯◯ Very low a,b,c | IMPORTANT |
| Binary Restenosis (follow-up: range 6 months to 9 months; assessed with: QCA) | ||||||||||||
| 3 | randomised trials | not serious | very serious d | Serious e | Serious c | none | 458 | 469 | - | MD 2.66 % higher (8.41 lower to 13.72 higher) | ⨁◯◯◯ Very low c,d,e | IMPORTANT |
| Minimal Lumen Diameter (follow-up: range 6 months to 9 months; assessed with: QCA) | ||||||||||||
| 3 | randomised trials | not serious | very serious f | Serious g | Serious h | none | 458 | 469 | - | MD 0.11 mm lower (0.38 lower to 0.17 higher) | ⨁◯◯◯ Very low f,g,h | IMPORTANT |
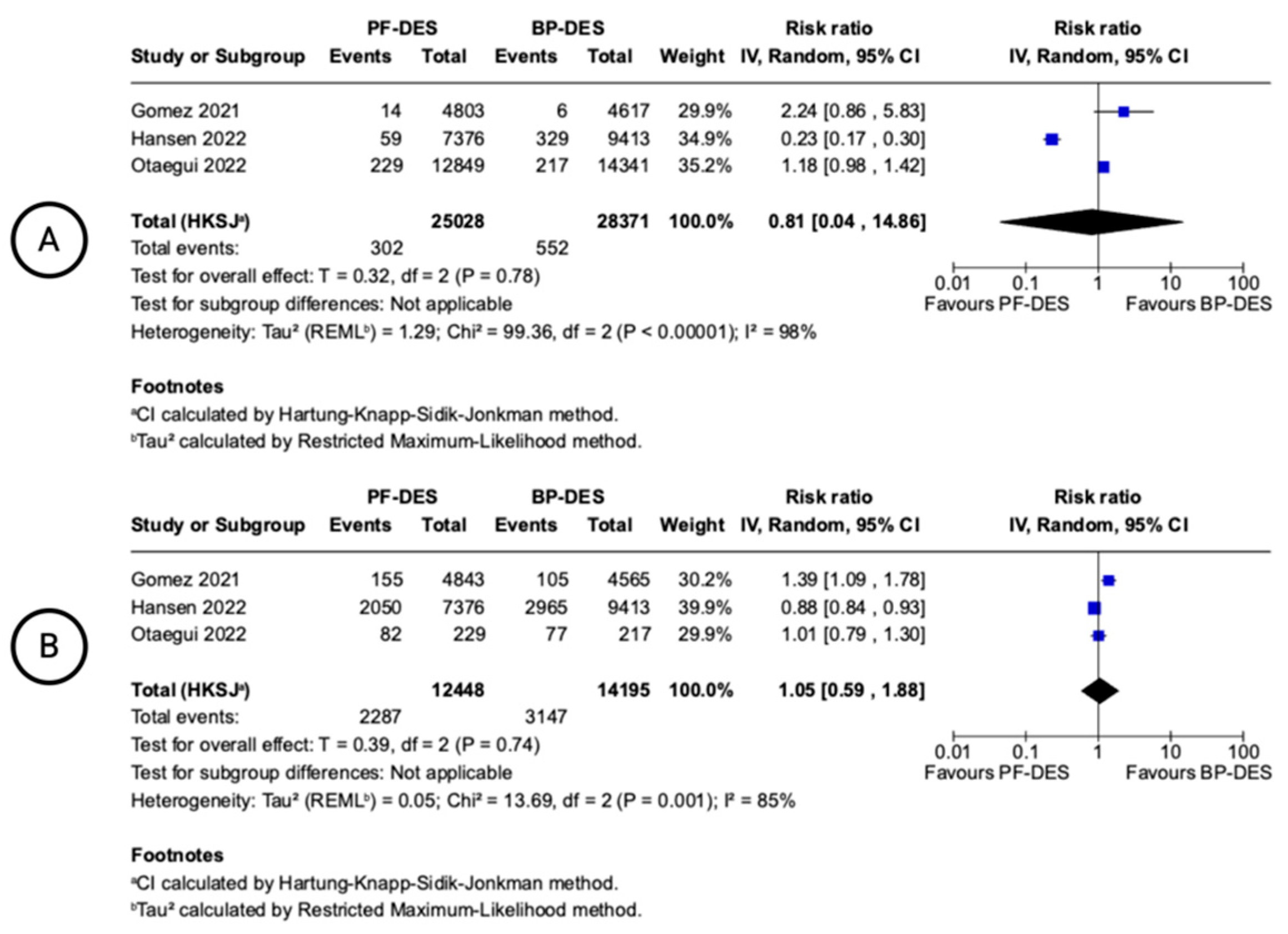
| Malapposed Struts (follow-up: range 1 months to 6 months; assessed with: OCT) | ||||||||||||
| 3 | randomised trials | not serious | very serious i | Serious j | Serious k | none | 302/25028 (1.2%) | 552/28371 (1.9%) | RR 0.81 (0.04 to 14.86) | 4 fewer per 1.000 (from 19 fewer to 270 more) | ⨁◯◯◯ Very low i,j,k | NOT IMPORTANT |
| Uncovered Struts (follow-up: range 1 months to 6 months; assessed with: OCT) | ||||||||||||
| 3 | randomised trials | not serious | very serious l | Serious m | Serious n | none | 2247/12448 (18.1%) | 3147/14195 (22.2%) | RR 1.05 (0.59 to 1.88) | 11 more per 1.000 (from 91 fewer to 195 more) | ⨁◯◯◯ Very low l,m,n | NOT IMPORTANT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetta, M.; Sasso, S.; Paragliola, V.; Parato, A.G.; De Angelis, D.; Russo, G.; Albano, G.; Benedetto, D.; Moretti, F.; Valenti, F.; et al. Polymer-Free Versus Biodegradable Polymer Drug-Eluting Stents in Coronary Artery Disease: Updated Systematic Review and Meta-Analysis of Clinical, Angiographic, and OCT Outcomes. Biomedicines 2025, 13, 1470. https://doi.org/10.3390/biomedicines13061470
Marchetta M, Sasso S, Paragliola V, Parato AG, De Angelis D, Russo G, Albano G, Benedetto D, Moretti F, Valenti F, et al. Polymer-Free Versus Biodegradable Polymer Drug-Eluting Stents in Coronary Artery Disease: Updated Systematic Review and Meta-Analysis of Clinical, Angiographic, and OCT Outcomes. Biomedicines. 2025; 13(6):1470. https://doi.org/10.3390/biomedicines13061470
Chicago/Turabian StyleMarchetta, Marcello, Stefano Sasso, Vincenzo Paragliola, Andrea Giovanni Parato, Diego De Angelis, Giulio Russo, Giovanni Albano, Daniela Benedetto, Federico Moretti, Francesco Valenti, and et al. 2025. "Polymer-Free Versus Biodegradable Polymer Drug-Eluting Stents in Coronary Artery Disease: Updated Systematic Review and Meta-Analysis of Clinical, Angiographic, and OCT Outcomes" Biomedicines 13, no. 6: 1470. https://doi.org/10.3390/biomedicines13061470
APA StyleMarchetta, M., Sasso, S., Paragliola, V., Parato, A. G., De Angelis, D., Russo, G., Albano, G., Benedetto, D., Moretti, F., Valenti, F., Massaro, G., Chiricolo, G., Tesauro, M., & Sangiorgi, G. M. (2025). Polymer-Free Versus Biodegradable Polymer Drug-Eluting Stents in Coronary Artery Disease: Updated Systematic Review and Meta-Analysis of Clinical, Angiographic, and OCT Outcomes. Biomedicines, 13(6), 1470. https://doi.org/10.3390/biomedicines13061470









