Th1 Cytokines Inhibit Acinar Morphogenesis and Milk Protein Expression in 3D Mammary Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Immunoprecipitation and Western Blot Analysis
2.4. Immunofluorescence Microscopy
2.5. Cell Proliferation Assay
2.6. Live/Dead Cell Analysis
2.7. Statistical Analysis
3. Results
3.1. Combined Treatment with IFN-γ and TNF-α Inhibits Prolactin Signaling and Subsequent β-Casein Expression in Primary Mouse Mammary Epithelial Cells
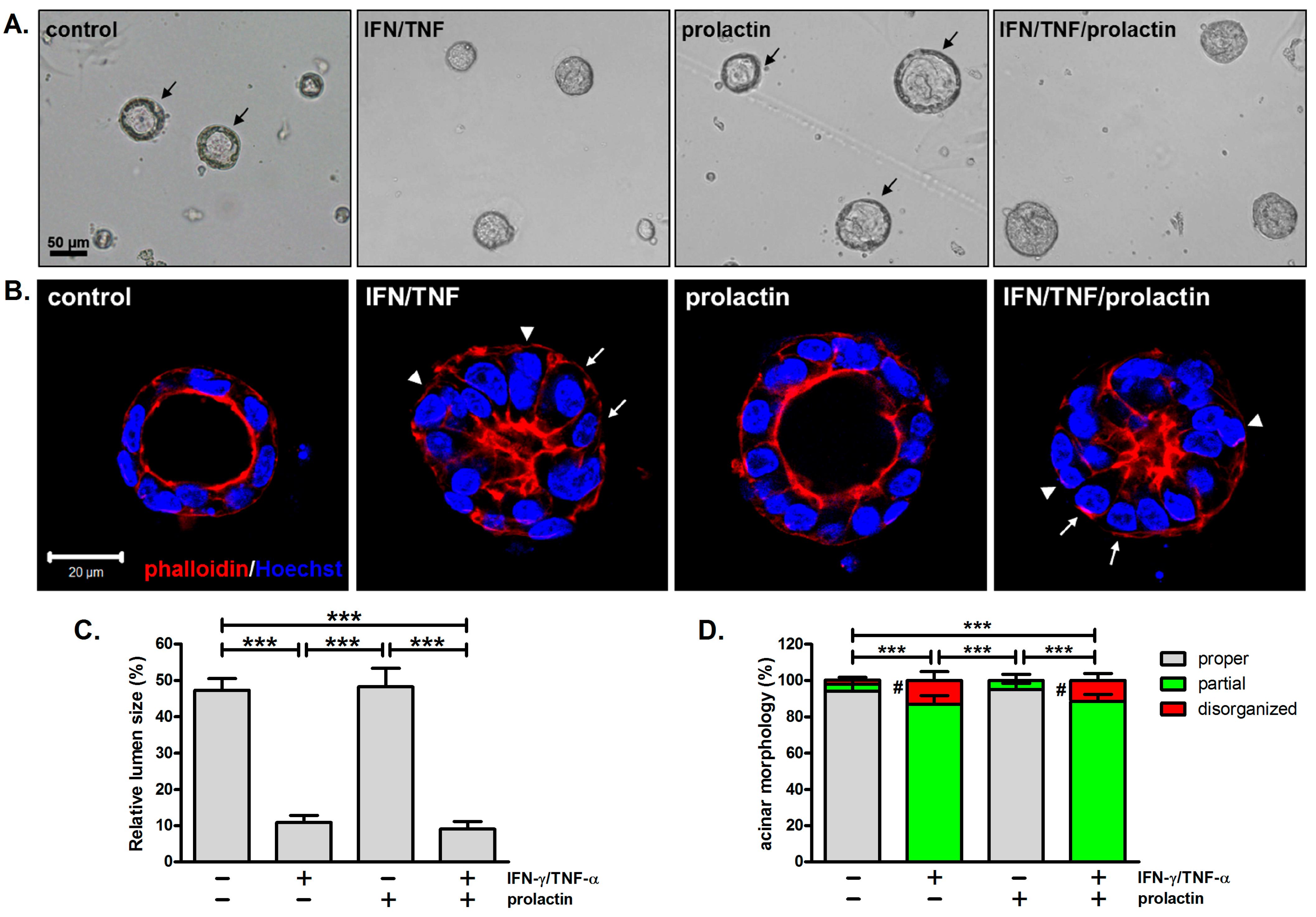
3.2. Combined Treatment with IFN-γ and TNF-α Alters Acinar Morphology
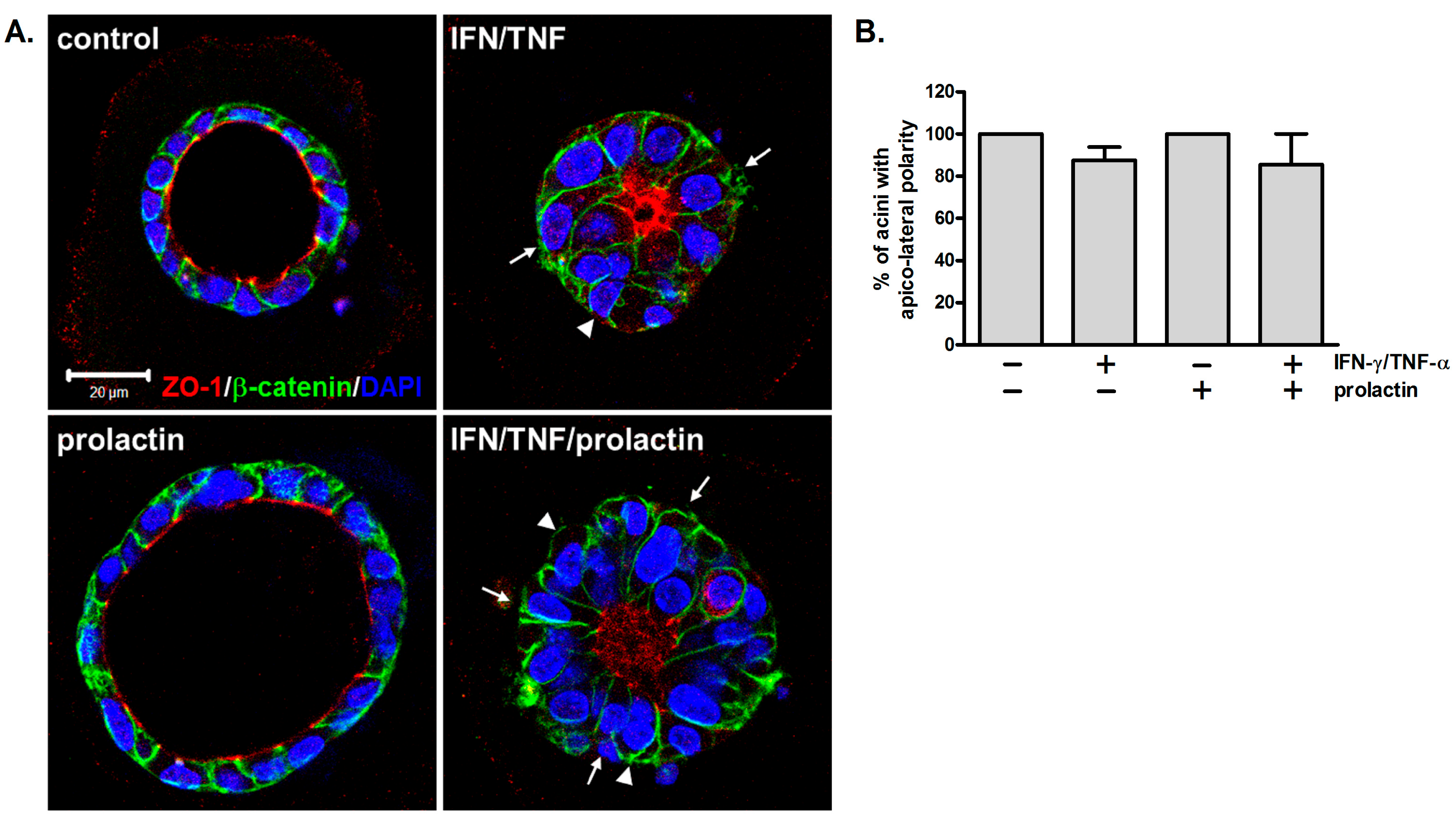
3.3. Apico-Lateral Polarity Is Largely Preserved in IFN-γ/TNF-α-Treated Acini
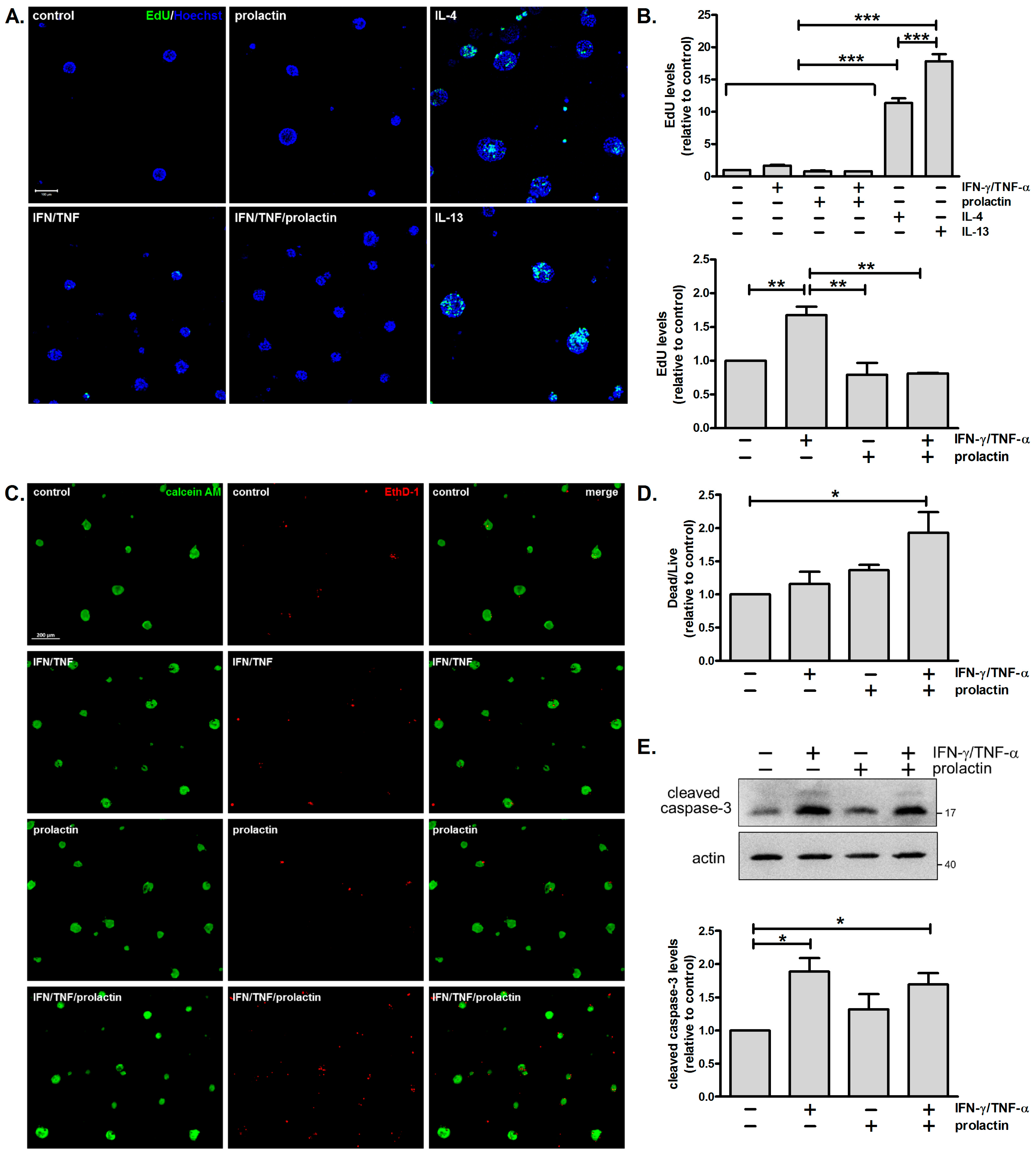
3.4. Combined Treatment with IFN-γ and TNF-α Moderately Enhances Cell Proliferation and Cell Death
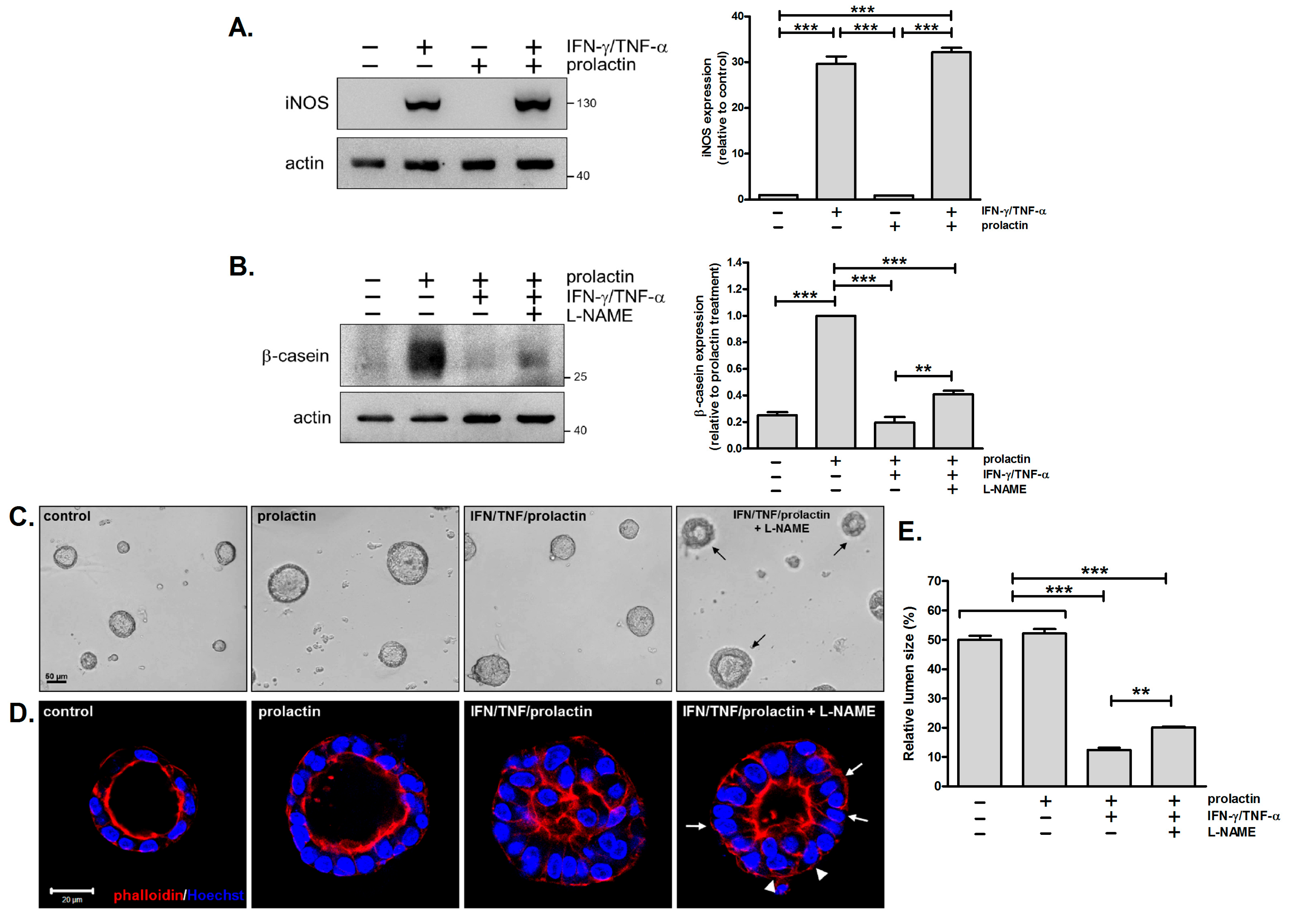
3.5. Inhibition of iNOS Partially Reverses the Detrimental Effects of IFN-γ/TNF-α on β-Casein Expression and Acinar Morphology
4. Discussion
4.1. Detrimental Effects of IFN-γ/TNF-α on Milk Protein Expression
4.2. Detrimental Effects of IFN-γ/TNF-α on Acinar Morphology
4.3. Involvement of iNOS in the Detrimental Effects of IFN-γ/TNF-α
4.4. Implications for Mastitis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| Th | T helper |
References
- Watson, C.J.; Khaled, W.T. Mammary development in the embryo and adult: New insights into the journey of morphogenesis and commitment. Development 2020, 147, 169862. [Google Scholar] [CrossRef]
- Kreuzaler, P.A.; Staniszewska, A.D.; Li, W.; Omidvar, N.; Kedjouar, B.; Turkson, J.; Poli, V.; Flavell, R.A.; Clarkson, R.W.; Watson, C.J. Stat3 controls lysosomal-mediated cell death in vivo. Nat. Cell Biol. 2011, 13, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Oliver, C.H.; Khaled, W.T. Cytokine signalling in mammary gland development. J. Reprod. Immunol. 2011, 88, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Muschler, J.; Streuli, C.H. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003202. [Google Scholar] [CrossRef]
- Dawson, C.A.; Visvader, J.E. The cellular organization of the mammary gland: Insights from microscopy. J. Mammary Gland Biol. Neoplasia 2021, 26, 71–85. [Google Scholar] [CrossRef]
- Vickers, R.; Porter, W. Immune cell contribution to mammary gland development. J. Mammary Gland Biol. Neoplasia 2024, 29, 16. [Google Scholar] [CrossRef]
- Plaks, V.; Boldajipour, B.; Linnemann, J.R.; Nguyen, N.H.; Kersten, K.; Wolf, Y.; Casbon, A.J.; Kong, N.; van den Bijgaart, R.J.; Sheppard, D.; et al. Adaptive immune regulation of mammary postnatal organogenesis. Dev. Cell 2015, 34, 493–504. [Google Scholar] [CrossRef]
- Stewart, T.A.; Hughes, K.; Hume, D.A.; Davis, F.M. Developmental stage-specific distribution of macrophages in mouse mammary gland. Front. Cell Dev. Biol. 2019, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.A.; Pal, B.; Vaillant, F.; Gandolfo, L.C.; Liu, Z.; Bleriot, C.; Ginhoux, F.; Smyth, G.K.; Lindeman, G.J.; Mueller, S.N.; et al. Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nat. Cell Biol. 2020, 22, 546–558. [Google Scholar] [CrossRef]
- Degnim, A.C.; Brahmbhatt, R.D.; Radisky, D.C.; Hoskin, T.L.; Stallings-Mann, M.; Laudenschlager, M.; Mansfield, A.; Frost, M.H.; Murphy, L.; Knutson, K.; et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res. Treat. 2014, 144, 539–549. [Google Scholar] [CrossRef]
- Corral, D.; Ansaldo, E.; Delaleu, J.; Pichler, A.C.; Kabat, J.; Oguz, C.; Teijeiro, A.; Yong, D.; Abid, M.; Rivera, C.A.; et al. Mammary intraepithelial lymphocytes promote lactogenesis and offspring fitness. Cell 2025, 188, 1662–1680. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.; Hughes, K.; Pensa, S.; Lloyd-Lewis, B.; Watson, C.J. The immune environment of the mammary gland fluctuates during post-lactational regression and correlates with tumour growth rate. Development 2022, 149, 200162. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Gülçek, E.; Aydoğdu, Y.F.; Emreol, U.; Bağrıaçık, E.; Akyürek, N. Investigation of pre and postoperative Th1/Th2 cytokine balance and novel cytokines in colorectal cancer patients. Clin. Exp. Med. 2024, 24, 211. [Google Scholar] [CrossRef]
- Khaled, W.T.; Read, E.K.; Nicholson, S.E.; Baxter, F.O.; Brennan, A.J.; Came, P.J.; Sprigg, N.; McKenzie, A.N.; Watson, C.J. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development 2007, 134, 2739–2750. [Google Scholar] [CrossRef]
- Hughes, K.; Watson, C.J. The mammary microenvironment in mastitis in humans, dairy ruminants, rabbits and rodents: A one health focus. J. Mammary Gland Biol. Neoplasia 2018, 23, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Ingman, W.V.; Glynn, D.J.; Hutchinson, M.R. Inflammatory mediators in mastitis and lactation insufficiency. J. Mammary Gland Biol. Neoplasia 2014, 19, 161–167. [Google Scholar] [CrossRef]
- Tuaillon, E.; Viljoen, J.; Dujols, P.; Cambonie, G.; Rubbo, P.A.; Nagot, N.; Bland, R.M.; Badiou, S.; Newell, M.L.; Van de Perre, P. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatr. Res. 2017, 81, 556–564. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Kruse, B.; Buzzai, A.C.; Shridhar, N.; Braun, A.D.; Gellert, S.; Knauth, K.; Pozniak, J.; Peters, J.; Dittmann, P.; Mengoni, M.; et al. CD4+ T cell-induced inflammatory cell death controls immune-evasive tumours. Nature 2023, 618, 1033–1040. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat.Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.T.; Mori, H.; Mott, J.; Bissell, M.J. Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J. Mammary Gland Biol. Neoplasia 2012, 17, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Wang, S.H.; Wu, C.C.; Su, Y.A.; Chiang, C.Y.; Lai, C.H.; Wang, T.H.; Cheng, T.L.; Kuo, J.Y.; Hsu, T.C.; et al. IL-4 and IL-13 promote proliferation of mammary epithelial cells through STAT6 and IRS-1. Int. J. Mol. Sci. 2021, 22, 12008. [Google Scholar] [CrossRef]
- Basu, A.; Ramamoorthi, G.; Albert, G.; Gallen, C.; Beyer, A.; Snyder, C.; Koski, G.; Disis, M.L.; Czerniecki, B.J.; Kodumudi, K. Differentiation and regulation of TH cells: A balancing act for cancer immunotherapy. Front. Immunol. 2021, 12, 669474. [Google Scholar] [CrossRef] [PubMed]
- Olabi, S.; Ucar, A.; Brennan, K.; Streuli, C.H. Integrin-Rac signalling for mammary epithelial stem cell self-renewal. Breast Cancer Res. 2018, 20, 128. [Google Scholar] [CrossRef]
- Xia, X.; Che, Y.; Gao, Y.; Zhao, S.; Ao, C.; Yang, H.; Liu, J.; Liu, G.; Han, W.; Wang, Y.; et al. Arginine supplementation recovered the IFN-γ-mediated decrease in milk protein and fat synthesis by inhibiting the GCN2/eIF2α pathway, which induces autophagy in primary bovine mammary epithelial cells. Mol. Cells 2016, 39, 410–417. [Google Scholar] [CrossRef]
- Kobayashi, K.; Matsunaga, K.; Tsugami, Y.; Wakasa, H.; Nishimura, T. IL-1β is a key inflammatory cytokine that weakens lactation-specific tight junctions of mammary epithelial cells. Exp. Cell Res. 2021, 409, 112938. [Google Scholar] [CrossRef]
- Streuli, C.H.; Schmidhauser, C.; Bailey, N.; Yurchenco, P.; Skubitz, A.P.; Roskelley, C.; Bissell, M.J. Laminin mediates tissue-specific gene expression in mammary epithelia. J. Cell Biol. 1995, 129, 591–603. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 2021, 184, 149–168. [Google Scholar] [CrossRef]
- Rankin, L.C.; Artis, D. Beyond host defense: Emerging functions of the immune system in regulating complex tissue physiology. Cell 2018, 173, 554–567. [Google Scholar] [CrossRef]
- Castro-Navarro, I.; Pace, R.M.; Williams, J.E.; Pace, C.D.W.; Kaur, H.; Piaskowski, J.; Aragón, A.; Rodríguez, J.M.; McGuire, M.A.; Fernandez, L.; et al. Immunological composition of human milk before and during subclinical and clinical mastitis. Front. Immunol. 2025, 15, 1532432. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Lalonde, C.; Olszanski, L.A.; Zhao, F.Q. Localized and systemic inflammatory mediators in a murine acute mastitis model. J. Inflamm. Res. 2021, 14, 4053–4067. [Google Scholar] [CrossRef]
- Connelly, L.; Barham, W.; Pigg, R.; Saint-Jean, L.; Sherrill, T.; Cheng, D.S.; Chodosh, L.A.; Blackwell, T.S.; Yull, F.E. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. J. Cell. Physiol. 2010, 222, 73–81. [Google Scholar] [CrossRef]
- Starr, R.; Willson, T.A.; Viney, E.M.; Murray, L.J.; Rayner, J.R.; Jenkins, B.J.; Gonda, T.J.; Alexander, W.S.; Metcalf, D.; Nicola, N.A.; et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997, 387, 917–921. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Wittlin, S.; Lada, H.; Naylor, M.J.; Santamaria, M.; Zhang, J.G.; Starr, R.; Hilton, D.J.; Alexander, W.S.; Ormandy, C.J.; et al. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev. 2001, 15, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Rajaram, R.D. Alveolar and lactogenic differentiation. J. Mammary Gland Biol. Neoplasia 2006, 11, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, D.; Na, R.; Feuermann, Y.; Pechhold, S.; Chen, W.; Robinson, G.W.; Hennighausen, L. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 2009, 23, 2382–2387. [Google Scholar] [CrossRef]
- Tecalco-Cruz, A.C.; Macías-Silva, M.; Ramírez-Jarquín, J.O.; Méndez-Ambrosio, B. Identification of genes modulated by interferon gamma in breast cancer cells. Biochem. Biophys. Rep. 2021, 27, 101053. [Google Scholar] [CrossRef]
- Stroka, K.M.; Vaitkus, J.A.; Aranda-Espinoza, H. Endothelial cells undergo morphological, biomechanical, and dynamic changes in response to tumor necrosis factor-α. Eur. Biophys. J. 2012, 41, 939–947. [Google Scholar] [CrossRef]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef]
- Recchia Luciani, G.; Barilli, A.; Visigalli, R.; Sala, R.; Dall’Asta, V.; Rotoli, B.M. IRF1 mediates growth arrest and the induction of a secretory phenotype in alveolar epithelial cells in response to inflammatory cytokines IFNγ/TNFα. Int. J. Mol. Sci. 2024, 25, 3463. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Shillingford, J.M.; Smith, G.H.; Grimm, S.L.; Wagner, K.U.; Oka, T.; Rosen, J.M.; Robinson, G.W.; Hennighausen, L. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 2001, 155, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.R.; Rickert, C.G.; Vermi, W.; Sheehan, K.C.; Arthur, C.; Allen, J.A.; White, J.M.; Archambault, J.; Lonardi, S.; McDevitt, T.M.; et al. Dysregulated STAT1-SOCS1 control of JAK2 promotes mammary luminal progenitor cell survival and drives ERα+ tumorigenesis. Cell Death Differ. 2014, 21, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.R.; Gallego-Ortega, D.; Stanford, P.M.; Junankar, S.; Au, W.W.Y.; Kikhtyak, Z.; von Korff, A.; Sergio, C.M.; Law, A.M.K.; Castillo, L.E.; et al. A mutation in the viral sensor 2′-5′-oligoadenylate synthetase 2 causes failure of lactation. PLoS Genet. 2017, 13, e1007072. [Google Scholar] [CrossRef]
- Wilson, G.J.; Fukuoka, A.; Vidler, F.; Graham, G.J. Diverse myeloid cells are recruited to the developing and inflamed mammary gland. Immunology 2022, 165, 206–218. [Google Scholar] [CrossRef]
- Wang, F.; Graham, W.V.; Wang, Y.; Witkowski, E.D.; Schwarz, B.T.; Turner, J.R. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am. J. Pathol. 2005, 166, 409–419. [Google Scholar] [CrossRef]
- Patrick, D.M.; Leone, A.K.; Shellenberger, J.J.; Dudowicz, K.A.; King, J.M. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol. 2006, 6, 2. [Google Scholar] [CrossRef]
- Barth, R.J., Jr.; Mulé, J.J.; Spiess, P.J.; Rosenberg, S.A. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J. Exp. Med. 1991, 173, 647–658. [Google Scholar] [CrossRef]
- Sun, Y.; Revach, O.Y.; Anderson, S.; Kessler, E.A.; Wolfe, C.H.; Jenney, A.; Mills, C.E.; Robitschek, E.J.; Davis, T.G.R.; Kim, S.; et al. Targeting TBK1 to overcome resistance to cancer immunotherapy. Nature 2023, 615, 158–167. [Google Scholar] [CrossRef]
- Braumüller, H.; Wieder, T.; Brenner, E.; Aßmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef]
- Homann, L.; Rentschler, M.; Brenner, E.; Böhm, K.; Röcken, M.; Wieder, T. IFN-γ and TNF induce senescence and a distinct senescence-associated secretory phenotype in melanoma. Cells 2022, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- López-García, L.; Castro-Manrreza, M.E. TNF-α and IFN-γ participate in improving the immunoregulatory capacity of mesenchymal stem/stromal cells: Importance of cell-cell contact and extracellular vesicles. Int. J. Mol. Sci. 2021, 22, 9531. [Google Scholar] [CrossRef] [PubMed]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef]
- Betts, C.B.; Pennock, N.D.; Caruso, B.P.; Ruffell, B.; Borges, V.F.; Schedin, P. Mucosal immunity in the female murine mammary gland. J. Immunol. 2018, 201, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef]
- Mazan, A.; Marusiak, A.A. Protocols for co-culture phenotypic assays with breast cancer cells and THP-1-derived macrophages. J. Mammary Gland Biol. Neoplasia 2024, 29, 4. [Google Scholar] [CrossRef]

| Antibody | Source | Concentration |
|---|---|---|
| β-casein | Santa Cruz Biotechnology, sc-17971 (Santa Cruz, CA, USA) | 0.4 μg/mL |
| phospho-STAT5 | Millipore, #05-495 (Temecula, CA, USA) | 1.5 μg/mL |
| STAT5 | Santa Cruz Biotechnology, sc-835 (Santa Cruz, CA, USA) | 0.6 μg/mL |
| iNOS | Santa Cruz Biotechnology, sc-650 (Santa Cruz, CA, USA) | 1.0 μg/mL |
| cleaved caspase-3 | Cell Signaling Biotechnology, #9661 (Beverly, MA, USA) | 1:500 |
| actin | Sigma-Aldrich, #A-5060 (St. Louis, MO, USA) | 1:1000 |
| ZO-1 | Invitrogen, #40-2200 (Camarillo, CA, USA) | 1:100 |
| β-catenin | BD Biosciences, #610153 (San Jose, CA, USA) | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-J.; Su, Y.-A.; Lin, T.-H.; Liao, W.-T.; Wu, C.-C.; Lin, C.-C.; Chen, C.-H.; Hsu, T.-C.; Yang, Y.-W.; Lee, Y.-J. Th1 Cytokines Inhibit Acinar Morphogenesis and Milk Protein Expression in 3D Mammary Cultures. Biomedicines 2025, 13, 1455. https://doi.org/10.3390/biomedicines13061455
Chen L-J, Su Y-A, Lin T-H, Liao W-T, Wu C-C, Lin C-C, Chen C-H, Hsu T-C, Yang Y-W, Lee Y-J. Th1 Cytokines Inhibit Acinar Morphogenesis and Milk Protein Expression in 3D Mammary Cultures. Biomedicines. 2025; 13(6):1455. https://doi.org/10.3390/biomedicines13061455
Chicago/Turabian StyleChen, Lih-Ju, Yi-An Su, Ting-Hui Lin, Wan-Ting Liao, Chun-Chi Wu, Chen-Chu Lin, Chang-Han Chen, Tsai-Ching Hsu, Ya-Wen Yang, and Yi-Ju Lee. 2025. "Th1 Cytokines Inhibit Acinar Morphogenesis and Milk Protein Expression in 3D Mammary Cultures" Biomedicines 13, no. 6: 1455. https://doi.org/10.3390/biomedicines13061455
APA StyleChen, L.-J., Su, Y.-A., Lin, T.-H., Liao, W.-T., Wu, C.-C., Lin, C.-C., Chen, C.-H., Hsu, T.-C., Yang, Y.-W., & Lee, Y.-J. (2025). Th1 Cytokines Inhibit Acinar Morphogenesis and Milk Protein Expression in 3D Mammary Cultures. Biomedicines, 13(6), 1455. https://doi.org/10.3390/biomedicines13061455







