Biomanufacturing and Curcumin-Loading of Human Choroid Plexus Organoid-Derived Extracellular Vesicles from a Vertical-Wheel Bioreactor to Alleviate Neuro-Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Undifferentiated hiPSC and hMSC Cultures
2.2. ChP Organoid Differentiation
2.3. ChP Organoid Derivation in Vertical-Wheel Bioreactors
2.4. Image Analysis for the ChP Organoids
2.5. Metabolite Analysis
2.6. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.7. Extracellular Vesicle Isolation
2.8. Nanoparticle Tracking Analysis (NTA)
2.9. Transmission Electron Microscopy (TEM)
2.10. Western Blot for Exosomal Markers
2.11. Loading EVs Using Sonication, Incubation, and Freeze–Thaw Methods
2.12. EV Lyophilization and Re-Hydration
2.13. EV Effects on Amyloid Beta (Aβ) 42 Oligomer-Stimulated Cells
2.14. Live/Dead Assay
2.15. Statistical Analysis
3. Results
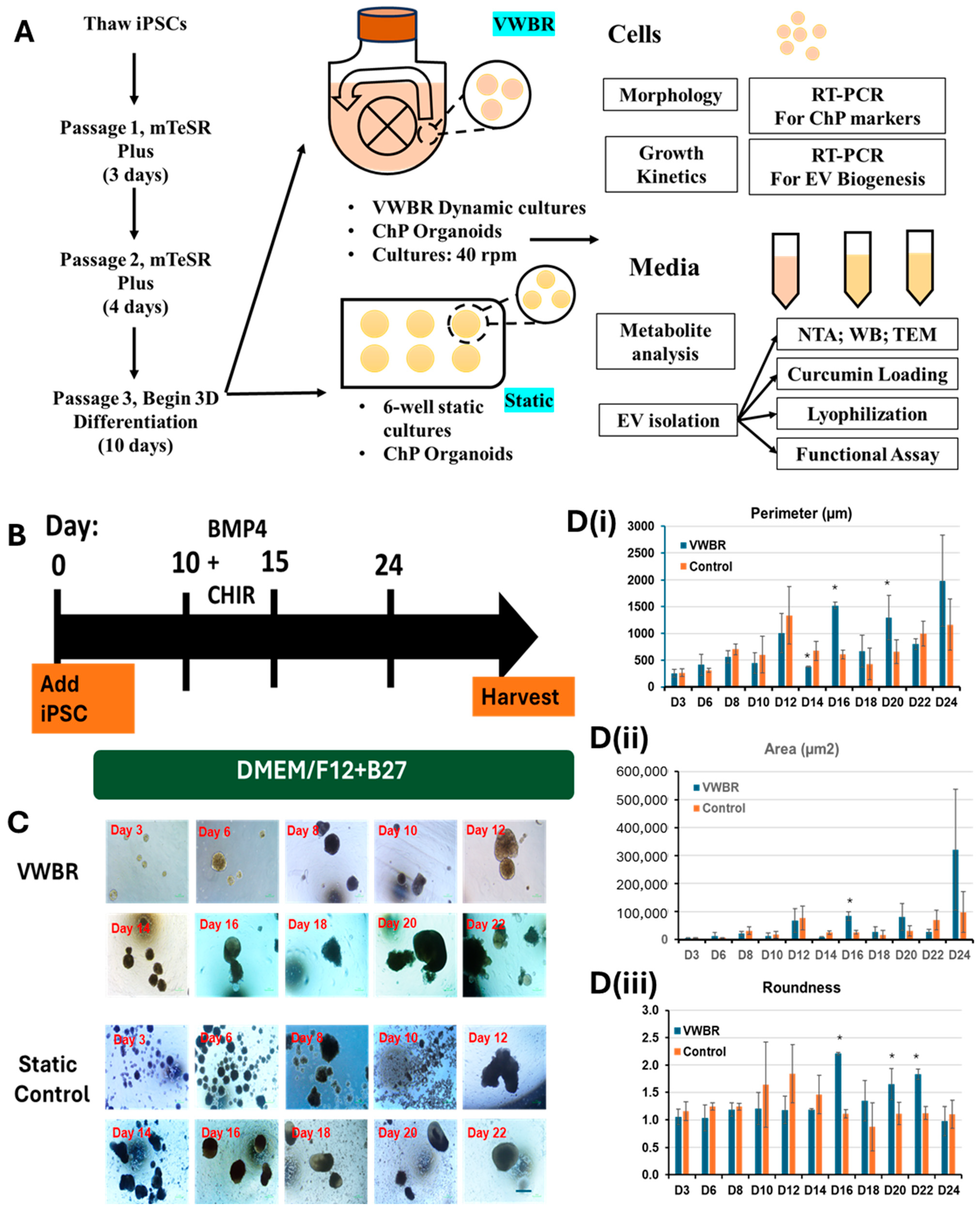
3.1. ChP Organoid Differentiation in VWBR and Characterization
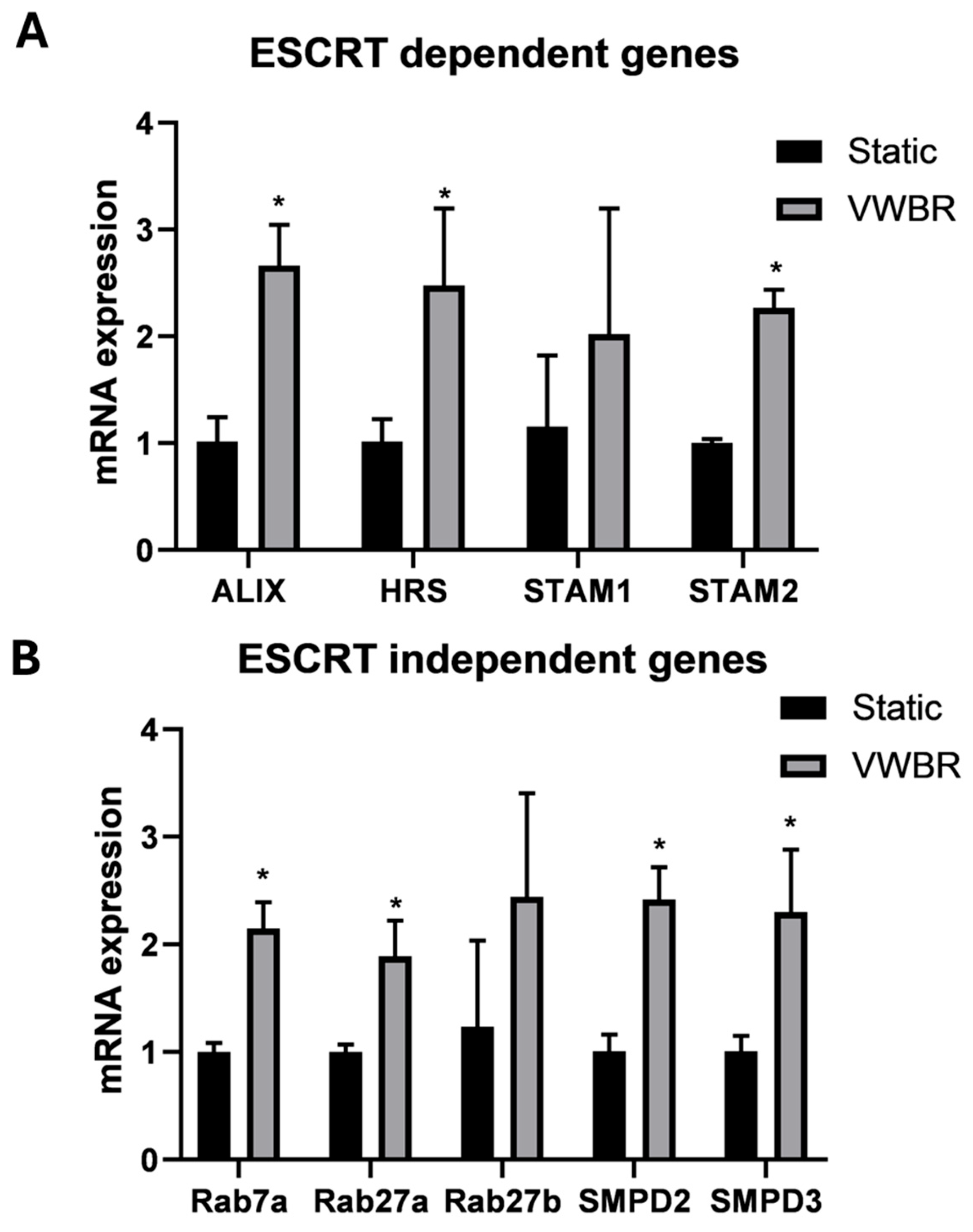
3.2. EV Biogenesis and Secretion of ChP Organoid EVs
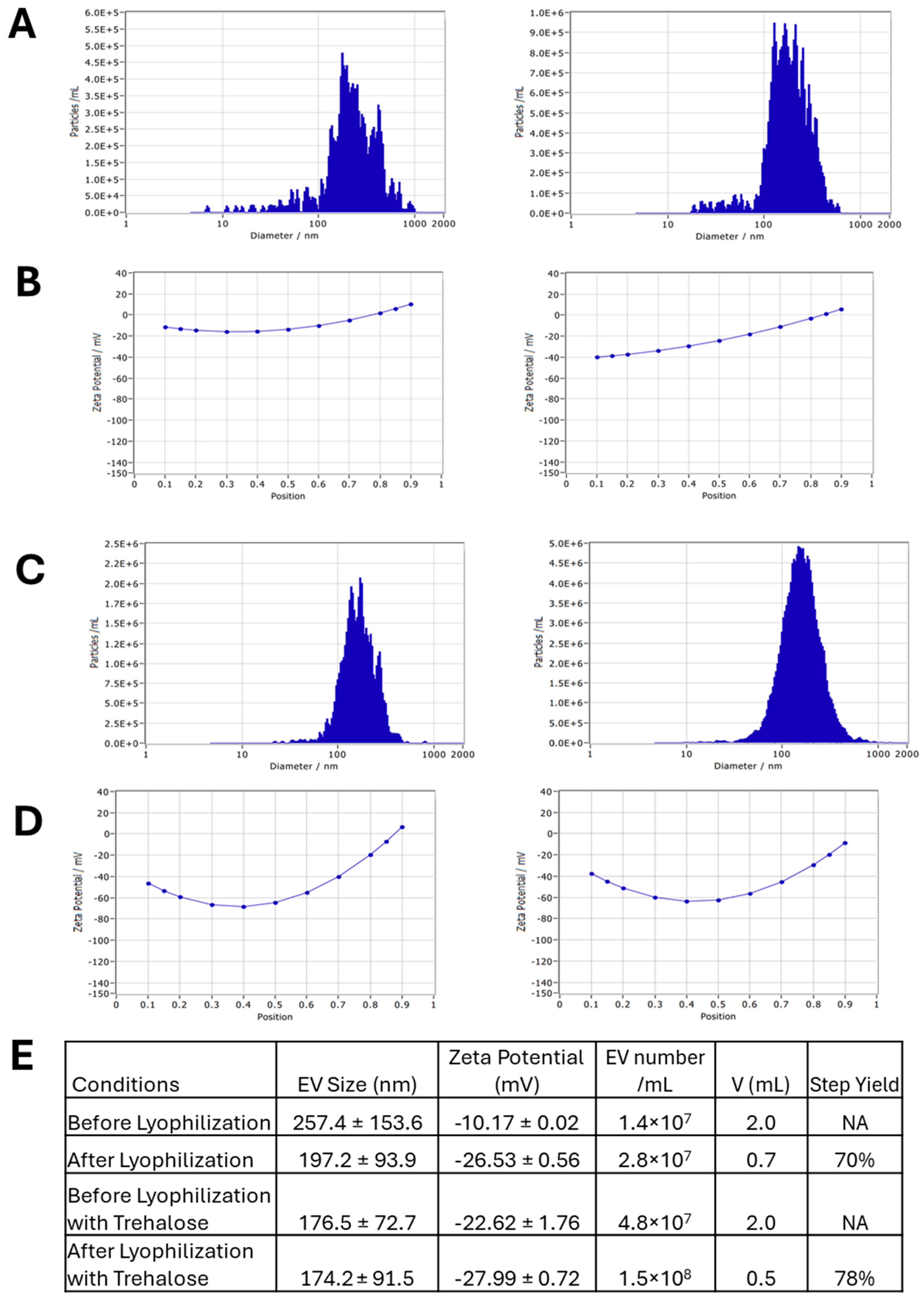
3.3. EV Loading with Curcumin and the EV Lyophilization
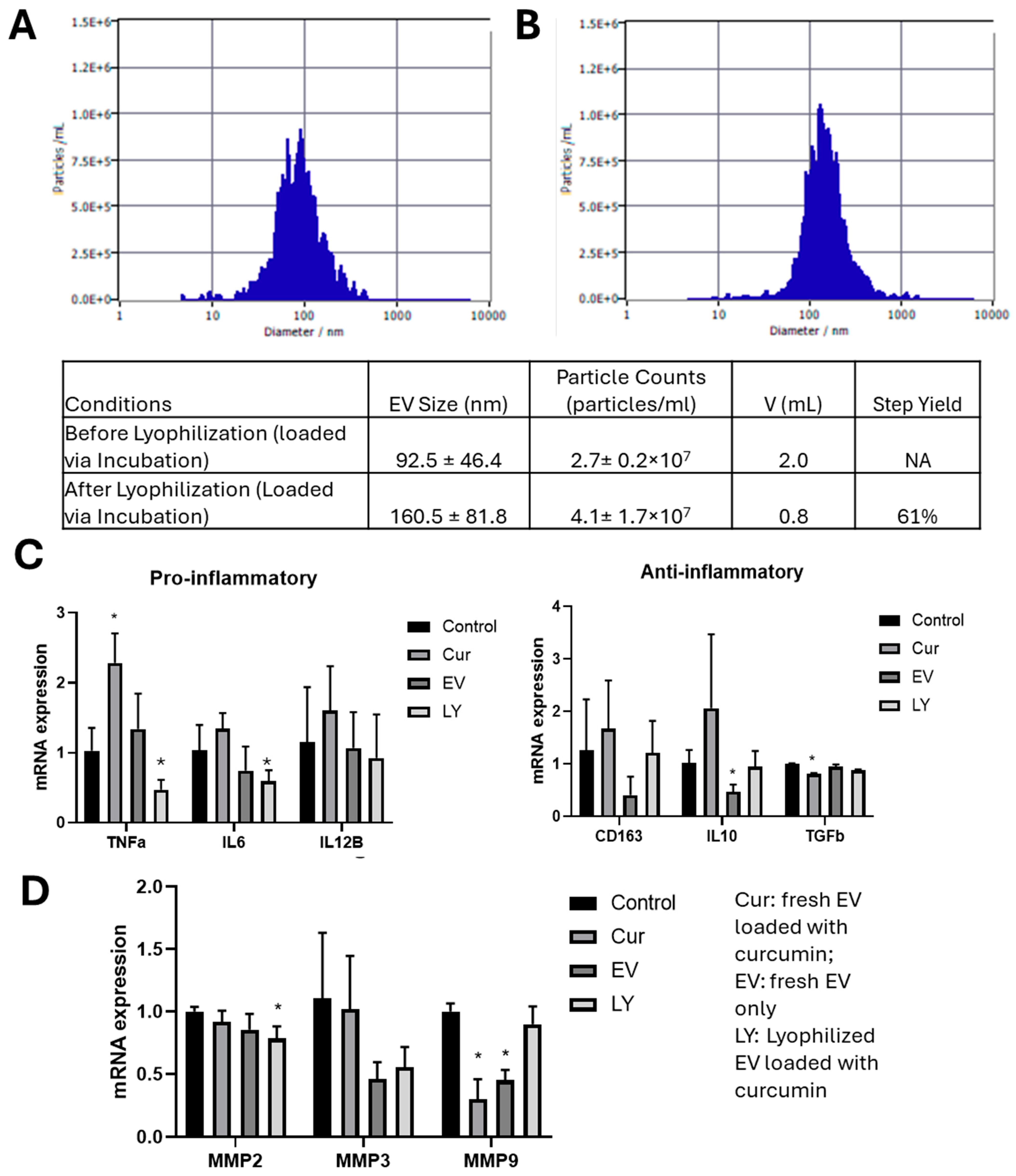
3.4. Functional Testing for Lyophilized Curcumin-Loaded ChP Organoid EVs
4. Discussion
4.1. ChP Organoid Differentiation in VWBR
4.2. EV Biogenesis of ChP Organoids in VWBR
4.3. Loaded ChP Organoid EVs as Neurological Therapeutics
4.4. EV Lyophilization for Preservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lehtinen, M.K.; Walsh, C.A. Neurogenesis at the brain-cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 2011, 27, 653–679. [Google Scholar] [CrossRef]
- Fame, R.M.; Lehtinen, M.K. Emergence and Developmental Roles of the Cerebrospinal Fluid System. Dev. Cell 2020, 52, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A. Development of the choroid plexus and blood-CSF barrier. Front. Neurosci. 2015, 9, 32. [Google Scholar] [CrossRef]
- Lehtinen, M.K.; Bjornsson, C.S.; Dymecki, S.M.; Gilbertson, R.J.; Holtzman, D.M.; Monuki, E.S. The choroid plexus and cerebrospinal fluid: Emerging roles in development, disease, and therapy. J. Neurosci. 2013, 33, 17553–17559. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Girgenti, M.J.; Banasr, M.; Stone, K.; Bruce, C.; Guilchicek, E.; Wilczak-Havill, K.; Nairn, A.; Williams, K.; Sass, S.; et al. A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl. Psychiatry 2012, 2, e139. [Google Scholar] [CrossRef]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef]
- Muok, L.; Liu, C.; Chen, X.; Esmonde, C.; Arthur, P.; Wang, X.; Singh, M.; Driscoll, T.P.; Li, Y. Inflammatory response and exosome biogenesis of choroid plexus organoids derived from human pluripotent stem cells. Int. J. Mol. Sci. 2023, 24, 7660. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Ene, J.; Esmonde, C.; Kanekiyo, T.; Zeng, C.; Sun, L.; Li, Y. Engineering Extracellular Vesicles Secreted by Human Brain Organoids with Different Regional Identity. ACS Appl. Mater. Interfaces 2025, 17, 15145–15162. [Google Scholar] [CrossRef]
- Carney, R.P.; Mizenko, R.R.; Bozkurt, B.T.; Lowe, N.; Henson, T.; Arizzi, A.; Wang, A.; Tan, C.; George, S.C. Harnessing extracellular vesicle heterogeneity for diagnostic and therapeutic applications. Nat. Nanotechnol. 2024, 20, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Die Pharm. -Int. J. Pharm. Sci. 2021, 76, 61–67. [Google Scholar] [CrossRef]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Processes 2021, 9, 356. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef]
- Ali, B.H.; Marrif, H.; Noureldayem, S.A.; Bakheit, A.O.; Blunden, G. Some Biological Properties of Curcumin: A Review. Nat. Prod. Commun. 2006, 1, 1934578X0600100613. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Stockl, S.; Lukas, C.; Herrmann, M.; Brochhausen, C.; Konig, M.A.; Johnstone, B.; Grassel, S. Curcumin-primed human BMSC-derived extracellular vesicles reverse IL-1beta-induced catabolic responses of OA chondrocytes by upregulating miR-126-3p. Stem Cell Res. Ther. 2021, 12, 252. [Google Scholar] [CrossRef]
- Rohani, L.; Borys, B.S.; Razian, G.; Naghsh, P.; Liu, S.; Johnson, A.A.; Machiraju, P.; Holland, H.; Lewis, I.A.; Groves, R.A.; et al. Stirred suspension bioreactors maintain naive pluripotency of human pluripotent stem cells. Commun. Biol. 2020, 3, 492. [Google Scholar] [CrossRef]
- Nogueira, D.E.S.; Cabral, J.M.S.; Rodrigues, C.A.V. Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering 2021, 8, 68. [Google Scholar] [CrossRef]
- Stephenson, M.; Grayson, W. Recent advances in bioreactors for cell-based therapies. F1000Research 2018, 7, F1000-Faculty. [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Sung, L.; Sun, L.; Li, Y. Upscaling Human Mesenchymal Stem Cell Production in a Novel Vertical Wheel Bioreactor Enhances Extracellular Vesicle Secretion and Cargo Profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar]
- Muok, L.; Sun, L.; Esmonde, C.; Worden, H.; Vied, C.; Duke, L.; Ma, S.; Zeng, O.Z.; Driscoll, T.P.; Jung, S.; et al. Extracellular Vesicle Biogenesis of Three-dimensional Human Pluripotent Stem Cells in a Novel Vertical-Wheel Bioreactor. J. Extracell. Biol. 2024, 3, e133. [Google Scholar] [CrossRef]
- Cuesta-Gomez, N.; Verhoeff, K.; Dadheech, N.; Dang, T.; Jasra, I.T.; de Leon, M.B.; Pawlick, R.; Marfil-Garza, B.; Anwar, P.; Razavy, H.; et al. Suspension culture improves iPSC expansion and pluripotency phenotype. Stem Cell Res. Ther. 2023, 14, 154. [Google Scholar] [CrossRef]
- Nogueira, D.E.S.; Rodrigues, C.A.V.; Carvalho, M.S.; Miranda, C.C.; Hashimura, Y.; Jung, S.; Lee, B.; Cabral, J.M.S. Strategies for the expansion of human induced pluripotent stem cells as aggregates in single-use Vertical-Wheel bioreactors. J. Biol. Eng. 2019, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.; Kandoi, K.; Sun, L.; Kalvala, A.; Kuthleria, S.; Bhattacharaya, S.; Kulkarni, T.; Nimma, R.; Li, Y.; Lamba, D.A.; et al. Biophysical, Molecular and Proteomic profiling of Human Retinal Organoids derived Exosomes. Pharm. Res. 2023, 40, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Nathani, A.; Sun, L.; Khan, I.; Aare, M.; Bagde, A.; Li, Y.; Singh, M. Combined role of interleukin-15 stimulated natural killer cell-derived extracellular vesicles and carboplatin in Osimertinib resistant H1975 lung cancer cells with EGFR mutation. Pharmaceutics 2024, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, L.; Worden, H.; Ene, J.; Zeng, O.Z.; Bhagu, J.; Grant, S.C.; Bao, X.; Jung, S.; Li, Y. Profiling biomanufactured extracellular vesicles of human forebrain spheroids in a Vertical Wheel bioreactor. J. Extracell. Biol. 2024, 3, e70002. [Google Scholar] [CrossRef]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef]
- Si-Tayeb, K.; Noto, F.K.; Sepac, A.; Sedlic, F.; Bosnjak, Z.J.; Lough, J.W.; Duncan, S.A. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev. Biol. 2010, 10, 81. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef]
- Marzano, M.; Bejoy, J.; Cheerathodi, M.; Sun, L.; York, S.; Zhao, J.; Kanekiyo, T.; Bu, G.; Meckes, D.G., Jr.; Li, Y. Differential effects of extracellular vesicles of lineage-specific human pluripotent stem cells on cellular behaviours of isogenic cortical spheroids. Cells 2019, 8, 993–1014. [Google Scholar] [CrossRef]
- Marzano, M.; Bou-Dargham, M.J.; Cone, A.S.; York, S.; Helsper, S.; Grant, S.C.; Meckes, D.G., Jr.; Sang, Q.X.; Li, Y. Biogenesis of Extracellular Vesicles Produced from Human Stem Cell-Derived Cortical Spheroids Exposed to Iron Oxides. ACS Biomater. Sci. Eng. 2021, 7, 1111–1122. [Google Scholar] [CrossRef]
- Cvjetkovic, A.; Lötvall, J.; Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef]
- Reiner, A.T.; Witwer, K.W.; Van Balkom, B.W.; De Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; De Kleijn, D. Concise review: Developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pekkanen-Mattila, M.; Shahsavani, M.; Falk, A.; Teixeira, A.I.; Herland, A. A 3D Alzheimer’s disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials 2014, 35, 1420–1428. [Google Scholar] [CrossRef]
- Yan, Y.; Bejoy, J.; Xia, J.; Guan, J.; Zhou, Y.; Li, Y. Neural patterning of human induced pluripotent stem cells in 3-D cultures for studying biomolecule-directed differential cellular responses. Acta Biomater. 2016, 42, 114–126. [Google Scholar] [CrossRef]
- Jeske, R.; Bejoy, J.; Marzano, M.; Li, Y. Human Pluripotent Stem Cell-Derived Extracellular Vesicles: Characteristics and Applications. Tissue Eng. Part B Rev. 2020, 26, 129–144. [Google Scholar] [CrossRef]
- Vandendriessche, C.; Balusu, S.; Van Cauwenberghe, C.; Brkic, M.; Pauwels, M.; Plehiers, N.; Bruggeman, A.; Dujardin, P.; Van Imschoot, G.; Van Wonterghem, E.; et al. Importance of extracellular vesicle secretion at the blood–cerebrospinal fluid interface in the pathogenesis of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 143. [Google Scholar] [CrossRef]
- Blanchette, C.R.; Rodal, A.A. Mechanisms for biogenesis and release of neuronal extracellular vesicles. Curr. Opin. Neurobiol. 2020, 63, 104–110. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chretien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef]
- Mendhe, B.; Khan, M.B.; Dunwody, D.; El Baradie, K.B.Y.; Smith, K.; Zhi, W.; Sharma, A.; Lee, T.J.; Hamrick, M.W. Lyophilized Extracellular Vesicles from Adipose-Derived Stem Cells Increase Muscle Reperfusion but Degrade Muscle Structural Proteins in a Mouse Model of Hindlimb Ischemia-Reperfusion Injury. Cells 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Neupane, Y.R.; Huang, C.; Wang, X.; Chng, W.H.; Venkatesan, G.; Zharkova, O.; Wacker, M.G.; Czarny, B.; Storm, G.; Wang, J.W.; et al. Lyophilization Preserves the Intrinsic Cardioprotective Activity of Bioinspired Cell-Derived Nanovesicles. Pharmaceutics 2021, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Gorgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Golan, M.E.; Stice, S.L. Extracellular vesicle lyophilization for enhanced distribution to the point of care. Extracell. Vesicle 2024, 3, 100041. [Google Scholar] [CrossRef]
- Dadheech, N.; Leon, M.; Cuesta-Gomez, N.; Jasra, I.; Pawlick, R.; Marfil-Garza, B.; Verhoeff, K.; Sapkota, S.; Razavy, H.; Anwar, P.; et al. Complete Suspension Differentiation of Human Pluripotent Stem Cells into Pancreatic Islets Using Vertical-Wheel Bioreactors; Cold Spring Harbor Laboratory, Biorxiv: New York, NY, USA, 2023. [Google Scholar]
- Silva, T.; Sousa-Luís, R.; Fernandes, T.; Bekman, E.; Rodrigues, C.; Vaz, S.; Moreira, L.; Hashimura, Y.; Jung, S.; Lee, B.; et al. Transcriptome profiling of human pluripotent stem cell-derived cerebellar organoids reveals faster commitment under dynamic conditions. Biotechnol. Bioeng. 2021, 118, 2781–2803. [Google Scholar] [CrossRef]
- Ene, J.; Liu, C.; Syed, F.; Sun, L.; Berry, D.; Durairaj, P.; Liu, Z.L.; Zeng, C.; Jung, S.; Li, Y. Biomanufacturing and Lipidomics Analysis of Extracellular Vesicles Secreted by Human Blood Vessel Organoids in a Vertical Wheel Bioreactor. Stem Cell Res. Ther. 2025, in press. [Google Scholar] [CrossRef]
- Barcena, A.J.R.; Mishra, A.; Bolinas, D.K.M.; Martin, B.M.; Melancon, M.P. Integration of Electrospun Scaffolds and Biological Polymers for Enhancing the Delivery and Efficacy of Mesenchymal Stem/Stromal Cell Therapies. Front. Biosci. Landmark Ed. 2024, 29, 228. [Google Scholar] [CrossRef]
- Nazari, H.; Cho, A.N.; Goss, D.; Thiery, J.P.; Ebrahimi Warkiani, M. Impact of brain organoid-derived sEVs on metastatic adaptation and invasion of breast carcinoma cells through a microphysiological system. Lab Chip 2024, 24, 3434–3455. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, S.; Wang, N.; Wang, J.; Wu, Y.; Duan, Y.; Ni, P.; Zhang, J.; Yu, S. Cerebral-Organoid-Derived Exosomes Alleviate Oxidative Stress and Promote LMX1A-Dependent Dopaminergic Differentiation. Int. J. Mol. Sci. 2023, 24, 11048. [Google Scholar] [CrossRef]
- Abdollahi, S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol. Bioeng. 2021, 118, 1029–1049. [Google Scholar] [CrossRef]
- Thompson, W.; Papoutsakis, E. The role of biomechanical stress in extracellular vesicle formation, composition and activity. Biotechnol. Adv. 2023, 66, 108158. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, K.; Yu, N.; An, S.; Lee, S.; Kwon, K. Fluid Shear Stress Regulates the Landscape of microRNAs in Endothelial Cell-Derived Small Extracellular Vesicles and Modulates the Function of Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 1314. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Azimi, I.; Monteith, G.; Bebawy, M. Ca 2+ mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J. Extracell. Vesicles 2020, 9, 1734326. [Google Scholar] [CrossRef]
- Kang, H.; Bae, Y.h.; Kwon, Y.; Kim, S.; Park, J. Extracellular Vesicles Generated Using Bioreactors and their Therapeutic Effect on the Acute Kidney Injury Model. Adv. Healthc. Mater. 2021, 11, 2101606. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Joshi, B.S.; Ortiz, D.; Zuhorn, I.S. Converting extracellular vesicles into nanomedicine: Loading and unloading of cargo. Mater. Today Nano 2021, 16, 100148. [Google Scholar] [CrossRef]
- Nowak, M.; Górczyńska, J.; Kołodzińska, K.; Rubin, J.; Choromańska, A. Extracellular Vesicles as Drug Transporters. Int. J. Mol. Sci. 2023, 24, 10267. [Google Scholar] [CrossRef]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.A.; Gazeau, F. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Pirmoradi, S.; Fathi, E.; Farahzadi, R.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Curcumin Affects Adipose Tissue-Derived Mesenchymal Stem Cell Aging Through TERT Gene Expression. Drug Res. 2018, 68, 213–221. [Google Scholar] [CrossRef]
- Rankin-Turner, S.; Vader, P.; O’Driscoll, L.; Giebel, B.; Heaney, L.M.; Davies, O.G. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv. Drug Deliv. Rev. 2021, 173, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Nizamudeen, Z.A.; Xerri, R.; Parmenter, C.; Suain, K.; Markus, R.; Chakrabarti, L.; Sottile, V. Low-Power Sonication Can Alter Extracellular Vesicle Size and Properties. Cells 2021, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- El Baradie, K.B.Y.; Nouh, M.; O’Brien Iii, F.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles From Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef]
- Manno, M.; Bongiovanni, A.; Margolia, L.; Bergese, P.; Arosio, P. The physico-chemical landscape of extracellular vesicles. Nat. Rev. Bioeng. 2024, 3, 68–82. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv. Healthc. Mater. 2022, 11, e2100538. [Google Scholar] [CrossRef]
- Frank, J.; Richter, M.; de Rossi, C.; Lehr, C.M.; Fuhrmann, K.; Fuhrmann, G. Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci. Rep. 2018, 8, 12377. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ene, J.; Muok, L.; Gonzalez, V.; Sanchez, N.; Nathani, A.; Syed, F.; Liu, Z.L.; Singh, M.; Driscoll, T.; Li, Y. Biomanufacturing and Curcumin-Loading of Human Choroid Plexus Organoid-Derived Extracellular Vesicles from a Vertical-Wheel Bioreactor to Alleviate Neuro-Inflammation. Biomedicines 2025, 13, 1069. https://doi.org/10.3390/biomedicines13051069
Ene J, Muok L, Gonzalez V, Sanchez N, Nathani A, Syed F, Liu ZL, Singh M, Driscoll T, Li Y. Biomanufacturing and Curcumin-Loading of Human Choroid Plexus Organoid-Derived Extracellular Vesicles from a Vertical-Wheel Bioreactor to Alleviate Neuro-Inflammation. Biomedicines. 2025; 13(5):1069. https://doi.org/10.3390/biomedicines13051069
Chicago/Turabian StyleEne, Justice, Laureana Muok, Vanessa Gonzalez, Nicolas Sanchez, Aakash Nathani, Falak Syed, Zixiang Leonardo Liu, Mandip Singh, Tristan Driscoll, and Yan Li. 2025. "Biomanufacturing and Curcumin-Loading of Human Choroid Plexus Organoid-Derived Extracellular Vesicles from a Vertical-Wheel Bioreactor to Alleviate Neuro-Inflammation" Biomedicines 13, no. 5: 1069. https://doi.org/10.3390/biomedicines13051069
APA StyleEne, J., Muok, L., Gonzalez, V., Sanchez, N., Nathani, A., Syed, F., Liu, Z. L., Singh, M., Driscoll, T., & Li, Y. (2025). Biomanufacturing and Curcumin-Loading of Human Choroid Plexus Organoid-Derived Extracellular Vesicles from a Vertical-Wheel Bioreactor to Alleviate Neuro-Inflammation. Biomedicines, 13(5), 1069. https://doi.org/10.3390/biomedicines13051069







