Prevalence of Myofascial Trigger Points in Patients with Radiating and Non-Radiating Low Back Pain: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search and Article Management Resources
2.3. Search Strategy
- -
- Population: Individuals with a diagnosis of non-specific LBP (no underlying medical condition) with or without radiating pain to the lower extremity.
- -
- Intervention: No applicable.
- -
- Comparison: Asymptomatic individuals or comparisons between painful or dominant sides.
- -
2.4. Eligibility Criteria
2.5. Methodological Quality Assessment
2.6. Screening and Data Extraction
3. Results
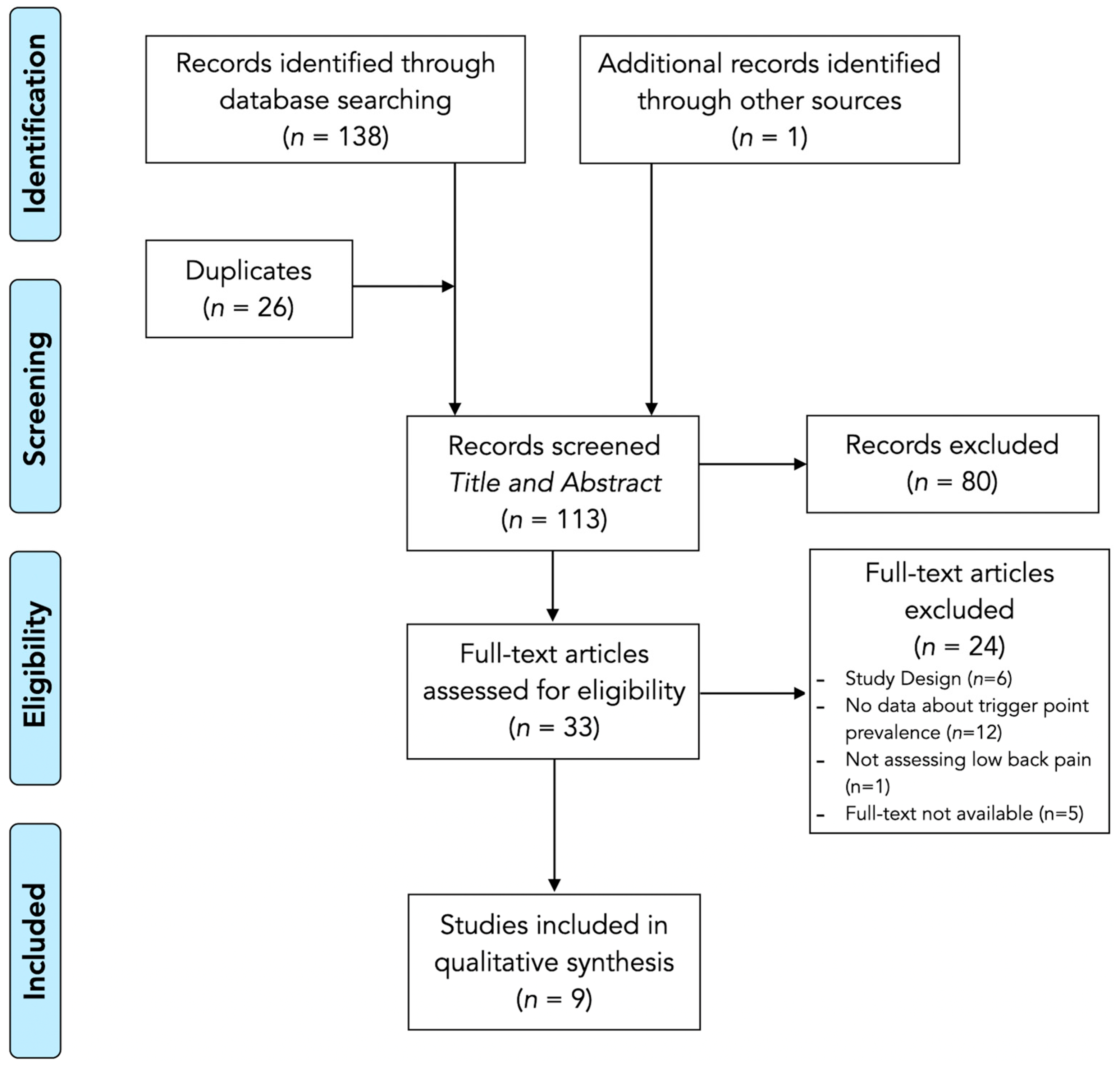
3.1. Study Selection
3.2. Study Characteristics
3.3. Methodological Quality
3.4. Results Synthesis: Prevalence of Active Trigger Points
3.5. Results Synthesis: Prevalence of Latent Trigger Points
4. Discussion
4.1. Future Resarch
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBP | Low back pain |
| MeSH | Medical Subject Headings |
| MTrP | Myofascial trigger point |
| PICO | Population, Intervention, Comparison, Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low Back Pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.W.C.; Chenot, J.F.; van Tulder, M.; Koes, B.W. Clinical Practice Guidelines for the Management of Non-Specific Low Back Pain in Primary Care: An Updated Overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Salman, D.; McGregor, A.H. Recent Clinical Practice Guidelines for the Management of Low Back Pain: A Global Comparison. BMC Musculoskelet. Disord. 2024, 25, 344. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Apeldoorn, A.; Hallegraeff, H.; Clark, J.; Smeets, R.; Malfliet, A.; Girbes, E.L.; De Kooning, M.; Ickmans, K. Low Back Pain: Guidelines for the Clinical Classification of Predominant Neuropathic, Nociceptive, or Central Sensitization Pain. Pain Physician 2015, 18, E333–E346. [Google Scholar] [CrossRef]
- Medrano-Escalada, Y.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Valera-Calero, J.A. Structural, Functional and Neurochemical Cortical Brain Changes Associated with Chronic Low Back Pain. Tomography 2022, 8, 2153–2163. [Google Scholar] [CrossRef]
- Petersen, T.; Laslett, M.; Juhl, C. Clinical Classification in Low Back Pain: Best-Evidence Diagnostic Rules Based on Systematic Reviews. BMC Musculoskelet. Disord. 2017, 18, 188. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-Specific Low Back Pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Grunau, G.L.; Darlow, B.; Flynn, T.; O’Sullivan, K.; O’Sullivan, P.B.; Forster, B.B. Red Flags or Red Herrings? Redefining the Role of Red Flags in Low Back Pain to Reduce Overimaging. Br. J. Sports Med. 2018, 52, 488–489. [Google Scholar] [CrossRef]
- George, S.Z.; Fritz, J.M.; Silfies, S.P.; Schneider, M.J.; Beneciuk, J.M.; Lentz, T.A.; Gilliam, J.R.; Hendren, S.; Norman, K.S. Interventions for the Management of Acute and Chronic Low Back Pain: Revision 2021. J. Orthop. Sports Phys. Ther. 2021, 51, CPG1–CPG60. [Google Scholar] [CrossRef]
- Nicodemus, C.L.; Sikorskii, A.; Epstein, J. Revisiting Chronic Low Back Pain: Evidence That It Is Not Non-Specific. J. Osteopath. Med. 2022, 123, 143–149. [Google Scholar] [CrossRef]
- Abd Rahman, N.A.; Li, S.; Schmid, S.; Shaharudin, S. Biomechanical Factors Associated with Non-Specific Low Back Pain in Adults: A Systematic Review. Phys. Ther. Sport 2023, 59, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, A.; Clijsen, R.; Fernandez-De-Las-Penas, C.; Barbero, M. Prevalence of Myofascial Trigger Points in Spinal Disorders: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2016, 97, 316–337. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Dommerholt, J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef]
- Simons, D.G. Review of Enigmatic MTrPs as a Common Cause of Enigmatic Musculoskeletal Pain and Dysfunction. J. Electromyogr. Kinesiol. 2004, 14, 95–107. [Google Scholar] [CrossRef]
- Lam, C.; Francio, V.T.; Gustafson, K.; Carroll, M.; York, A.; Chadwick, A.L. Myofascial Pain—A Major Player in Musculoskeletal Pain. Best Pract. Res. Clin. Rheumatol. 2024, 38, 101944. [Google Scholar] [CrossRef]
- Adelmanesh, F.; Jalali, A.; Shirvani, A.; Pakmanesh, K.; Pourafkari, M.; Raissi, G.R.; Shir, Y. The Diagnostic Accuracy of Gluteal Trigger Points to Differentiate Radicular From Nonradicular Low Back Pain. Clin. J. Pain 2016, 32, 666–672. [Google Scholar] [CrossRef]
- Iglesias-González, J.J.; Muñoz-García, M.T.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F.; Fernández-de-Las-Peñas, C. Myofascial Trigger Points, Pain, Disability, and Sleep Quality in Patients with Chronic Nonspecific Low Back Pain. Pain Med. 2013, 14, 1964–1970. [Google Scholar] [CrossRef]
- Duarte, F.C.K.; West, D.W.D.; Linde, L.D.; Hassan, S.; Kumbhare, D.A. Re-Examining Myofascial Pain Syndrome: Toward Biomarker Development and Mechanism-Based Diagnostic Criteria. Curr. Rheumatol. Rep. 2021, 23, 69. [Google Scholar] [CrossRef]
- Barbero, M.; Schneebeli, A.; Koetsier, E.; Maino, P. Myofascial Pain Syndrome and Trigger Points: Evaluation and Treatment in Patients with Musculoskeletal Pain. Curr. Opin. Support. Palliat. Care 2019, 13, 270–276. [Google Scholar] [CrossRef]
- Wong, C.K.; Mak, R.Y.; Kwok, T.S.; Tsang, J.S.; Leung, M.Y.; Funabashi, M.; Macedo, L.G.; Dennett, L.; Wong, A.Y. Prevalence, Incidence, and Factors Associated With Non-Specific Chronic Low Back Pain in Community-Dwelling Older Adults Aged 60 Years and Older: A Systematic Review and Meta-Analysis. J. Pain 2022, 23, 509–534. [Google Scholar] [CrossRef]
- Lluch, E.; Nijs, J.; De Kooning, M.; Van Dyck, D.; Vanderstraeten, R.; Struyf, F.; Roussel, N.A. Prevalence, Incidence, Localization, and Pathophysiology of Myofascial Trigger Points in Patients With Spinal Pain: A Systematic Literature Review. J. Manip. Physiol. Ther. 2015, 38, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, R.D.; Shannon, S.; Hong, C.-Z.; Hubbard, D.; Gevirtz, R. Interrater Reliability in Myofascial Trigger Point Examination. Pain 1997, 69, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Harris, J.D.; Quatman, C.E.; Manring, M.M.; Siston, R.A.; Flanigan, D.C. How to Write a Systematic Review. Am. J. Sports Med. 2014, 42, 2761–2768. [Google Scholar] [CrossRef]
- Meade, M.; DiCiurcio, W.; Radack, T.; Michael, M.; Woods, B. Reference Managers. Clin. Spine Surg. 2024, 37, 77–78. [Google Scholar] [CrossRef]
- De la Rosa, D.; Sanz-Ayan, P.; Alcantara, A.; Margarit, C.; Sanchez, J.; Sanfeliu, M.; Aleo, A. Consensus on Functional Assessment of Chronic Pain in Primary Care: A Delphi Study. Curr. Med. Res. Opin. 2021, 37, 2125–2132. [Google Scholar] [CrossRef]
- Salom-Moreno, J.; Gil-López, P.P.; Truyols-Domínguez, S.; Palacios-Ceña, M.; Ortega-Santiago, R.; Fernández-de-las-Peñas, C. Puntos Gatillo Miofasciales En El Músculo Glúteo Medio En Pacientes Con Lumbalgia Mecánica: Análisis Topográfico. Fisioterapia 2015, 37, 9–14. [Google Scholar] [CrossRef]
- Hua, N.K.; Van der Does, E. The Occurrence and Inter-Rater Reliability of Myofascial Trigger Points in the Quadratus Lumborum and Gluteus Medius: A Prospective Study in Non-Specific Low Back Pain Patients and Controls in General Practice. Pain 1994, 58, 317–323. [Google Scholar] [CrossRef]
- Álvarez Delgado, S.; Velázquez Saornil, J.; Sánchez Milá, Z.; Jaén Crespo, G.; Campón Chekroun, A.; Barragán Casas, J.M.; Frutos Llanes, R.; Rodríguez Sanz, D. Effectiveness of Dry Needling and Ischemic Trigger Point Compression in the Gluteus Medius in Patients with Non-Specific Low Back Pain: A Randomized Short-Term Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 12468. [Google Scholar] [CrossRef]
- Holm-Jensen, A.; Kjaer, P.; Schiøttz-Christensen, B.; Ziegler, D.S.; Andersen, S.; Myburgh, C. The Interexaminer Reproducibility and Prevalence of Lumbar and Gluteal Myofascial Trigger Points in Patients With Radiating Low Back Pain. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Carroll, M.; Ellis, R.; Kohut, S.; Garrett, N.; Fernández-de-Las-Peñas, C. Associations Between Gluteus Medius Trigger Points With Hip Passive Range of Movement and Muscle Strength in Adults With Chronic Nonspecific Low Back Pain: A Cross-Sectional Study. J. Manip. Physiol. Ther. 2022, 45, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Saeidian, S.R.; Pipelzadeh, M.R.; Rasras, S.; Zeinali, M. Effect of Trigger Point Injection on Lumbosacral Radiculopathy Source. Anesth. Pain Med. 2014, 4, e15500. [Google Scholar] [CrossRef] [PubMed]
- Adelmanesh, F.; Jalali, A.; Jazayeri Shooshtari, S.M.; Raissi, G.R.; Ketabchi, S.M.; Shir, Y. Is There an Association between Lumbosacral Radiculopathy and Painful Gluteal Trigger Points? A Cross-Sectional Study. Am. J. Phys. Med. Rehabil. 2015, 94, 784–791. [Google Scholar] [CrossRef]
- Dach, F.; Ferreira, K.S. Treating Myofascial Pain with Dry Needling: A Systematic Review for the Best Evidence-Based Practices in Low Back Pain. Arq. Neuropsiquiatr. 2023, 81, 1169–1178. [Google Scholar] [CrossRef]
- Lara-Palomo, I.C.; Gil-Martínez, E.; López-Fernández, M.D.; González González, L.M.; Querol-Zaldívar, M.d.L.Á.; Castro-Sánchez, A.M. Efficacy of Dry Needling for Chronic Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Altern. Ther. Health Med. 2023, 29, 110–120. [Google Scholar]
- Liu, L.; Huang, Q.-M.; Liu, Q.-G.; Thitham, N.; Li, L.-H.; Ma, Y.-T.; Zhao, J.-M. Evidence for Dry Needling in the Management of Myofascial Trigger Points Associated With Low Back Pain: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 144–152.e2. [Google Scholar] [CrossRef]
- Hu, H.-T.; Gao, H.; Ma, R.-J.; Zhao, X.-F.; Tian, H.-F.; Li, L. Is Dry Needling Effective for Low Back Pain?: A Systematic Review and PRISMA-Compliant Meta-Analysis. Medicine 2018, 97, e11225. [Google Scholar] [CrossRef]
- Simons, D.G. Muscle Pain Syndromes—Part I. Am. J. Phys. Med. 1975, 54, 289–311. [Google Scholar]
- Simons, D.G. Muscle Pain Syndromes—Part II. Am. J. Phys. Med. 1976, 55, 15–42. [Google Scholar]
- Valera-Calero, J.A.; Varol, U.; Ortega-Santiago, R.; Navarro-Santana, M.J.; Díaz-Arribas, M.J.; Buffet-García, J.; Plaza-Manzano, G. MyofAPPcial: Construct Validity of a Novel Technological Aid for Improving Clinical Reasoning in the Management of Myofascial Pain Syndrome. Eur. J. Clin. Investig. 2024, 54, e14313. [Google Scholar] [CrossRef]
- Ramsook, R.R.; Malanga, G.A. Myofascial Low Back Pain. Curr. Pain Headache Rep. 2012, 16, 423–432. [Google Scholar] [CrossRef] [PubMed]
- López-Redondo, M.; Vicente-Campos, D.; Álvarez-González, J.; Roldán-Ruiz, A.; Sánchez-Jorge, S.; Buffet-García, J.; Rabanal-Rodríguez, G.; Valera-Calero, J.A. Association of Quadratus Lumborum Muscle Stiffness with Chronic Low Back Pain Features: An Observational Study. Medicina 2025, 61, 270. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.J.; Hinrichs, R.N. The Biomechanical and Clinical Significance of the Lumbar Erector Spinae Flexion-Relaxation Phenomenon: A Review of Literature. J. Manip. Physiol. Ther. 2005, 28, 623–631. [Google Scholar] [CrossRef]
- Sadler, S.; Cassidy, S.; Peterson, B.; Spink, M.; Chuter, V. Gluteus Medius Muscle Function in People with and without Low Back Pain: A Systematic Review. BMC Musculoskelet. Disord. 2019, 20, 463. [Google Scholar] [CrossRef]
- Choi, J. Il Chicken and Egg: Peripheral Nerve Entrapment or Myofascial Trigger Point? Korean J. Pain 2014, 27, 186–188. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Nijs, J.; Cagnie, B.; Gerwin, R.D.; Plaza-Manzano, G.; Valera-Calero, J.A.; Arendt-Nielsen, L. Myofascial Pain Syndrome: A Nociceptive Condition Comorbid with Neuropathic or Nociplastic Pain. Life 2023, 13, 694. [Google Scholar] [CrossRef]
- Peduzzi de Castro, M.; de Brito Fontana, H.; Fóes, M.C.; Santos, G.M.; Ruschel, C.; Roesler, H. Activation of the Gluteus Maximus, Gluteus Medius and Tensor Fascia Lata Muscles during Hip Internal and External Rotation Exercises at Three Hip Flexion Postures. J. Bodyw. Mov. Ther. 2021, 27, 487–492. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, Y.-J.; Koo, H.-M. Activation of the Gluteus Medius According to Load during Horizontal Hip Abduction in a One-Leg Stance. J. Phys. Ther. Sci. 2015, 27, 2601–2603. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Hong, C.Z.; Adams, A.H.; Platt, K.J.; Danielson, C.D.; Hoehler, F.K.; Tobis, J.S. Interexaminer Reliability of the Palpation of Trigger Points in the Trunk and Lower Limb Muscles. Arch. Phys. Med. Rehabil. 2000, 81, 258–264. [Google Scholar] [CrossRef]
| Reference | Study Design | Demographic Characteristics | Clinical Characteristics | Complementary MTrP Identification Procedures | Muscles Assessed |
|---|---|---|---|---|---|
| Adelmanesh et al., 2015 [33] | Case–Control | n = 271 Males 43.2% Females 56.8% | Radiating Low Back Pain | Pain Pressure Thresholds | Gluteus region (no muscles specified) |
| Adelmanesh et al., 2016 [16] | Diagnostic accuracy | n = 185 Males 47% Females 53% | Radiating Low Back Pain and Non-Radiating Low Back Pain | Pain Pressure Thresholds | Gluteus region (no muscles specified) |
| Álvarez-Delgado et al., 2022 [29] | Randomized Clinical Trial | n = 87 n = 80 with MTrPs n = 7 without MTrPs | Non-Radiating Low Back Pain | Pain Pressure Thresholds | Gluteus medius |
| Carroll et al., 2022 [31] | Observational | n = 42 Males 17% Females 83% | Non-Radiating Low Back Pain | No | Quadratus lumborum |
| Holm-Jensen et al., 2020 [30] | Inter-examiner reliability | n = 32 Males 53% Females 47% | Radiating Low Back Pain and Non-Radiating Low Back Pain | No | Quadratus lumborum Gluteus medius Gluteus minimus Piriformis |
| Iglesias-González et al., 2013 [17] | Observational | n = 42 Males 50% Females 50% | Non-Radiating Low Back Pain | No | Quadratus lumborum Lumbar iliocostalis Psoas Piriformis Gluteus medius Gluteus minimus |
| Hua et al., 1994 [28] | Case–Control | n = 124 Males 52.4% Females 47.6% | Non-Radiating Low Back Pain | No | Quadratus lumborum Gluteus medius |
| Saeidian et al., 2014 [32] | Quasi-experimental | n = 98 n = 64 with MTrPs n = 34 without MTrPs | Radiating Low Back Pain | No | Adductor longus Medial head of the gastrocnemius Tibialis anterior and posterior Short head of the biceps femoris Lumbar paraspinal muscles |
| Salom-Moreno et al., 2015 [27] | Case series | n = 13 Males 23.1% Females 76.9% | Non-Radiating Low Back Pain | Pain Pressure Thresholds | Gluteus medius |
| Reference | Patient Group Description | Control Group Description | Selection Bias | Exposure | Blinded Measurement of Exposure | Confounders | Results | Score |
|---|---|---|---|---|---|---|---|---|
| Adelmanesh et al., 2015 [33] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6/7 |
| Adelmanesh et al., 2016 [16] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6/7 |
| Álvarez-Delgado et al., 2022 [29] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6/7 |
| Carroll et al., 2022 [31] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 5/7 |
| Holm-Jensen et al., 2020 [30] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 5/7 |
| Iglesias-González et al., 2013 [17] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 5/7 |
| Hua et al., 1994 [28] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 5/7 |
| Saeidian et al., 2014 [32] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 4/7 |
| Salom- Moreno et al., 2015 [27] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 5/7 |
| Active Myofascial Trigger Points | Latent Myofascial Trigger Points | References | |||
|---|---|---|---|---|---|
| Radiating Low Back Pain | Non-Radiating Low Back Pain | Radiating Low Back Pain | Non-Radiating Low Back Pain | Asymptomatic Subjects | |
| Quadratus lumborum | |||||
| - | 36% | - | - | - | Hua et al., 1994 [28] |
| 30% | - | - | - | - | Holm-Jensen et al., 2020 [30] |
| - | 55% a–45% b | - | 14% a–17% b | 0% c–10% d | Iglesias-González et al., 2013 [17] |
| Gluteal region (no specific muscles reported) | |||||
| 76.4% | - | - | - | - | Adelmanesh et al., 2015 [33] |
| 74.1% | 8.5% | - | - | - | Adelmanesh et al., 2016 [16] |
| Gluteus medius | |||||
| - | - | - | 91% | - | Álvarez-Delgado et al., 2022 [29] |
| - | 39–93% | - | - | - | Salom-Moreno et al., 2015 [27] |
| - | 43–45% | - | 74% | - | Carroll et al., 2022 [31] |
| - | 34% | - | - | - | Hua et al., 1994 [28] |
| 35% | - | - | - | - | Holm-Jensen et al., 2020 [30] |
| - | 35% a–38% b | - | 12% a–17% b | 5% | Iglesias-González et al., 2013 [17] |
| Gluteus minimus | |||||
| - | 12% a–5% b | - | 7% a–12% b | 12 c–10% d | Iglesias-González et al., 2013 [17] |
| 42% | - | - | - | - | Holm-Jensen et al., 2020 [28] |
| Piriformis | |||||
| 42% | - | - | - | - | Holm-Jensen et al., 2020 [30] |
| - | 35% a–28% b | - | 22% a–19% b | 0% c–7% d | Iglesias-González et al., 2013 [17] |
| Psoas | |||||
| - | 10% a–5% b | - | 26% a–36% b | 19% c–26% d | Iglesias-González et al., 2013 [17] |
| Iliocostalis | |||||
| - | 38% a–33% b | - | 19% | 0% c–5% d | Iglesias-González et al., 2013 [17] |
| Lower Extremity (no specific muscles reported) | |||||
| - | 65% a | - | - | - | Saeidian et al., 2014 [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monclús-Díez, G.; Díaz-Arribas, M.J.; Fernández-de-las-Peñas, C.; Kosson, D.; Kołacz, M.; Kobylarz, M.D.; Sánchez-Jorge, S.; Valera-Calero, J.A. Prevalence of Myofascial Trigger Points in Patients with Radiating and Non-Radiating Low Back Pain: A Systematic Review. Biomedicines 2025, 13, 1453. https://doi.org/10.3390/biomedicines13061453
Monclús-Díez G, Díaz-Arribas MJ, Fernández-de-las-Peñas C, Kosson D, Kołacz M, Kobylarz MD, Sánchez-Jorge S, Valera-Calero JA. Prevalence of Myofascial Trigger Points in Patients with Radiating and Non-Radiating Low Back Pain: A Systematic Review. Biomedicines. 2025; 13(6):1453. https://doi.org/10.3390/biomedicines13061453
Chicago/Turabian StyleMonclús-Díez, Germán, María José Díaz-Arribas, César Fernández-de-las-Peñas, Dariusz Kosson, Marcin Kołacz, Mateusz D. Kobylarz, Sandra Sánchez-Jorge, and Juan Antonio Valera-Calero. 2025. "Prevalence of Myofascial Trigger Points in Patients with Radiating and Non-Radiating Low Back Pain: A Systematic Review" Biomedicines 13, no. 6: 1453. https://doi.org/10.3390/biomedicines13061453
APA StyleMonclús-Díez, G., Díaz-Arribas, M. J., Fernández-de-las-Peñas, C., Kosson, D., Kołacz, M., Kobylarz, M. D., Sánchez-Jorge, S., & Valera-Calero, J. A. (2025). Prevalence of Myofascial Trigger Points in Patients with Radiating and Non-Radiating Low Back Pain: A Systematic Review. Biomedicines, 13(6), 1453. https://doi.org/10.3390/biomedicines13061453