Emerging Chemotherapy Targets: Insights from Advances in Glioma Treatment
Abstract
1. Introduction
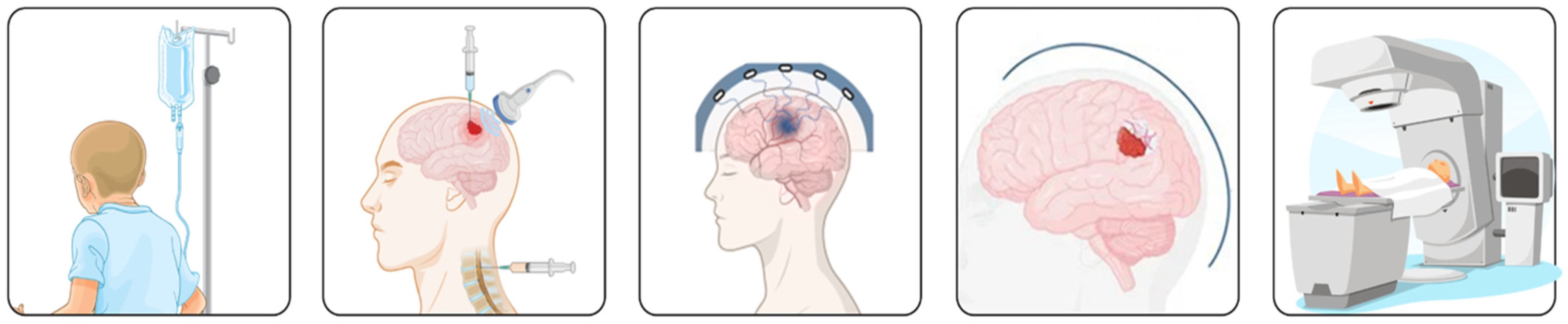
2. Current Challenges in Chemotherapy for Gliomas
2.1. Blood–Brain Barrier
2.2. Molecular Resistance
2.3. Immunomodulation
2.4. Toxicity
3. Emerging Targets in Glioma Chemotherapy
| Study ID | Drug | Results |
|---|---|---|
| 29492119 | Gefitinib | Gefitinib significantly improved outcomes in patients with the EGFR mutation and wild-type PTEN, achieving a progression-free survival (PFS) of approximately 9 months and an overall survival (OS) of about 20 months. Gefitinib demonstrated poorer outcomes in GBM patients with concurrent EGFR and PTEN mutations, with PFS and OS of 6 months and 9 months, respectively [58]. |
| 31119479 | Imatinib | The median progression-free survival (PFS) with imatinib was approximately 4 months, and the overall survival (OS) was about 16.6 months, compared to 8 months in the control group. Patients with the 1p/19q codeletion demonstrated a higher OS of 19.2 months. Of the participants, 61% experienced adverse effects, including fatigue, hemorrhage, gastrointestinal issues, and hypophosphatemia. These findings warrant validation in a larger cohort [59]. |
| 30115593 | Bevacizumab | Between 2011 and 2015, 155 patients received either monotherapy of temozolomide (n = 77) or combination therapy of temozolomide and bevacizumab (n = 78). At 12 months, overall survival was 61% for monotherapy and 55% for combination therapy. Grade 3 or 4 hematological toxicity was higher in the combination group (33% vs. 23%). Common adverse events included nervous system disorders, fatigue, and nausea, all more frequent with combination therapy. Infections were also higher in the combination group (38% vs. 23%), with one treatment-related death reported [60]. |
| 37316802 | Bevacizumab | Bevacizumab (BEV) improves progression-free survival, palliation, and cognitive function in recurrent glioblastoma (rGBM), but overall survival benefits lack strong evidence. BEV combined with lomustine, or radiotherapy is more effective than monotherapy. Better responses are predicted by IDH mutation, large tumor burden, and double-positive signs. Low-dose BEV is as effective as the standard dose, but optimal timing remains unclear. Further studies are needed [61]. |
| 28426845 | CAR-T Cells | Seventeen patients with progressive HER2-positive glioblastoma (10 adults, 7 children) received autologous HER2-CAR VST infusions without prior lymphodepletion. Infusions were well tolerated, with no dose-limiting toxic effects, and HER2-CAR VSTs were detectable in peripheral blood for up to 12 months. Of 16 evaluable patients, 1 had a partial response for over 9 months, 7 had stable disease (8 weeks to 29 months), and 8 progressed. Three patients with stable disease remained progression-free for 24–29 months. Table 1: Overview of Study Results for the Specified Drug Agents. The median overall survival for the cohort was 11.1 months from the first infusion and 24.5 months from diagnosis. Phase 2b study is warranted [51]. |
| 260959190 | CAR-T Cells | The study demonstrates the feasibility of producing autologous CTL clones expressing an IL13 (E13Y)-zetakine CAR for targeted HLA-independent glioma treatment. Intracranial infusion of these CTLs into three patients with recurrent glioblastoma was well-tolerated, with manageable brain inflammation. Two patients showed transient anti-glioma responses, and one patient’s tumor tissue analysis revealed reduced IL13Rα2 expression post-treatment. MRI of another patient showed increased tumor necrosis at the infusion site [52]. |
| 28724573 | CAR-T Cells | The infusion of EGFRvIII-directed CAR T cells in recurrent glioblastoma (GBM) patients was well-tolerated and showed temporary T cell expansion in the blood. Post-treatment tumor analysis revealed antigen loss in five patients, but adaptive resistance mechanisms emerged, including upregulation of inhibitory molecules and increased regulatory T cell infiltration [62]. |
| 35129069 | Gamitrinib | The study showed that Gamitrinib exhibits significant anticancer activity in various cancer cell lines, including colon adenocarcinoma, breast adenocarcinoma, and melanoma. It effectively inhibits cancer cell growth, both as a monotherapy and in combination with other therapies, with favorable pharmacokinetics and minimal toxicity in preclinical models (rats and beagle dogs). Toxicity studies found no major adverse effects on the tested doses [54]. |
| 37272516 | Vorasidenib | In a study of 331 patients, 168 received vorasidenib and 163 received a placebo. After a median follow-up of 14.2 months, progression-free survival was significantly longer in the vorasidenib group (27.7 months) compared to the placebo group (11.1 months). Grade 3 or higher adverse events occurred in 22.8% of patients receiving vorasidenib, compared to 13.5% in the placebo group [56]. |
| PMC9354202 | BMX-01 (BMX-HGG) | Fifteen patients (ages 19–80) with WHO grade 4 glioblastomas underwent neurocognitive testing before and after radiation therapy (RT). Most had neurocognitive impairment, with deficits ranging from 46.7% to 80% on specific tests. However, at two months (n = 15) and six months (n = 9) after treatment, most patients showed improved neurocognitive performance. Neurocognitive function can be maintained or improved in patients receiving concurrent radiation therapy and temozolomide, along with BMX-001 treatment [63]. |
4. Overcoming Possible Challenges
4.1. BBB and Physiological Barriers
4.2. Intrinsic Tumor Barriers
4.3. Systemic Challenges
4.4. Prognosis and Long-Term Survivorship
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma multiforme |
| BBB | Blood–brain barrier |
| EC | Endothelial cells |
| DCE-MR | Dynamic contrast-enhanced magnetic resonance |
| CSC | Cancer stem cells |
| miRNAs | microRNAs |
| lncRNAs | Long non-coding RNAs |
| VEGF | Vascular endothelial growth factor |
| VM | Vascular mimicry |
| PD-L1 | Programmed death-ligand 1 |
| TGF-β | Tumor growth factor β |
| CLDN 4 | Claudin-4 |
| TME | Tumor microenvironment |
| TK | Tyrosine kinase |
| EGFR | Epidermal growth factor receptor |
| PDGFR | Platelet-derived growth factor |
| CAR-T | Chimeric antigen receptor T-cells |
| PDHA | Pyruvate dehydrogenase alpha |
| OGDH | Alpha ketoglutarate dehydrogenase |
| Hsp90 | Heat shock protein 1 |
| TRAP1 | TNF receptor associated protein 1 |
| IDH | Isocitrate dehydrogenase |
| 2-HG | d-2-hydroxyglutarate |
| PFS | Progression free survival |
| OS | Overall survival |
References
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, R.W.; Zhang, P.; Lin, R.; Schiapparelli, P.; Quinones-Hinojosa, A.; Cui, H. Nanotherapeutic systems for local treatment of brain tumors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1479. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.L.; Alshamsan, B.; Ng, T.L. Temozolomide Chronotherapy in Glioma: A Systematic Review. Curr. Oncol. 2023, 30, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Jezierzański, M.; Nafalska, N.; Stopyra, M.; Furgoł, T.; Miciak, M.; Kabut, J.; Gisterek-Grocholska, I. Temozolomide (TMZ) in the Treatment of Glioblastoma Multiforme—A Literature Review and Clinical Outcomes. Curr. Oncol. 2024, 31, 3994–4002. [Google Scholar] [CrossRef]
- Qi, X.; Jha, S.K.; Jha, N.K.; Dewanjee, S.; Dey, A.; Deka, R.; Pritam, P.; Ramgopal, K.; Liu, W.; Hou, K. Antioxidants in brain tumors: Current therapeutic significance and future prospects. Mol. Cancer 2022, 21, 204. [Google Scholar] [CrossRef]
- Chen, C.; Xu, T.; Lu, Y.; Chen, J.; Wu, S. The efficacy of temozolomide for recurrent glioblastoma multiforme. Eur. J. Neurol. 2013, 20, 223–230. [Google Scholar] [CrossRef]
- Frisella, S.; Bonosi, L.; Ippolito, M.; Giammalva, G.R.; Ferini, G.; Viola, A.; Marchese, V.A.; Umana, G.E.; Iacopino, D.G.; Giarratano, A.; et al. Palliative Care and End-of-Life Issues in Patients with Brain Cancer Admitted to ICU. Medicina 2023, 59, 288. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Kacimi, R.; Giffard, R.G.; Yenari, M.A. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J. Inflamm. 2011, 8, 7. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.D.; Limoli, C.L. Breaking barriers: Neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free Radic. Biol. Med. 2022, 178, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.N.; Yang, Z.H.; Liu, Y.H.; Ying, H.Q.; Zhang, H.; Xue, Y.X. Vascular endothelial growth factor increases permeability of the blood-tumor barrier via caveolae-mediated transcellular pathway. J. Mol. Neurosci. 2011, 44, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.-J.; Lee, K.-J.; Peng, I.-C.; Hsu, C.-H.; Lin, J.-H.; Chen, K.-H.; Tien, Y.-W.; Hsieh, P.C.H. Inducing a Transient Increase in Blood-Brain Barrier Permeability for Improved Liposomal Drug Therapy of Glioblastoma Multiforme. ACS Nano 2019, 13, 97–113. [Google Scholar] [CrossRef]
- Morris, E.K.; Daignault-Mill, S.; Stehbens, S.J.; Genovesi, L.A.; Lagendijk, A.K. Addressing blood-brain-tumor-barrier heterogeneity in pediatric brain tumors with innovative preclinical models. Front. Oncol. 2023, 13, 1101522. [Google Scholar] [CrossRef]
- Rathi, S.; Griffith, J.I.; Zhang, W.; Zhang, W.; Oh, J.-H.; Talele, S.; Sarkaria, J.N.; Elmquist, W.F. The influence of the blood-brain barrier in the treatment of brain tumours. J. Intern. Med. 2022, 292, 3–30. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; Swanson, K.R. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Dymova, M.A.; Kuligina, E.V.; Richter, V.A. Molecular Mechanisms of Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 6385. [Google Scholar] [CrossRef]
- Wallenborn, M.; Xu, L.-X.; Kirsten, H.; Rohani, L.; Rudolf, D.; Ahnert, P.; Schmidt, C.; Schulz, R.M.; Richter, M.; Krupp, W.; et al. Molecular analyses of glioblastoma stem-like cells and glioblastoma tissue. PLoS ONE 2020, 15, e0234986. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Li, W.; Gao, P.; Zhang, N. HER2-targeting CAR-T cells show highly efficient anti-tumor activity against glioblastoma both in vitro and in vivo. Genes Immun. 2024, 25, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Beylerli, O.; Liang, Y.; Xiang, H.; Liu, C.; Xu, X.; Yuan, C.; Ahmad, A.; Yang, G. The Role of MicroRNAs in Therapeutic Resistance of Malignant Primary Brain Tumors. Front. Cell Dev. Biol. 2021, 9, 740303. [Google Scholar] [CrossRef]
- Xiao, B.; Zhou, X.; Ye, M.; Lv, S.; Wu, M.; Liao, C.; Han, L.; Kang, C.; Zhu, X. MicroRNA-566 modulates vascular endothelial growth factor by targeting Von Hippel-Landau in human glioblastoma in vitro and in vivo. Mol. Med. Rep. 2016, 13, 379–385. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53. [Google Scholar] [CrossRef]
- Balandeh, E.; Mohammadshafie, K.; Mahmoudi, Y.; Hossein Pourhanifeh, M.; Rajabi, A.; Bahabadi, Z.R.; Mohammadi, A.H.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Roles of Non-coding RNAs and Angiogenesis in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 716462. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ali, M.M. Recent Treatment Strategies and Molecular Pathways in Resistance Mechanisms of Antiangiogenic Therapies in Glioblastoma. Cancers 2024, 16, 2975. [Google Scholar] [CrossRef]
- Chen, G.; Lin, T.; Wu, M.; Cai, G.; Wu, C.; Ding, Q.; Xu, J.; Chen, H.; Li, W.; Xu, G.; et al. Causal Association of Cytokines and Growth Factors with Stroke and Its Subtypes: A Mendelian Randomization Study. Mol. Neurobiol. 2024, 61, 3212–3222. [Google Scholar] [CrossRef]
- Leone, P.; Malerba, E.; Susca, N.; Favoino, E.; Perosa, F.; Brunori, G.; Prete, M.; Racanelli, V. Endothelial cells in tumor microenvironment: Insights and perspectives. Front. Immunol. 2024, 15, 1367875. [Google Scholar] [CrossRef]
- Himes, B.T.; Geiger, P.A.; Ayasoufi, K.; Bhargav, A.G.; Brown, D.A.; Parney, I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021, 11, 770561. [Google Scholar] [CrossRef]
- Masood, A.B.; Batool, S.; Bhatti, S.N.; Ali, A.; Valko, M.; Jomova, K.; Kuca, K. Plasma PD-L1 as a biomarker in the clinical management of glioblastoma multiforme-a retrospective cohort study. Front. Immunol. 2023, 14, 1202098. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Widodo, S.S.; Dinevska, M.; Furst, L.M.; Stylli, S.S.; Mantamadiotis, T. IL-10 in glioma. Br. J. Cancer 2021, 125, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Crane, C.A.; Kaur, R.; Safaee, M.; Rutkowski, M.J.; Parsa, A.T. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 2013, 19, 3165–3175. [Google Scholar] [CrossRef]
- Yan, T.; Tan, Y.; Deng, G.; Sun, Z.; Liu, B.; Wang, Y.; Yuan, F.; Sun, Q.; Hu, P.; Gao, L.; et al. TGF-β induces GBM mesenchymal transition through upregulation of CLDN4 and nuclear translocation to activate TNF-α/NF-κB signal pathway. Cell Death Dis. 2022, 13, 339. [Google Scholar] [CrossRef]
- Gangoso, E.; Southgate, B.; Bradley, L.; Bradley, L.; Rus, S.; Galvez-Cancino, F.; McGivern, N.; Güç, E.; Kapourani, C.-A.; Byron, A.; et al. Glioblastomas acquire myeloid-affiliated transcriptional programs via epigenetic immunoediting to elicit immune evasion. Cell 2021, 184, 2454–2470.e26. [Google Scholar] [CrossRef]
- Montoya, M.; Collins, S.A.; Chuntova, P.; Patel, T.S.; Nejo, T.; Yamamichi, A.; Kasahara, N.; Okada, H. Interferon regulatory factor 8-driven reprogramming of the immune microenvironment enhances antitumor adaptive immunity and reduces immunosuppression in murine glioblastoma. Neuro-Oncology 2024, 26, 2272–2287. [Google Scholar] [CrossRef]
- Khan, F.; Pang, L.; Dunterman, M.; Lesniak, M.S.; Heimberger, A.B.; Chen, P. Macrophages and microglia in glioblastoma: Heterogeneity, plasticity, and therapy. J. Clin. Investig. 2023, 133, e163446. [Google Scholar] [CrossRef]
- Lofiego, M.F.; Piazzini, F.; Caruso, F.P.; Marzani, F.; Solmonese, L.; Bello, E.; Celesti, F.; Costa, M.C.; Noviello, T.; Mortarini, R.; et al. Epigenetic remodeling to improve the efficacy of immunotherapy in human glioblastoma: Pre-clinical evidence for development of new immunotherapy approaches. J. Transl. Med. 2024, 22, 223. [Google Scholar] [CrossRef]
- Tirgar, F.; Azizi, Z.; Hosseindoost, S.; Hadjighassem, M. Preclinical gene therapy in glioblastoma multiforme: Using olfactory ensheathing cells containing a suicide gene. Life Sci. 2022, 311 Pt A, 121132. [Google Scholar] [CrossRef]
- Wesolowski, J.R.; Rajdev, P.; Mukherji, S.K. Temozolomide (Temodar). Am. J. Neuroradiol. 2010, 31, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Park, M.J.; Lee, M.M.; Kim, T.M.; Lee, S.-H.; Cho, S.Y.; Kim, Y.-H.; Kim, Y.J.; Park, C.-K.; Kim, C.-Y. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. J. Korean Med. Sci. 2014, 29, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef]
- Ezzati, S.; Salib, S.; Balasubramaniam, M.; Aboud, O. Epidermal Growth Factor Receptor Inhibitors in Glioblastoma: Current Status and Future Possibilities. Int. J. Mol. Sci. 2024, 25, 2316. [Google Scholar] [CrossRef]
- Kast, R.E.; Focosi, D. Three paths to better tyrosine kinase inhibition behind the blood-brain barrier in treating chronic myelogenous leukemia and glioblastoma with imatinib. Transl. Oncol. 2010, 3, 13–15. [Google Scholar] [CrossRef]
- Chou, Y.T.; Bivona, T.G. Inhibition of SHP2 as an approach to block RAS-driven cancers. Adv. Cancer Res. 2022, 153, 205–236. [Google Scholar] [CrossRef]
- Sang, Y.; Hou, Y.; Cheng, R.; Zheng, L.; Alvarez, A.A.; Hu, B.; Cheng, S.-Y.; Zhang, W.; Li, Y.; Feng, H. Targeting PDGFRα-activated glioblastoma through specific inhibition of SHP-2-mediated signaling. Neuro-Oncology 2019, 21, 1423–1435. [Google Scholar] [CrossRef]
- Chinot, O.L.; de La Motte Rouge, T.; Moore, N.; Zeaiter, A.; Das, A.; Phillips, H.; Modrusan, Z.; Cloughesy, T. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv. Ther. 2011, 28, 334–340. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bappy, M.H.; Clocchiatti-Tuozzo, S.; Cheeti, S.; Chowdhury, S.; Patel, V. Current Advances in Immunotherapy for Glioblastoma Multiforme and Future Prospects. Cureus 2021, 13, e20604. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.-C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Kamgar, M.; Aboukameel, A.; Bannoura, S.; Chung, B.Y.; Li, Y.; Al Hallak, M.N.; Philip, P.A.; Tsai, S.; Luther, S.; et al. Targeting Cellular Metabolism With CPI-613 Sensitizes Pancreatic Cancer Cells to Radiation Therapy. Adv. Radiat. Oncol. 2022, 8, 101122. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Elliott, G.T.; Olszanski, A.J.; Altieri, D.C. Feasibility and safety of targeting mitochondria for cancer therapy—Preclinical characterization of gamitrinib, a first-in-class, mitochondriaL-targeted small molecule Hsp90 inhibitor. Cancer Biol. Ther. 2022, 23, 117–126. [Google Scholar] [CrossRef]
- Konteatis, Z.; Artin, E.; Nicolay, B.; Straley, K.; Padyana, A.K.; Jin, L.; Chen, Y.; Narayaraswamy, R.; Tong, S.; Wang, F.; et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma. ACS Med. Chem. Lett. 2020, 11, 101–107. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; van den Bent, M.J.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef]
- Arif, S.H.; Pandith, A.A.; Tabasum, R.; Ramzan, A.; Singh, S.; Siddiqi, M.; Bhat, A. Significant Effect of Anti-tyrosine Kinase Inhibitor (Gefitinib) on Overall Survival of the Glioblastoma Multiforme Patients in the Backdrop of Mutational Status of Epidermal Growth Factor Receptor and PTEN Genes. Asian J. Neurosurg. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Jaeckle, K.A.; Anderson, S.K.; Twohy, E.L.; Dixon, J.G.; Giannini, C.; Jenkins, R.; Egorin, M.J.; Sarkaria, J.N.; Brown, P.D.; Flynn, P.J.; et al. Phase I–II trial of imatinib mesylate (Gleevec; STI571) in treatment of recurrent oligodendroglioma and mixed oligoastrocytoma. North central cancer treatment group study N0272 (ALLIANCE/NCCTG). J. Neuro-Oncol. 2019, 143, 573–581. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Klein, M.; Smits, M.; Reijneveld, J.C.; French, P.J.; Clement, P.P.; de Vos, F.Y.F.; Wick, A.; Mulholland, P.J.; Taphoorn, P.M.J.B.; et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): A randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018, 19, 1170–1179. [Google Scholar] [CrossRef]
- Fu, M.; Zhou, Z.; Huang, X.; Chen, Z.; Zhang, L.; Zhang, J.; Hua, W.; Mao, Y. Use of Bevacizumab in recurrent glioblastoma: A scoping review and evidence map. BMC Cancer 2023, 23, 544. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef]
- Peters, K.; Kirkpatrick, J.; Batinic-Haberle, I.; Affronti, M.L.; Woodring, S.; Lipp, E.; Herndon, J.; Boyd, K.; Spasojevic, I.; Penchev, S.; et al. SPCR-03 Neurocognitive Outcomes from Phase 1 Trial of BMX-001 in Combination with Concurrent Radiation Therapy and Temozolomide in Newly Diagnosed High-Grade Glioma Patients. Neuro-Oncol. Adv. 2022, 4 (Suppl. S1), i20. [Google Scholar] [CrossRef]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95. [Google Scholar] [CrossRef]
- Blakeley, J. Drug delivery to brain tumors. Curr. Neurol. Neurosci. Rep. 2008, 8, 235–241. [Google Scholar] [CrossRef]
- Meairs, S.; Alonso, A. Ultrasound, microbubbles and the blood-brain barrier. Prog. Biophys. Mol. Biol. 2007, 93, 354–362. [Google Scholar] [CrossRef]
- Chen, P.Y.; Hsieh, H.Y.; Huang, C.Y.; Lin, C.Y.; Wei, K.C.; Liu, H.L. Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: A preclinical feasibility study. J. Transl. Med. 2015, 13, 93. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Ye, J.; Li, D.; Jie, Y.; Luo, H.; Zhang, W.; Qiu, C. Exosome-based nanoparticles and cancer immunotherapy. Biomed. Pharmacother. 2024, 179, 117296. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Vogelbaum, M.A.; Barnett, G.H.; Jalali, R.; Ahluwalia, M.S. Molecular targeted therapy in recurrent glioblastoma: Current challenges and future directions. Expert Opin. Investig. Drugs 2012, 21, 1247–1266. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R. Food and Drug Administration Drug approval summary: Temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005, 11, 6767–6771. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.J.; Willke, R.J.; Garrison, L.P., Jr. A Health Economics Approach to US Value Assessment Frameworks—Introduction: An ISPOR Special Task Force Report [1]. Value Health 2018, 21, 119–123. [Google Scholar] [CrossRef]
- Messali, A.; Villacorta, R.; Hay, J.W. A review of the economic burden of glioblastoma and the cost effectiveness of pharmacologic treatments. Pharmacoeconomics 2014, 32, 1201–1212. [Google Scholar] [CrossRef]
- Chandana, S.R.; Movva, S.; Arora, M.; Singh, T. Primary brain tumors in adults. Am. Fam. Physician 2008, 77, 1423–1430. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezk, R.; Hanna, A.G.; Hutchinson, H.; Farag, M.; Lucke-Wold, B. Emerging Chemotherapy Targets: Insights from Advances in Glioma Treatment. Biomedicines 2025, 13, 1452. https://doi.org/10.3390/biomedicines13061452
Rezk R, Hanna AG, Hutchinson H, Farag M, Lucke-Wold B. Emerging Chemotherapy Targets: Insights from Advances in Glioma Treatment. Biomedicines. 2025; 13(6):1452. https://doi.org/10.3390/biomedicines13061452
Chicago/Turabian StyleRezk, Rogina, Abanob George Hanna, Hunter Hutchinson, Mariam Farag, and Brandon Lucke-Wold. 2025. "Emerging Chemotherapy Targets: Insights from Advances in Glioma Treatment" Biomedicines 13, no. 6: 1452. https://doi.org/10.3390/biomedicines13061452
APA StyleRezk, R., Hanna, A. G., Hutchinson, H., Farag, M., & Lucke-Wold, B. (2025). Emerging Chemotherapy Targets: Insights from Advances in Glioma Treatment. Biomedicines, 13(6), 1452. https://doi.org/10.3390/biomedicines13061452







