Glucocorticoid Insensitivity: Is It a Question of Time and Place?
Abstract
1. Introduction
2. The Glucocorticoid Receptor
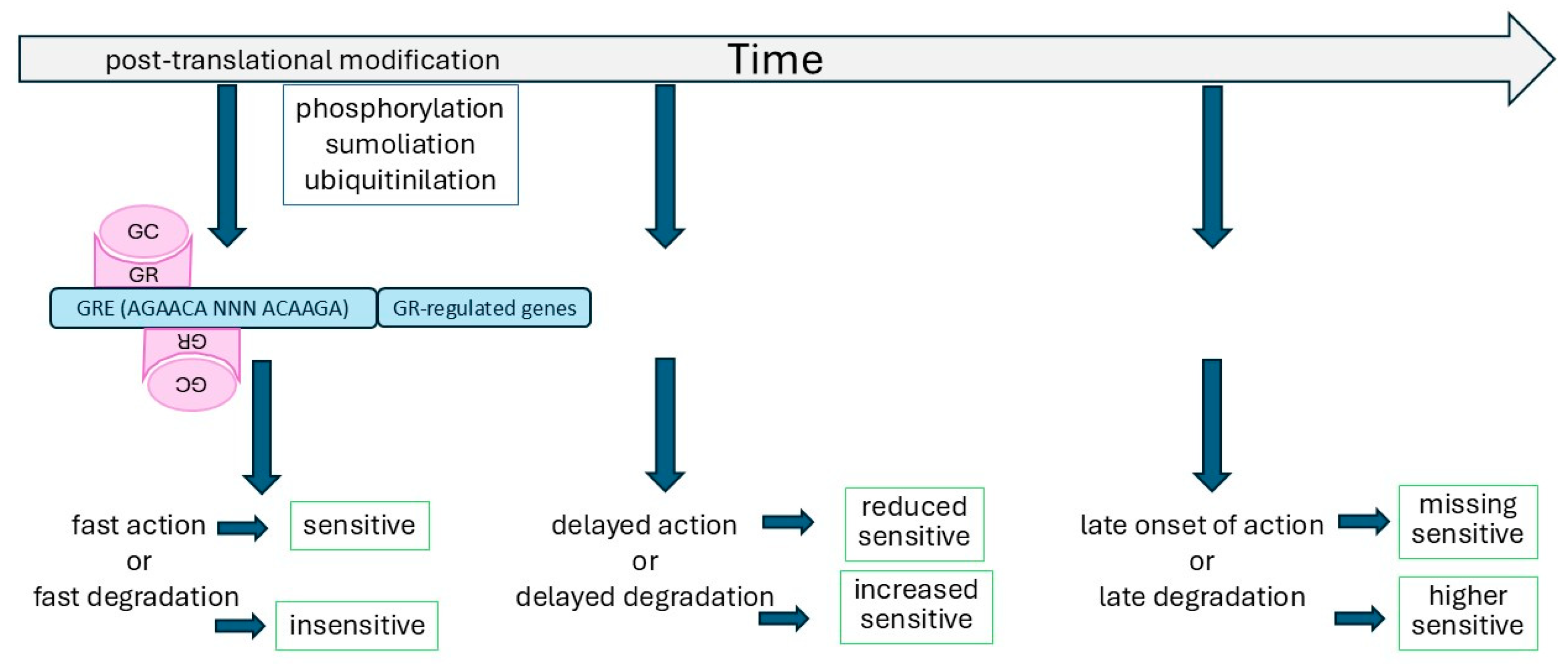
3. The Regulation of GR Function by Time
4. The Chromatin Structure Regulates Steroid Response
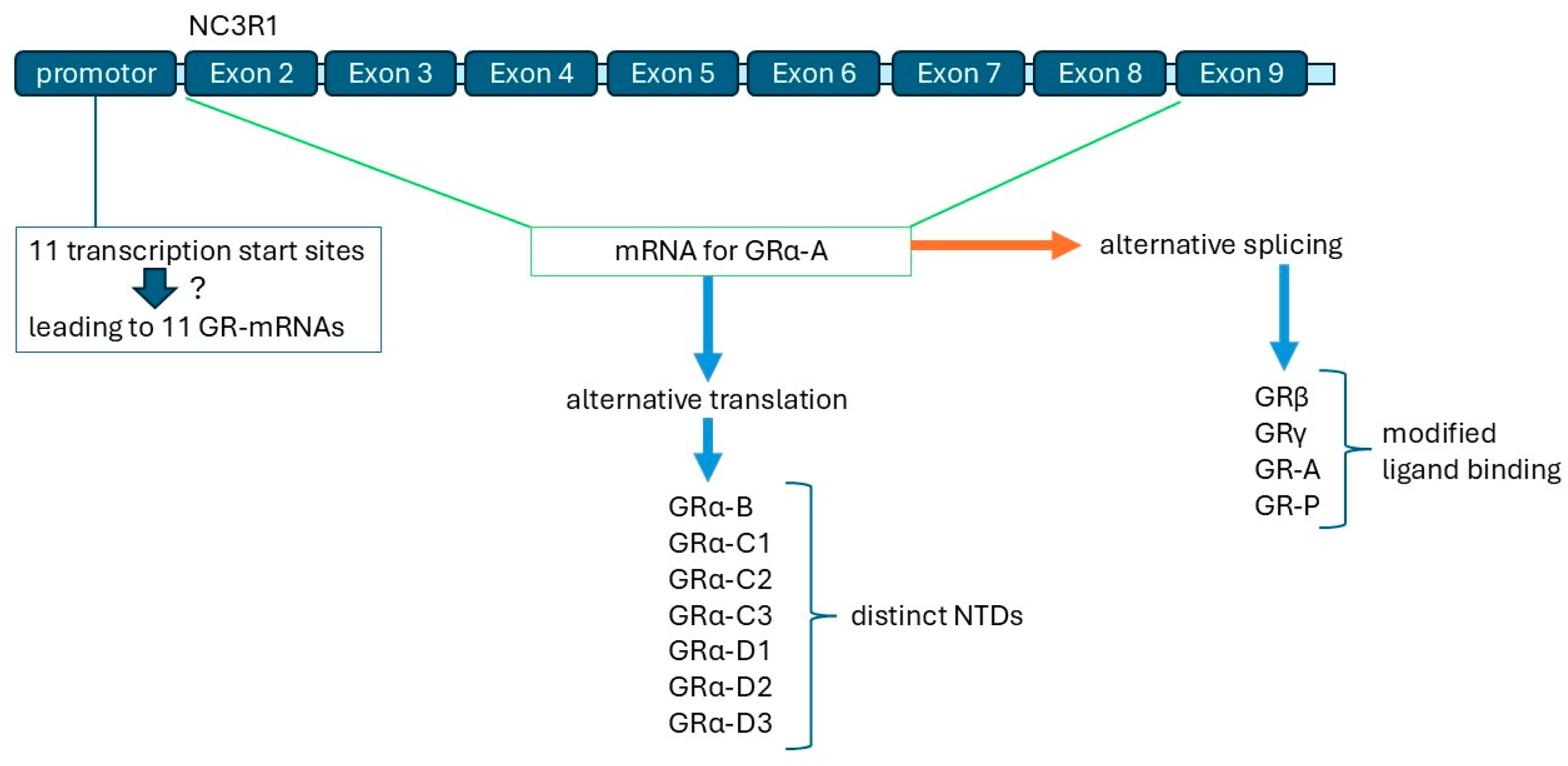
5. GR Isoforms: Their Origin and Regulation
6. GR Interaction and Regulation by Other Transcription Factors
7. Steroid Availability and Its Regulation
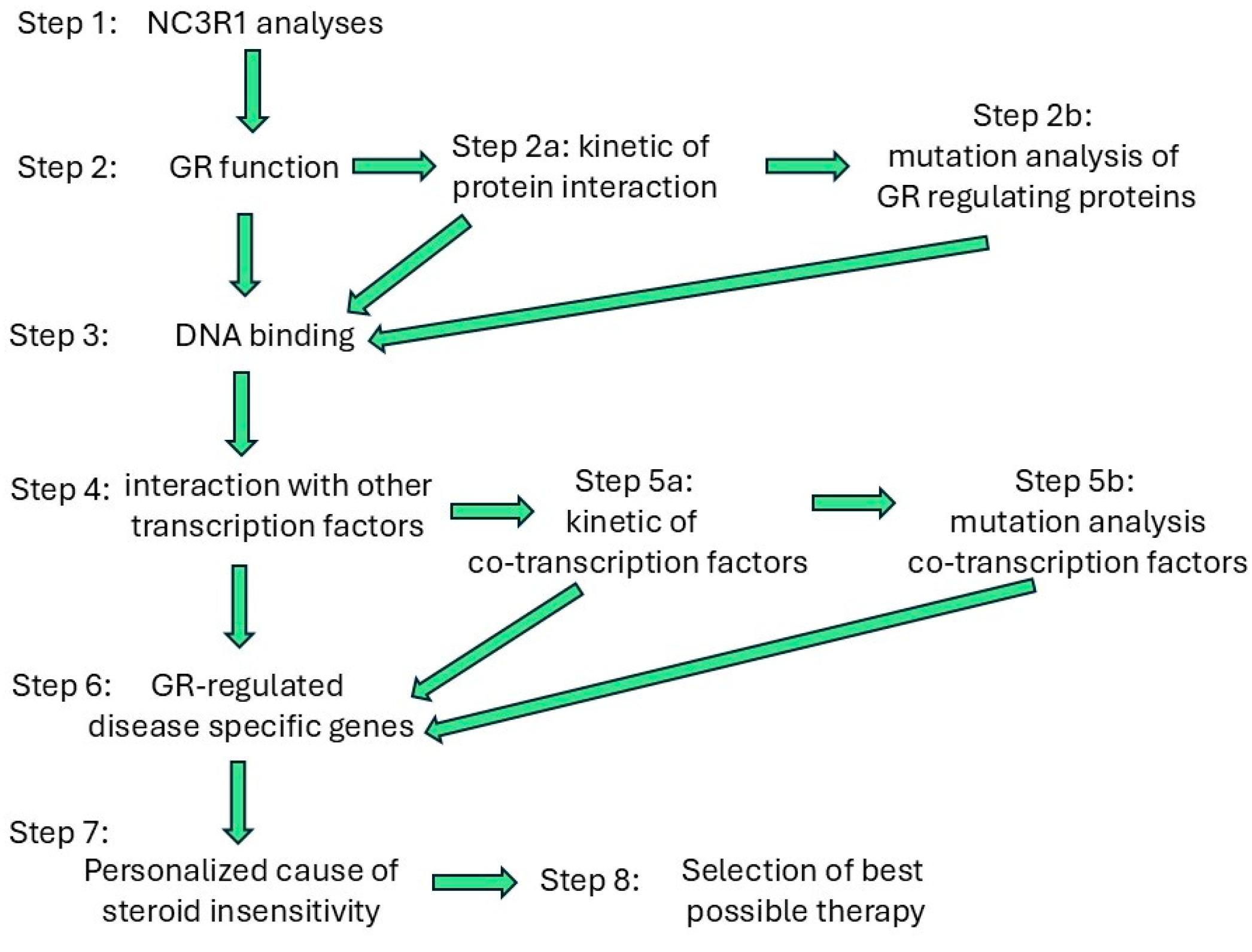
8. Discussion
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-1 | Activator protein 1 |
| CBG | Corticosteroid-binding globulin |
| C/EBP | CCAAT enhancer-binding protein |
| COPD | Chronic obstructive pulmonary disease |
| CREBP | Cyclic AMP-binding protein |
| DNA | Deoxyribonucleic acid |
| DOAJ | Directory of open-access journals |
| FKBP | FK506-binding proteins |
| GC | Glucocorticoid |
| GR | Glucocorticoid receptor |
| GRE | Glucocorticoid response element |
| HSP | Heat shock protein |
| mRNA | Messenger ribonucleic acid |
| NFkB | Nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| SNP | Single-nucleotide polymorphism |
| TAC1 | Th2 high-inflammatory signature protein 1 |
References
- Global Initiative for Asthma: 2024 GINA Main Report. Available online: https://ginasthma.org/2024-report/ (accessed on 3 March 2025).
- American Lung Association: Asthma Trends and Burden. Available online: https://www.lung.org/research/trends-in-lung-disease/asthma-trends-brief/trends-and-burden (accessed on 3 March 2025).
- Oh, J.; Lee, M.; Kim, M.; Kim, H.J.; Lee, S.W.; Rhee, S.Y.; Koyanagi, A.; Smith, L.; Kim, M.S.; Lee, H.; et al. Incident allergic diseases in post-COVID-19 condition: Multinational cohort studies from South Korea, Japan and the UK. Nat. Commun. 2024, 15, 2830. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes de Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef]
- Henderson, I.; Caiazzo, E.; McSharry, C.; Guzik, T.J.; Maffia, P. Why do some asthma patients respond poorly to glucocorticoid therapy? Pharmacol. Res. 2020, 160, 105189. [Google Scholar] [CrossRef]
- Jacques, M.R.; Kuhn, B.T.; Albertson, T.E. Update on the pharmacological treatment of chronic obstructive pulmonary disease. Expert Opin. Pharmacother. 2024, 25, 1903–1922. [Google Scholar] [CrossRef]
- Sayers, I.; John, C.; Chen, J.; Hall, I.P. Genetics of chronic respiratory disease. Nat. Rev. Genet. 2024, 25, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Quax, R.A.; Manenschijn, L.; Koper, J.W.; Hazes, J.M.; Lamberts, S.W.; van Rossum, E.F.; Feelders, R.A. Glucocorticoid sensitivity in health and disease. Nat. Rev. Endocrinol. 2013, 9, 670–686. [Google Scholar] [CrossRef]
- Blackhurst, G.; McElroy, P.K.; Fraser, R.; Swan, R.L.; Connell, J.M. Seasonal variation in glucocorticoid receptor binding characteristics in human mononuclear leucocytes. Clin. Endocrinol. 2001, 55, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, J.; Pretorius, C.J.; Ungerer, J.P. Biological and diurnal variation in glucocorticoid sensitivity detected with a sensitive in vitro dexamethasone suppression of cytokine production assay. J. Clin. Endocrinol. Metab. 2010, 95, 3657–3663. [Google Scholar] [CrossRef]
- Barnes, P.J.; Adcock, I.M. Glucocorticoid resistance in inflammatory diseases. Lancet 2009, 373, 1905–1917. [Google Scholar] [CrossRef]
- Martinez, G.J.; Appleton, M.; Kipp, Z.A.; Loria, A.S.; Min, B.; Hinds, T.D., Jr. Glucocorticoids, their uses, sexual dimorphisms, and diseases: New concepts, mechanisms, and discoveries. Physiol. Rev. 2024, 104, 473–532. [Google Scholar] [CrossRef]
- Brattsand, R.; Selroos, O. May a different kinetic mode explain the high efficacy/safety profile of inhaled budesonide? Pulm. Pharmacol. Ther. 2022, 77, 102167. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Bareille, P.J.; Rousell, V.M. Fluticasone furoate, a novel inhaled corticosteroid, demonstrates prolonged lung absorption kinetics in man compared with inhaled fluticasone propionate. Clin. Pharmacokinet. 2013, 52, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G. Pharmacokinetics/pharmacodynamics of glucocorticoids: Modeling the glucocorticoid receptor dynamics and dose/response of commonly prescribed glucocorticoids. ADMET DMPK 2024, 12, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.M.; Rider, C.F.; Shah, S.; Traves, S.L.; Gordon, P.M.K.; Miller-Larsson, A.; Leigh, R.; Newton, R. Glucocorticoid-driven transcriptomes in human airway epithelial cells: Commonalities, differences and functional insight from cell lines and primary cells. BMC Med. Genom. 2019, 12, 29. [Google Scholar] [CrossRef]
- Mars, U.; d’Argy, R.; Hallbeck, K.; Miller-Larsson, A.; Edsbäcker, S. Tissue accumulation kinetics of ciclesonide-active metabolite and budesonide in mice. Basic Clin. Pharmacol. Toxicol. 2013, 112, 401–411. [Google Scholar] [CrossRef]
- Reichardt, S.D.; Amouret, A.; Muzzi, C.; Vettorazzi, S.; Tuckermann, J.P.; Lühder, F.; Reichardt, H.M. The Role of Glucocorticoids in Inflammatory Diseases. Cells 2021, 10, 2921. [Google Scholar] [CrossRef]
- Caramori, G.; Nucera, F.; Mumby, S.; Lo Bello, F.; Adcock, I.M. Corticosteroid resistance in asthma: Cellular and molecular mechanisms. Mol. Asp. Med. 2022, 85, 100969. [Google Scholar] [CrossRef]
- Lockett, J.; Inder, W.J.; Clifton, V.L. The Glucocorticoid Receptor: Isoforms, Functions, and Contribution to Glucocorticoid Sensitivity. Endocr. Rev. 2024, 45, 593–624. [Google Scholar] [CrossRef]
- Clarisse, D.; Van Moortel, L.; Van Leene, C.; Gevaert, K.; De Bosscher, K. Glucocorticoid receptor signaling: Intricacies and therapeutic opportunities. Trends Biochem. Sci. 2024, 49, 431–444. [Google Scholar] [CrossRef]
- Moessmer, P.; Suren, T.; Majdic, U.; Dahiya, V.; Rutz, D.; Buchner, J.; Rief, M. Active unfolding of the glucocorticoid receptor by the Hsp70/Hsp40 chaperone system in single-molecule mechanical experiments. Proc. Natl. Acad. Sci. USA 2022, 119, e2119076119. [Google Scholar] [CrossRef]
- Hay, C.W.; Shanley, L.; Davidson, S.; Cowie, P.; Lear, M.; McGuffin, P.; Riedel, G.; McEwan, I.J.; MacKenzie, A. Functional effects of polymorphisms on glucocorticoid receptor modulation of human anxiogenic substance-P gene promoter activity in primary amygdala neurones. Psychoneuroendocrinology 2014, 47, 43–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinelli, S.; Hafner, K.; Koedel, M.; Knauer-Arloth, J.; Gassen, N.C.; Binder, E.B. Differential Dynamics and Roles of FKBP51 Isoforms and Their Implications for Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 12318. [Google Scholar] [CrossRef]
- National Institute of Health, National Center for Biotechnological Information. Available online: https://www.ncbi.nlm.nih.gov/gene/?term=NR3C1 (accessed on 3 March 2025).
- Koper, J.W.; van Rossum, E.F.; van den Akker, E.L. Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids 2014, 92, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Degtyareva, A.O.; Antontseva, E.V.; Merkulova, T.I. Regulatory SNPs: Altered Transcription Factor Binding Sites Implicated in Complex Traits and Diseases. Int. J. Mol. Sci. 2021, 22, 6454. [Google Scholar] [CrossRef] [PubMed]
- Gebru, N.T.; Hill, S.E.; Blair, L.J. Genetically engineered mouse models of FK506-binding protein 5. J. Cell. Biochem. 2024, 125, e30374. [Google Scholar] [CrossRef]
- Singh, S.; Kularia, S.; Shukla, S.; Singh, M.; Kumar, M.; Sharma, A.K. A current review on animal models of anti-asthmatic drugs screening. Front. Pharmacol. 2025, 16, 1508460. [Google Scholar] [CrossRef]
- Fröhlich, E. Animals in Respiratory Research. Int. J. Mol. Sci. 2024, 25, 2903. [Google Scholar] [CrossRef]
- Johnson, J.L. FKBP51 functions in the regulation of circadian rhythm and Alzheimer’s disease. Cell Stress Chaperones 2025, 30, 81–83. [Google Scholar] [CrossRef]
- Gebru, N.T.; Beaulieu-Abdelahad, D.; Gulick, D.; Blair, L.J. FKBP51 overexpression in the corticolimbic system stabilizes circadian rhythms. Cell Stress Chaperones 2025, 30, 22–32. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, G.E.; Chae, C.W.; Lim, J.R.; Jung, Y.H.; Yoon, J.H.; Park, J.Y.; Han, H.J. Melatonin-mediated FKBP4 downregulation protects against stress-induced neuronal mitochondria dysfunctions by blocking nuclear translocation of GR. Cell Death Dis. 2023, 14, 146. [Google Scholar] [CrossRef]
- Preston, R.; Chrisp, R.; Dudek, M.; Morais, M.R.P.T.; Tian, P.; Williams, E.; Naylor, R.W.; Davenport, B.; Pathiranage, D.R.J.; Benson, E.; et al. The glomerular circadian clock temporally regulates basement membrane dynamics and the podocyte glucocorticoid response. Kidney Int. 2025, 107, 99–115. [Google Scholar] [CrossRef]
- Welter, H.; Kreitmair, N.; Schneider, M.; Schneider, J.; Petkov, S.; Stepanov, Y.; Köhn, F.M.; Pickl, U.; Trottmann, M.; Fröhlich, T.; et al. Dexamethasone is a regulator of clock genes in testicular peritubular cells. Andrology 2024. online ahead of print. [Google Scholar] [CrossRef]
- Akinborewa, O.; Quattrocelli, M. Glucocorticoid receptor epigenetic activity in the heart. Epigenetics 2025, 20, 2468113. [Google Scholar] [CrossRef]
- Francia, M.; Bot, M.; Boltz, T.; De la Hoz, J.F.; Boks, M.; Kahn, R.S.; Ophoff, R.A. Fibroblasts as an in vitro model of circadian genetic and genomic studies. Mamm. Genome 2024, 35, 432–444. [Google Scholar] [CrossRef]
- Sundar, I.K.; Yao, H.; Sellix, M.T.; Rahman, I. Circadian clock-coupled lung cellular and molecular functions in chronic airway diseases. Am. J. Respir. Cell Mol. Biol. 2015, 53, 285–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 2013, 34, 518–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraft, M.; Vianna, E.; Martin, R.J.; Leung, D.Y. Nocturnal asthma is associated with reduced glucocorticoid receptor binding affinity and decreased steroid responsiveness at night. J. Allergy Clin. Immunol. 1999, 103 Pt 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Hamid, Q.; Chrousos, G.P.; Martin, R.J.; Leung, D.Y. Decreased steroid responsiveness at night in nocturnal asthma. Is the macrophage responsible? Am. J. Respir. Crit. Care Med. 2001, 163, 1219–1225. [Google Scholar] [CrossRef]
- Burioka, N.; Fukuoka, Y.; Takata, M.; Endo, M.; Miyata, M.; Chikumi, H.; Tomita, K.; Kodani, M.; Touge, H.; Takeda, K.; et al. Circadian rhythms in the CNS and peripheral clock disorders: Function of clock genes: Influence of medication for bronchial asthma on circadian gene. J. Pharmacol. Sci. 2007, 103, 144–149. [Google Scholar] [CrossRef]
- Hayasaka, N.; Yaita, T.; Kuwaki, T.; Honma, S.; Honma, K.; Kudo, T.; Shibata, S. Optimization of dosing schedule of daily inhalant dexamethasone to minimize phase shifting of clock gene expression rhythm in the lungs of the asthma mouse model. Endocrinology 2007, 148, 3316–3326. [Google Scholar] [CrossRef]
- Gibbs, J.E.; Beesley, S.; Plumb, J.; Singh, D.; Farrow, S.; Ray, D.W.; Loudon, A.S. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 2009, 150, 268–276. [Google Scholar] [CrossRef]
- Shimba, A.; Ikuta, K. Glucocorticoids Regulate Circadian Rhythm of Innate and Adaptive Immunity. Front. Immunol. 2020, 11, 2143. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Lemmer, B.; Reinberg, A.E. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv. Drug Deliv. Rev. 2007, 59, 852–882. [Google Scholar] [CrossRef]
- Lee, B.H.; Stallcup, M.R. Glucocorticoid receptor binding to chromatin is selectively controlled by the coregulator Hic-5 and chromatin remodeling enzymes. J. Biol. Chem. 2017, 292, 9320–9334. [Google Scholar] [CrossRef]
- Dendoncker, K.; Timmermans, S.; Van Looveren, K.; De Cauwer, L.; De Bosscher, K.; Libert, C. The nature of the GRE influences the screening for GR-activity enhancing modulators. PLoS ONE 2017, 12, e0181101. [Google Scholar] [CrossRef]
- Neophytou, A.M.; Lutzker, L.; Good, K.M.; Mann, J.K.; Noth, E.M.; Holm, S.M.; Costello, S.; Tyner, T.; Nadeau, K.C.; Eisen, E.A.; et al. Associations between prenatal and early-life air pollution exposure and lung function in young children: Exploring influential windows of exposure on lung development. Environ. Res. 2023, 222, 115415. [Google Scholar] [CrossRef]
- Damiani, I.; Solberg, E.H.; Iyer, M.; Cheng, P.; Weldy, C.S.; Kim, J.B. Environmental pollutants and atherosclerosis: Epigenetic mechanisms linking genetic risk and disease. Atherosclerosis 2025, 404, 119131. [Google Scholar] [CrossRef]
- Noguchi, T.; Makino, S.; Matsumoto, R.; Nakayama, S.; Nishiyama, M.; Terada, Y.; Hashimoto, K. Regulation of glucocorticoid receptor transcription and nuclear translocation during single and repeated immobilization stress. Endocrinology 2010, 151, 4344–4355. [Google Scholar] [CrossRef]
- Stortz, M.; Pecci, A.; Presman, D.M.; Levi, V. Unraveling the molecular interactions involved in phase separation of glucocorticoid receptor. BMC Biol. 2020, 18, 59. [Google Scholar] [CrossRef]
- Frank, F.; Liu, X.; Ortlund, E.A. Glucocorticoid receptor condensates link DNA-dependent receptor dimerization and transcriptional transactivation. Proc. Natl. Acad. Sci. USA 2021, 118, e2024685118. [Google Scholar] [CrossRef]
- Fang, L.; Wuptram, K.; Chen, D.; Li, H.; Huang, S.K.; Jin, C.; Yokoyama, K.K. Environmental-stress-induced Chromatin Regulation and its Heritability. J. Carcinog. Mutagen. 2014, 5, 22058. [Google Scholar] [CrossRef] [PubMed]
- Abell, N.S.; DeGorter, M.K.; Gloudemans, M.J.; Greenwald, E.; Smith, K.S.; He, Z.; Montgomery, S.B. Multiple causal variants underlie genetic associations in humans. Science 2022, 375, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-González, L.; Ruiz-Herrera, A. Evolution of 3D Chromatin Folding. Annu. Rev. Anim. Biosci. 2025, 13, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhao, M.; Deng, F.; Xu, T.; Ji, B.; Wang, X.; Zhang, M.; Sun, M.; Gao, Q. Prenatal dexamethasone exposure impaired vascular reactivity in adult male offspring cerebral arteries. J. Mol. Cell Cardiol. 2023, 181, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.; Cao, H.; Tian, F.; Huang, Y.; Jiang, M.; Zhao, H.; Tai, X.; Xu, W.; Kosasih, H.J.; Kealy, D.J.; et al. PU.1 eviction at lymphocyte-specific chromatin domains mediates glucocorticoid response in acute lymphoblastic leukemia. Nat. Commun. 2024, 15, 9697. [Google Scholar] [CrossRef]
- Takao, T.; Matsui, A.; Kikutake, C.; Kan-O, K.; Inoue, A.; Suyama, M.; Okamoto, I.; Ito, M. Maternal asthma imprints fetal lung ILC2s via glucocorticoid signaling leading to worsened allergic airway inflammation in murine adult offspring. Nat. Commun. 2025, 16, 631. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, J.; Pei, L.; He, Z.; Luo, H.; Magdalou, J.; Chen, L.; Wang, H. Articular damages in multi-generational female offspring due to prenatal caffeine exposure correlates with H3K9 deacetylation of TGFβ signaling pathway. Toxicology 2020, 442, 152533. [Google Scholar] [CrossRef]
- Burd, C.J.; Archer, T.K. Chromatin architecture defines the glucocorticoid response. Mol. Cell Endocrinol. 2013, 380, 25–31. [Google Scholar] [CrossRef]
- Bothe, M.; Buschow, R.; Meijsing, S.H. Glucocorticoid signaling induces transcriptional memory and universally reversible chromatin changes. Life Sci. Alliance 2021, 4, e202101080. [Google Scholar] [CrossRef] [PubMed]
- Lovem, M.I.; Huska, M.R.; Jurk, M.; Schöpflin, R.; Starick, S.R.; Schwahn, K.; Cooper, S.B.; Yamamoto, K.R.; Thomas-Chollier, M.; Vingron, M.; et al. Role of the chromatin landscape and sequence in determining cell type-specific genomic glucocorticoid receptor binding and gene regulation. Nucleic Acids Res. 2017, 45, 1805–1819. [Google Scholar] [CrossRef]
- Jubb, A.W.; Boyle, S.; Hume, D.A.; Bickmore, W.A. Glucocorticoid Receptor Binding Induces Rapid and Prolonged Large-Scale Chromatin Decompaction at Multiple Target Loci. Cell Rep. 2017, 21, 3022–3031. [Google Scholar] [CrossRef]
- Wagh, K.; Stavreva, D.A.; Hager, G.L. Transcription dynamics and genome organization in the mammalian nucleus: Recent advances. Mol. Cell 2025, 85, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Stortz, M.; Oses, C.; Lafuente, A.L.; Presman, D.M.; Levi, V. Catching the glucocorticoid receptor in the act: Lessons from fluorescence fluctuation methods. Biochem. Biophys. Res. Commun. 2025, 748, 151327. [Google Scholar] [CrossRef] [PubMed]
- Ibello, E.; Saracino, F.; Delle Cave, D.; Buonaiuto, S.; Amoroso, F.; Andolfi, G.; Corona, M.; Guardiola, O.; Colonna, V.; Sainz, B., Jr.; et al. Three-dimensional environment sensitizes pancreatic cancer cells to the anti-proliferative effect of budesonide by reprogramming energy metabolism. J. Exp. Clin. Cancer Res. 2024, 43, 165. [Google Scholar] [CrossRef]
- Jing, D.; Huang, Y.; Liu, X.; Sia, K.C.S.; Zhang, J.C.; Tai, X.; Wang, M.; Toscan, C.E.; McCalmont, H.; Evans, K.; et al. Lymphocyte-Specific Chromatin Accessibility Pre-determines Glucocorticoid Resistance in Acute Lymphoblastic Leukemia. Cancer Cell 2018, 34, 906–921.e8. [Google Scholar] [CrossRef]
- Borin, C.; Pieters, T.; Serafin, V.; Ntziachristos, P. Emerging Epigenetic and Posttranslational Mechanisms Controlling Resistance to Glucocorticoids in Acute Lymphoblastic Leukemia. Hemasphere 2023, 7, e916. [Google Scholar] [CrossRef]
- Stolz, D.; Papakonstantinou, E.; Pascarella, M.; Jahn, K.; Siebeneichler, A.; Darie, A.M.; Herrmann, M.J.; Strobel, W.; Salina, A.; Grize, L.; et al. Airway smooth muscle area to predict steroid responsiveness in COPD patients receiving triple therapy (HISTORIC): A randomised, placebo-controlled, double-blind, investigator-initiated trial. Eur. Respir. J. 2023, 62, 2300218. [Google Scholar] [CrossRef]
- Zhou, Y.; Ampon, M.R.; Abramson, M.J.; James, A.L.; Maguire, G.P.; Wood-Baker, R.; Johns, D.P.; Marks, G.B.; Reddel, H.K.; Toelle, B.G. Prevalence and characteristics of adults with preserved ratio impaired spirometry (PRISm): Data from the BOLD Australia study. Chron. Respir. Dis. 2025, 22, 14799731241312687. [Google Scholar] [CrossRef]
- Buhumaid, R.; Alzaabi, A.; Mahboub, B.; Iqbal, M.N.; Alhameli, H.A.; Al-Mafrachi, M.G.; Dittrich, K.C.; Jaiganesh, T. The need for implementing a standardized, evidence-based emergency department discharge plan for optimizing adult asthma patient outcomes in the UAE, expert meeting report. Int. J. Emerg. Med. 2024, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- van der Mark, L.B.; Lyklema, P.H.; Geskus, R.B.; Mohrs, J.; Bindels, P.J.; van Aalderen, W.M.; Ter Riet, G. A systematic review with attempted network meta-analysis of asthma therapy recommended for five to eighteen year olds in GINA steps three and four. BMC Pulm. Med. 2012, 12, 63. [Google Scholar] [CrossRef]
- Ortega, V.E.; Meyers, D.A.; Bleecker, E.R. Asthma pharmacogenetics and the development of genetic profiles for personalized medicine. Pharmgenomics Pers. Med. 2015, 8, 9–22. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cidlowski, J.A. The human glucocorticoid receptor: One gene, multiple proteins and diverse responses. Steroids 2005, 70, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.Z.; Cidlowski, J.A. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell 2005, 18, 331–342. [Google Scholar] [CrossRef]
- Leventhal, S.M.; Lim, D.; Green, T.L.; Cantrell, A.E.; Cho, K.; Greenhalgh, D.G. Uncovering a multitude of human glucocorticoid receptor variants: An expansive survey of a single gene. BMC Genet. 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Escoter-Torres, L.; Greulich, F.; Quagliarini, F.; Wierer, M.; Uhlenhaut, N.H. Anti-inflammatory functions of the glucocorticoid receptor require DNA binding. Nucleic Acids Res. 2020, 48, 8393–8407. [Google Scholar] [CrossRef]
- Vahedi, G. Remodeling the chromatin landscape in T lymphocytes by a division of labor among transcription factors. Immunol. Rev. 2021, 300, 167–180. [Google Scholar] [CrossRef]
- Oh, K.S.; Patel, H.; Gottschalk, R.A.; Lee, W.S.; Baek, S.; Fraser, I.D.C.; Hager, G.L.; Sung, M.H. Anti-Inflammatory Chromatinscape Suggests Alternative Mechanisms of Glucocorticoid Receptor Action. Immunity 2017, 47, 298–309.e5. [Google Scholar] [CrossRef]
- Chang, W.T.; Hong, M.Y.; Chen, C.L.; Hwang, C.Y.; Tsai, C.C.; Chuang, C.C. Mutant glucocorticoid receptor binding elements on the interleukin-6 promoter regulate dexamethasone effects. BMC Immunol. 2021, 22, 24. [Google Scholar] [CrossRef]
- Oakley, R.H.; Ramamoorthy, S.; Foley, J.F.; Busada, J.T.; Lu, N.Z.; Cidlowski, J.A. Glucocorticoid receptor isoform-specific regulation of development, circadian rhythm, and inflammation in mice. FASEB J. 2018, 32, 5258–5271. [Google Scholar] [CrossRef]
- Duma, D.; Cidlowski, J.A. Generating diversity in glucocorticoid receptor signaling: Mechanisms, receptor isoforms, and post-translational modifications. Horm. Mol. Biol. Clin. Investig. 2010, 3, 319–328. [Google Scholar] [CrossRef]
- Oakley, R.H.; Sar, M.; Cidlowski, J.A. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 1996, 271, 9550–9559. [Google Scholar] [PubMed]
- Buoso, E.; Galasso, M.; Ronfani, M.; Serafini, M.M.; Lanni, C.; Corsini, E.; Racchi, M. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol. Res. 2017, 120, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Chollier, M.; Watson, L.C.; Cooper, S.B.; Pufall, M.A.; Liu, J.S.; Borzym, K.; Vingron, M.; Yamamoto, K.R.; Meijsing, S.H. A naturally occurring insertion of a single amino acid rewires transcriptional regulation by glucocorticoid receptor isoforms. Proc. Natl. Acad. Sci. USA 2013, 110, 17826–17831. [Google Scholar] [CrossRef]
- Sinclair, D.; Webster, M.J.; Wong, J.; Weickert, C.S. Dynamic molecular and anatomical changes in the glucocorticoid receptor in human cortical development. Mol. Psychiatry 2011, 16, 504–515. [Google Scholar] [CrossRef]
- He, B.; Cruz-Topete, D.; Oakley, R.H.; Xiao, X.; Cidlowski, J.A. Human Glucocorticoid Receptor β Regulates Gluconeogenesis and Inflammation in Mouse Liver. Mol. Cell Biol. 2015, 36, 714–730. [Google Scholar] [CrossRef] [PubMed]
- de Lange, P.; Segeren, C.M.; Koper, J.W.; Wiemer, E.; Sonneveld, P.; Brinkmann, A.O.; White, A.; Brogan, I.J.; de Jong, F.H.; Lamberts, S.W. Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res. 2001, 61, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Saif, Z.; Hodyl, N.A.; Stark, M.J.; Fuller, P.J.; Cole, T.; Lu, N.; Clifton, V.L. Expression of eight glucocorticoid receptor isoforms in the human preterm placenta vary with fetal sex and birthweight. Placenta 2015, 36, 723–730. [Google Scholar] [CrossRef]
- Young, S.L.; Saif, Z.; Meakin, A.S.; McMaster, E.S.; Hayes, N.; Gallo, L.A.; Reid, N.; Moritz, K.M.; Clifton, V.L. Alterations to Placental Glucocorticoid Receptor Expression with Alcohol Consumption. Reprod. Sci. 2021, 28, 1390–1402. [Google Scholar] [CrossRef]
- Morgan, D.J.; Poolman, T.M.; Williamson, A.J.; Wang, Z.; Clark, N.R.; Ma’ayan, A.; Whetton, A.D.; Brass, A.; Matthews, L.C.; Ray, D.W. Glucocorticoid receptor isoforms direct distinct mitochondrial programs to regulate ATP production. Sci. Rep. 2016, 6, 26419. [Google Scholar] [CrossRef]
- Beger, C.; Gerdes, K.; Lauten, M.; Tissing, W.J.; Fernandez-Munoz, I.; Schrappe, M.; Welte, K. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br. J. Haematol. 2003, 122, 245–252. [Google Scholar] [CrossRef]
- Haarman, E.G.; Kaspers, G.J.; Pieters, R.; Rottier, M.M.; Veerman, A.J. Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia 2004, 18, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Duma, D.; Jewell, C.M.; Cidlowski, J.A. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J. Steroid Biochem. Mol. Biol. 2006, 102, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Blind, R.D.; Garabedian, M.J. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J. Steroid Biochem. Mol. Biol. 2008, 109, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Tello, A.; Halwani, R.; Hamid, Q.; Al-Muhsen, S. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells. J. Clin. Immunol. 2013, 33, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Wang, Y.; Mo, B.; Wei, J.; Ma, L.; Rao, L.; Wang, J.; Yao, D.; Huang, J.; et al. E4BP4 facilitates glucocorticoid sensitivity of human bronchial epithelial cells via down-regulation of glucocorticoid receptor-beta. Cell Immunol. 2018, 334, 31–37. [Google Scholar] [CrossRef]
- Bansal, A.; Kooi, C.; Kalyanaraman, K.; Gill, S.; Thorne, A.; Chandramohan, P.; Necker-Brown, A.; Mostafa, M.M.; Milani, A.; Leigh, R.; et al. Synergy between Interleukin-1β, Interferon-γ, and Glucocorticoids to Induce TLR2 Expression Involves NF-κB, STAT1, and the Glucocorticoid Receptor. Mol. Pharmacol. 2023, 105, 23–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Le, Y.; Ji, Y.; Yarde, S.; Yu, X.; Cheng, X. Activation of activator protein-1-fibroblast growth factor 21 signaling attenuates Cisplatin hepatotoxicity. Biochem. Pharmacol. 2021, 194, 114823. [Google Scholar] [CrossRef]
- Sevilla, L.M.; Jiménez-Panizo, A.; Alegre-Martí, A.; Estébanez-Perpiñá, E.; Caelles, C.; Pérez, P. Glucocorticoid Resistance: Interference between the Glucocorticoid Receptor and the MAPK Signalling Pathways. Int. J. Mol. Sci. 2021, 22, 10049. [Google Scholar] [CrossRef]
- Rüdiger, J.J.; Roth, M.; Bihl, M.P.; Cornelius, B.C.; Johnson, M.; Ziesche, R.; Block, L.H. Interaction of C/EBPalpha and the glucocorticoid receptor in vivo and in nontransformed human cells. FASEB J. 2002, 16, 177–184. [Google Scholar] [CrossRef]
- Petty, E.; Pillus, L. Balancing chromatin remodeling and histone modifications in transcription. Trends Genet. 2013, 29, 621–629. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Xu, L.; Chen, J.Y.; Chong, J.; Oh, J.; Leschziner, A.E.; Fu, X.D.; Wang, D. Cockayne syndrome B protein acts as an ATP-dependent processivity factor that helps RNA polymerase II overcome nucleosome barriers. Proc. Natl. Acad. Sci. USA 2020, 117, 25486–25493. [Google Scholar] [CrossRef] [PubMed]
- Mustafi, P.; Hu, M.; Kumari, S.; Das, C.; Li, G.; Kundu, T.K. Phosphorylation-dependent association of human chromatin protein PC4 to linker histone H1 regulates genome organization and transcription. Nucleic Acids Res. 2022, 50, 6116–6136. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Johnson, P.R.; Rüdiger, J.J.; King, G.G.; Ge, Q.; Burgess, J.K.; Anderson, G.; Tamm, M.; Black, J.L. Interaction between glucocorticoids and beta2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet 2002, 360, 1293–1299. [Google Scholar] [CrossRef]
- Usmani, O.S.; Ito, K.; Maneechotesuwan, K.; Ito, M.; Johnson, M.; Barnes, P.J.; Adcock, I.M. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am. J. Respir. Crit. Care Med. 2005, 172, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Oliver, B.G.; Johnston, S.L.; Baraket, M.; Burgess, J.K.; King, N.J.; Roth, M.; Lim, S.; Black, J.L. Increased proinflammatory responses from asthmatic human airway smooth muscle cells in response to rhinovirus infection. Respir. Res. 2006, 7, 71. [Google Scholar] [CrossRef]
- Miglino, N.; Roth, M.; Tamm, M.; Borger, P. House dust mite extract downregulates C/EBPα in asthmatic bronchial smooth muscle cells. Eur. Respir. J. 2011, 38, 50–58. [Google Scholar] [CrossRef]
- Yang, J.Q.; Rüdiger, J.J.; Hughes, J.M.; Goulet, S.; Gencay-Cornelson, M.M.; Borger, P.; Tamm, M.; Roth, M. Cell density and serum exposure modify the function of the glucocorticoid receptor C/EBP complex. Am. J. Respir. Cell Mol. Biol. 2008, 38, 414–422. [Google Scholar] [CrossRef]
- Lambers, C.; Kornauth, C.; Oberndorfer, F.; Boehm, P.M.; Tamm, M.; Klepetko, W.; Roth, M. Mechanism of anti-remodelling action of treprostinil in human pulmonary arterial smooth muscle cells. PLoS ONE 2018, 13, e0205195. [Google Scholar] [CrossRef]
- Roth, M.; Johnson, P.R.; Borger, P.; Bihl, M.P.; Rüdiger, J.J.; King, G.G.; Ge, Q.; Hostettler, K.; Burgess, J.K.; Black, J.L.; et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N. Engl. J. Med. 2004, 351, 560–574. [Google Scholar] [CrossRef]
- Carceller-Zazo, E.; Sevilla, L.M.; Pons-Alonso, O.; Chiner-Oms, Á.; Amazit, L.; An Vu, T.; Vitellius, G.; Viengchareun, S.; Comas, I.; Jaszczyszyn, Y.; et al. The mineralocorticoid receptor modulates timing and location of genomic binding by glucocorticoid receptor in response to synthetic glucocorticoids in keratinocytes. FASEB J. 2023, 37, e22709. [Google Scholar] [CrossRef]
- Haque, R.; Hakim, A.; Moodley, T.; Torrego, A.; Essilfie-Quaye, S.; Jazrawi, E.; Johnson, M.; Barnes, P.J.; Adcock, I.M.; Usmani, O.S. Inhaled long-acting β2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. J. Allergy Clin. Immunol. 2013, 132, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, F.; Mir, A.A.; Balazs, K.; Wierer, M.; Dyar, K.A.; Jouffe, C.; Makris, K.; Hawe, J.; Heinig, M.; Filipp, F.V.; et al. Cistromic Reprogramming of the Diurnal Glucocorticoid Hormone Response by High-Fat Diet. Mol. Cell 2019, 76, 531–545.e5. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joëls, M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol. Cell Endocrinol. 2012, 350, 299–309. [Google Scholar] [CrossRef]
- Trebble, P.J.; Woolven, J.M.; Saunders, K.A.; Simpson, K.D.; Farrow, S.N.; Matthews, L.C.; Ray, D.W. A ligand-specific kinetic switch regulates glucocorticoid receptor trafficking and function. J. Cell Sci. 2013, 126, 3159–3169. [Google Scholar] [CrossRef]
- Medar, M.L.; Andric, S.A.; Kostic, T.S. Stress-induced glucocorticoids alter the Leydig cells’ timing and steroidogenesis-related systems. Mol. Cell Endocrinol. 2021, 538, 111469. [Google Scholar] [CrossRef]
- Fadel, L.; Dacic, M.; Fonda, V.; Sokolsky, B.A.; Quagliarini, F.; Rogatsky, I.; Uhlenhaut, N.H. Modulating glucocorticoid receptor actions in physiology and pathology: Insights from coregulators. Pharmacol. Ther. 2023, 251, 108531. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.C.; Bleecker, E.R.; Curran-Everett, D.; Erzurum, S.C.; Ameredes, B.T.; Bacharier, L.; Calhoun, W.J.; Castro, M.; Chung, K.F.; Clark, M.P.; et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J. Allergy Clin. Immunol. 2007, 119, 405–413. [Google Scholar] [CrossRef]
- Thomson, N.C.; Chaudhuri, R.; Livingston, E. Asthma and cigarette smoking. Eur. Respir. J. 2004, 24, 822–833. [Google Scholar] [CrossRef]
- Wu, X.; Jia, B.; Luo, X.; Wang, J.; Li, M. Glucocorticoid Alleviates Mechanical Stress-Induced Airway Inflammation and Remodeling in COPD via Transient Receptor Potential Canonical 1 Channel. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 1837–1851. [Google Scholar] [CrossRef]
- Zhou, L.; Roth, M.; Papakonstantinou, E.; Tamm, M.; Stolz, D. Expression of glucocorticoid receptor and HDACs in airway smooth muscle cells is associated with response to steroids in COPD. Respir. Res. 2024, 25, 227. [Google Scholar] [CrossRef]
- Faiz, A.; Pavlidis, S.; Kuo, C.H.; Rowe, A.; Hiemstra, P.S.; Timens, W.; Berg, M.; Wisman, M.; Guo, Y.K.; Djukanović, R.; et al. Th2 high and mast cell gene signatures are associated with corticosteroid sensitivity in COPD. Thorax 2023, 78, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L.; Smith, C.L.; Goping, I.S.; Underhill, D.A.; Harley, M.J.; Reventos, J.; Musto, N.A.; Gunsalus, G.L.; Bardin, C.W. Primary structure of human corticosteroid binding globulin, deduced from hepatic and pulmonary cDNAs, exhibits homology with serine protease inhibitors. Proc. Natl. Acad. Sci. USA 1987, 84, 5153–5157. [Google Scholar] [CrossRef] [PubMed]
- Gardill, B.R.; Vogl, M.R.; Lin, H.Y.; Hammond, G.L.; Muller, Y.A. Corticosteroid-binding globulin: Structure-function implications from species differences. PLoS ONE 2012, 7, e52759. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, P.K.; Murai, J.T.; Hammond, G.L.; Nisker, J.A.; Raymoure, W.J.; Kuhn, R.W. The serum transport of steroid hormones. Recent Prog. Horm. Res. 1982, 38, 457–510. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Lin, H.Y.; Muller, Y.A.; Hammond, G.L. Molecular and structural basis of steroid hormone binding and release from corticosteroidbinding globulin. Mol. Cell. Endocrinol. 2010, 316, 3–12. [Google Scholar] [CrossRef]
- Bartalena, L.; Hammond, G.L.; Farsetti, A.; Flink, I.L.; Robbins, J. Interleukin-6 inhibits corticosteroid-binding globulin synthesis by human hepatoblastoma-derived (hep G2) cells. Endocrinology 1993, 133, 291–296. [Google Scholar] [CrossRef]
- Garrel, D.R. Corticosteroid-binding globulin during inflammation and burn injury: Nutritional modulation and clinical implications. Horm. Res. 1996, 45, 245–251. [Google Scholar] [CrossRef]
- Simard, M.; Hill, L.A.; Lewis, J.G.; Hammond, G.L. Naturally occurring mutations of human corticosteroid-binding globulin. J. Clin. Endocrinol. Metab. 2015, 100, E129–E139. [Google Scholar] [CrossRef]
- Lin, H.Y.; Underhill, C.; Lei, J.H.; Helander-Claesson, A.; Lee, H.Y.; Gardill, B.R.; Muller, Y.A.; Wang, H.; Hammond, G.L. High frequency of SERPINA6 polymorphisms that reduce plasma corticosteroid-binding globulin activity in Chinese subjects. J. Clin. Endocrinol. Metab. 2012, 97, E678–E686. [Google Scholar] [CrossRef]
- Meyer, E.J.; Spangenberg, L.; Ramírez, M.J.; De Sousa, S.M.C.; Raggio, V.; Torpy, D.J. CBG Montevideo: A Clinically Novel SERPINA6 Mutation Leading to Haploinsufficiency of Corticosteroid-binding Globulin. J. Endocr. Soc. 2021, 5, bvab115. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Schuelter, U.; Hesse, J.; Moser, D.; Phillips, D.I.; Hellhammer, D.; Meyer, J. Haploinsufficiency of the SERPINA6 gene is associated with severe muscle fatigue: A de novo mutation in corticosteroid-binding globulin deficiency. J. Neural. Transm 2007, 114, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Torpy, D.J.; Lundgren, B.A.; Ho, J.T.; Lewis, J.G.; Scott, H.S.; Mericq, V. CBG Santiago: A novel CBG mutation. J. Clin. Endocrinol. Metab. 2012, 97, E151–E155. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Nucera, F.; Rosani, U.; Brun, P.; Gnemmi, I.; Maniscalco, M.; D’Anna, S.E.; Leonardi, A.; Carriero, V.; Bertolini, F.; et al. Impaired SERPIN-Protease Balance in the Peripheral Lungs of Stable COPD Patients. Int. J. Mol. Sci. 2025, 26, 2832. [Google Scholar] [CrossRef]
- Navara, K.J. Programming of offspring sex ratios by maternal stress in humans: Assessment of physiological mechanisms using a comparative approach. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2010, 180, 785–796. [Google Scholar] [CrossRef]
- Hammond, G.L. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef]
- Fries, G.R.; Gassen, N.C.; Rein, T. The FKBP51 Glucocorticoid Receptor Co-Chaperone: Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2017, 18, 2614. [Google Scholar] [CrossRef]
- Petta, I.; Dejager, L.; Ballegeer, M.; Lievens, S.; Tavernier, J.; De Bosscher, K.; Libert, C. The Interactome of the Glucocorticoid Receptor and Its Influence on the Actions of Glucocorticoids in Combatting Inflammatory and Infectious Diseases. Microbiol. Mol. Biol. Rev. 2016, 80, 495–522. [Google Scholar] [CrossRef]
- Chalfun, G.; Reis, M.M.; de Oliveira, M.B.G.; de Araújo Brasil, A.; Dos Santos Salú, M.; da Cunha, A.J.L.A.; Prata-Barbosa, A.; de Magalhães-Barbosa, M.C. Perinatal stress and methylation of the NR3C1 gene in newborns: Systematic review. Epigenetics 2022, 17, 1003–1019. [Google Scholar] [CrossRef]
- Lesovaya, E.A.; Chudakova, D.; Baida, G.; Zhidkova, E.M.; Kirsanov, K.I.; Yakubovskaya, M.G.; Budunova, I.V. The long winding road to the safer glucocorticoid receptor (GR) targeting therapies. Oncotarget 2022, 13, 408–424. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambers, C.; Roth, M. Glucocorticoid Insensitivity: Is It a Question of Time and Place? Biomedicines 2025, 13, 1418. https://doi.org/10.3390/biomedicines13061418
Lambers C, Roth M. Glucocorticoid Insensitivity: Is It a Question of Time and Place? Biomedicines. 2025; 13(6):1418. https://doi.org/10.3390/biomedicines13061418
Chicago/Turabian StyleLambers, Christopher, and Michael Roth. 2025. "Glucocorticoid Insensitivity: Is It a Question of Time and Place?" Biomedicines 13, no. 6: 1418. https://doi.org/10.3390/biomedicines13061418
APA StyleLambers, C., & Roth, M. (2025). Glucocorticoid Insensitivity: Is It a Question of Time and Place? Biomedicines, 13(6), 1418. https://doi.org/10.3390/biomedicines13061418





