Immunological Markers of Cardiovascular Pathology in Older Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Approval
2.3. Sample Processing
2.4. Immunophenotyping
2.4.1. Multicolor Staining for Cell Surface Antigens and Intracellular Cytokines
2.4.2. Flow Cytometry Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Groups
3.2. Immunophenotyping of Blood Lymphocytes
Intracellular Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, J.L.; Yang, J.S.; Jin, Q.; Yang, J.; Xue, Q.; Guang, X.F. Novel Diagnostic Biomarkers Related to Necroptosis and Immune Infiltration in Coronary Heart Disease. J. Inflamm. Res. 2024, 17, 4525–4548. [Google Scholar] [CrossRef] [PubMed]
- Sobhon, P.; Savedvanich, G.; Weerakiet, S. Oxidative stress and inflammation: The root causes of aging. Explor. Med. 2023, 4, 127–156. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S. Obesity and aging: Molecular mechanisms and therapeutic approaches. Aging Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef]
- Caruso, C.; Ligotti, M.E.; Accardi, G.; Aiello, A.; Candore, G. An immunologist’s guide to immunosenescence and its treatment. Expert. Rev. Clin. Immunol. 2022, 18, 961–981. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, M. Cytokines in age-related eye diseases: Pathogenesis and potential targets for innovative therapies. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Carrasco, E.; Gómez de las Heras, M.M.; Gabandé-Rodríguez, E.; Desdín-Micó, G.; Aranda, J.F.; Mittelbrunn, M. The role of T cells in age-related diseases. Nat. Rev. Immunol. 2022, 22, 97–111. [Google Scholar] [CrossRef]
- Adamo, L.; Rocha- Resende, C.; Mann, D.L. The Emerging Role of B Lymphocytes in Cardiovascular Disease. Annu. Rev. Immunol. 2020, 38, 99–121. [Google Scholar] [CrossRef]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef]
- Pawelec, G.; Bronikowski, A.; Cunnane, S.C.; Ferrucci, L.; Franceschi, C.; Fulop, T. The conundrum of human immune system “senescence”. Mech. Ageing Dev. 2020, 192, 111357. [Google Scholar] [CrossRef]
- Talley, S.; Rademacher, D.J.; Campbell, E.M. Inflammasome activation occurs in CD4+ and CD8+ T cells during graft-versus-host disease. Cell Death Dis. 2023, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.E.; Dinh, H.Q.; Wu, R. Naive CD8 + T Cells Expressing CD95 Increase Human Cardiovascular Disease Severity. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e383. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Liu, K.Q.; Ying, Z.M. Effect of CD14 polymorphisms on the risk of cardiovascular disease: Evidence from a meta-analysis. Lipids Health Dis. 2019, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Sharygin, D.; Koniaris, L.G.; Wells, C.; Zimmers, T.A.; Hamidi, T. Role of CD14 in human disease. Immunology 2023, 169, 260–270. [Google Scholar] [CrossRef]
- Li, H.Z.; Wang, Q.; Zhang, Y.Y.; Wang, J.D.; Wu, H.J. Onset of Coronary Heart Disease is Associated with HCMV Infection and Increased CD14+CD16+ Monocytes in a Population of Weifang, China. Biomed. Environ. Sci. 2020, 33, 573–582. [Google Scholar] [CrossRef]
- Liu, J.; Moosa, V.; Tan, I. The Role of Immune Cell Types in Ischemic Heart Disease Progression: A Systematic Review. URNCST J. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Zou, Y.; Fan, Y.; Feng, P.; Fu, X.; Li, K.; Zhang, J.; Dong, Y.; Yan, S.; et al. Peripheral blood CD19 positive B lymphocytes increase after ischemic stroke and correlate with carotid atherosclerosis. Front. Neurol. 2023, 14, 1308041. [Google Scholar] [CrossRef]
- Kumrić, M.; Kurir, T.T.; Borovac, J.A. The Role of Natural Killer (NK) Cells in Acute Coronary Syndrome: A Comprehensive Review. J. Biomol. 2020, 10, 1514. [Google Scholar] [CrossRef]
- Cacciatore, F.; Amarelli, C.; Sabia, C. Effect on Long-Term Mortality of HLA-DR Matching in Heart Transplantation. J. Card. Fail. 2019, 25, 409–411. [Google Scholar] [CrossRef]
- Runzi Zheng, R.; Zhang, Y. The Complement System, Aging, and Aging-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8689. [Google Scholar] [CrossRef]
- Weinstock, C. Association of Blood Group Antigen CD59 with Disease. Transfus. Med. Chemother. 2022, 49, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; da Rocha, A.L.; Kohama, E.B.; Gaspar, R.C.; Simabuco, F.M.; Frantz, F.G.; de Moura, L.P.; Pauli, J.R.; E Cintra, D.; Ropelle, E.R.; et al. Exhaustive acute exercise-induced ER stress is attenuated in IL-6-knockout mice. J. Endocrinol. 2019, 240, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Kazusa, I.; Marie Pouzolles, M.; Chien, C.D. Perforin-deficient CAR T cells recapitulate late-onset inflammatory toxicities observed in patients. J. Clin. Investig. 2020, 130, 5425–5443. [Google Scholar] [CrossRef]
- Tylutka, A.; Zembron-Lacny, A. Level of IL-6, TNF, and IL-1β and age-related diseases: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1330386. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The role of interleukin-10 family members in cardiovascular diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Larsson, S.C.; Michaëlsson, K.; Burgess, S. IGF-1 and cardiometabolic diseases: A Mendelian randomization study. Diabetologia 2020, 63, 1775–1782. [Google Scholar] [CrossRef]
- Yingzi Chen, Y.; Li, F.; Hua, M. Role of GM-CSF in lung balance and disease. Front. Immunol. 2023, 14, 1158859. [Google Scholar] [CrossRef]
- Shiomi, A.; Usui, T. Pivotal Roles of GM-CSF in Autoimmunity and Inflammation. Mediat. Inflamm. 2015, 2015, 568543. [Google Scholar] [CrossRef]
- Achuthan, A.A.; Lee, K.M.C.; Hamilton, J.A. Targeting GM-CSF in inflammatory and autoimmune disorders. Semin. Immunol. 2021, 54, 101523. [Google Scholar] [CrossRef]
- Suleimenova, M.; Abzaliyev, K.; Mansurova, M.; Abzaliyeva, S.; Kurmanova, A.; Tokhtakulinova, G.; Bugibayeva, A.; Sundetova, D.; Abdykassymova, M.; Sagalbayeva, U.; et al. A Predictive Model of Cardiovascular Aging by Clinical and Immunological Markers Using Machine Learning. Diagnostics 2025, 15, 850. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, S.G.; Buzkova, P.; Shitole, S.C.; Reiner, A.P.; Garg, P.K.; Gottdiener, J.S.; Psaty, B.M.; Kizer, J.R. Soluble CD14 and Risk of Heart Failure and Its Subtypes in Older Adults. J. Card. Fail. 2020, 26, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Chumakova, S.P.; Urazova, O.I.; Denisenko, O.A.; Pogonchenkova, D.A.; Shipulin, V.M.; Pryakhin, A.S.; Nevskaya, K.V.; Gladkovskaya, M.V. Blood monocytes in maintaining the balance of vascular endothelial injury and repair process in ischemic cardiomyopathy. Complex Issues Cardiovasc. Dis. 2022, 11, 84–96. (In Russian) [Google Scholar] [CrossRef]
- DeConne, T.M.; Sitlani, C.M.; Decker, K.P.; Delaney, J.A.; Psaty, B.M.; Doyle, M.F.; Buzkova, P.; Landay, A.L.; Huber, S.A.; Hughes, T.M.; et al. Associations of circulating T-cell subsets with endothelial function: The Multi-Ethnic Study of Atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H1374–H1379. [Google Scholar] [CrossRef]
- Kahan, S.M.; Bakshi, R.K.; Ingram, J.T.; Hendrickson, R.C.; Lefkowitz, E.J.; Crossman, D.K.; Harrington, L.E.; Weaver, C.T.; Zajac, A.J. Intrinsic IL-2 production by effector CD8 T cells affects IL-2 signaling and promotes fate decisions, stemness, and protection. Sci. Immunol. 2022, 7, eabl6322. [Google Scholar] [CrossRef]
- Zhao, T.; Zeng, J.; Zhang, R.; Fan, W.; Guan, Q.; Wang, H.; Pu, L.; Jiang, Y.; Yang, H.; Wang, X. Serum olink proteomics-based identification of protein biomarkers associated with the immune response in ischemic stroke. J. Proteome Res. 2024, 23, 1118–1128. [Google Scholar] [CrossRef]
- Mazidi, M.; Wright, N.; Yao, P.; Kartsonaki, C.; Millwood, I.Y.; Fry, H.; Said, S.; Pozarickij, A.; Pei, P.; Chen, Y. Risk prediction of ischemic heart disease using plasma proteomics, conventional risk factors and polygenic scores in Chinese and European adults. Eur. J. Epidemiol. 2024, 39, 1229–1240. [Google Scholar] [CrossRef]
- Hu, S.; Cai, J.; Chen, S.; Wang, Y.; Ren, L. Identification of novel biomarkers and immune infiltration characteristics of ischemic stroke based on comprehensive bioinformatic analysis and machine learning. Biochem. Biophys. Rep. 2023, 37, 101595. [Google Scholar] [CrossRef]
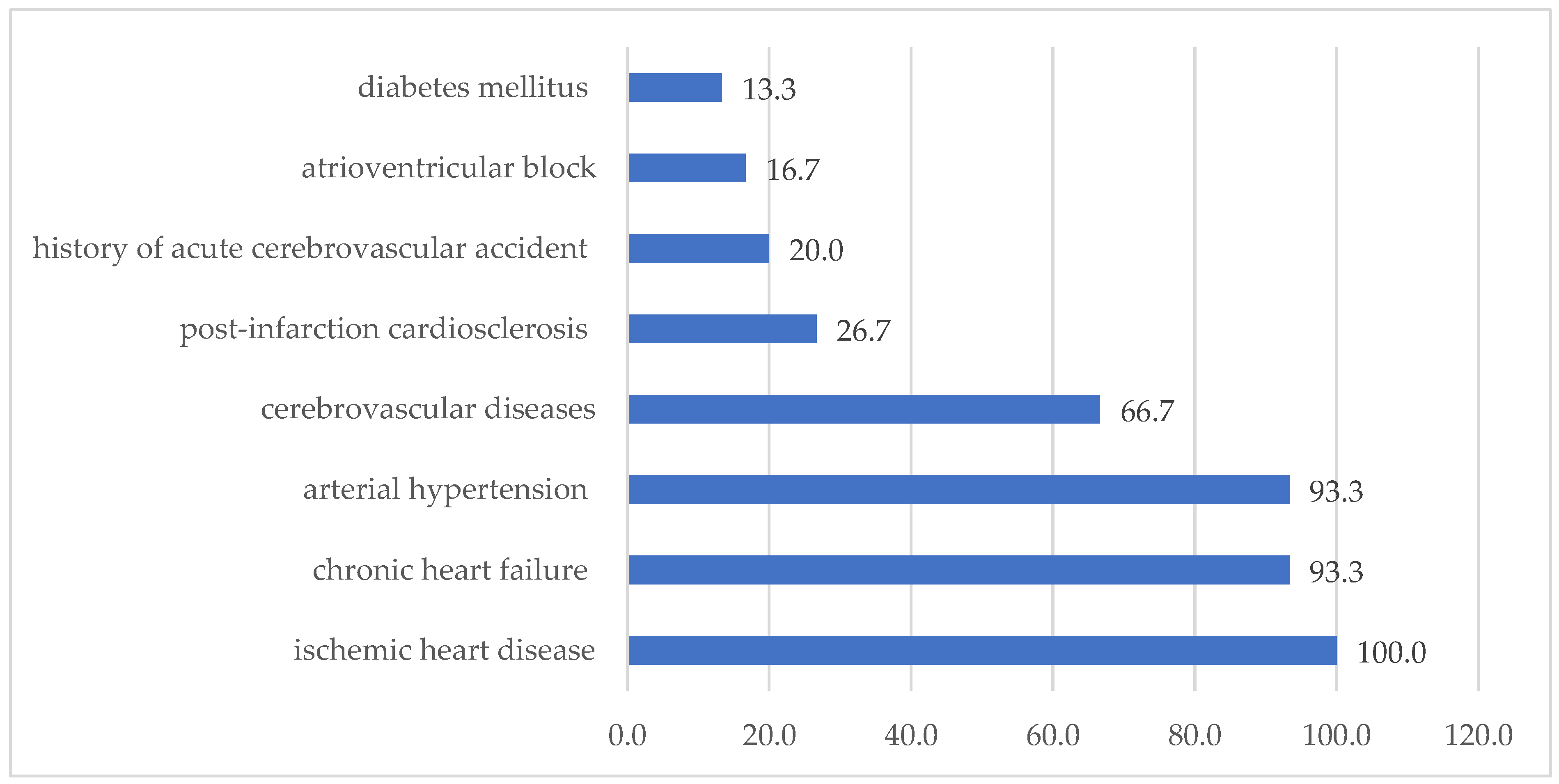
| Characteristic | with CVD (abs, %), n = 30 | Without CVD (abs, %), n = 22 | p Value |
|---|---|---|---|
| Man | 13 (43.3%) | 5 (22.7%) * | 0.004 |
| Woman | 17 (77.3%) | 17 (56.7%) | 0.845 |
| Married | 17 (56.7%) | 9 (40.9%) * | 0.002 |
| Widow/widower | 11 (36.7%) | 12 (54.5%) * | 0.003 |
| Kazakhs | 20 (66.7%) | 9 (40.9%) * | 0.005 |
| Russian | 7 (23.3%) | 9 (40.9%) | 0.647 |
| Uigur | 1 (3.3%) | 0 (0.0%) | 0.011 |
| Other | 2 (6.7%) | 4 (18.2%) | |
| Disability: Yes | 6 (20.0%) | 4 (18.2%) | |
| Disability: No | 24 (80.0%) | 18 (81.8%) |
| Characteristic | with CVD (abs, %), n = 30 | Without CVD (abs, %), n = 22 | p Value |
|---|---|---|---|
| Smoking (abs, %) | 7 (23.3%) | 4 (18.2%) | 0.011 * |
| Alcohol abuse (abs, %) | 2 (6.7%) | 4 (18.2%) | 0.714 |
| Body mass index (kg/m2: M ± m) | 25.7 ± 0.7 0 | 22.0 ± 0.51 | 0.000 * |
| Low physical activity < 30 min (M ± m) | 12.5 ± 1.75 | 20.06 ± 12.7 | 0.358 |
| Systolic blood pressure (M ± m) | 136.33 ± 2.1 | 126.14 ± 1.5 | 0.000 * |
| Diastolic blood pressure (M ± m) | 81.17 ± 1.5 | 80.23 ± 1.6 | 0.831 |
| Heart rate (M ± m) | 81.40 ± 1.4 | 76.86 ± 1.2 | 0.040 * |
| Comorbidity | |||
| Bronchial asthma | 1 (3.3%) | 0 (0%) | 0.000 * |
| Chronic obstructive pulmonary disease | 9 (30.0%) | 10 (45.5%) | 0.394 |
| Oncopathology | 3 (10.0%) | 1 (0%) | 0.354 |
| Stroke | 6 (20.0%) | 0 (0%) | 0.043 * |
| Osteoarthritis | 18 (60.0%) | 15 (68.2%) | 0.754 |
| Iron deficiency anemia | 3 (10.0%) | 4 (18.2%) | 0.658 |
| Cerebrovascular diseases | 20 (66.7%) | 12 (54.5%) | 0.549 |
| Discirculatory encephalopathy | 23 (76.7%) | 9 (40.9%) | 0.020 * |
| Chronic renal failure | 8 (26.7%) | 3 (13.6%) | 0.428 |
| Characteristic | Group 1 with CVD (n = 30) | Group 2 without CVD (n = 22) | p | ||
|---|---|---|---|---|---|
| Me | IQR | Me | IQR | ||
| Subpopulation peripheral blood lymphocyte profile (surface receptor markers) | |||||
| CD4+ | 34.57 | 31.59–42.77 | 36.77 | 32.84–41.8 | 0.578 |
| CD8+ | 27.26 | 25.56–31.41 | 30.23 | 27.93–30.92 | 0.046 * |
| CD14+ | 4.08 | 2.68–4.91 | 3.02 | 2.46–3.83 | 0.014 * |
| CD19+ | 12.93 | 11–16.09 | 11.97 | 11.43–16.27 | 0.978 |
| CD16+ | 31.36 | 27.54–33.6 | 30.59 | 25.08–33.63 | 0.448 |
| CD56+ | 90.31 | 81.58–96.34 | 82.82 | 78.63–94.81 | 0.232 |
| CD59+ | 90.30 | 79.02–96.17 | 82.82 | 80.82–91.18 | 0.232 |
| HLA-DR+ | 24.0 | 21.62–31.04 | 24.6 | 22.15–28.42 | 0.553 |
| CD95+ | 71.46 | 55.63–77.84 | 70.23 | 57.79–81.33 | 0.963 |
| Intracellular production of cytokines and growth factors | |||||
| TNF | 3.88 | 2.49–5.86 | 4.68 | 3.16–6.07 | 0.195 |
| IL-10 | 2.14 | 1.66–2.57 | 1.9 | 1.58–2.47 | 0.295 |
| Perforin | 4,7350 | 3.01–5.1 | 4355 | 3.52–4.59 | 0.259 |
| IGF | 86.39 | 80.13–92.74 | 80.3 | 78.65–90.01 | 0.122 |
| VEGF2 | 72.05 | 66.87–74.84 | 69.09 | 65.94–72.92 | 0.195 |
| GM-CSF | 1.63 | 0.96–2.32 | 2.32 | 1.7–3.5 | 0.013 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugibayeva, A.; Kurmanova, A.; Abzaliyev, K.; Abzaliyeva, S.; Kurmanova, G.; Sundetova, D.; Abdykassymova, M.; Bitemirova, R.; Sagalbayeva, U.; Absatarova, K.; et al. Immunological Markers of Cardiovascular Pathology in Older Patients. Biomedicines 2025, 13, 1392. https://doi.org/10.3390/biomedicines13061392
Bugibayeva A, Kurmanova A, Abzaliyev K, Abzaliyeva S, Kurmanova G, Sundetova D, Abdykassymova M, Bitemirova R, Sagalbayeva U, Absatarova K, et al. Immunological Markers of Cardiovascular Pathology in Older Patients. Biomedicines. 2025; 13(6):1392. https://doi.org/10.3390/biomedicines13061392
Chicago/Turabian StyleBugibayeva, Akbota, Almagul Kurmanova, Kuat Abzaliyev, Symbat Abzaliyeva, Gaukhar Kurmanova, Diana Sundetova, Merei Abdykassymova, Raushan Bitemirova, Ulzas Sagalbayeva, Karashash Absatarova, and et al. 2025. "Immunological Markers of Cardiovascular Pathology in Older Patients" Biomedicines 13, no. 6: 1392. https://doi.org/10.3390/biomedicines13061392
APA StyleBugibayeva, A., Kurmanova, A., Abzaliyev, K., Abzaliyeva, S., Kurmanova, G., Sundetova, D., Abdykassymova, M., Bitemirova, R., Sagalbayeva, U., Absatarova, K., & Suleimenova, M. (2025). Immunological Markers of Cardiovascular Pathology in Older Patients. Biomedicines, 13(6), 1392. https://doi.org/10.3390/biomedicines13061392






