Plasma Fatty Acid Profiling and Mathematical Estimation of the Omega-3 Index: Toward Diagnostic Tools in Atherosclerosis and Statin Therapy Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Biological Samples
2.3. Histological Evaluation of Atherosclerotic Plaques
2.4. Reagents
2.5. Sample Preparation and HPLC-MS/MS Analysis
2.6. Data Processing and Statistics
3. Results
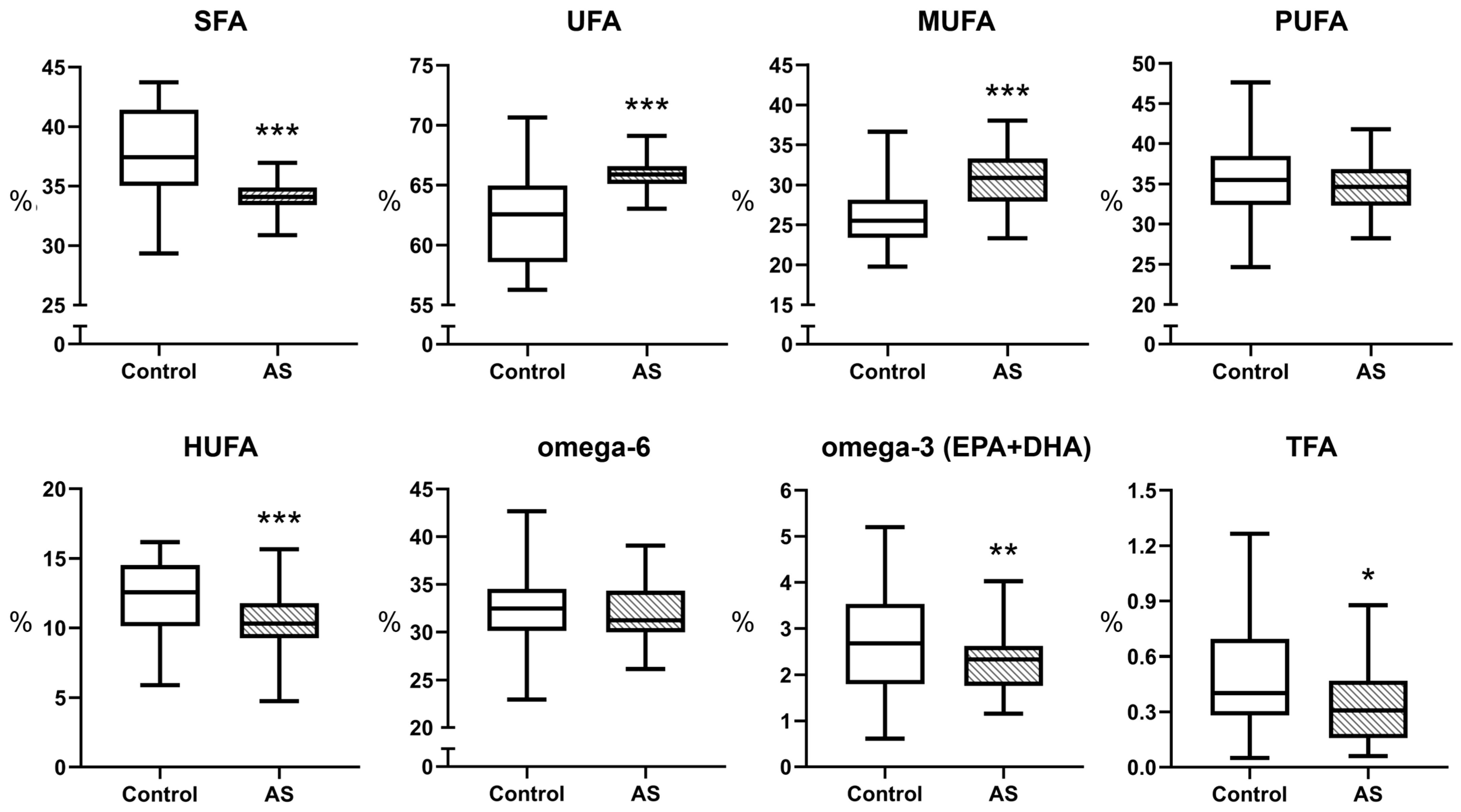
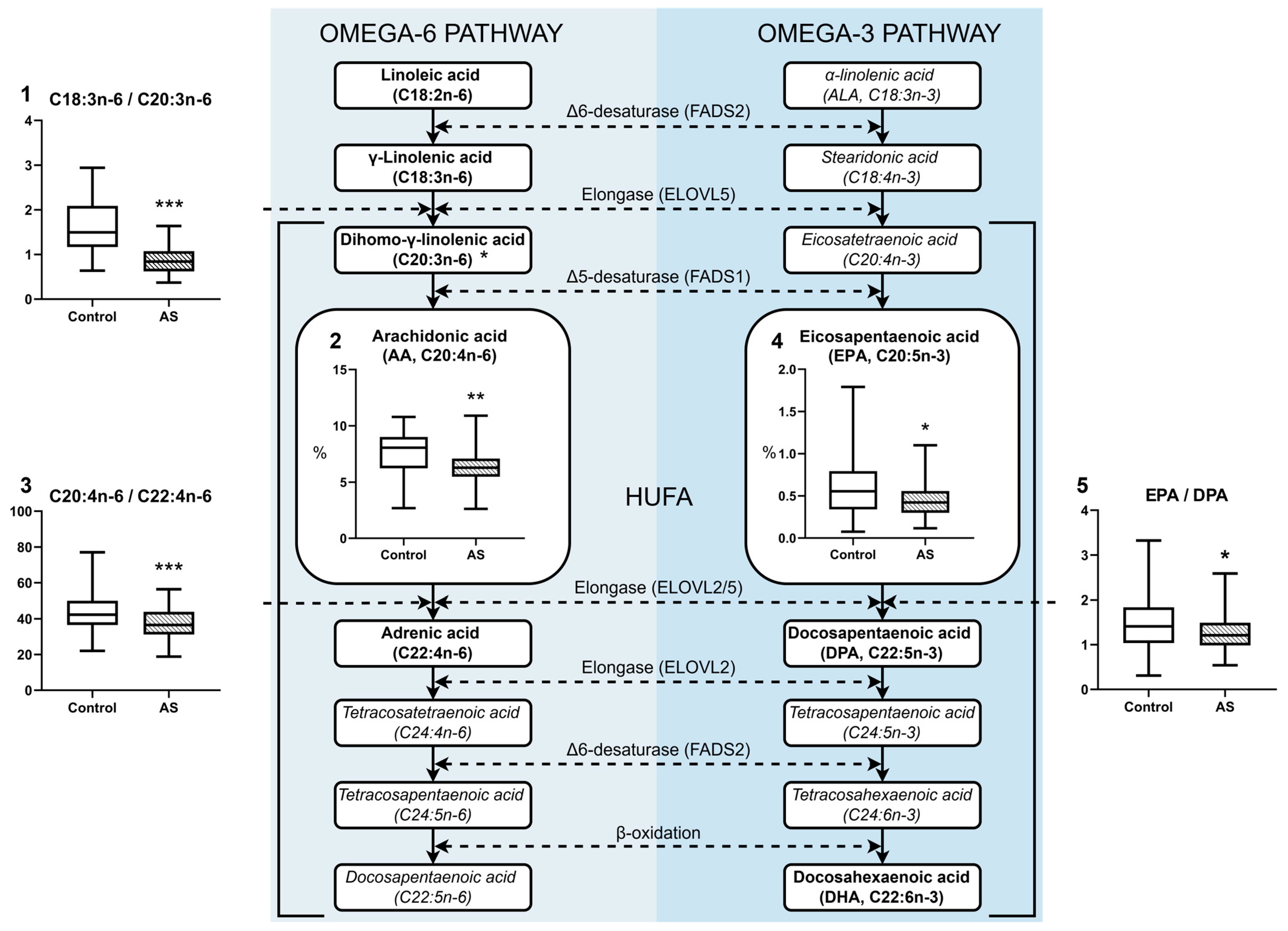
3.1. Fatty Acid Profile of Blood Plasma in Atherosclerosis
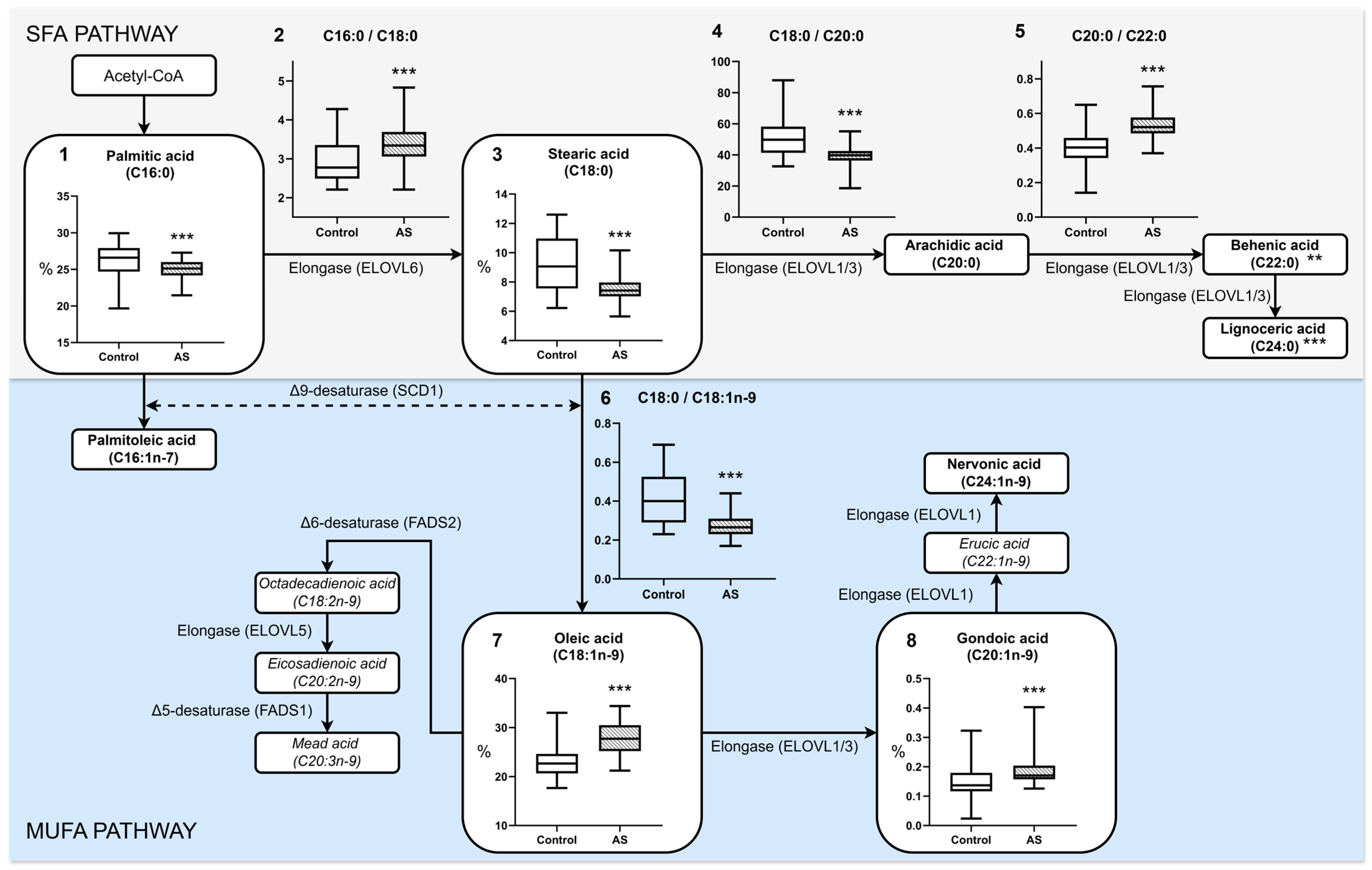
3.2. Fatty Acid Profile of Atherosclerotic Plaques
3.3. Effect of Statin Therapy on Plasma Fatty Acid Composition
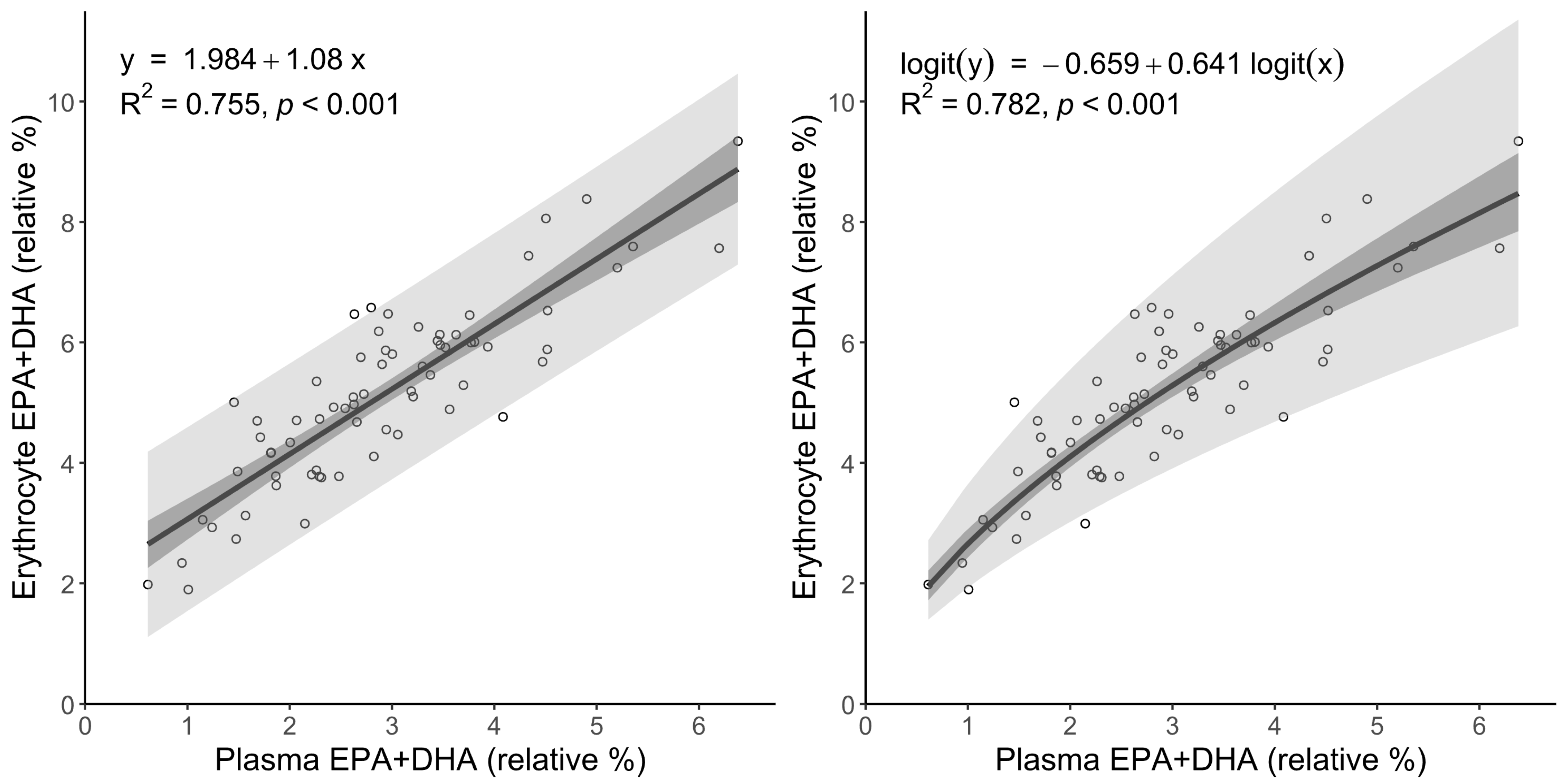
3.4. Linear and Logit Regression Models for Translating Plasma to Erythrocyte Omega-3 Index
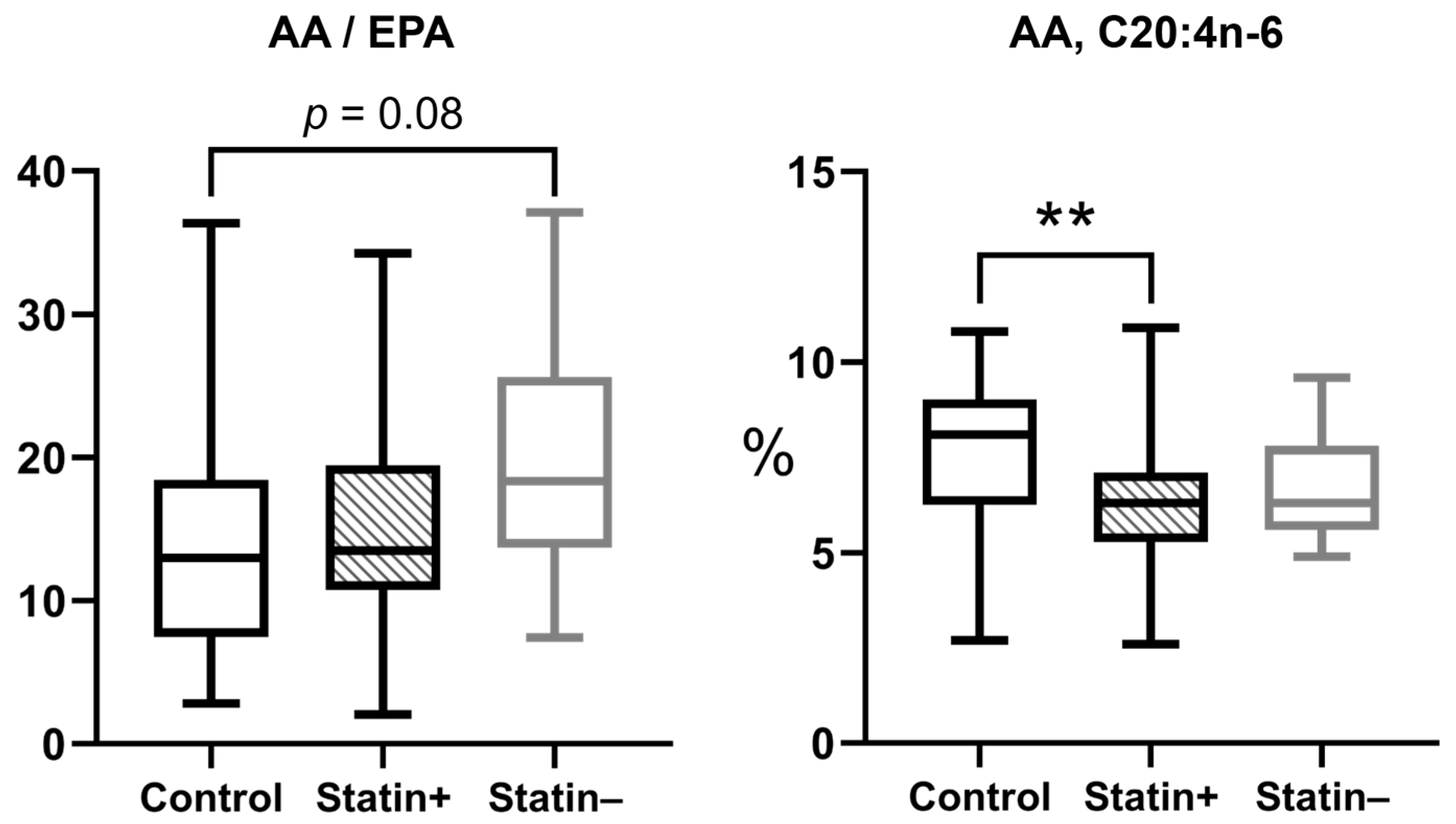
3.5. Association Between Statin Efficacy and Omega-3 PUFAs
3.6. Concomitant Use of Statins and β-Blockers
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfaddagh, A.; Elajami, T.K.; Saleh, M.; Mohebali, D.; Bistrian, B.R.; Welty, F.K. An Omega-3 Fatty Acid Plasma Index ≥4% Prevents Progression of Coronary Artery Plaque in Patients with Coronary Artery Disease on Statin Treatment. Atherosclerosis 2019, 285, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Omega-3 (n-3) Fatty Acid–Statin Interaction: Evidence for a Novel Therapeutic Strategy for Atherosclerotic Cardiovascular Disease. Nutrients 2024, 16, 962. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Park, S.K.; Park, T.S.; Kim, J.H.; Yun, E.; Kim, S.P.; Lee, H.W.; Oh, J.H.; Choi, J.H.; Cha, K.S.; et al. Effect of N-3 Polyunsaturated Fatty Acids on Regression of Coronary Atherosclerosis in Statin Treated Patients Undergoing Percutaneous Coronary Intervention. Korean Circ. J. 2016, 46, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Hande, L.N.; Thunhaug, H.; Enebakk, T.; Ludviksen, J.; Pettersen, K.; Hovland, A.; Lappegård, K.T. Addition of Marine Omega-3 Fatty Acids to Statins in Familial Hypercholesterolemia Does Not Affect in Vivo or in Vitro Endothelial Function. J. Clin. Lipidol. 2019, 13, 762–770. [Google Scholar] [CrossRef]
- Umemoto, N.; Ishii, H.; Kamoi, D.; Aoyama, T.; Sakakibara, T.; Takahashi, H.; Tanaka, A.; Yasuda, Y.; Suzuki, S.; Matsubara, T.; et al. Reverse Association of Omega-3/Omega-6 Polyunsaturated Fatty Acids Ratios with Carotid Atherosclerosis in Patients on Hemodialysis. Atherosclerosis 2016, 249, 65–69. [Google Scholar] [CrossRef]
- Preston Mason, R. New Insights into Mechanisms of Action for Omega-3 Fatty Acids in Atherothrombotic Cardiovascular Disease. Curr. Atheroscler. Rep. 2019, 21, 2. [Google Scholar] [CrossRef]
- Fleischhauer, F.J.; Yan, W.-D.; Fischell, T.A. Fish Oil Improves Endothelium-Dependent Coronary Vasodilation in Heart Transplant Recipients. J. Am. Coll. Cardiol. 1993, 21, 982–989. [Google Scholar] [CrossRef]
- Pisaniello, A.D.; Psaltis, P.J.; King, P.M.; Liu, G.; Gibson, R.A.; Tan, J.T.M.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Omega-3 Fatty Acids Ameliorate Vascular Inflammation: A Rationale for Their Atheroprotective Effects. Atherosclerosis 2021, 324, 27–37. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Campana, M.C.; De Caterina, R. Omega-3 Fatty Acids, Inflammation and Angiogenesis: Basic Mechanisms behind the Cardioprotective Effects of Fish and Fish Oils. Cell. Mol. Biol. 2010, 56, 59–82. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing Inflammation. Open Heart 2018, 5, e000946. [Google Scholar] [CrossRef]
- Loukil, I.; Mutch, D.M.; Plourde, M. Genetic Association between FADS and ELOVL Polymorphisms and the Circulating Levels of EPA/DHA in Humans: A Scoping Review. Genes. Nutr. 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, L.; Wang, L.; Ji, C.; Xia, R.; Yang, Y. A Systematic Review and Meta-Analysis on the Efficacy of Statins in the Treatment of Atherosclerosis. Ann. Palliat. Med. 2021, 10, 6793–6803. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, A.; Gognieva, D.; Suvorov, A.; Silantyev, A.; Abasheva, A.; Vasina, Y.; Abdullaev, M.; Nartova, A.; Eroshchenko, N.; Kazakova, V.; et al. The Blood Plasma Lipidomic Profile in Atherosclerosis of the Brachiocephalic Arteries. Biomedicines 2024, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Cerrato, M.; Ceseri, M.; DeFina, L.F.; Delgado, G.E.; Gellert, S.; Hahn, A.; Howard, B.V.; Kadota, A.; Kleber, M.E.; et al. Red Blood Cell Fatty Acid Patterns from 7 Countries: Focus on the Omega-3 Index. Prostaglandins Leukot. Essent. Fat. Acids 2022, 179, 102418. [Google Scholar] [CrossRef]
- Strandjord, S.E.; Lands, B.; Hibbeln, J.R. Validation of an Equation Predicting Highly Unsaturated Fatty Acid (HUFA) Compositions of Human Blood Fractions from Dietary Intakes of Both HUFAs and Their Precursors. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 171–176. [Google Scholar] [CrossRef]
- Stark, K.D.; Aristizabal Henao, J.J.; Metherel, A.H.; Pilote, L. Translating Plasma and Whole Blood Fatty Acid Compositional Data into the Sum of Eicosapentaenoic and Docosahexaenoic Acid in Erythrocytes. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Hu, X.F.; Sandhu, S.K.; Harris, W.S.; Chan, H.M. Conversion Ratios of N-3 Fatty Acids between Plasma and Erythrocytes: A Systematic Review and Meta-Regression. Br. J. Nutr. 2017, 117, 1162–1173. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Virmani, R.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Finn, A.V.; Gold, H.K. Pathologic Assessment of the Vulnerable Human Coronary Plaque. Heart 2004, 90, 1385–1391. [Google Scholar] [CrossRef]
- Eroshchenko, N.N.; Veselov, V.V.; Pirogov, A.V.; Danilova, E.Y.; Kirushin, A.N.; Paravyan, A.L.; Cravotto, G. Development and Validation of a HPLC-MS/MS Method for the Analysis of Fatty Acids—In the Form of FAME Ammonium Adducts—In Human Whole Blood and Erythrocytes to Determine Omega-3 Index. J. Chromatogr. B 2023, 1227, 123799. [Google Scholar] [CrossRef]
- Mazidi, M.; Gao, H.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Kengne, A.P. The Relationship of Plasma Trans Fatty Acids with Dietary Inflammatory Index among US Adults. Lipids Health Dis. 2017, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Guillou, H.; Zadravec, D.; Martin, P.G.P.; Jacobsson, A. The Key Roles of Elongases and Desaturases in Mammalian Fatty Acid Metabolism: Insights from Transgenic Mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Macášek, J.; Zeman, M.; Žák, A.; Staňková, B.; Vecka, M. Altered Indices of Fatty Acid Elongases ELOVL6, ELOVL5, and ELOVL2 Activities in Patients with Impaired Fasting Glycemia. Metab. Syndr. Relat. Disord. 2021, 19, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Bond, L.M.; Miyazaki, M.; O’Neill, L.M.; Ding, F.; Ntambi, J.M. Fatty Acid Desaturation and Elongation in Mammals. In Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 185–208. [Google Scholar]
- Paton, C.M.; Ntambi, J.M. Biochemical and Physiological Function of Stearoyl-CoA Desaturase. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef]
- Butler, L.M.; Yuan, J.-M.; Huang, J.Y.; Su, J.; Wang, R.; Koh, W.-P.; Ong, C.-N. Plasma Fatty Acids and Risk of Colon and Rectal Cancers in the Singapore Chinese Health Study. npj Precis. Oncol. 2017, 1, 38. [Google Scholar] [CrossRef]
- Moon, Y.-A.; Hammer, R.E.; Horton, J.D. Deletion of ELOVL5 Leads to Fatty Liver through Activation of SREBP-1c in Mice. J. Lipid Res. 2009, 50, 412–423. [Google Scholar] [CrossRef]
- Sassa, T.; Wakashima, T.; Ohno, Y.; Kihara, A. Lorenzo’s Oil Inhibits ELOVL1 and Lowers the Level of Sphingomyelin with a Saturated Very Long-Chain Fatty Acid. J. Lipid Res. 2014, 55, 524–530. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.-M.; Hu, G.-R.; Li, F.-L. Biosynthesis of Nervonic Acid and Perspectives for Its Production by Microalgae and Other Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3027–3035. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Watanabe, T.; Kohri, T.; Yamasaki, M.; Watanabe, R.; Baba, K.; Shibata, K.; et al. Lower Estimates of δ-5 Desaturase and Elongase Activity Are Related to Adverse Profiles for Several Metabolic Risk Factors in Young Japanese Women. Nutr. Res. 2008, 28, 816–824. [Google Scholar] [CrossRef]
- Martinelli, N.; Girelli, D.; Malerba, G.; Guarini, P.; Illig, T.; Trabetti, E.; Sandri, M.; Friso, S.; Pizzolo, F.; Schaeffer, L.; et al. FADS Genotypes and Desaturase Activity Estimated by the Ratio of Arachidonic Acid to Linoleic Acid Are Associated with Inflammation and Coronary Artery Disease. Am. J. Clin. Nutr. 2008, 88, 941–949. [Google Scholar] [CrossRef]
- de Antueno, R.J.; Knickle, L.C.; Smith, H.; Elliot, M.L.; Allen, S.J.; Nwaka, S.; Winther, M.D. Activity of Human Δ5 and Δ6 Desaturases on Multiple N-3 and N-6 Polyunsaturated Fatty Acids. FEBS Lett. 2001, 509, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Very Long-Chain Fatty Acids: Elongation, Physiology and Related Disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Polreis, J. Measurement of the Omega-3 Index in Dried Blood Spots. Ann. Clin. Lab. Res. 2016, 4, 4. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Lee, M.W.; Park, J.K.; Hong, J.W.; Kim, K.J.; Shin, D.Y.; Ahn, C.W.; Song, Y.D.; Cho, H.K.; Park, S.W.; Lee, E.J. Beneficial Effects of Omega-3 Fatty Acids on Low Density Lipoprotein Particle Size in Patients with Type 2 Diabetes Already under Statin Therapy. Diabetes Metab. J. 2013, 37, 207–211. [Google Scholar] [CrossRef][Green Version]
- Vasandani, C.; Kafrouni, A.I.; Caronna, A.; Bashmakov, Y.; Gotthardt, M.; Horton, J.D.; Spady, D.K. Upregulation of Hepatic LDL Transport by N-3 Fatty Acids in LDL Receptor Knockout Mice. J. Lipid Res. 2002, 43, 772–784. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Irfan, A.; Haider, S.H.; Nasir, A.; Larik, M.O.; Naz, T. Assessing the Efficacy of Omega-3 Fatty Acids + Statins vs. Statins Only on Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of 40,991 Patients. Curr. Probl. Cardiol. 2024, 49, 102245. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, X.; Li, J.; Lin, Z.; Huang, Z.; Chang, J.; Zhang, Y.; Wang, J.; Zhang, C.; et al. An LC-MS/MS Method for Comparing the Stability of Ethanol’s Non-Oxidative Metabolites in Dried Blood Spots during 90 Days. Alcohol 2020, 83, 29–35. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Risé, P.; Ghezzi, S.; Carissimi, R.; Mastromauro, F.; Petroni, A.; Galli, C. Δ5 Desaturase MRNA Levels Are Increased by Simvastatin via SREBP-1 at Early Stages, Not via PPARα, in THP-1 Cells. Eur. J. Pharmacol. 2007, 571, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, W.J. Unsaturated Fatty Acids, Desaturases, and Human Health. J. Med. Food 2014, 17, 189–197. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids and Cardiovascular Disease: The Epidemiological Evidence. Environ. Health Prev. Med. 2002, 6, 203–209. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Paassilta, M.; Kuusela, E.; Korppi, M.; Lemponen, R.; Kaila, M.; Nikkari, S.T. Food Allergy in Small Children Carries a Risk of Essential Fatty Acid Deficiency, as Detected by Elevated Serum Mead Acid Proportion of Total Fatty Acids. Lipids Health Dis. 2014, 13, 180. [Google Scholar] [CrossRef]
- Stegemann, C.; Drozdov, I.; Shalhoub, J.; Humphries, J.; Ladroue, C.; Didangelos, A.; Baumert, M.; Allen, M.; Davies, A.H.; Monaco, C.; et al. Comparative Lipidomics Profiling of Human Atherosclerotic Plaques. Circ. Cardiovasc. Genet. 2011, 4, 232–242. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and Protectin D1 Activate Inflammation-Resolution Programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine N−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Torrissen, M.; Ytteborg, E.; Svensen, H.; Stoknes, I.; Nilsson, A.; Ostbye, T.K.; Berge, G.M.; Bou, M.; Ruyter, B. Investigation of the Functions of N-3 Very-Long-Chain PUFAs in Skin Using in Vivo Atlantic Salmon and in Vitro Human and Fish Skin Models. Br. J. Nutr. 2023, 130, 1915–1931. [Google Scholar] [CrossRef]
- Hopiavuori, B.R.; Anderson, R.E.; Agbaga, M.P. ELOVL4: Very Long-Chain Fatty Acids Serve an Eclectic Role in Mammalian Health and Function. Prog. Retin. Eye Res. 2019, 69, 137–158. [Google Scholar] [CrossRef]
- Ding, N.; Chen, G.; Hoffman, R.; Loughran, P.A.; Sodhi, C.P.; Hackam, D.J.; Billiar, T.R.; Neal, M.D. Toll-Like Receptor 4 Regulates Platelet Function and Contributes to Coagulation Abnormality and Organ Injury in Hemorrhagic Shock and Resuscitation. Circ. Cardiovasc. Genet. 2014, 7, 615–624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, S.; Cao, X.; Gao, J. C24:0 Avoids Cold Exposure-Induced Oxidative Stress and Fatty Acid β-Oxidation Damage. iScience 2021, 24, 103409. [Google Scholar] [CrossRef]
- Yatoh, S.; Totsuka-Mizuma, K.; Matsuzaka, T.; Sekiya, M.; Suzuki, H.; Shimano, H. Estimated Stearoyl-CoA-Desaturase (SCD)-1 and Elongase of Very Long Chain Fatty Acids (Elovl) 6 Activities From Serum Fatty Acids Are Reciprocally Associated with Visceral Fat Area in Type 2 Diabetic Patients. J. Diabetes Obes. 2018, 5, 41–47. [Google Scholar] [CrossRef]
- Tanaka, T.; Shen, J.; Abecasis, G.R.; Kisialiou, A.; Ordovas, J.M.; Guralnik, J.M.; Singleton, A.; Bandinelli, S.; Cherubini, A.; Arnett, D.; et al. Genome-Wide Association Study of Plasma Polyunsaturated Fatty Acids in the InCHIANTI Study. PLoS Genet. 2009, 5, e1000338. [Google Scholar] [CrossRef]
- Bangalore, S.; Makani, H.; Radford, M.; Thakur, K.; Toklu, B.; Katz, S.D.; DiNicolantonio, J.J.; Devereaux, P.J.; Alexander, K.P.; Wetterslev, J.; et al. Clinical Outcomes with β-Blockers for Myocardial Infarction: A Meta-Analysis of Randomized Trials. Am. J. Med. 2014, 127, 939–953. [Google Scholar] [CrossRef]
- Boquist, S.; Ruotolo, G.; Hellénius, M.-L.; Danell-Toverud, K.; Karpe, F.; Hamsten, A. Effects of a Cardioselective β-Blocker on Postprandial Triglyceride-Rich Lipoproteins, Low Density Lipoprotein Particle Size and Glucose-Insulin Homeostasis in Middle-Aged Men with Modestly Increased Cardiovascular Risk. Atherosclerosis 1998, 137, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A. Effect of Beta Blockers on Blood Lipid Profile. Am. Heart J. 1985, 109, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Harvengt, C.; Heller, F.R.; Martial, P.; VAN Nieuwenhuyze, Y. Short-Term Effects of Beta Blockers Atenolol, Nadolol, Pindolol, and Propranolol on Lipoprotein Metabolism in Normolipemic Subjects. J. Clin. Pharmacol. 1987, 27, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.B.; Guerra, I.M.S.; Melo, T.; Rocha, H.; Moreira, A.S.P.; Paiva, A.; Domingues, M.R. Dried Blood Spots in Clinical Lipidomics: Optimization and Recent Findings. Anal. Bioanal. Chem. 2022, 414, 7085–7101. [Google Scholar] [CrossRef]
- Simmonds, L.A.; Yelland, L.N.; Best, K.P.; Liu, G.; Gibson, R.A.; Makrides, M. Translating N-3 Polyunsaturated Fatty Acid Status from Whole Blood to Plasma and Red Blood Cells during Pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 2022, 176, 102367. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K.C. The Arcsine Is Asinine: The Analysis of Proportions in Ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global Survey of the Omega-3 Fatty Acids, Docosahexaenoic Acid and Eicosapentaenoic Acid in the Blood Stream of Healthy Adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Cormier, H.; Rudkowska, I.; Thifault, E.; Lemieux, S.; Couture, P.; Vohl, M.-C. Polymorphisms in Fatty Acid Desaturase (FADS) Gene Cluster: Effects on Glycemic Controls Following an Omega-3 Polyunsaturated Fatty Acids (PUFA) Supplementation. Genes 2013, 4, 485–498. [Google Scholar] [CrossRef]
| Variables n (%) | Group 1—Control n = 50 | Group 2—AS n = 52 | p-Value |
|---|---|---|---|
| Overall (Total n = 102) | 50 (49.0%) | 52 (51.0%) | >0.9999 |
| Demographic | |||
| Age, year (Mean ± SD) | 46 ± 11.6 | 67 ± 8.4 | <0.0001 |
| Obese (BMI ≥ 30 kg/m2) | 9 (18.0%) | 14 (26.9%) | 0.3462 |
| Male | 28 (56.0%) | 40 (76.9%) | 0.0353 |
| Female | 22 (44.0%) | 12 (23.1%) | 0.0353 |
| Comorbidities | |||
| Smokers | 6 (12.0%) | 25 (48.1%) | <0.0001 |
| DM | 2 (4.0%) | 12 (23.1%) | 0.0078 |
| HTN | 12 (24.0%) | 47 (90.4%) | <0.0001 |
| IHD | 0 (0.0%) | 18 (34.6%) | <0.0001 |
| IS | 0 (0.0%) | 16 (30.8%) | <0.0001 |
| HFrEF | 0 (0.0%) | 4 (7.7%) | 0.1179 |
| Paroxysmal atrial fibrillation | 0 (0.0%) | 1 (1.9%) | >0.9999 |
| CKD | 3 (6.0%) | 27 (51.9%) | <0.0001 |
| Medication Use | |||
| Statin therapy | 0 (0.0%) | 40 (76.9%) | <0.0001 |
| Statin therapy + BB (out of statin therapy group n = 40) | - | 25 (62.5%) | - |
| Thiazide diuretic | 0 (0.0%) | 3 (5.8%) | 0.2429 |
| Loop diuretic | 0 (0.0%) | 2 (3.8%) | 0.4952 |
| Indapamide (thiazide-like diuretic) | 1 (2.0%) | 6 (11.5%) | 0.1125 |
| Xanthine oxidase inhibitors | 0 (0.0%) | 5 (9.6%) | 0.0566 |
| Parameter, % | Plasma | Plaques | ||
|---|---|---|---|---|
| Control (n = 50) | AS (n = 52) | Stable (n = 28) | Unstable (n = 24) | |
| Broad FA classes and omega-3 index | ||||
| SFA | 37.61 ± 3.88 *** | 34.04 ± 1.38 | 33.97 ± 6.40 | 31.66 ± 5.16 |
| UFA | 62.40 ± 3.88 *** | 65.96 ± 1.38 | 66.03 ± 6.40 | 68.34 ± 5.16 |
| MUFA | 26.15 ± 3.64 *** | 30.94 ± 3.50 | 30.90 ± 4.15 # | 33.62 ± 5.11 |
| PUFA | 35.77 ± 4.47 | 34.80 ± 3.11 | 36.04 [30.68–38.66] | 34.68 [32.80–35.95] |
| HUFA | 12.55 [10.12–14.52] *** | 10.31 [9.25–11.78] | 11.91 ± 1.79 # | 10.68 ± 1.74 |
| omega-6 | 32.68 ± 4.07 | 32.12 ± 3.13 | 32.72 ± 4.03 | 32.31 ± 2.63 |
| EPA + DHA | 2.70 ± 1.07 ** | 2.23 ± 0.58 | 1.90 [1.54–2.48] # | 1.76 [1.34–1.90] |
| omega-3 index | 4.92 ± 1.29 ** | 4.35 ± 0.72 | - | - |
| TFA | 0.40 [0.28–0.69] *** | 0.31 [0.16–0.47] | 0.33 ± 0.13 | 0.35 ± 0.15 |
| Selected FA: SFA, MUFA pathway | ||||
| C16:0 | 26.60 [24.70–27.91] *** | 25.12 [24.17–26.00] | 23.07 ± 3.23 | 22.03 ± 3.15 |
| C18:0 | 9.27 ± 1.83 *** | 7.48 ± 0.97 | 7.59 [5.99–10.18] | 6.92 [5.87–8.24] |
| C20:0 | 0.19 [0.13–0.23] | 0.19 [0.17–0.20] | 0.14 ± 0.05 # | 0.11 ± 0.04 |
| C22:0 | 0.43 [0.33–0.56] *** | 0.36 [0.32–0.42] | 0.41 ± 0.13 # | 0.35 ± 0.08 |
| C24:0 | 0.33 [0.28–0.44] *** | 0.28 [0.24–0.33] | 0.54 ± 0.16 # | 0.46 ± 0.14 |
| C16:1n-7 | 2.19 [1.71–2.76] | 2.12 [1.74–2.81] | 2.79 [2.16–3.33] # | 3.02 [2.56–3.79] |
| C18:1n-9 | 23.14 ± 3.38 *** | 27.92 ± 3.24 | 26.86 ± 3.62 # | 29.36 ± 4.31 |
| C20:1n-9 | 0.14 [0.12–0.18] *** | 0.17 [0.16–0.20] | 0.26 ± 0.11 # | 0.33 ± 0.15 |
| Selected FA: omega-6, omega-3 pathway | ||||
| C20:3n-6 | 1.31 [1.10–1.54] * | 1.14 [0.94–1.45] | 1.69 [1.06–1.99] | 1.50 [1.12–1.95] |
| C20:4n-6 | 8.06 [6.25–9.01] ** | 6.29 [5.48–7.10] | 7.02 ± 0.97 ## | 6.17 ± 1.23 |
| DPA, C22:5n-3 | 0.37 [0.31–0.45] | 0.34 [0.30–0.38] | 0.38 [0.27–0.47] # | 0.26 [0.23–0.36] |
| EPA, C20:5n-3 | 0.56 [0.34–0.79] ** | 0.42 [0.30–0.56] | 0.57 [0.37–0.78] | 0.48 [0.34–0.62] |
| DHA, C22:6n-3 | 2.06 [1.34–2.55] | 1.80 [1.40–2.06] | 1.29 [1.16–1.62] # | 1.11 [0.96–1.35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eroshchenko, N.; Danilova, E.; Lomonosova, A.; Antonik, A.; Lebedeva, S.; Gognieva, D.; Shchekochikhin, D.; Demura, T.; Krot, M.; Gogiberidze, N.; et al. Plasma Fatty Acid Profiling and Mathematical Estimation of the Omega-3 Index: Toward Diagnostic Tools in Atherosclerosis and Statin Therapy Monitoring. Biomedicines 2025, 13, 1383. https://doi.org/10.3390/biomedicines13061383
Eroshchenko N, Danilova E, Lomonosova A, Antonik A, Lebedeva S, Gognieva D, Shchekochikhin D, Demura T, Krot M, Gogiberidze N, et al. Plasma Fatty Acid Profiling and Mathematical Estimation of the Omega-3 Index: Toward Diagnostic Tools in Atherosclerosis and Statin Therapy Monitoring. Biomedicines. 2025; 13(6):1383. https://doi.org/10.3390/biomedicines13061383
Chicago/Turabian StyleEroshchenko, Nikolay, Elena Danilova, Anastasiia Lomonosova, Alexey Antonik, Svetlana Lebedeva, Daria Gognieva, Dmitry Shchekochikhin, Tatiana Demura, Marina Krot, Nana Gogiberidze, and et al. 2025. "Plasma Fatty Acid Profiling and Mathematical Estimation of the Omega-3 Index: Toward Diagnostic Tools in Atherosclerosis and Statin Therapy Monitoring" Biomedicines 13, no. 6: 1383. https://doi.org/10.3390/biomedicines13061383
APA StyleEroshchenko, N., Danilova, E., Lomonosova, A., Antonik, A., Lebedeva, S., Gognieva, D., Shchekochikhin, D., Demura, T., Krot, M., Gogiberidze, N., Syrkin, A., & Kopylov, P. (2025). Plasma Fatty Acid Profiling and Mathematical Estimation of the Omega-3 Index: Toward Diagnostic Tools in Atherosclerosis and Statin Therapy Monitoring. Biomedicines, 13(6), 1383. https://doi.org/10.3390/biomedicines13061383








