Role of Cav1.3 Channels in Brain–Heart Interactions: An Unexpected Journey
Abstract
1. Introduction
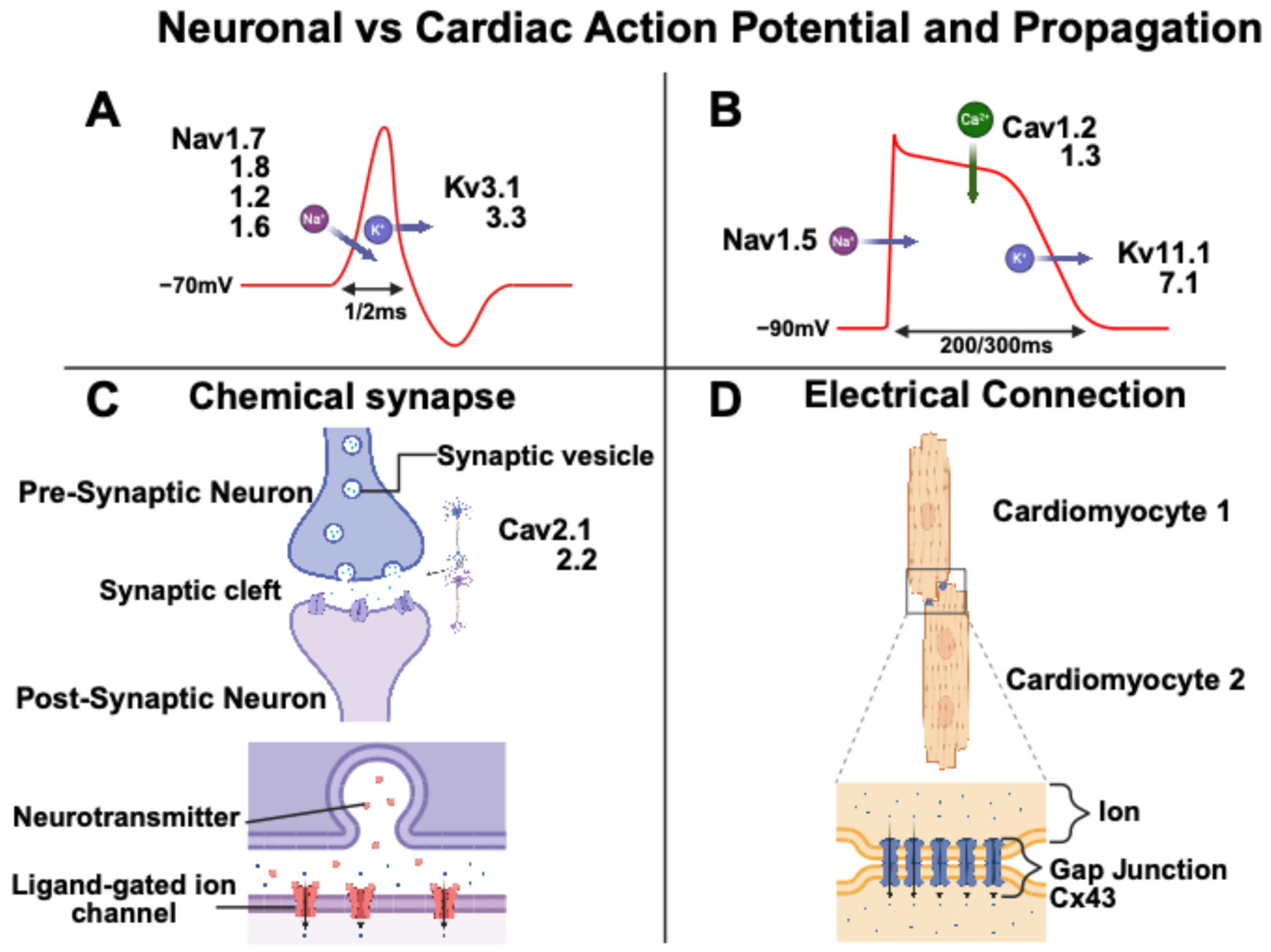
2. Neuronal and Cardiomyocyte Action Potential
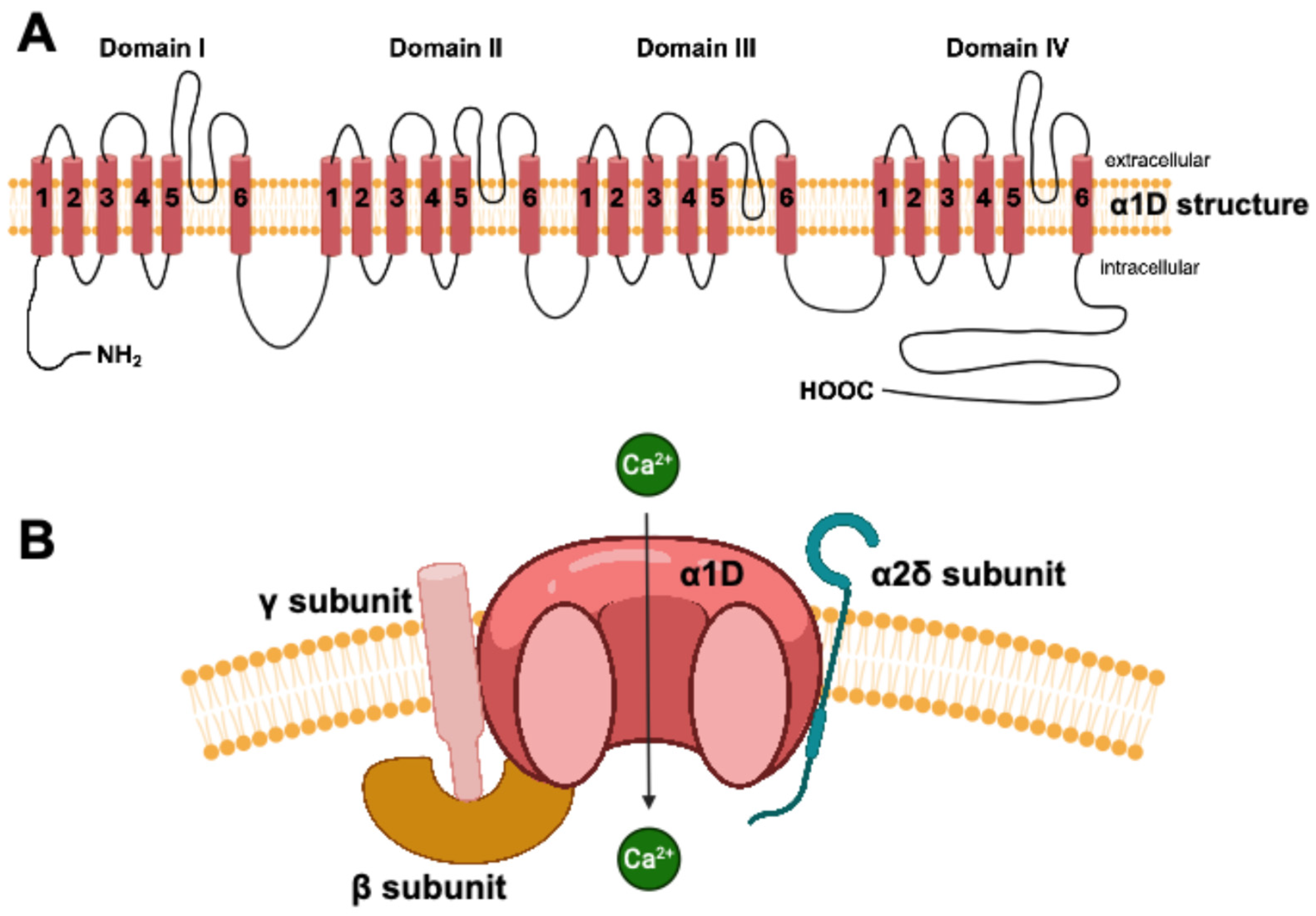
3. The Cav1.3 Channel in the Central Nervous System
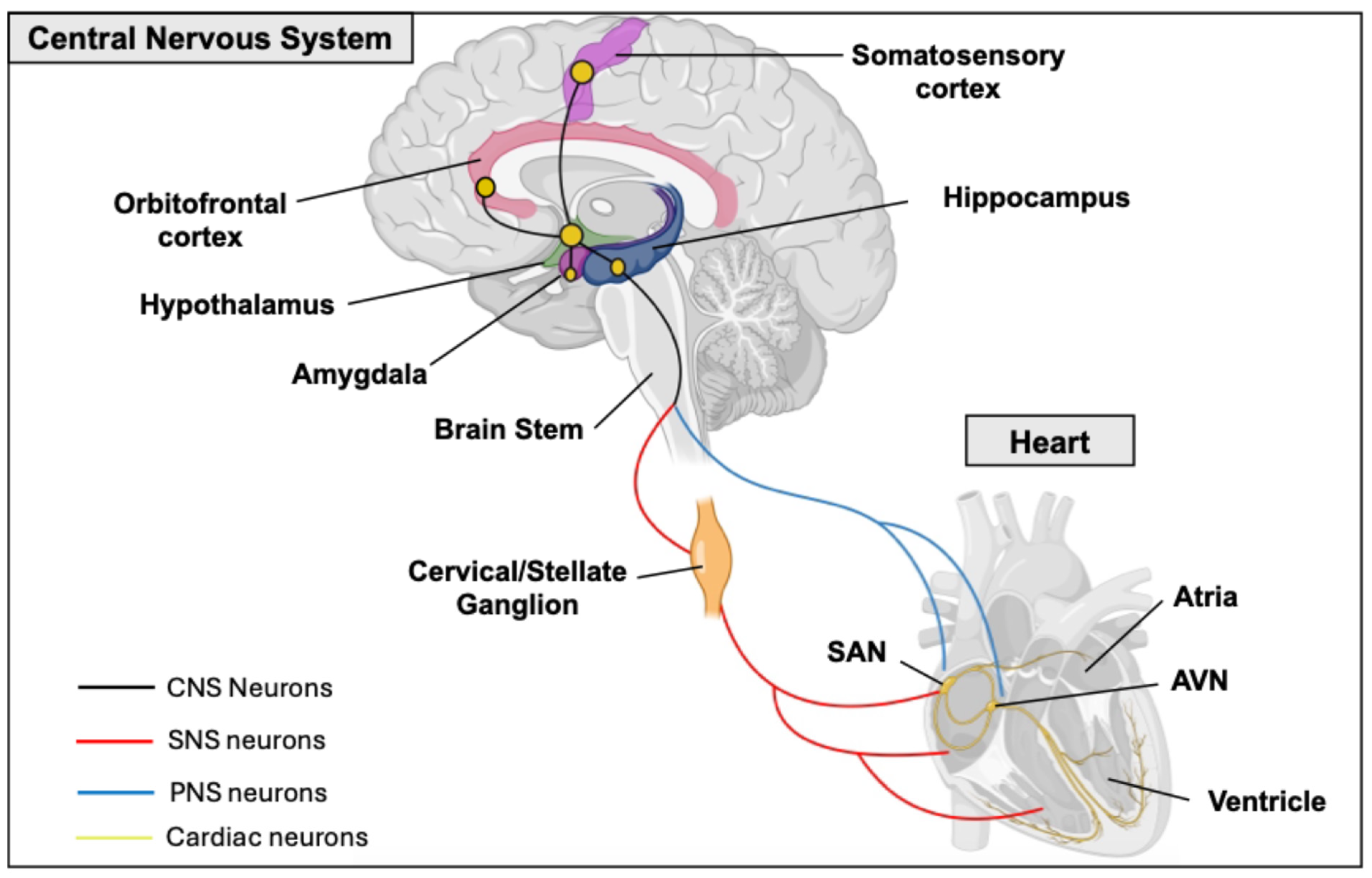
Heart–Brain Interactions
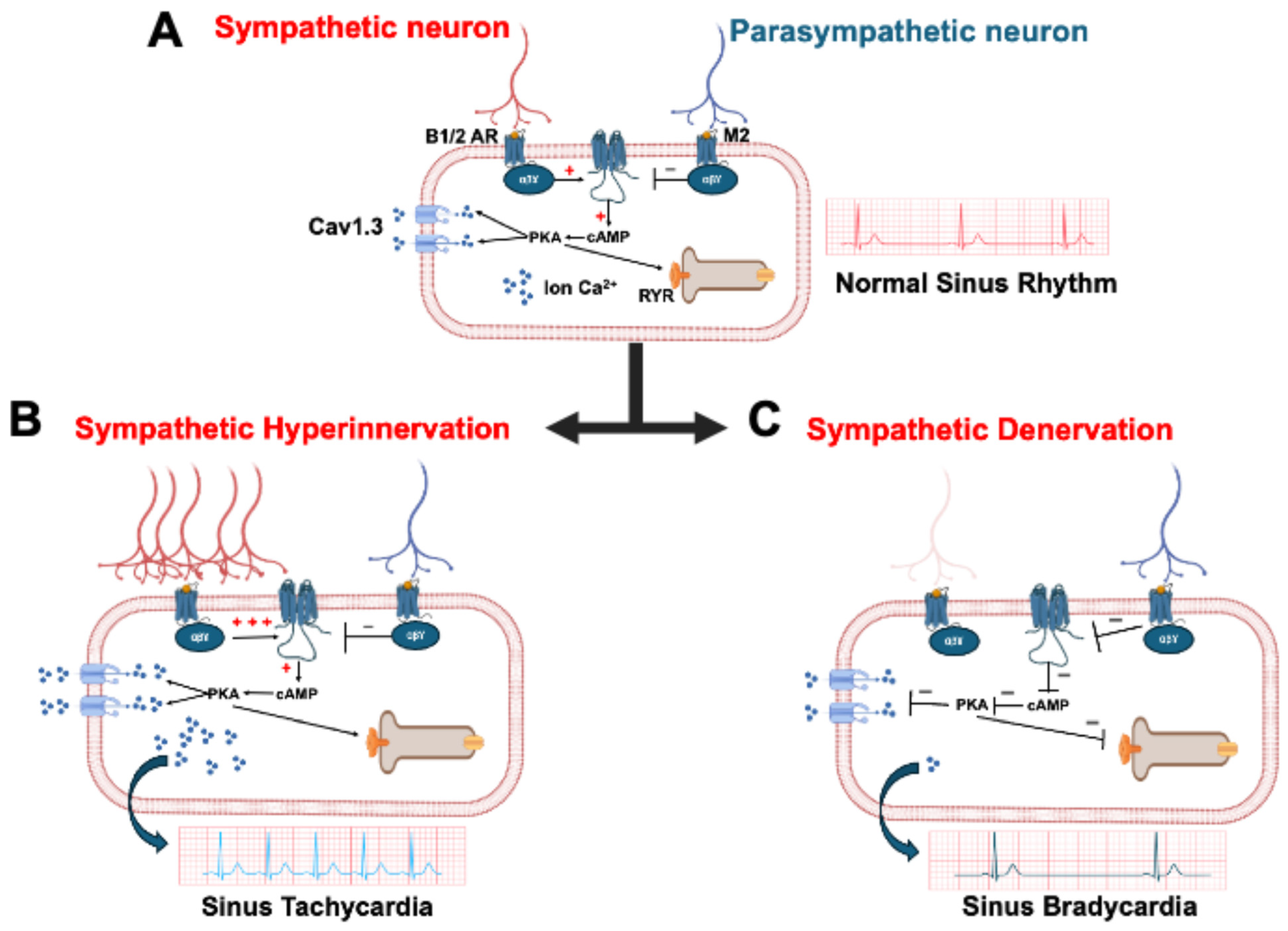
4. The Cav1.3 Channel in Heart
5. Neuromuscular Experimental Modalities and Developments
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| ANS | Autonomic Nervous System |
| AP | Action Potential |
| AV | Atrioventricular |
| CM | Cardiomyocyte |
| CNS | Central Nervous System |
| DG | Dentate Gyrus |
| hiPSC | Human Induced Pluripotent Stem Cell |
| HRV | Heart Rate Variability |
| ICN | Intracardiac Neuron |
| LTCC | L-Type Calcium Channel |
| NA | Noradrenaline |
| PNS | Parasympathetic Nervous System |
| PKA | Protein Kinase A |
| PVN | Paraventricular Nucleus |
| SAN | Sinoatrial Node |
| SN | Sympathetic Neuron |
| SNS | Sympathetic Nervous System |
References
- Ajijola, O.A.; Aksu, T.; Arora, R.; Biaggioni, I.; Chen, P.-S.; Ferrari, G.D.; Dusi, V.; Fudim, M.; Goldberger, J.J.; Green, A.L.; et al. Clinical neurocardiology: Defining the value of neuroscience-based cardiovascular therapeutics—2024 update. J. Physiol. 2025, 603, 1781–1839. [Google Scholar] [CrossRef] [PubMed]
- Zaglia, T.; Mongillo, M. Cardiac sympathetic innervation, from a different point of (re)view. J. Physiol. 2017, 595, 3919–3930. [Google Scholar] [CrossRef] [PubMed]
- Winbo, A.; Paterson, D.J. The Brain-Heart Connection in Sympathetically Triggered Inherited Arrhythmia Syndromes. Heart Lung Circ. 2020, 29, 529–537. [Google Scholar] [CrossRef]
- Baltogiannis, G.G.; Lysitsas, D.N.; di Giovanni, G.; Ciconte, G.; Sieira, J.; Conte, G.; Kolettis, T.M.; Chierchia, G.-B.; de Asmundis, C.; Brugada, P. CPVT: Arrhythmogenesis, Therapeutic Management, and Future Perspectives. A Brief Review of the Literature. Front. Cardiovasc. Med. 2019, 6, 92. [Google Scholar] [CrossRef]
- Cao, J.M.; Fishbein, M.C.; Han, J.B.; Lai, W.W.; Lai, A.C.; Wu, T.J.; Czer, L.; Wolf, P.L.; Denton, T.A.; Shintaku, I.P.; et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000, 101, 1960–1969. [Google Scholar] [CrossRef]
- Gardner, R.T.; Ripplinger, C.M.; Myles, R.C.; Habecker, B.A. Molecular Mechanisms of Sympathetic Remodeling and Arrhythmias. Circ. Arrhythmia Electrophysiol. 2016, 9, e001359. [Google Scholar] [CrossRef] [PubMed]
- Salamon, R.J.; Halbe, P.; Kasberg, W.; Bae, J.; Audhya, A.; Mahmoud, A.I. Parasympathetic and sympathetic axons are bundled in the cardiac ventricles and undergo physiological reinnervation during heart regeneration. iScience 2023, 26, 107709. [Google Scholar] [CrossRef]
- Shanks, J.; Ramchandra, R. Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension. Int. J. Mol. Sci. 2021, 22, 12305. [Google Scholar] [CrossRef]
- Olshansky, B.; Sabbah, H.N.; Hauptman, P.J.; Colucci, W.S. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008, 118, 863–871. [Google Scholar] [CrossRef]
- Khan, A.A.; Lip, G.Y.H.; Shantsila, A. Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system. Eur. J. Clin. Investig. 2019, 49, e13174. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef] [PubMed]
- Herron, T.J.; Rocha, A.M.D.; Campbell, K.F.; Ponce-Balbuena, D.; Willis, B.C.; Guerrero-Serna, G.; Liu, Q.; Klos, M.; Musa, H.; Zarzoso, M.; et al. Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function. Circ. Arrhythm Electrophysiol. 2016, 9, e003638. [Google Scholar] [CrossRef]
- Teed, A.R.; Feinstein, J.S.; Puhl, M.; Lapidus, R.C.; Upshaw, V.; Kuplicki, R.T.; Bodurka, J.; Ajijola, O.A.; Kaye, W.H.; Thompson, W.K.; et al. Association of Generalized Anxiety Disorder With Autonomic Hypersensitivity and Blunted Ventromedial Prefrontal Cortex Activity During Peripheral Adrenergic Stimulation: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, A.; Mora, F. Neurotransmitters and prefrontal cortex-limbic system interactions: Implications for plasticity and psychiatric disorders. J. Neural Transm. 2009, 116, 941–952. [Google Scholar] [CrossRef]
- Arapova, Y.Y.; Popov, I.A.; Shikhliarova, A.I.; Rostorguev, E.E.; Kuznetsova, N.S.; Protasova, T.P. Studies of Cognitive Functions and the Organization of Brain Bioelectrical Activity during Waking and Sleep in Patients with Frontal Lobe Tumors. Neurosci. Behav. Phys. 2022, 52, 994–998. [Google Scholar] [CrossRef]
- Kovner, R.; Oler, J.A.; Kalin, N.H. Cortico-Limbic Interactions Mediate Adaptive and Maladaptive Responses Relevant to Psychopathology. Am. J. Psychiatry 2019, 176, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.M.; Bartsch, D. The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 2014, 357, 463–476. [Google Scholar] [CrossRef]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Trafficking and stability of voltage-gated calcium channels. Cell. Mol. Life Sci. 2012, 69, 843–856. [Google Scholar] [CrossRef]
- Yao, X.; Gao, S.; Yan, N. Structural basis for pore blockade of human voltage-gated calcium channel Cav1.3 by motion sickness drug cinnarizine. Cell Res. 2022, 32, 946–948. [Google Scholar] [CrossRef]
- Baig, S.M.; Koschak, A.; Lieb, A.; Gebhart, M.; Dafinger, C.; Nürnberg, G.; Ali, A.; Ahmad, I.; Sinnegger-Brauns, M.J.; Brandt, N.; et al. Loss of Cav1.3 (CACNA1D) function in a human channelopathy with bradycardia and congenital deafness. Nat. Neurosci. 2011, 14, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rinné, S.; Stallmeyer, B.; Pinggera, A.; Netter, M.F.; Matschke, L.A.; Dittmann, S.; Kirchhefer, U.; Neudorf, U.; Opp, J.; Striessnig, J.; et al. Whole Exome Sequencing Identifies a Heterozygous Variant in the Cav1.3 Gene CACNA1D Associated with Familial Sinus Node Dysfunction and Focal Idiopathic Epilepsy. Int. J. Mol. Sci. 2022, 23, 14215. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, K.; Schrauwen, I.; Raza, S.I.; Lee, K.; Hussain, S.; Chakchouk, I.; Nasir, A.; Acharya, A.; Abbe, I.; Umair, M.; et al. Identification of CACNA1D variants associated with sinoatrial node dysfunction and deafness in additional Pakistani families reveals a clinical significance. J. Hum. Genet. 2019, 64, 153–160. [Google Scholar] [CrossRef]
- Zaveri, S.; Srivastava, U.; Qu, Y.S.; Chahine, M.; Boutjdir, M. Pathophysiology of Cav1.3 L-type calcium channels in the heart. Front. Physiol. 2023, 14, 1144069. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M.; Kass, R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 2005, 85, 1205–1253. [Google Scholar] [CrossRef]
- Fujishiro, A.; Kaneko, H.; Kawashima, T.; Ishida, M.; Kawano, T. In vivo neuronal action potential recordings via three-dimensional microscale needle-electrode arrays. Sci. Rep. 2014, 4, 4868. [Google Scholar] [CrossRef]
- Zhang, M.; Man, M.; Ma, G.; Ye, M.; Liu, S. Research on action behavior of neuron system in case of single pulse stimulus. Sci. Rep. 2020, 10, 1240. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, W.; Gao, T.; Liu, J.; Lieber, C.M. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 2016, 11, 776–782. [Google Scholar] [CrossRef]
- Pallien, T.; Klussmann, E. New aspects in cardiac L-type Ca2+ channel regulation. Biochem. Soc. Trans. 2020, 48, 39–49. [Google Scholar] [CrossRef]
- Mesirca, P.; Bidaud, I.; Mangoni, M.E. Rescuing cardiac automaticity in L-type Cav1.3 channelopathies and beyond. J. Physiol. 2016, 594, 5869–5879. [Google Scholar] [CrossRef]
- Moore, S.J.; Murphy, G.G. The Role of L-Type Calcium Channels in Neuronal Excitability and Aging. Neurobiol. Learn. Mem. 2020, 173, 107230. [Google Scholar] [CrossRef] [PubMed]
- Rubi, L.; Schandl, U.; Lagler, M.; Geier, P.; Spies, D.; Gupta, K.D.; Boehm, S.; Kubista, H. Raised Activity of L-Type Calcium Channels Renders Neurons Prone to Form Paroxysmal Depolarization Shifts. Neuromol. Med. 2013, 15, 476–492. [Google Scholar] [CrossRef]
- Gamelli, A.E.; McKinney, B.C.; White, J.A.; Murphy, G.G. Deletion of the L-type calcium channel CaV1.3 but not CaV1.2 results in a diminished sAHP in mouse CA1 pyramidal neurons. Hippocampus 2011, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.E.; Manfredsson, F.P.; Steece-Collier, K. The Role of Striatal Cav1.3 Calcium Channels in Therapeutics for Parkinson’s Disease. Handb. Exp. Pharmacol. 2023, 279, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, F.; Von Moers, A.; Bittigau, P.; Lange, J.; Wiegand, S.; Török, F.; Stölting, G.; Striessnig, J.; Motazacker, M.M.; Broekema, M.F.; et al. A Novel De Novo Gain-of-Function CACNA1D Variant in Neurodevelopmental Disease With Congenital Tremor, Seizures, and Hypotonia. Neurol. Genet. 2024, 10, e200186. [Google Scholar] [CrossRef]
- Pinggera, A.; Mackenroth, L.; Rump, A.; Schallner, J.; Beleggia, F.; Wollnik, B.; Striessnig, J. New gain-of-function mutation shows CACNA1D as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum. Mol. Genet. 2017, 26, 2923–2932. [Google Scholar] [CrossRef]
- McKinney, B.C.; Sze, W.; Lee, B.; Murphy, G.G. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of CaV1.3 knockout mice. Neurobiol. Learn. Mem. 2009, 92, 519–528. [Google Scholar] [CrossRef]
- Olson, P.A.; Tkatch, T.; Hernandez-Lopez, S.; Ulrich, S.; Ilijic, E.; Mugnaini, E.; Zhang, H.; Bezprozvanny, I.; Surmeier, D.J. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J. Neurosci. 2005, 25, 1050–1062. [Google Scholar] [CrossRef]
- Ma, H.; Cohen, S.; Li, B.; Tsien, R.W. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci. Rep. 2012, 33, e00009. [Google Scholar] [CrossRef]
- Ortner, N.J.; Sah, A.; Paradiso, E.; Shin, J.; Stojanovic, S.; Hammer, N.; Haritonova, M.; Hofer, N.T.; Marcantoni, A.; Guarina, L.; et al. The human channel gating-modifying A749G CACNA1D (Cav1.3) variant induces a neurodevelopmental syndrome-like phenotype in mice. JCI Insight 2023, 8, e162100. [Google Scholar] [CrossRef] [PubMed]
- Pinggera, A.; Lieb, A.; Benedetti, B.; Lampert, M.; Monteleone, S.; Liedl, K.R.; Tuluc, P.; Striessnig, J. CACNA1D De Novo Mutations in Autism Spectrum Disorders Activate Cav1.3 L-Type Calcium Channels. Biol. Psychiatry 2015, 77, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Leitch, B.; Szostek, A.; Lin, R.; Shevtsova, O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience 2009, 164, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Lauffer, M.; Wen, H.; Myers, B.; Plumb, A.; Parker, K.; Williams, A. Deletion of the voltage-gated calcium channel, CaV1.3, causes deficits in motor performance and associative learning. Genes Brain Behav. 2022, 21, e12791. [Google Scholar] [CrossRef]
- Marschallinger, J.; Sah, A.; Schmuckermair, C.; Unger, M.; Rotheneichner, P.; Kharitonova, M.; Waclawiczek, A.; Gerner, P.; Jaksch-Bogensperger, H.; Berger, S.; et al. The L-type calcium channel Cav1.3 is required for proper hippocampal neurogenesis and cognitive functions. Cell Calcium 2015, 58, 606–616. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, Y.-R.; Lee, B.; Choi, B.; Kim, H.; Kim, C.-H. Reduction of Cav1.3 channels in dorsal hippocampus impairs the development of dentate gyrus newborn neurons and hippocampal-dependent memory tasks. PLoS ONE 2017, 12, e0181138. [Google Scholar] [CrossRef]
- Zhai, J.; Navakkode, S.; Yeow, S.Q.Z.; Krishna-K, K.; Liang, M.C.; Koh, J.H.; Wong, R.X.; Yu, W.P.; Sajikumar, S.; Huang, H.; et al. Loss of CaV1.3 RNA editing enhances mouse hippocampal plasticity, learning, and memory. Proc. Natl. Acad. Sci. USA 2022, 119, e2203883119. [Google Scholar] [CrossRef]
- Huang, H.; Tan, B.Z.; Shen, Y.; Tao, J.; Jiang, F.; Sung, Y.Y.; Ng, C.K.; Raida, M.; Köhr, G.; Higuchi, M.; et al. RNA editing of the IQ domain in Cav1.3 channels modulates their Ca2+-dependent inactivation. Neuron 2012, 73, 304–316. [Google Scholar] [CrossRef]
- Steece-Collier, K.; Stancati, J.A.; Collier, N.J.; Sandoval, I.M.; Mercado, N.M.; Sortwell, C.E.; Collier, T.J.; Manfredsson, F.P. Genetic silencing of striatal CaV1.3 prevents and ameliorates levodopa dyskinesia. Mov. Disord. 2019, 34, 697–707. [Google Scholar] [CrossRef]
- Schuster, S.; Doudnikoff, E.; Rylander, D.; Berthet, A.; Aubert, I.; Ittrich, C.; Bloch, B.; Cenci, M.A.; Surmeier, D.J.; Hengerer, B.; et al. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol. Psychiatry 2009, 65, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Z.; Jiang, F.; Tan, M.Y.; Yu, D.; Huang, H.; Shen, Y.; Soong, T.W. Functional characterization of alternative splicing in the C terminus of L-type CaV1.3 channels. J. Biol. Chem. 2011, 286, 42725–42735. [Google Scholar] [CrossRef]
- Anthony, T.E.; Dee, N.; Bernard, A.; Lerchner, W.; Heintz, N.; Anderson, D.J. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 2014, 156, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. The amygdala: Functional organization and involvement in neurologic disorders. Neurology 2015, 84, 313–324. [Google Scholar] [CrossRef]
- Palmiter, R.D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018, 41, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, S.V.; Kunert, K.; Rüttiger, L.; Zuccotti, A.; Schönig, K.; Friauf, E.; Knipper, M.; Bartsch, D.; Nothwang, H.G. Retrocochlear function of the peripheral deafness gene Cacna1d. Hum. Mol. Genet. 2012, 21, 3896–3909. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, J.J.; Boesen, M.; Braun, N.; Deitmer, J.W.; Kramer, F.; Lohr, C.; Müller, B.; Nothwang, H.G.; Striessnig, J.; Löhrke, S.; et al. Cav1.3 Calcium Channels Are Required for Normal Development of the Auditory Brainstem. J. Neurosci. 2011, 31, 8280–8294. [Google Scholar] [CrossRef]
- Baudot, M.; Torre, E.; Bidaud, I.; Louradour, J.; Torrente, A.G.; Fossier, L.; Talssi, L.; Nargeot, J.; Barrère-Lemaire, S.; Mesirca, P.; et al. Concomitant genetic ablation of L-type Cav1.3 (α1D) and T-type Cav3.1 (α1G) Ca2+ channels disrupts heart automaticity. Sci. Rep. 2020, 10, 18906. [Google Scholar] [CrossRef] [PubMed]
- Louradour, J.; Bortolotti, O.; Torre, E.; Bidaud, I.; Lamb, N.; Fernandez, A.; Le Guennec, J.-Y.; Mangoni, M.E.; Mesirca, P. L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells. Cells 2022, 11, 1114. [Google Scholar] [CrossRef]
- Qu, Y.; Baroudi, G.; Yue, Y.; El-Sherif, N.; Boutjdir, M. Localization and modulation of α1D (Cav1.3) L-type Ca channel by protein kinase A. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2123–H2130. [Google Scholar] [CrossRef]
- Hafez, O.A.; Chang, R.B. Regulation of Cardiac Function by the Autonomic Nervous System. Physiology 2025, 40, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Scott-Solomon, E.; Boehm, E.; Kuruvilla, R. The sympathetic nervous system in development and disease. Nat. Rev. Neurosci. 2021, 22, 685–702. [Google Scholar] [CrossRef]
- Schwartz, P.J.; De Ferrari, G.M. Sympathetic-parasympathetic interaction in health and disease: Abnormalities and relevance in heart failure. Heart Fail. Rev. 2011, 16, 101–107. [Google Scholar] [CrossRef]
- Grégoire, J.-M.; Gilon, C.; Carlier, S.; Bersini, H. Autonomic nervous system assessment using heart rate variability. Acta Cardiol. 2023, 78, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef]
- McCorry, L.K. Physiology of the Autonomic Nervous System. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Sjörs Dahlman, A.; Jonsdottir, I.H.; Hansson, C. The hypothalamo-pituitary-adrenal axis and the autonomic nervous system in burnout. Handb. Clin. Neurol. 2021, 182, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. Mental Stress and Cardiovascular Health—Part I. J. Clin. Med. 2022, 11, 3353. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Davidson, R.J.; McEwen, B.S. Social influences on neuroplasticity: Stress and interventions to promote well-being. Nat. Neurosci. 2012, 15, 689–695. [Google Scholar] [CrossRef]
- Verberne, A.J.; Owens, N.C. Cortical modulation of the cardiovascular system. Prog. Neurobiol. 1998, 54, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, W.; Liu, Y.; Hua, T.; Yang, M.; Liu, Y. Heart-brain interactions: Clinical evidence and mechanisms based on critical care medicine. Front. Cardiovasc. Med. 2024, 11, 1483482. [Google Scholar] [CrossRef] [PubMed]
- Melville, K.I.; Blum, B.; Shister, H.E.; Silver, M.D. Cardiac ischemic changes and arrhythmias induced by hypothalamic stimulation. Am. J. Cardiol. 1963, 12, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, I.E.; McGovern, A.E.; Driessen, A.K.; Kerr, N.F.; Mills, P.C.; Mazzone, S.B. Hippocampal modulation of cardiorespiratory function. Respir. Physiol. Neurobiol. 2018, 252–253, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Savić, B.; Murphy, D.; Japundžić-Žigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef]
- Allen, A.M. Inhibition of the Hypothalamic Paraventricular Nucleus in Spontaneously Hypertensive Rats Dramatically Reduces Sympathetic Vasomotor Tone. Hypertension 2002, 39, 275–280. [Google Scholar] [CrossRef]
- Busnardo, C.; Fassini, A.; Lopes-Azevedo, S.; Omena-Giatti, L.; Goulart, M.T.; Antunes-Rodrigues, J.; Alves, F.H.F.; Corrêa, F.M.A.; Crestani, C.C. Endocannabinoid system in the paraventricular nucleus of the hypothalamus modulates autonomic and cardiovascular changes but not vasopressin response in a rat hemorrhagic shock model. Shock 2024, 61, 294–303. [Google Scholar] [CrossRef]
- Hetzenauer, A.; Sinnegger-Brauns, M.J.; Striessnig, J.; Singewald, N. Brain activation pattern induced by stimulation of L-type Ca2+-channels: Contribution of CaV1.3 and CaV1.2 isoforms. Neuroscience 2006, 139, 1005–1015. [Google Scholar] [CrossRef]
- Punzi, M.; Sestieri, C.; Picerni, E.; Chiarelli, A.M.; Padulo, C.; Delli Pizzi, A.; Tullo, M.G.; Tosoni, A.; Granzotto, A.; Della Penna, S.; et al. Atrophy of hippocampal subfields and amygdala nuclei in subjects with mild cognitive impairment progressing to Alzheimer’s disease. Heliyon 2024, 10, e27429. [Google Scholar] [CrossRef]
- Waraczynski, M.; Abbott, S.; Schultz, A.V. CaV1.3 channel blockade in the extended amygdala has a delayed effect on the reward efficacy of medial forebrain bundle stimulation. Behav. Brain Res. 2017, 317, 485–493. [Google Scholar] [CrossRef]
- Benarroch, E.E. Physiology and Pathophysiology of the Autonomic Nervous System. Contin. Lifelong Learn. Neurol. 2020, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Koschak, A.; Sinnegger-Brauns, M.J.; Hetzenauer, A.; Nguyen, N.K.; Busquet, P.; Pelster, G.; Singewald, N. Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem. Soc. Trans. 2006, 34, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, S.K.; Yin, C.; Weber, C.; Godinho-Silva, C.; Veiga-Fernandes, H.; Xu, Q.J.; Chang, R.B.; Habenicht, A.J.R. Cardiovascular Brain Circuits. Circ. Res. 2023, 132, 1546–1565. [Google Scholar] [CrossRef]
- Martín-Gallego, A.; González-García, L.; Carrasco-Brenes, A.; Segura-Fernández-Nogueras, M.; Delgado-Babiano, A.; Ros-Sanjuán, A.; Romero-Moreno, L.; Domínguez-Páez, M.; Dawid-Milner, M.S.; Arráez-Sánchez, M.A. Brainstem and Autonomic Nervous System Dysfunction: A Neurosurgical Point of View. Acta Neurochir. Suppl. 2017, 124, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sattin, D.; Leonardi, M.; Picozzi, M. The autonomic nervous system and the brainstem: A fundamental role or the background actors for consciousness generation? Hypothesis, evidence, and future directions for rehabilitation and theoretical approaches. Brain Behav. 2020, 10, e01474. [Google Scholar] [CrossRef]
- Eckrich, S.; Hecker, D.; Sorg, K.; Blum, K.; Fischer, K.; Münkner, S.; Wenzel, G.; Schick, B.; Engel, J. Cochlea-Specific Deletion of Cav1.3 Calcium Channels Arrests Inner Hair Cell Differentiation and Unravels Pitfalls of Conditional Mouse Models. Front. Cell. Neurosci. 2019, 13, 225. [Google Scholar] [CrossRef]
- Platzer, J.; Engel, J.; Schrott-Fischer, A.; Stephan, K.; Bova, S.; Chen, H.; Zheng, H.; Striessnig, J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 2000, 102, 89–97. [Google Scholar] [CrossRef]
- Parsons, R.L. Mammalian Cardiac Ganglia as Local Integration Centers: Histochemical and Electrophysiological Evidence. In Neural Mechanisms of Cardiovascular Regulation; Dun, N.J., Machado, B.H., Pilowsky, P.M., Eds.; Springer: Boston, MA, USA, 2004; pp. 335–356. ISBN 978-1-4419-9054-9. [Google Scholar]
- Armour, J.A. Potential clinical relevance of the “little brain” on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef]
- Richardson, R.J.; Grkovic, I.; Anderson, C.R. Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res. 2003, 314, 337–350. [Google Scholar] [CrossRef]
- Ashton, J.L.; Prince, B.; Sands, G.; Argent, L.; Anderson, M.; Smith, J.E.G.; Tedoldi, A.; Ahmad, A.; Baddeley, D.; Pereira, A.G.; et al. Electrophysiology and 3D-imaging reveal properties of human intracardiac neurons and increased excitability with atrial fibrillation. J. Physiol. 2025, 603, 1923–1939. [Google Scholar] [CrossRef]
- Jungen, C.; Scherschel, K.; Eickholt, C.; Kuklik, P.; Klatt, N.; Bork, N.; Salzbrunn, T.; Alken, F.; Angendohr, S.; Klene, C.; et al. Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias. Nat. Commun. 2017, 8, 14155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tu, H.; Wang, C.; Cao, L.; Muelleman, R.L.; Wadman, M.C.; Li, Y.-L. Correlation of Ventricular Arrhythmogenesis with Neuronal Remodeling of Cardiac Postganglionic Parasympathetic Neurons in the Late Stage of Heart Failure after Myocardial Infarction. Front. Neurosci. 2017, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, H.; Zheng, H.; Zhang, L.; Tran, T.P.; Muelleman, R.L.; Li, Y.-L. Alterations of calcium channels and cell excitability in intracardiac ganglion neurons from type 2 diabetic rats. Am. J. Physiol.-Cell Physiol. 2012, 302, C1119–C1127. [Google Scholar] [CrossRef]
- Tu, H.; Liu, J.; Zhang, D.; Zheng, H.; Patel, K.P.; Cornish, K.G.; Wang, W.-Z.; Muelleman, R.L.; Li, Y.-L. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am. J. Physiol.-Cell Physiol. 2014, 306, C132–C142. [Google Scholar] [CrossRef]
- Bescond, J.; Faivre, J.-F.; Jean, A.; Bois, P.; Chatelier, A. Ion channel expression in intrinsic cardiac neurons: New players in cardiac channelopathies? Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2025, 1872, 119983. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Karnabi, E.; Ramadan, O.; Yue, Y.; Chahine, M.; Boutjdir, M. Perinatal and postnatal expression of Cav1.3 α1D Ca2+ channel in the rat heart. Pediatr. Res. 2011, 69, 479–484. [Google Scholar] [CrossRef]
- Stanika, R.; Campiglio, M.; Pinggera, A.; Lee, A.; Striessnig, J.; Flucher, B.E.; Obermair, G.J. Splice variants of the CaV1.3 L-type calcium channel regulate dendritic spine morphology. Sci. Rep. 2016, 6, 34528. [Google Scholar] [CrossRef]
- Hofer, N.T.; Pinggera, A.; Nikonishyna, Y.V.; Tuluc, P.; Fritz, E.M.; Obermair, G.J.; Striessnig, J. Stabilization of negative activation voltages of Cav1.3 L-Type Ca2+-channels by alternative splicing. Channels 2021, 15, 38–52. [Google Scholar] [CrossRef]
- Singh, A.; Gebhart, M.; Fritsch, R.; Sinnegger-Brauns, M.J.; Poggiani, C.; Hoda, J.-C.; Engel, J.; Romanin, C.; Striessnig, J.; Koschak, A. Modulation of Voltage- and Ca2+-dependent Gating of CaV1.3 L-type Calcium Channels by Alternative Splicing of a C-terminal Regulatory Domain. J. Biol. Chem. 2008, 283, 20733–20744. [Google Scholar] [CrossRef]
- Kuzmenkina, E.; Novikova, E.; Jangsangthong, W.; Matthes, J.; Herzig, S. Single-Channel Resolution of the Interaction between C-Terminal CaV1.3 Isoforms and Calmodulin. Biophys. J. 2019, 116, 836–846. [Google Scholar] [CrossRef]
- Silvani, A.; Calandra-Buonaura, G.; Dampney, R.A.L.; Cortelli, P. Brain–heart interactions: Physiology and clinical implications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150181. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol.-Cell Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Dewenter, M.; von der Lieth, A.; Katus, H.A.; Backs, J. Calcium Signaling and Transcriptional Regulation in Cardiomyocytes. Circ. Res. 2017, 121, 1000–1020. [Google Scholar] [CrossRef]
- Scalco, A.; Moro, N.; Mongillo, M.; Zaglia, T. Neurohumoral Cardiac Regulation: Optogenetics Gets Into the Groove. Front. Physiol. 2021, 12, 726895. [Google Scholar] [CrossRef]
- Ramadan, O.; Qu, Y.; Wadgaonkar, R.; Baroudi, G.; Karnabi, E.; Chahine, M.; Boutjdir, M. Phosphorylation of the consensus sites of protein kinase A on α1D L-type calcium channel. J. Biol. Chem. 2009, 284, 5042–5049. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, G.; Qu, Y.; Ramadan, O.; Chahine, M.; Boutjdir, M. Protein kinase C activation inhibits Cav1.3 calcium channel at NH2-terminal serine 81 phosphorylation site. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1614–H1622. [Google Scholar] [CrossRef]
- Chahine, M.; Qu, Y.; Mancarella, S.; Boutjdir, M. Protein kinase C activation inhibits α1D L-type Ca channel: A single-channel analysis. Pflüg. Arch. 2008, 455, 913–919. [Google Scholar] [CrossRef]
- Qu, Y.; Baroudi, G.; Yue, Y.; Boutjdir, M. Novel Molecular Mechanism Involving α1D (Cav1.3) L-Type Calcium Channel in Autoimmune-Associated Sinus Bradycardia. Circulation 2005, 111, 3034–3041. [Google Scholar] [CrossRef]
- Mesirca, P.; Chemin, J.; Barrère, C.; Torre, E.; Gallot, L.; Monteil, A.; Bidaud, I.; Diochot, S.; Lazdunski, M.; Soong, T.W.; et al. Selective blockade of Cav1.2 (α1C) versus Cav1.3 (α1D) L-type calcium channels by the black mamba toxin calciseptine. Nat. Commun. 2024, 15, 54. [Google Scholar] [CrossRef]
- Striessnig, J.; Bolz, H.J.; Koschak, A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflüg. Arch. 2010, 460, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, U.; Jennings-Charles, R.; Qu, Y.S.; Sossalla, S.; Chahine, M.; Boutjdir, M. Novel re-expression of L-type calcium channel Cav1.3 in left ventricles of failing human heart. Heart Rhythm 2020, 17, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Mesirca, P.; Fedorov, V.V.; Hund, T.J.; Torrente, A.G.; Bidaud, I.; Mohler, P.J.; Mangoni, M.E. Pharmacologic Approach to Sinoatrial Node Dysfunction. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 757–778. [Google Scholar] [CrossRef]
- Gerritse, M.; van Ham, W.B.; Denning, C.; van Veen, T.A.B.; Maas, R.G.C. Characteristics and pharmacological responsiveness in hiPSC models of inherited cardiomyopathy. Pharmacol. Ther. 2025, 272, 108845. [Google Scholar] [CrossRef]
- Zhu, Z.; Huangfu, D. Human pluripotent stem cells: An emerging model in developmental biology. Development 2013, 140, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Chapotte-Baldacci, C.-A.; Pierre, M.; Djemai, M.; Pouliot, V.; Chahine, M. Biophysical properties of NaV1.5 channels from atrial-like and ventricular-like cardiomyocytes derived from human induced pluripotent stem cells. Sci. Rep. 2023, 13, 20685. [Google Scholar] [CrossRef]
- Ren, J.; Han, P.; Ma, X.; Farah, E.N.; Bloomekatz, J.; Zeng, X.-X.I.; Zhang, R.; Swim, M.M.; Witty, A.D.; Knight, H.G.; et al. Canonical Wnt5b Signaling Directs Outlying Nkx2.5+ Mesoderm into Pacemaker Cardiomyocytes. Dev. Cell 2019, 50, 729–743.e5. [Google Scholar] [CrossRef]
- Oh, Y.; Cho, G.-S.; Li, Z.; Hong, I.; Zhu, R.; Kim, M.-J.; Kim, Y.J.; Tampakakis, E.; Tung, L.; Huganir, R.; et al. Functional Coupling with Cardiac Muscle Promotes Maturation of hPSC-Derived Sympathetic Neurons. Cell Stem Cell 2016, 19, 95–106. [Google Scholar] [CrossRef]
- Winbo, A.; Ramanan, S.; Eugster, E.; Jovinge, S.; Skinner, J.R.; Montgomery, J.M. Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H927–H937. [Google Scholar] [CrossRef]
- Takayama, Y.; Kushige, H.; Akagi, Y.; Suzuki, Y.; Kumagai, Y.; Kida, Y.S. Selective Induction of Human Autonomic Neurons Enables Precise Control of Cardiomyocyte Beating. Sci. Rep. 2020, 10, 9464. [Google Scholar] [CrossRef]
- Bernardin, A.A.; Colombani, S.; Rousselot, A.; Andry, V.; Goumon, Y.; Delanoë-Ayari, H.; Pasqualin, C.; Brugg, B.; Jacotot, E.D.; Pasquié, J.-L.; et al. Impact of Neurons on Patient-Derived Cardiomyocytes Using Organ-On-A-Chip and iPSC Biotechnologies. Cells 2022, 11, 3764. [Google Scholar] [CrossRef] [PubMed]
- Häkli, M.; Jäntti, S.; Joki, T.; Sukki, L.; Tornberg, K.; Aalto-Setälä, K.; Kallio, P.; Pekkanen-Mattila, M.; Narkilahti, S. Human Neurons Form Axon-Mediated Functional Connections with Human Cardiomyocytes in Compartmentalized Microfluidic Chip. Int. J. Mol. Sci. 2022, 23, 3148. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.; Fedele, L.; Chakravarthy, P.; Leonov, V.; Tsansizi, L.; Gu, H.; Seyedmousavi, S.; Cosson, M.-V.; Bernardo, A.S.; Gorelik, J.; et al. Sympathetic neurons can modify the intrinsic structural and functional properties of human pluripotent stem cell-derived cardiomyocytes. J. Physiol. 2025, 603, 2089–2118. [Google Scholar] [CrossRef]
- Charoensook, S.N.; Williams, D.J.; Chakraborty, S.; Leong, K.W.; Vunjak-Novakovic, G. Bioreactor Model of Neuromuscular Junction with Electrical Stimulation for Pharmacological Potency Testing. Integr. Biol. 2017, 9, 956–967. [Google Scholar] [CrossRef]
- Schmid, J.; Schwarz, S.; Meier-Staude, R.; Sudhop, S.; Clausen-Schaumann, H.; Schieker, M.; Huber, R. A Perfusion Bioreactor System for Cell Seeding and Oxygen-Controlled Cultivation of Three-Dimensional Cell Cultures. Tissue Eng. Part C Methods 2018, 24, 585–595. [Google Scholar] [CrossRef]
- Happe, C.L.; Tenerelli, K.P.; Gromova, A.K.; Kolb, F.; Engler, A.J. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol. Biol. Cell 2017, 28, 1950–1958. [Google Scholar] [CrossRef]
- Santhanam, N.; Kumanchik, L.; Guo, X.; Sommerhage, F.; Cai, Y.; Jackson, M.; Martin, C.; Saad, G.; McAleer, C.W.; Wang, Y.; et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials 2018, 166, 64–78. [Google Scholar] [CrossRef]
- Agrawal, G.; Aung, A.; Varghese, S. Skeletal muscle-on-a-chip: An in vitro model to evaluate tissue formation and injury. Lab Chip 2017, 17, 3447–3461. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Araki, H.; Sakata, K.; Tonomura, W.; Hashida, M.; Konishi, S. Microfluidic devices for construction of contractile skeletal muscle microtissues. J. Biosci. Bioeng. 2015, 119, 212–216. [Google Scholar] [CrossRef]
- Visone, R.; Talò, G.; Lopa, S.; Rasponi, M.; Moretti, M. Enhancing all-in-one bioreactors by combining interstitial perfusion, electrical stimulation, on-line monitoring and testing within a single chamber for cardiac constructs. Sci. Rep. 2018, 8, 16944. [Google Scholar] [CrossRef]
- Sleiman, Y.; Reisqs, J.-B.; Reina, B.; Frank, C.; Mohamed, C.; Mohamed, B. Generation of an iPSC cell line (VANYHHi001-A) from a patient with cardiac arrythmias carrying CACNA1D, SCN5A, and DSP variants. Stem Cell Res. 2024, 81, 103608. [Google Scholar] [CrossRef] [PubMed]
| Variant | Effect | Pathology Associated | Reference |
|---|---|---|---|
| A749G | Gain of function | Parkinson | [35,41] |
| G407R | Gain of function | Autism | [42] |
| V401L | Gain of function | Autism | [37] |
| G1169D | Gain of function | Autism | [36] |
| R930H | Loss of function Gain of function | Sinus node Dysfunction Epilepsy | [22] |
| V259D | Gain of function | Sinoatrial dysfunction Deafness (SNND) | [21] |
| G403D | Gain of function | Sinoatrial dysfunction Deafness (SNND) | |
| F747L | Gain of function | Sinoatrial dysfunction Deafness (SNND) | |
| A376V | Loss of function | Sinoatrial dysfunction Deafness (SNND) | [23] |
| I770M | Shift of activation | Atrioventricular dysfunction | [24] |
| Organ | Area | Function | References | |
|---|---|---|---|---|
| Brain | Heart | |||
| Brain | Hippocampus | Neuronal excitability | [43,48] | |
| Striatum | Neuronal excitability | [41,50] | ||
| Hypothalamus Paraventricular nucleus | Undetermined | [52] | ||
| Amygdala | Neuronal excitability | [38,53,54] | ||
| Parabranchial nucleus | Undetermined | [55] | ||
| Brainstem | Undetermined Auditory function | [56,57] | ||
| Heart | Sinoatrial node | Pacemaker function Adrenergic response | [58,59] | |
| Atria | Action Potential shape | [24] | ||
| Atrioventricular node | Electrical conduction | [60] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reisqs, J.-B.; Sleiman, Y.; Cupelli, M.; Boutjdir, M. Role of Cav1.3 Channels in Brain–Heart Interactions: An Unexpected Journey. Biomedicines 2025, 13, 1376. https://doi.org/10.3390/biomedicines13061376
Reisqs J-B, Sleiman Y, Cupelli M, Boutjdir M. Role of Cav1.3 Channels in Brain–Heart Interactions: An Unexpected Journey. Biomedicines. 2025; 13(6):1376. https://doi.org/10.3390/biomedicines13061376
Chicago/Turabian StyleReisqs, Jean-Baptiste, Yvonne Sleiman, Michael Cupelli, and Mohamed Boutjdir. 2025. "Role of Cav1.3 Channels in Brain–Heart Interactions: An Unexpected Journey" Biomedicines 13, no. 6: 1376. https://doi.org/10.3390/biomedicines13061376
APA StyleReisqs, J.-B., Sleiman, Y., Cupelli, M., & Boutjdir, M. (2025). Role of Cav1.3 Channels in Brain–Heart Interactions: An Unexpected Journey. Biomedicines, 13(6), 1376. https://doi.org/10.3390/biomedicines13061376







