Bone Marrow-Derived Inducible Microglia-like Cells Promote Recovery of Chronic Ischemic Stroke Through Modulating Neuroinflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Model of Middle Cerebral Artery Occlusion (MCAO)
2.3. Isolation of Bone Marrow and Induction of BM-iMG Cells from Mice
2.4. Cytokine Assay
2.5. Immunocytochemistry Analysis
2.6. Intracranial Cell Injection of BM-iMG Cells for MCAO Mouse Model
2.7. Behavioral Tests
2.8. Histological Analysis
2.9. Quantitative Polymerase Chain Reaction Analysis
2.10. Measurement of Cerebral Blood Flow
2.11. Statistical Analysis
3. Results
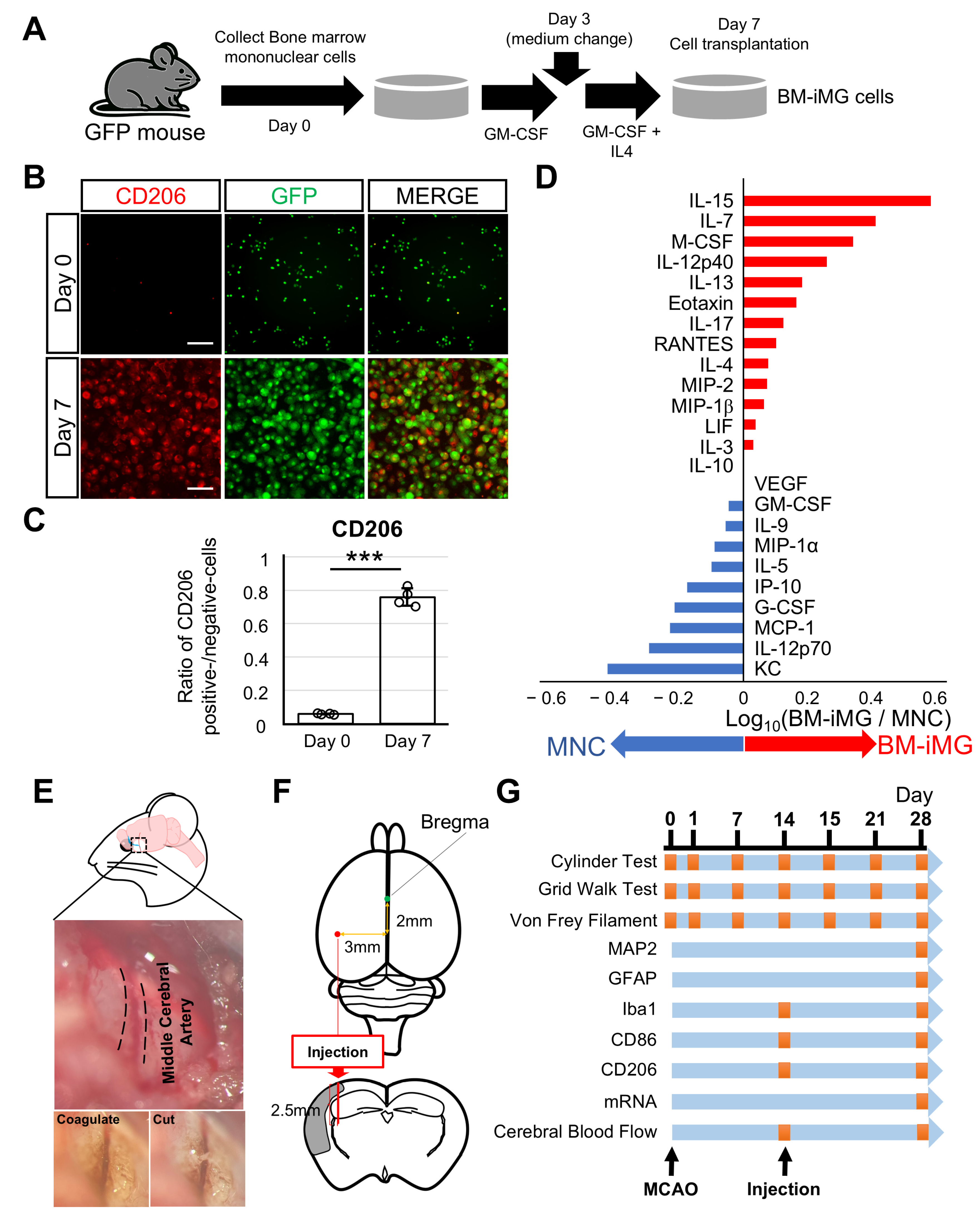
3.1. Induction of Neuroprotective BM-iMG Cells and Their Therapeutic Application in the Chronic MCAO Mouse Model
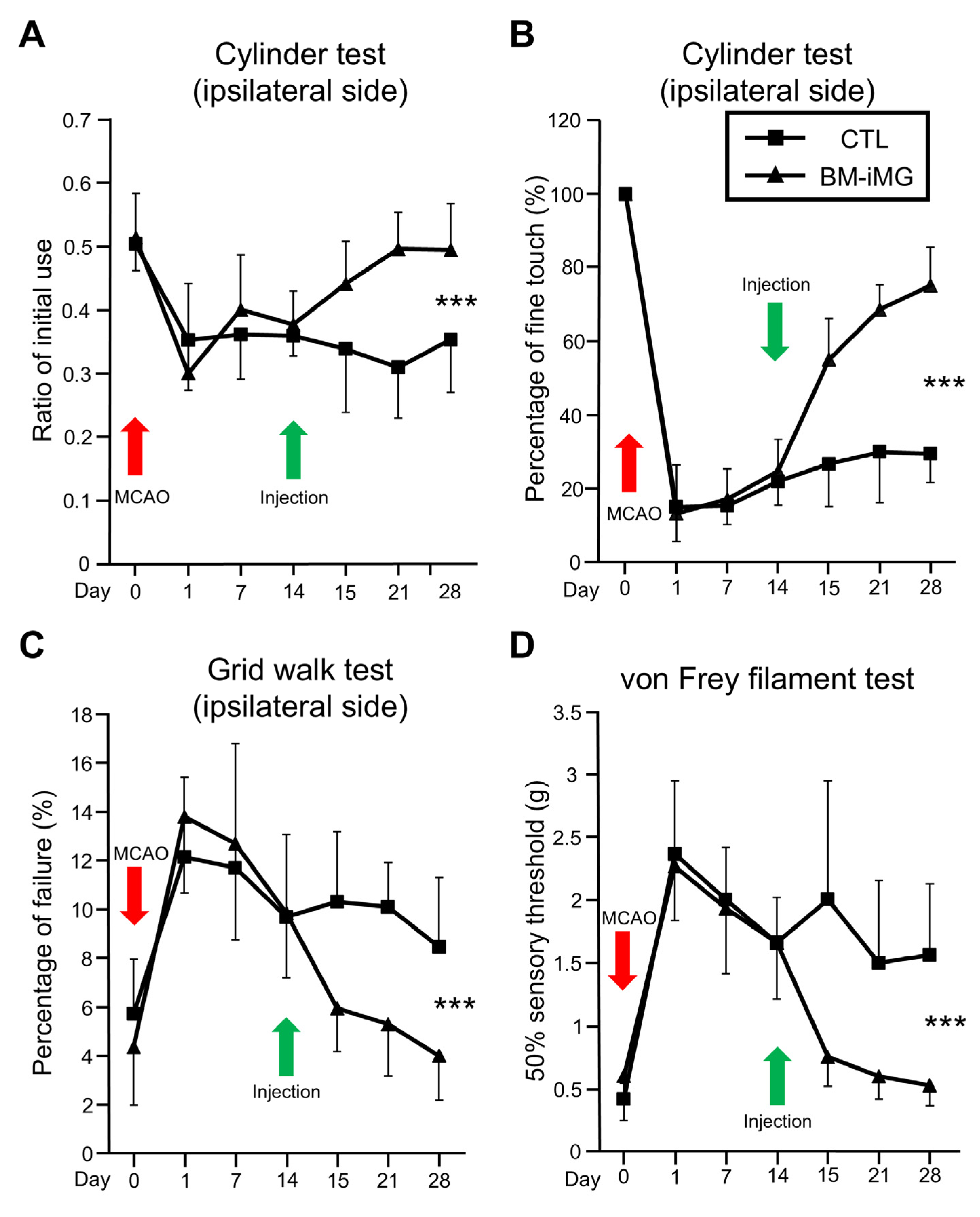
3.2. Effects of Intracranial BM-iMG Cell Injection on Neurobehavioral Function and Brain Volume in Chronic MCAO Mice
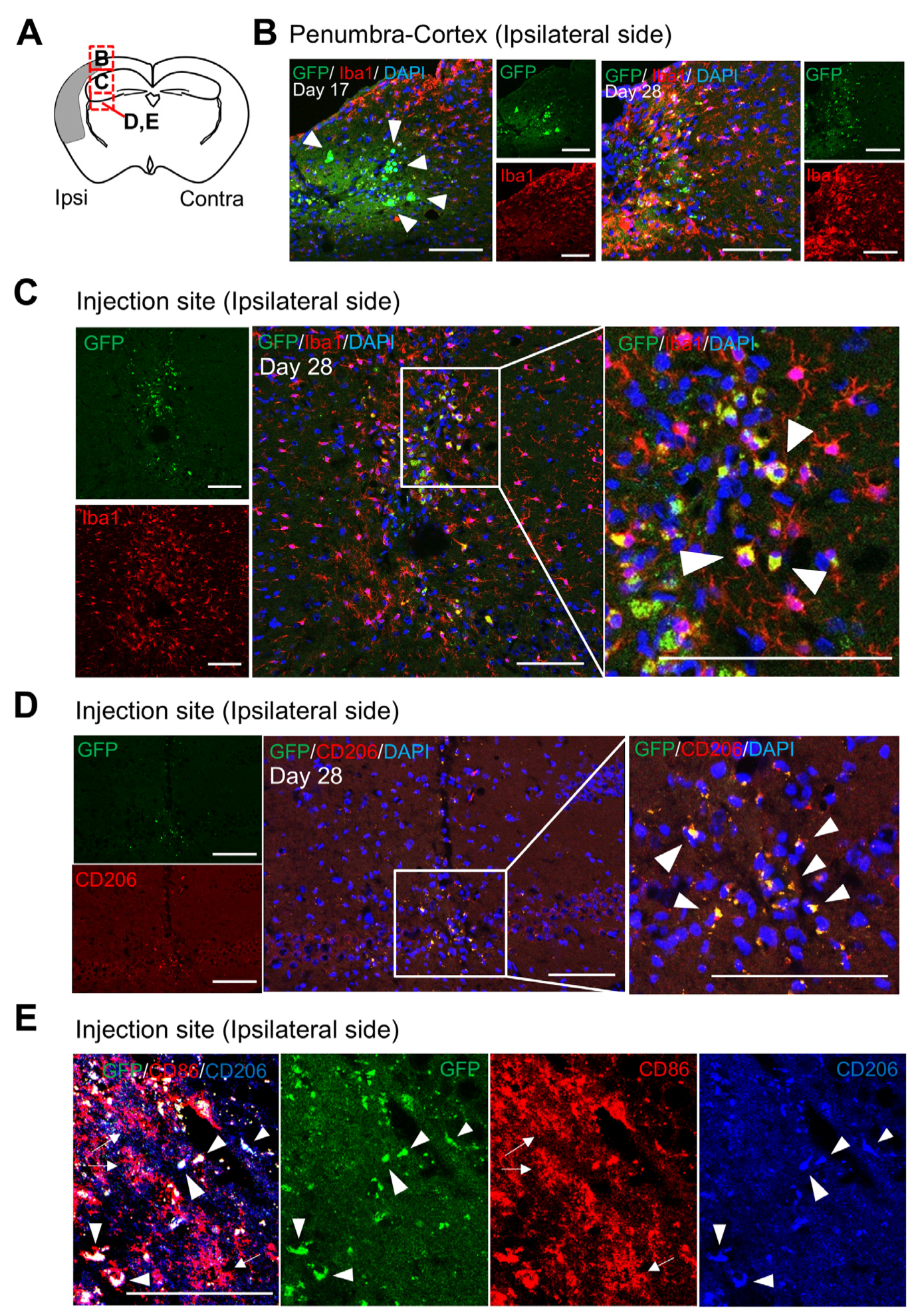
3.3. Localization and Characteristics of BM-iMG Cells Post-Injection in the Infarcted Hemisphere of Chronic MCAO Mice
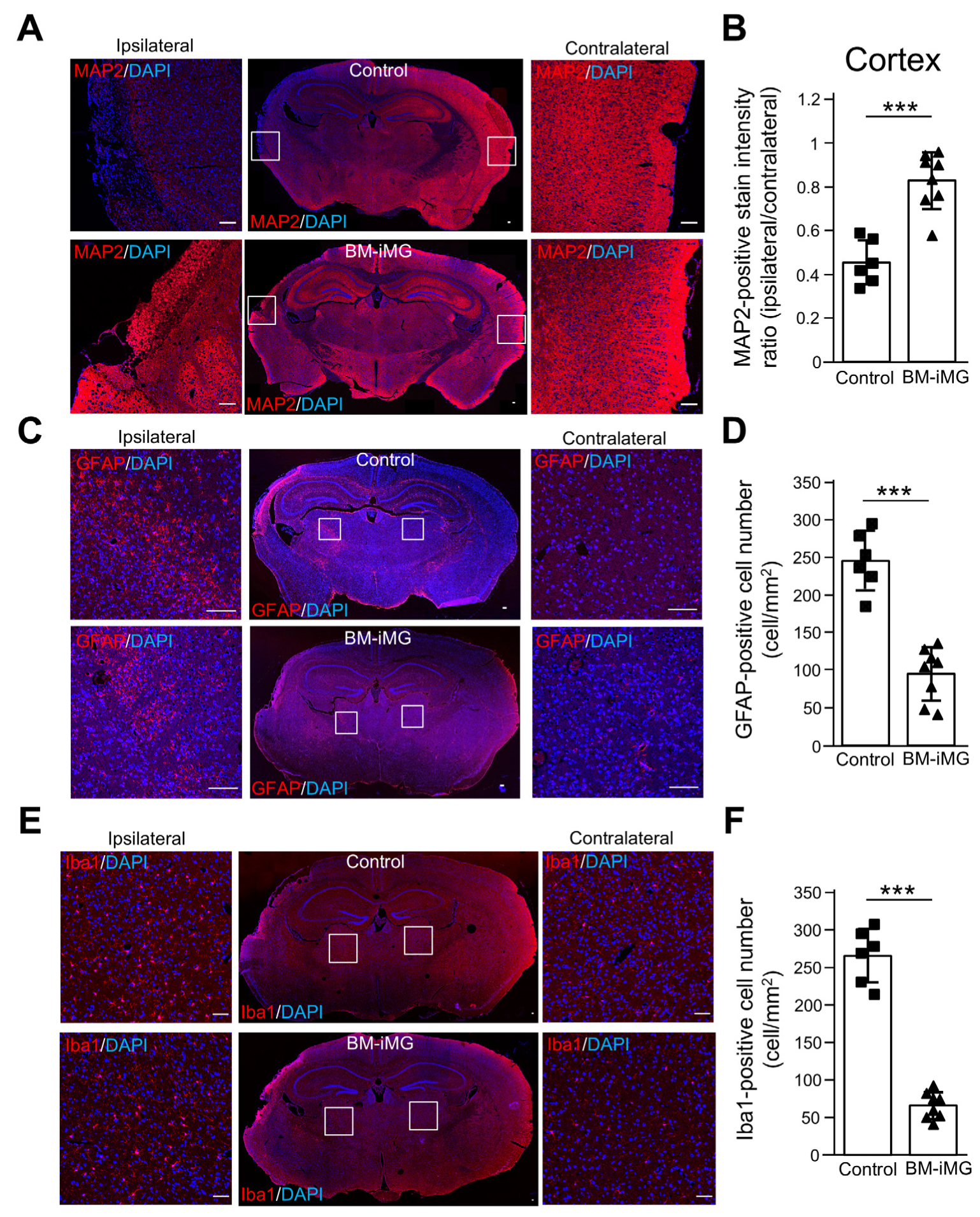
3.4. BM-iMG Cells Suppressed Neuronal Cell Loss and Exerted Anti-Inflammatory Effects by Suppressing Neuroinflammatory Astrocytic and Microglial Response
3.5. Histochemical Characters of BM-iMG Cells After Injection in MCAO Mice
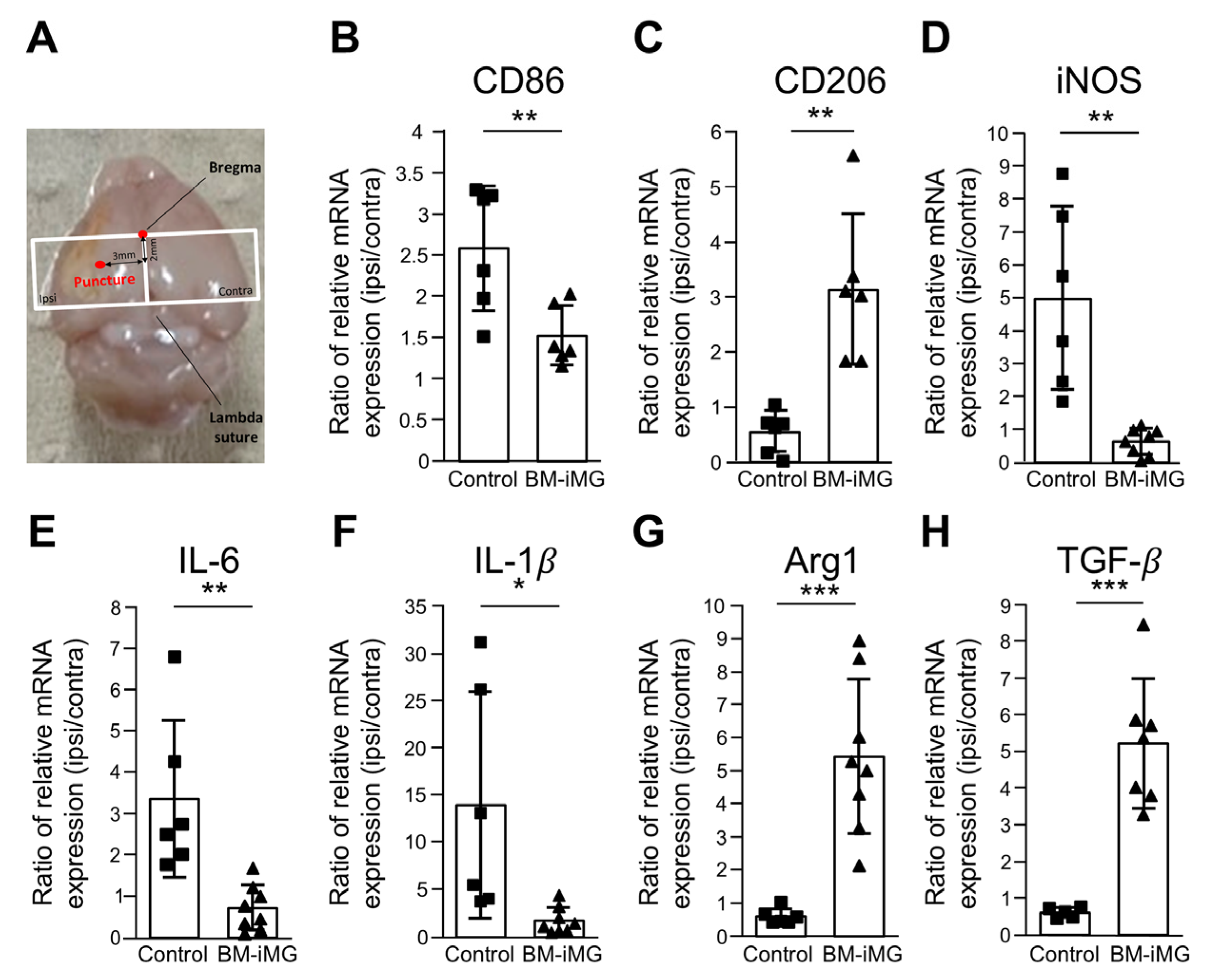
3.6. Administration of BM-iMG Cells Decreased Proinflammatory and Increased Anti-Inflammatory Gene Expression in Chronic Stage of Ischemic Stroke
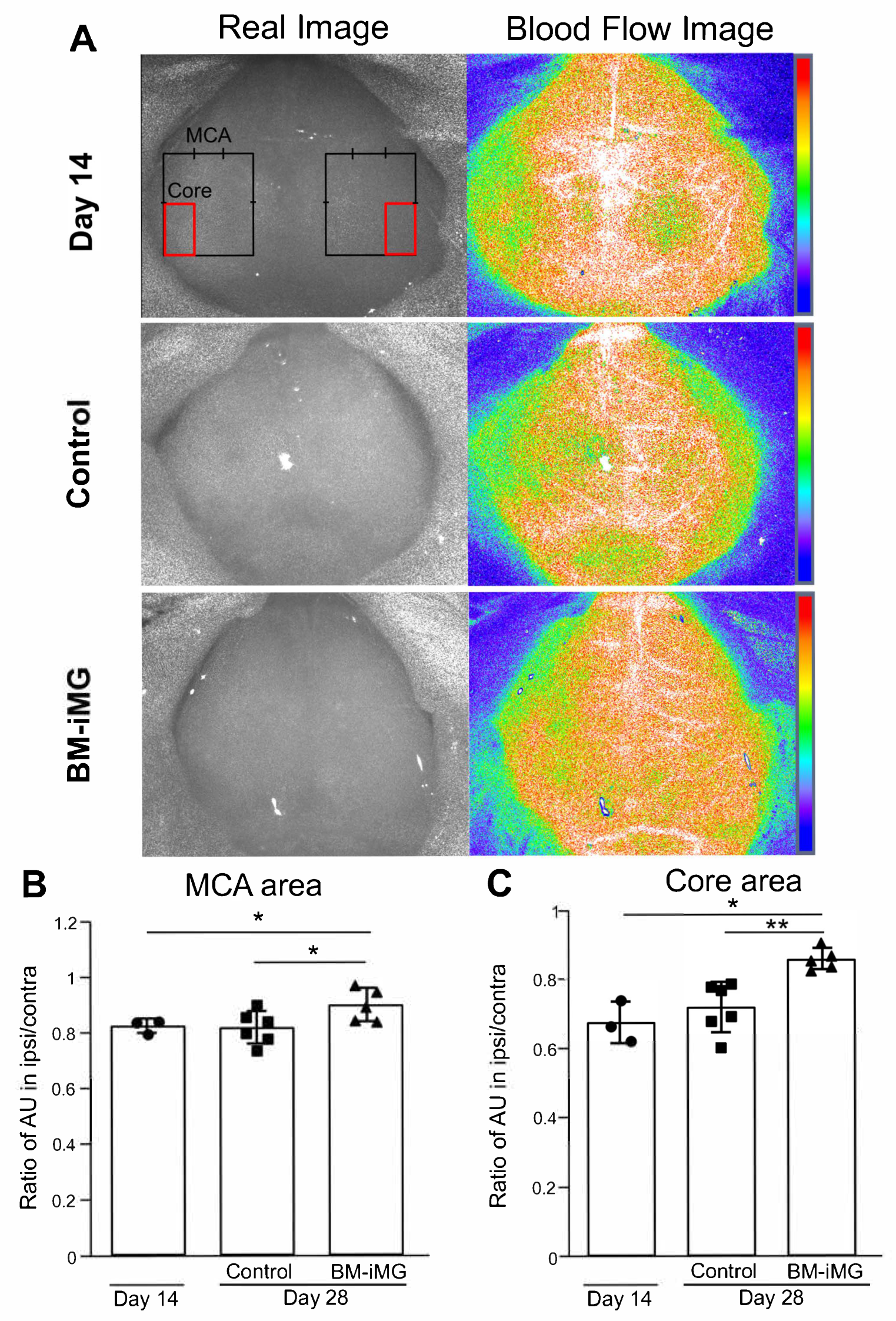
3.7. Improvement in Cerebral Blood Flow During Ischemic Stroke Recovery by Administration of BM-iMG Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| Arg1 | Arginase 1 |
| BM-iMGs | Bone marrow-derived inducible microglia-like cells |
| BM-MNCs | Bone marrow-derived mononuclear cells |
| CBF | Cerebral blood flow |
| EDTA | Ethylenediaminetetraacetic acid |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GFAP | Glial fibrillary acidic protein |
| GFP | Green fluorescence protein |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| MAP2 | Microtubule-associated protein 2 |
| MCAO | Middle cerebral artery occlusion |
| MSCs | Mesenchymal stem cells |
| PBS | Phosphate-buffered saline |
| qPCR | Quantitative polymerase chain reaction |
| SCID | Severe combined immunodeficiency |
| TGF-β | Transforming growth factor-β |
References
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef]
- Di Carlo, A. Human and economic burden of stroke. Age Ageing 2008, 38, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef] [PubMed]
- Emmrich, J.V.; Knauss, S.; Endres, M. Stroke–Global challenges, local solutions, version 1.0: Current advances, challenges, and opportunities in stroke research, management, and care. NAL-live 2021, 2, 1–16. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Thomas, W.E. Brain macrophages: Evaluation of microglia and their functions. Brain Res. Rev. 1992, 17, 61–74. [Google Scholar] [CrossRef]
- Lalancette-Hébert, M.; Gowing, G.; Simard, A.; Weng, Y.C.; Kriz, J. Selective Ablation of Proliferating Microglial Cells Exacerbates Ischemic Injury in the Brain. J. Neurosci. 2007, 27, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, L.; Zhang, S.; Nanda, A.; Li, G. Role of Inflammation and Its Mediators in Acute Ischemic Stroke. J. Cardiovasc. Transl. Res. 2013, 6, 834–851. [Google Scholar] [CrossRef]
- Lai, A.Y.; Todd, K.G. Microglia in cerebral ischemia: Molecular actions and interactions. Can. J. Physiol. Pharmacol. 2006, 84, 49–59. [Google Scholar] [CrossRef]
- Endres, M.; Moro, M.A.; Nolte, C.H.; Dames, C.; Buckwalter, M.S.; Meisel, A. Immune Pathways in Etiology, Acute Phase, and Chronic Sequelae of Ischemic Stroke. Circ. Res. 2022, 130, 1167–1186. [Google Scholar] [CrossRef]
- Pál, G.; Vincze, C.; Renner, É.; Wappler, E.A.; Nagy, Z.; Lovas, G.; Dobolyi, A. Time Course, Distribution and Cell Types of Induction of Transforming Growth Factor Betas following Middle Cerebral Artery Occlusion in the Rat Brain. PLoS ONE 2012, 7, e46731. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Jin, J.-L.; Ge, H.-M.; Yin, K.-L.; Chen, X.; Han, L.-J.; Chen, Y.; Qian, L.; Li, X.-X.; Xu, Y. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARγ-dependent manner. J. Neuroinflammation 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Li, F.; Ma, Q.; Zhao, H.; Wang, R.; Tao, Z.; Fan, Z.; Zhang, S.; Li, G.; Luo, Y. L-3-n-Butylphthalide reduces ischemic stroke injury and increases M2 microglial polarization. Metab. Brain Dis. 2018, 33, 1995–2003. [Google Scholar] [CrossRef]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef]
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of microglia activation and polarization in ischemic stroke. Mol. Med. Rep. 2020, 21, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Goodman, G.W.; Do, T.H.; Tan, C.; Ritzel, R.M. Drivers of Chronic Pathology Following Ischemic Stroke: A Descriptive Review. Cell. Mol. Neurobiol. 2023, 44, 1–15. [Google Scholar] [CrossRef]
- Kobashi, S.; Terashima, T.; Katagi, M.; Urushitani, M.; Kojima, H. Bone marrow-derived inducible microglia-like cells ameliorate motor function and survival in a mouse model of amyotrophic lateral sclerosis. Cytotherapy 2022, 24, 789–801. [Google Scholar] [CrossRef]
- Kitamura, T.; Terashima, T.; Katagi, M.; Ohashi, N.; Nozaki, K.; Tsuji, A. Bone marrow-derived mononuclear cells ameliorate neurological function in chronic cerebral infarction model mice via improvement of cerebral blood flow. Cytotherapy 2023, 25, 1186–1199. [Google Scholar] [CrossRef]
- Taguchi, A.; Kasahara, Y.; Nakagomi, T.; Stern, D.M.; Fukunaga, M.; Ishikawa, M.; Matsuyama, T. A Reproducible and Simple Model of Permanent Cerebral Ischemia in CB-17 and SCID Mice. J. Exp. Stroke Transl. Med. 2010, 3, 28–33. [Google Scholar] [CrossRef]
- Aykan, S.A.; Xie, H.; Zheng, Y.; Chung, D.Y.; Kura, S.; Lai, J.H.; Erdogan, T.D.; Morais, A.; Tamim, I.; Yagmur, D.; et al. Rho-Kinase Inhibition Improves the Outcome of Focal Subcortical White Matter Lesions. Stroke 2022, 53, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Tennant, K.A.; Jones, T.A. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J. Neurosci. Methods 2009, 181, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Roome, R.B.; Vanderluit, J.L. Paw-Dragging: A Novel, Sensitive Analysis of the Mouse Cylinder Test. J. Vis. Exp. 2015, e52701. [Google Scholar] [CrossRef]
- Matsumura, H.; Marushima, A.; Ishikawa, H.; Toyomura, J.; Ohyama, A.; Watanabe, M.; Takaoka, S.; Bukawa, H.; Matsumura, A.; Matsumaru, Y.; et al. Induced Neural Cells from Human Dental Pulp Ameliorate Functional Recovery in a Murine Model of Cerebral Infarction. Stem Cell Rev. Rep. 2021, 18, 595–608. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef]
- Ohshima, M.; Tsuji, M.; Taguchi, A.; Kasahara, Y.; Ikeda, T. Cerebral blood flow during reperfusion predicts later brain damage in a mouse and a rat model of neonatal hypoxic–ischemic encephalopathy. Exp. Neurol. 2012, 233, 481–489. [Google Scholar] [CrossRef]
- Fan, J.; Li, X.; Yu, X.; Liu, Z.; Jiang, Y.; Fang, Y.; Zong, M.; Suo, C.; Man, Q.; Xiong, L. Global Burden, Risk Factor Analysis, and Prediction Study of Ischemic Stroke, 1990–2030. Neurology 2023, 101, E137–E150. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Rege, S.V.; Wang, M.; Si, G.; Zhou, Y.; Wang, S.; Griffin, J.H.; A Goldman, S.; Zlokovic, B.V. 3K3A–activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat. Med. 2016, 22, 1050–1055. [Google Scholar] [CrossRef]
- Gómez-Nicola, D.; Valle-Argos, B.; Pallas-Bazarra, N.; Nieto-Sampedro, M. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol. Biol. Cell. 2011, 22, 1960–1970. [Google Scholar] [CrossRef]
- I Mhairi, M. New models of focal cerebral ischaemia. Br. J. Clin. Pharmacol. 1992, 34, 302–308. [Google Scholar] [CrossRef]
- Ng, Y.S.; Stein, J.; Ning, M.; Black-Schaffer, R.M. Comparison of Clinical Characteristics and Functional Outcomes of Ischemic Stroke in Different Vascular Territories. Stroke 2007, 38, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Fluri, F.; Schuhmann, M. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J. Animal models of stroke. Anim. Model. Exp. Med. 2021, 4, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Braeuninger, S.; Kleinschnitz, C. Rodent models of focal cerebral ischemia: Procedural pitfalls and translational problems. Exp. Transl. Stroke Med. 2009, 1, 8. [Google Scholar] [CrossRef]
- Macrae, I. Preclinical stroke research–advantages and disadvantages of the most common rodent models of focal ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef]
- Nishie, H.; Nakano-Doi, A.; Sawano, T.; Nakagomi, T. Establishment of a Reproducible Ischemic Stroke Model in Nestin-GFP Mice with High Survival Rates. Int. J. Mol. Sci. 2021, 22, 12997. [Google Scholar] [CrossRef]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren-Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef]
- Lam, J.; Lowry, W.E.; Carmichael, S.T.; Segura, T. Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Adv. Funct. Mater. 2014, 24, 7053–7062. [Google Scholar] [CrossRef]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef]
- Liesz, A.; Hu, X.; Kleinschnitz, C.; Offner, H. Functional Role of Regulatory Lymphocytes in Stroke. Stroke 2015, 46, 1422–1430. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, D.; Guo, C.; Liu, M.; Tao, Y.; Zhou, J.; Wang, F.; Zhao, Y.; Wei, J.; Zhang, Y.; et al. Berberine Facilitates Angiogenesis Against Ischemic Stroke Through Modulating Microglial Polarization via AMPK Signaling. Cell. Mol. Neurobiol. 2019, 39, 751–768. [Google Scholar] [CrossRef]
- Huang, M.; Wan, Y.; Mao, L.; He, Q.; Xia, Y.; Li, M.; Li, Y.; Jin, H.; Hu, B. Inhibiting the Migration of M1 Microglia at Hyperacute Period Could Improve Outcome of tMCAO Rats. CNS Neurosci. Ther. 2016, 23, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, J.; Hu, X.; Pu, H.; Shi, Y.; Hassan, S.H.; Xu, M.; Leak, R.K.; Stetler, R.A.; Gao, Y.; Chen, J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp. Neurol. 2015, 272, 109–119. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/Macrophage Polarization Dynamics Reveal Novel Mechanism of Injury Expansion After Focal Cerebral Ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Wysoczynki, M.; Khan, A.; Bolli, R. New Paradigms in Cell Therapy. Circ. Res. 2018, 123, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Jeong, C.H.; Jun, J.A.; Kim, S.M.; Ryu, C.H.; Hou, Y.; Oh, W.; Chang, J.W.; Jeun, S.-S. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res. Ther. 2011, 2, 38. [Google Scholar] [CrossRef]
- Hurst, R.W.; Bosch, E.P.; Morris, J.M.; Dyck, P.J.; Reeves, R.K. Inflammatory hypertrophic cauda equina following intrathecal neural stem cell injection. Muscle Nerve 2013, 48, 831–835. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow–Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef]
- Chen, D.-C.; Lin, S.-Z.; Fan, J.-R.; Lin, C.-H.; Lee, W.; Lin, C.-C.; Liu, Y.-J.; Tsai, C.-H.; Chen, J.-C.; Cho, D.-Y.; et al. Intracerebral Implantation of Autologous Peripheral Blood Stem Cells in Stroke Patients: A Randomized Phase II Study. Cell Transplant. 2014, 23, 1599–1612. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 628940. [Google Scholar] [CrossRef]
- Ahnstedt, H.; Patrizz, A.; Chauhan, A.; Roy-O’Reilly, M.; Furr, J.W.; Spychala, M.S.; D’Aigle, J.; Blixt, F.W.; Zhu, L.; Alegria, J.B.; et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav. Immun. 2020, 87, 556–567. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, B.N.; Kitamura, T.; Kobashi, S.; Urushitani, M.; Terashima, T. Bone Marrow-Derived Inducible Microglia-like Cells Promote Recovery of Chronic Ischemic Stroke Through Modulating Neuroinflammation in Mice. Biomedicines 2025, 13, 1347. https://doi.org/10.3390/biomedicines13061347
Nguyen BN, Kitamura T, Kobashi S, Urushitani M, Terashima T. Bone Marrow-Derived Inducible Microglia-like Cells Promote Recovery of Chronic Ischemic Stroke Through Modulating Neuroinflammation in Mice. Biomedicines. 2025; 13(6):1347. https://doi.org/10.3390/biomedicines13061347
Chicago/Turabian StyleNguyen, Bach Ngoc, Tomoaki Kitamura, Shuhei Kobashi, Makoto Urushitani, and Tomoya Terashima. 2025. "Bone Marrow-Derived Inducible Microglia-like Cells Promote Recovery of Chronic Ischemic Stroke Through Modulating Neuroinflammation in Mice" Biomedicines 13, no. 6: 1347. https://doi.org/10.3390/biomedicines13061347
APA StyleNguyen, B. N., Kitamura, T., Kobashi, S., Urushitani, M., & Terashima, T. (2025). Bone Marrow-Derived Inducible Microglia-like Cells Promote Recovery of Chronic Ischemic Stroke Through Modulating Neuroinflammation in Mice. Biomedicines, 13(6), 1347. https://doi.org/10.3390/biomedicines13061347






