Abstract
Background/Objectives: The clinical forms of coronavirus disease 2019 (COVID-19) vary widely in severity, ranging from asymptomatic or moderate cases to severe pneumonia that can lead to acute respiratory failure, acute respiratory distress syndrome, multiple organ dysfunction syndrome, and death. Our main objective was to determine the prevalence of bacterial and fungal secondary infections in an intensive care unit (ICU). Secondary objectives included analyzing the impact of these infections on mortality and medical resource utilization, as well as assessing antimicrobial resistance in this context. Methods: We conducted a retrospective cohort study that included critically ill severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients treated in an ICU and analyzed the prevalence of co-infections and superinfections. Results: A multivariate analysis of mortality found that the presence of superinfections increased the odds of death by more than 15-fold, while the Sequential Organ Failure Assessment (SOFA) score and C-reactive protein (adjusted for confounders) increased the odds of mortality by 51% and 13%, respectively. The antibiotic resistance profile of microorganisms indicated a high prevalence of resistant strains. Carbapenems, glycopeptides, and oxazolidinones were the most frequently used classes of antibiotics. Among patients, 27.9% received a single antibiotic, 47.5% received two from different classes, and 24.4% were treated with three or more. Conclusions: The incidence and spectrum of bacterial and fungal superinfections are higher in critically ill ICU patients, leading to worse outcomes in COVID-19 cases. Multidrug-resistant pathogens present significant challenges for ICU and public health settings. Early screening, accurate diagnosis, and minimal use of invasive devices are essential to reduce risks and improve patient outcomes.
1. Introduction
Since the identification of the first case of coronavirus disease 2019 (COVID-19) in Wuhan, China, in December 2019, a total of 772,166,517 confirmed cases, 6,981,263 deaths, and 13,595,583,125 vaccine doses administered had been reported by December 2023 [1,2]. Clinical forms of COVID-19 vary significantly in severity, ranging from asymptomatic or moderate cases to severe types that require hospitalization in intensive care units (ICUs) and may necessitate invasive or noninvasive mechanical ventilation [3]. Acute respiratory failure (ARF) often progresses to acute respiratory distress syndrome (ARDS) or multiorgan dysfunction syndrome (MODS), which can ultimately lead to death [4]. The mortality rate of COVID-19 patients in ICUs during the initial months of the pandemic ranged from 40% to 45%, depending on the ICU level, the use of mechanical ventilation, and the characteristics of the study population [5]. Key risk factors for mortality among COVID-19 patients in ICUs include advanced age, male gender, hematologic and oncologic malignancies, and secondary bacterial infections [6]. Given the critical condition of ICU patients and the presence of these risk factors, they are highly susceptible to infections and superinfections due to their compromised immune status and the invasive therapeutic procedures they undergo [7]. The incidence of superinfections in COVID-19 patients in ICU settings varies widely, from 9.3% [8] to 86.6% [9], depending on geographical factors and the population studied. While many studies have investigated COVID-19-related superinfections, data on their prevalence and impact in Romania remain limited. This variability highlights the need to understand the specific risks faced by ICU patients, particularly since the risk of ventilator-associated pneumonia in COVID-19 patients is estimated to range from 31% to 45.4% [10]. Bacterial and fungal superinfections pose significant complications for critically ill patients with viral respiratory infections, resulting in increased morbidity and mortality [11,12]. Superinfections are particularly common among ICU patients, with incidence rates reaching as high as 45% [13,14,15].
Among the most common infectious complications are respiratory infections, including pneumonia, which affect more than 50% of ICU patients, followed by bloodstream infections (BSIs), which occur in up to 34% of cases [16]. These complications are exacerbated by the need for invasive procedures such as orotracheal intubation, tracheostomy, and the placement of catheters (peripheral, central venous, dialysis, feeding, and urinary), all of which increase the risk of superinfection.
In addition to these procedural risks, critically ill patients are often immunocompromised, have multiple comorbidities, and undergo various treatments, including anti-inflammatory, antibiotic, antiviral, immunomodulatory, and corticosteroid therapies. All these factors increase their susceptibility to bacterial and fungal infections [17,18]. This increased vulnerability is of particular concern in the case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), where community-acquired bacteria, such as Streptococcus pneumoniae, Haemophilus influenzae, or Staphylococcus aureus, frequently cause co-infections. Additionally, hospital-acquired superinfections are often driven by multidrug-resistant (MDR) bacteria and fungi, which pose a significant challenge in treating critically ill patients [4,19].
The empirical use of broad-spectrum antibiotics in these patients also exacerbates the problem, increasing the likelihood of microorganisms developing antibiotic resistance [20,21]. This contributes to poorer patient outcomes, as co-infections and superinfections significantly worsen the prognosis. Although respiratory failure caused by SARS-CoV-2 remains the primary cause of death in this patient group, these secondary infections increase morbidity and mortality and further strain healthcare systems by escalating treatment costs [22]. Secondary bacterial infections, often influenced by factors such as the duration of intubation, ICU stay, and catheter insertion, complicate the clinical course [23]. Additionally, the use of corticosteroids and anti-cytokine therapies affects the immune response and facilitates the development of these secondary infections [24].
Given the complexity of managing bacterial complications in critically ill COVID-19 patients and the growing concern over bacterial resistance profiles, it is crucial to investigate the impact of these infections further. In this light, we aimed to retrospectively analyze these factors in critically ill patients admitted to the COVID ICU of Mureș County Clinical Hospital (MCCH) to better understand the prevalence and implications of such infections within this population.
Our primary objective was to determine the prevalence of bacterial and fungal secondary infections in critically ill COVID-19 patients. Secondary objectives included evaluating the impact of these infections on mortality and healthcare resource utilization and assessing antimicrobial resistance patterns in this context.
2. Materials and Methods
2.1. Hospital Characteristics
MCCH, located in the central region of Romania, is a tertiary care facility with a total capacity of 1181 beds. Approximately 4% of these beds are allocated to the ICU, which includes 26 beds for general intensive care and 18 beds in the intermediate ICU. In February 2020, by order number 555 of the Romanian Ministry of Health, the hospital was officially designated as a COVID-19 facility, permitting the admission of patients with confirmed SARS-CoV-2 infections and those requiring medical and surgical emergencies. During the second and third waves of the COVID-19 pandemic, a provisional satellite ICU was established under the coordination of the Mureș Clinical Emergency Hospital. However, our study focused exclusively on patients admitted to the general ICU of MCCH during its designation as a COVID-19 facility. This reorganization resulted in a 50% reduction in incoming patients during 2020–2021 compared with previous years.
2.2. Study Design
We conducted a retrospective cohort study that included critically ill patients requiring treatment in the ICU who had a positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) test upon admission to the ICU. We collected and analyzed data from 344 patients diagnosed with ARF due to SARS-CoV-2 infection admitted to the ICU between 1 March 2021 and 28 February 2022.
The criteria for inclusion in this study were
- Positive SARS-CoV-2 RT-PCR test;
- Severe form of COVID-19;
- ARDS;
- Required mechanical ventilation or other intensive care support;
- Admission between 1 March 2021, and 28 February 2022.
2.3. Data Collection
We created a database that includes demographic data (age, sex, and urban or rural background), number of ICU hospitalization days, comorbidities, type of ventilatory support provided, number of hours of mechanical ventilation, and Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores during the first 24 h of ICU admission. We also recorded microbiological data (type of sample collected, identified pathogen, and antibiogram), antibiotic and antifungal treatments administered to patients with infections, immunomodulatory treatments provided (anakinra and tocilizumab), corticosteroid therapy, organ dysfunctions during hospitalization, and patient status at discharge (alive or deceased). The biological samples we studied included blood, urine, pharyngeal secretions, tracheal or bronchial secretions, and stool. All samples were analyzed using standard cultures, although a lack of uniformity may affect data comparability. The variables selected for analysis were based on the prior literature, clinical relevance, and statistical significance in a univariate analysis. Incomplete reports and data heterogeneity may have impacted the retrospective data collection.
We divided patients into the following groups:
Group I—SARS-CoV-2 patients with associated infections, with three subgroups:
- Subgroup IA—COVID-19 with bacterial superinfection;
- Subgroup IB—COVID-19 with fungal superinfection;
- Subgroup IC—COVID-19 with fungal and bacterial superinfections.
Group II—SARS-CoV-2 patients without concurrent infections.
2.4. Definitions
Infection diagnosis was based on clinical symptoms and the isolation of pathogenic microorganisms. SARS-CoV-2 infection was diagnosed from clinical symptoms and confirmed by RT-PCR. ICU admission was defined by the need for mechanical ventilation due to ARF, the presence of one or more organ dysfunctions, or septic or other shock in a confirmed COVID-19 patient. Healthcare-associated respiratory infections were categorized as hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP). HAP is pneumonia occurring 48 h or more after hospital admission, without signs of incubation at admission. In contrast, VAP develops more than 48 h after endotracheal intubation [20,25].
Depending on the infection’s location, the types of infections were defined as follows:
- Respiratory infection was defined, according to guidelines, as the growth of pathogenic microorganisms in bronchial aspirate or sputum in patients with clinical and radiological signs of infection. Bronchoalveolar lavage was not routinely used due to its limited application in our unit.
- BSI was identified by the isolation of bacteria or fungi in one or more blood cultures.
- Catheter-associated urinary tract infections (UTIs) occurred more than 48 h after urinary bladder catheterization. All patients in the study were catheterized [26].
- Clostridioides difficile infection (CDI) was defined as the presence of three or more unformed diarrheal stools within 24 h and positive tests for Clostridioides difficile toxins A and B [27].
2.5. Statistical Analysis
The collected data were compiled in a Microsoft Excel 2016 database. Qualitative data were coded using a binary system, and both absolute and relative frequencies were calculated for each category. Histograms were used to describe quantitative data, and the mean, median, standard deviation (SD) with 95% confidence interval (CI), interquartile range (IQR), and minimum and maximum values were reported. Associations between qualitative variables were analyzed using the chi-squared test (if all the contingency table values exceeded 10), the chi-squared test with Yates’s correction (if any value was between 5 and 10), or Fisher’s exact test (if any value was below 5). Odds ratios (ORs) were also calculated.
Numerical variables were tested for a parametric (Gaussian) distribution using the Shapiro–Wilk test. Variables with a non-Gaussian distribution were reported as median and IQR, and those with a Gaussian distribution were reported as mean ± SD.
To compare numerical values between two groups, we used the t-test for parametric data and the Mann–Whitney U test for non-parametric data. ANOVA was applied to compare more than two groups when the data had a parametric distribution; otherwise, the Kruskal–Wallis test was used. Post hoc pairwise comparisons were performed using Dunn’s test with Bonferroni correction.
Binary logistic regression was used to develop a model for predicting mortality in patients with superinfections. The model’s predictive capacity was assessed using IBM SPSS 26 with a cutoff value of 0.5, and its goodness of fit was validated using the Hosmer–Lemeshow test.
Data were statistically analyzed using IBM SPSS Statistics 29. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Patient Data
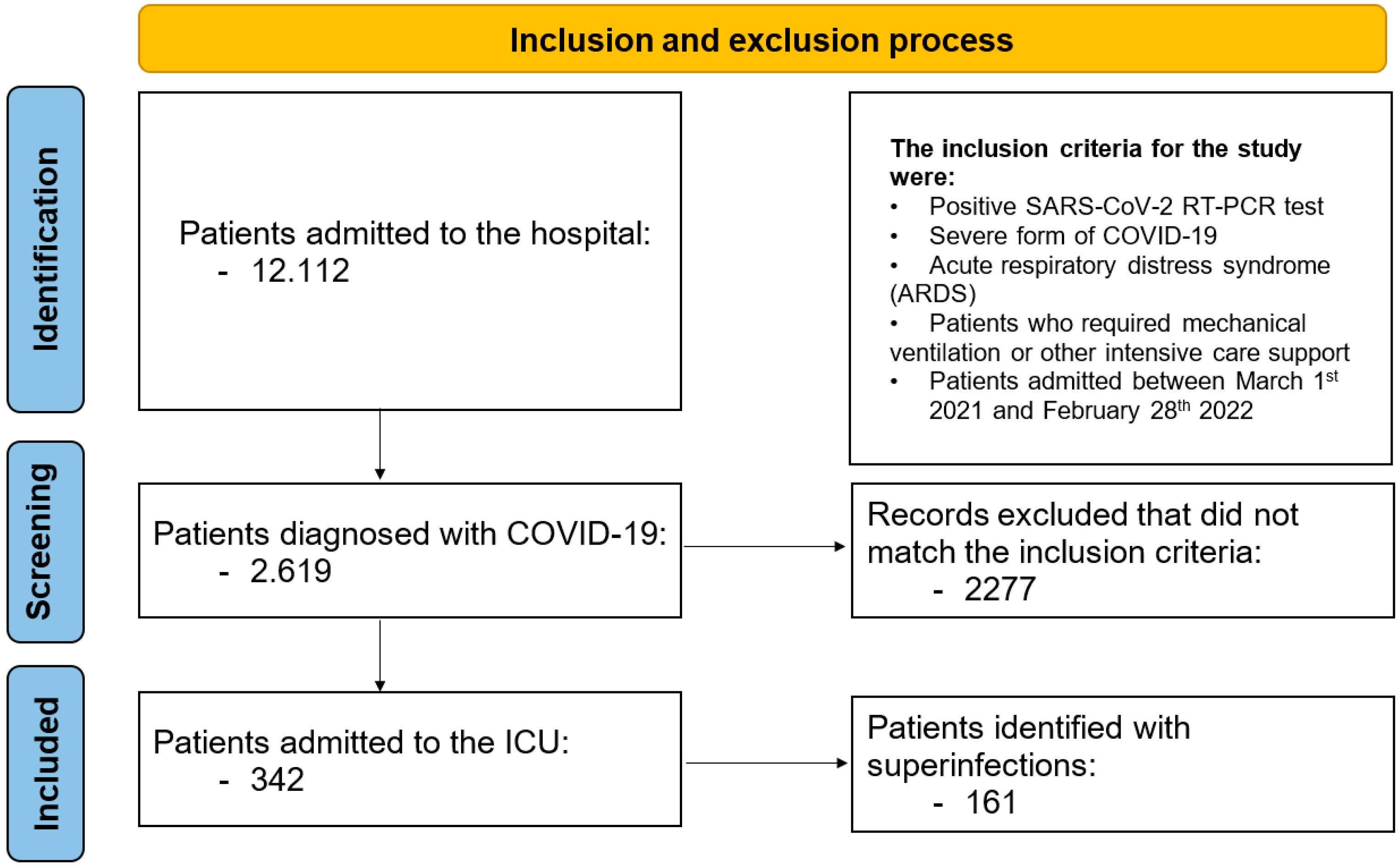
During the monitored period, 12,112 patients were admitted to the hospital, of whom 2619 were diagnosed with COVID-19. Among the COVID-19 patients, 342 (13.0%) were admitted to the ICU; their general characteristics are presented in Table 1. We identified 161 (47.0%) patients with bacterial, fungal, or combined (bacterial and fungal) superinfections not limited to the respiratory tract. Patients in Group I (with superinfections) had a median age of 72 years (IQR: 63.5–79); 78 patients (48%) were male, and 80 (49.6%) resided in rural areas. The median (IQR) APACHE II score at admission was 22.3 ± 6.3, and the median (IQR) SOFA score was 10 (8–11). A flowchart of patient inclusion is presented in Figure 1.

Table 1.
Characteristics of COVID-19 patients regarding superinfections.
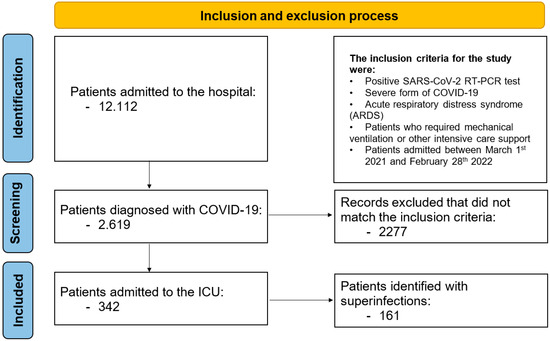
Figure 1.
Inclusion and exclusion process.
The characteristics of patients in Groups IA, IB, and IC are shown in Table 2. We identified 80 patients (49.6%) with bacterial superinfections (Group IA), 24 (14.9%) with fungal superinfections (Group IB), and 57 (35.4%) with both types (Group IC). The median (IQR) ages of patients in Groups IA, IB, and IC were 73.5 (64–79), 70 (59.25–78), and 73 (64–79) years, respectively.

Table 2.
Characteristics of COVID-19 patients with bacterial and/or fungal superinfections.
The median (IQR) APACHE II scores were 21 (17–27) in Group IA, 26 (19–30) in Group IB, and 22.5 (17.75–25) in Group IC. The corresponding SOFA scores were 10 (8–11), 13 (9–15), and 10 (8–11.5), respectively.
A statistically significant difference in ICU length of stay was observed among the three patient groups (p = 0.016). Post hoc pairwise comparisons with Bonferroni correction revealed a statistically significant difference between Groups IA and IC (p = 0.018).
Mechanical ventilation hours also differed significantly between groups (p = 0.011), with significant differences between Groups IB and IC (p = 0.04) and Groups IA and IC (p = 0.03).
The ferritin levels at admission were significantly different among groups (p = 0.02), with a significant post hoc difference between Groups IA and IC.
No significant statistical associations were found between mortality and corticosteroid administration or the type of superinfection.
3.2. The Prevalence of Superinfections in COVID-19 Patients
Table 2 illustrates the prevalence of bacterial and fungal superinfections. As shown, 47% of hospitalized patients experienced at least one infection during their ICU stay. Patients over the age of 50 had a statistically significant risk of superinfection (bacterial, fungal, or CDI; p = 0.036). Patients requiring ICU care for more than 10 days also faced a considerable risk of superinfection (p < 0.001). Additionally, patients with MODS had an elevated risk of superinfection (p < 0.001).
The mortality rate among COVID-19 patients with superinfections was 87.6%, compared with 68% among those without superinfections (p < 0.001; OR = 3.3244). Corticosteroid therapy, administered at a dose of 6 mg/day for 10 days according to therapeutic guidelines, did not significantly increase the incidence of superinfections (p = 0.07). Immunomodulatory therapy with anakinra and tocilizumab also did not significantly raise the risk of infection (p = 0.750 and p = 0.211, respectively).
Ferritin levels at admission were significantly higher in patients with superinfections (p = 0.049).
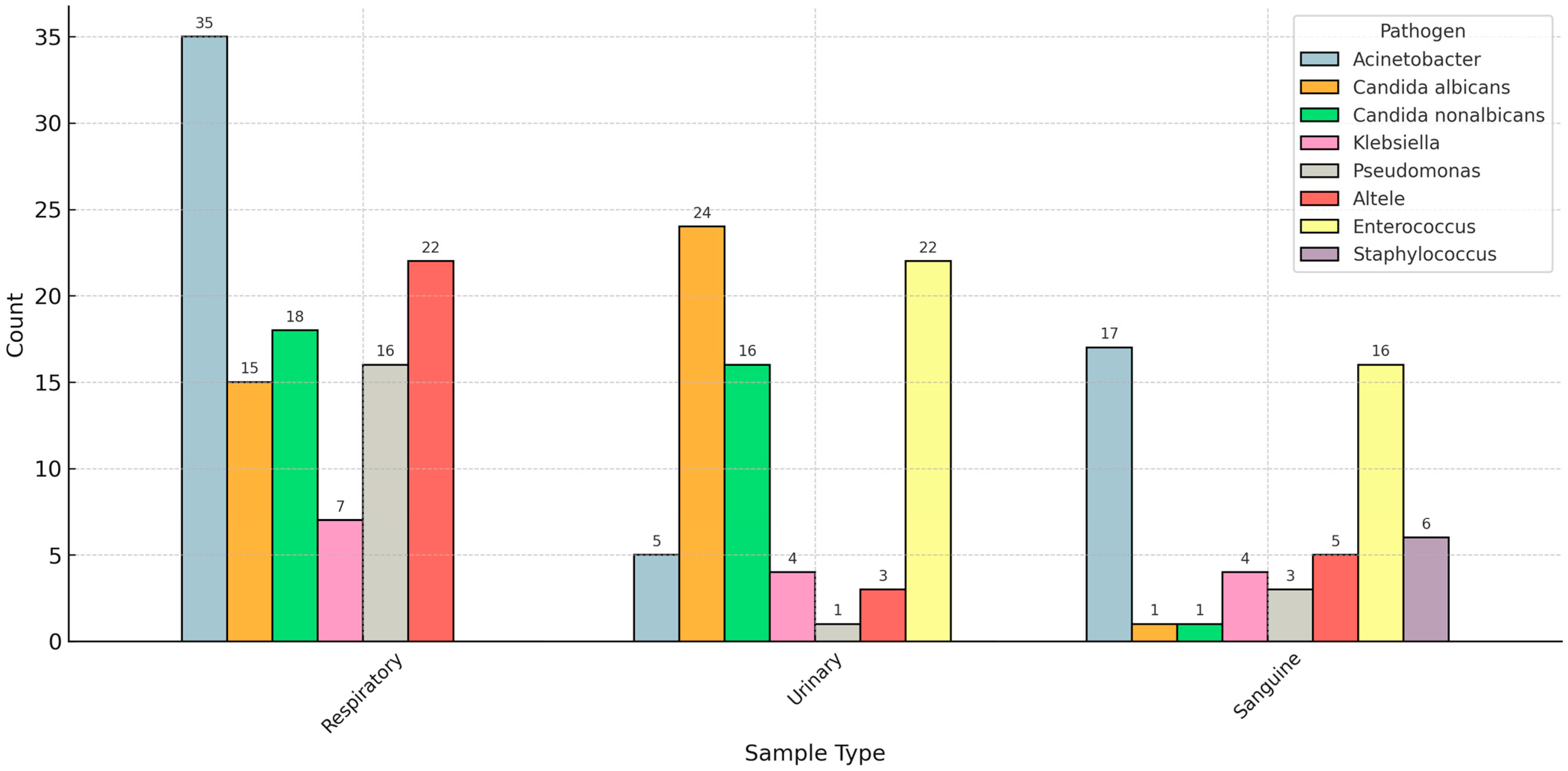
We analyzed the distribution of pathogen species based on the sample type used to diagnose the superinfection. Acinetobacter species was the most common bacterial pathogen in respiratory and bloodstream superinfections. In contrast, Candida (albicans and non-albicans) was the most prevalent fungal infection in respiratory and urinary samples (Figure 2).
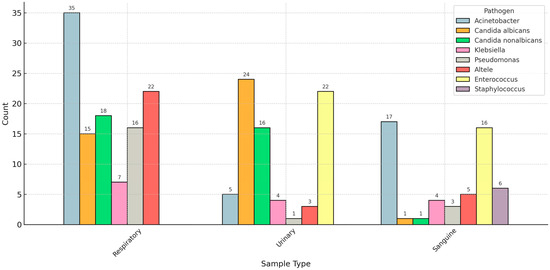
Figure 2.
Distribution of pathogens based on the sample type.
3.3. Classification of Infections and Their Incidence
Data on the classification of superinfections are presented in Table S1 (Supplementary Materials). Specifically, HAP was observed in 23 patients (14.2%), and VAP was diagnosed in 52 patients (23.1%) receiving mechanical ventilation. BSIs occurred in 40 patients (24.8%), and UTIs were identified in 63 patients (39.1%).
3.4. The Prevalence of Germs Involved in Superinfections
3.4.1. Microorganisms Involved in HAP
The most frequently implicated pathogens in HAP were non-albicans Candida species (23.33%), Acinetobacter species (23.2%), Candida albicans (13.3%), and Klebsiella species (10%). These details are summarized in Table 3.

Table 3.
Hospital-acquired pneumonia (HAP).
3.4.2. Ventilator-Associated Pneumonia (VAP)
The pathogens most frequently associated with VAP in COVID-19 patients were Acinetobacter spp. (33.7%), Pseudomonas aeruginosa (16.8%), Candida albicans (13.2%), and non-albicans Candida spp. (13.2%), as presented in Table 4.

Table 4.
Ventilator-associated pneumonia.
3.4.3. Prevalence of Pathogens Isolated in Urine
The most common pathogens isolated from the urine of COVID-19 patients were Candida albicans (24 patients, 32%), Enterococcus spp. (22, 29.3%), and non-albicans Candida spp. (16, 21.3%) (Table 5).

Table 5.
Urine-isolated pathogens.
3.4.4. Prevalence of Pathogens Isolated in Blood
The most common microorganisms isolated from blood cultures were Acinetobacter spp. (17 patients, 32%), Enterococcus spp. (16, 30.1%), coagulase-negative Staphylococcus spp. (6, 11.3%), Klebsiella pneumoniae (4, 7.5%), and Pseudomonas aeruginosa (3, 5.6%) (Table 6).

Table 6.
Blood-isolated pathogens (blood infection).
3.5. Global Analysis of Isolated Pathogens
The microorganisms were isolated from the following pathological samples in order of incidence: bronchial aspirate, 62 strains; blood, 40 strains; urine, 38 strains; feces, 37 strains; and sputum, 5 pathological strains. Fungi were isolated from the following pathological samples: bronchial aspirate, 41 strains; urine, 39 strains; pharyngeal exudate, 9 strains; and blood, 2 strains. Absolute and relative distributions are presented in Table 7. The highest incidences were noted for Acinetobacter spp. (26.4%), Enterococcus faecium (17.8%), Clostridioides difficile (16.8%), and Pseudomonas aeruginosa (13.2%).

Table 7.
Global analysis of isolated germs.
3.6. Analysis of Antibiotic-Resistant Pathogens
The antibiotic resistance profile of the microorganisms (Table 8) shows a high percentage of strains resistant to one or more antibiotics. Specifically, 34 (30.0%) strains of Acinetobacter baumannii and 20 (17.7%) strains of Acinetobacter junii were identified as MDR. In addition, 17 (15.0%) strains of Klebsiella pneumoniae exhibited multiple resistance mechanisms, including carbapenemase production, extended-spectrum beta-lactamase (ESBL) activity, and MDR. Similarly, 17 (15.0%) strains of Pseudomonas aeruginosa were classified as MDR.

Table 8.
Analysis of pathogen resistance.
3.7. Antibiotics Administered to Patients with COVID-19 Superinfections
Antibiotics from the carbapenem, glycopeptide, and oxazolidinone classes were the most frequently used. Of the patients, 27.97% received a single antibiotic, 47.5% received two antibiotics from different classes, and 24.4% received three or more antibiotics from different classes. The results are presented in Table S2 (Supplementary Materials).
3.8. Comorbidities
A total of 96% of patients admitted to the ICU had one or more comorbidities at the time of admission (Figure 2). The most common comorbidities among COVID-19 patients admitted to the intensive care unit included cardiovascular diseases, followed by obesity, diabetes mellitus, chronic pulmonary diseases, neurological or neuromuscular diseases, chronic kidney disease, immunosuppression, metabolic syndrome, and chronic liver diseases. The analysis of comorbidities and the occurrence of superinfections revealed no statistically significant correlation (Table 1).
The most common comorbidities in the group with superinfections were hypertension, 36.9%; obesity, 15.6%; diabetes mellitus, 11.0%; and COPD, 9.0% (Table 1).
3.9. Mortality
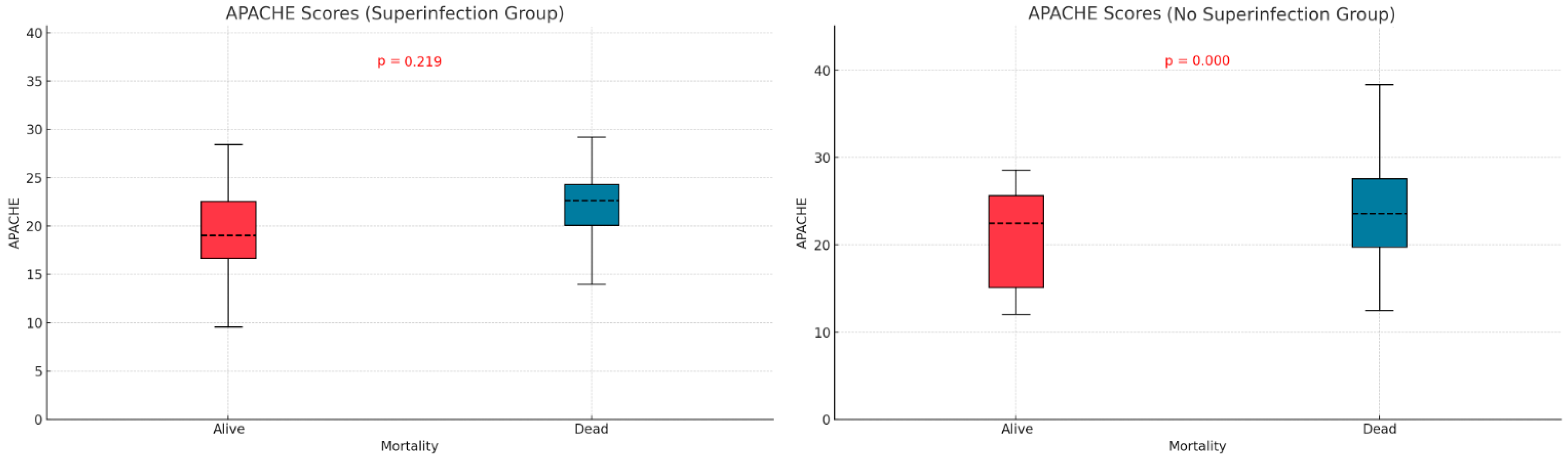
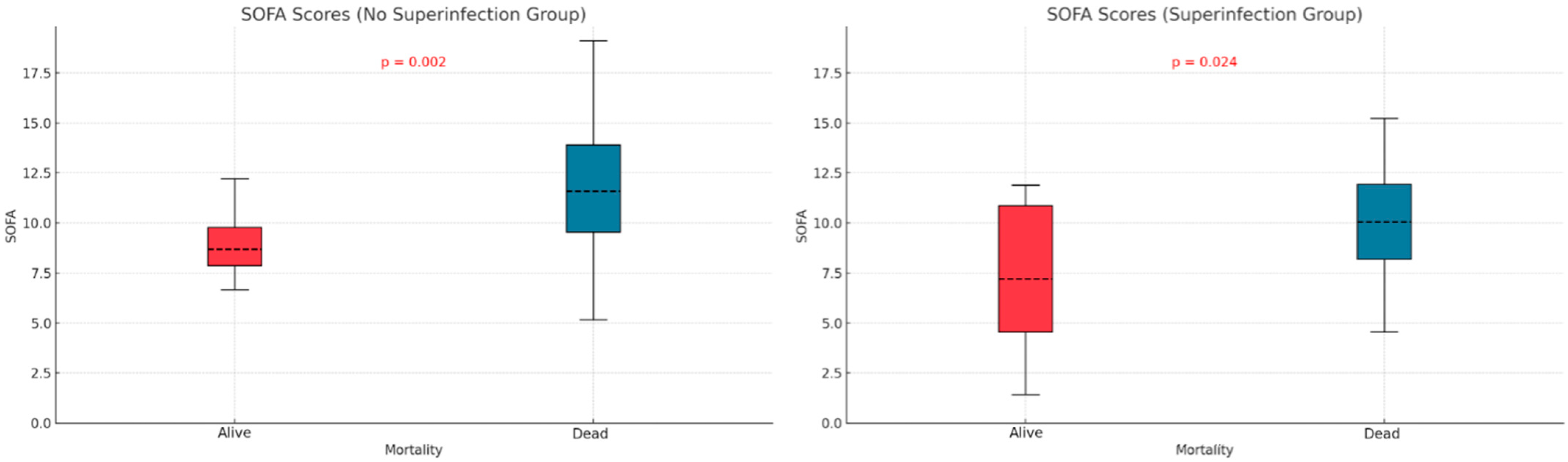
We examined the relationship between mortality and potential contributing variables by comparing APACHE II and SOFA scores in the two subgroups defined by superinfection status. The APACHE II score was significantly higher in non-survivors without superinfections (p < 0.001), but no significant difference was observed in the superinfection group (Figure 3). For SOFA scores, both groups—with or without superinfection—had significantly higher scores among non-survivors (p < 0.001, p < 0.013) (Figure 4).
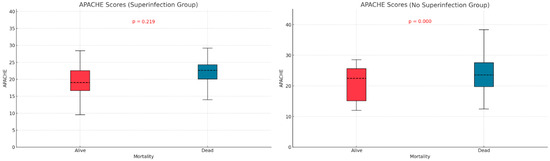
Figure 3.
Apache score differences based on mortality and superinfection status.
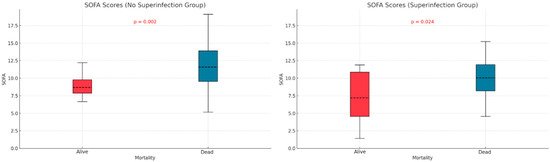
Figure 4.
SOFA score differences based on mortality and superinfection status.
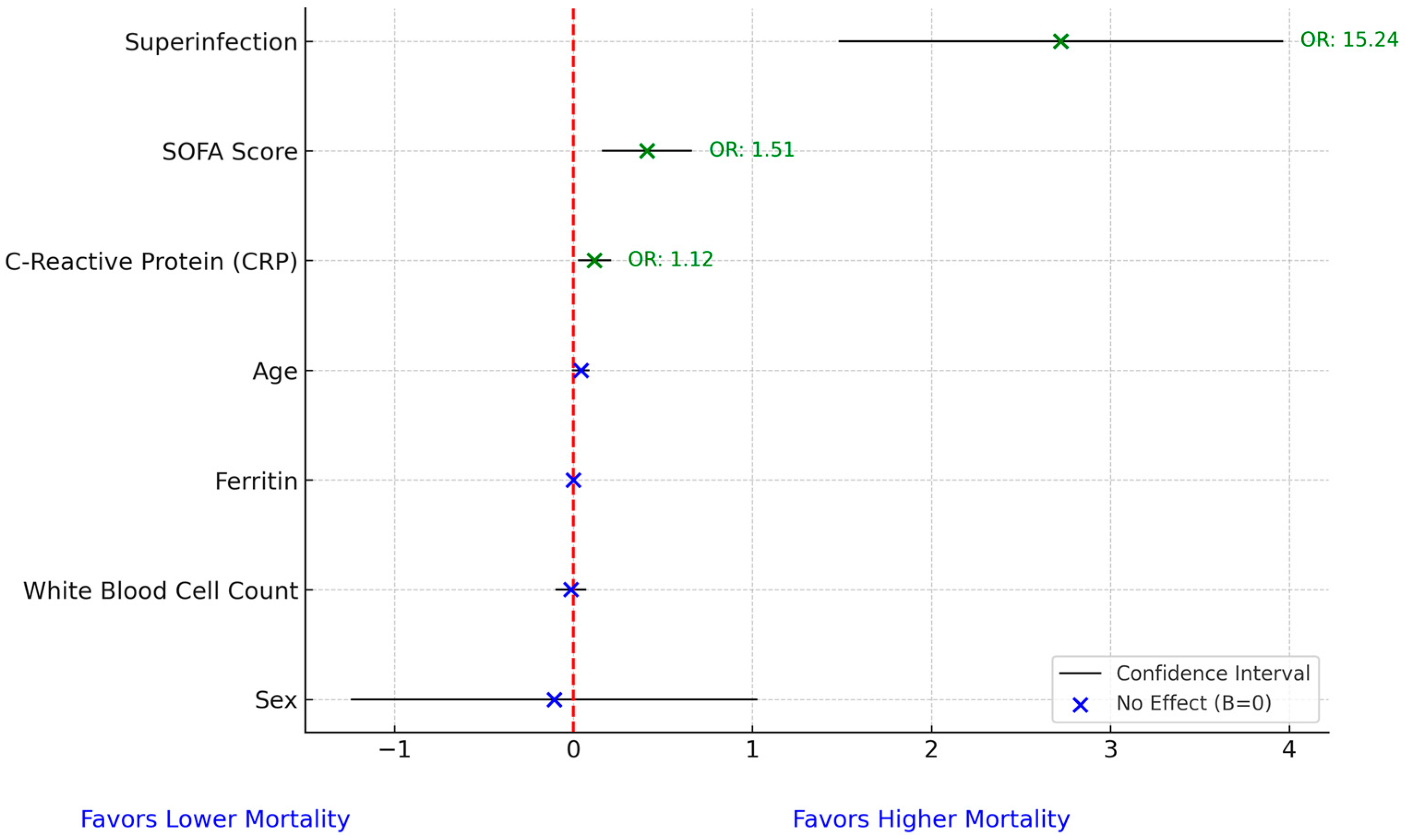
We conducted a multivariate analysis for mortality and found that superinfection increased mortality odds by more than 15-fold (OR = 15.24), while SOFA score and C-reactive protein increased mortality odds by 51% (OR = 1.51) and 13% (OR = 1.13), respectively. No other variables significantly influenced mortality odds. The Hosmer–Lemeshow test (p = 0.087) indicated an adequate fit for the model, with no evidence of poor calibration (Figure 5).
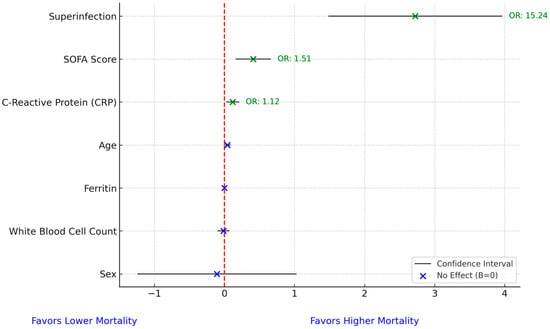
Figure 5.
Forest plot for mortality prediction model. In the figure, the red dashed vertical line represents the line of no effect, positioned at 0 on the x-axis. A value of zero implies no association between the predictor and mortality in the logistic regression model. Coefficients to the right of the line indicate a positive association with increased mortality risk, while those to the left suggest a negative association.
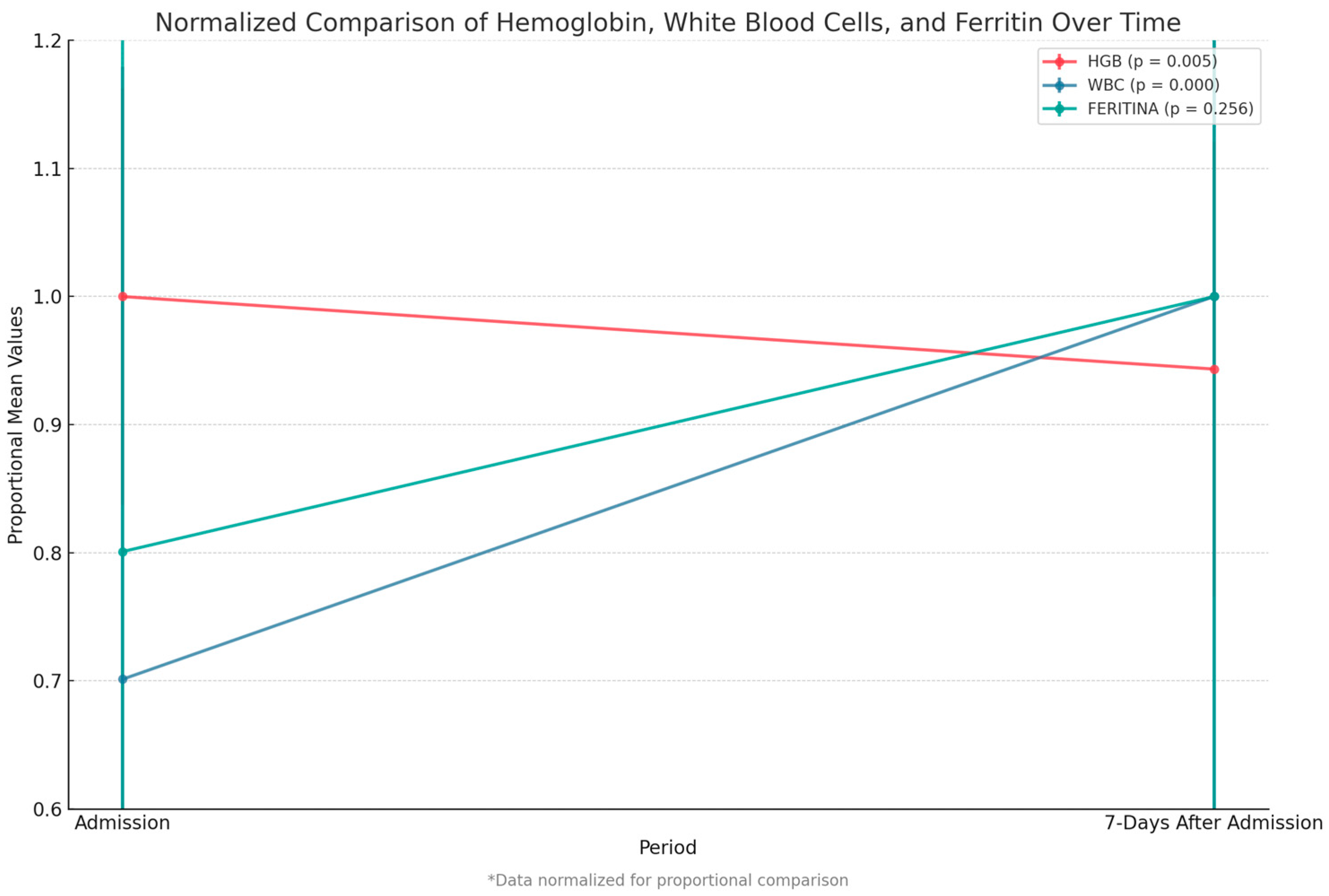
We compared key paraclinical variables—hemoglobin, white blood cell count, and ferritin level—between admission and 7 days post-admission. We observed a significant decrease in hemoglobin levels (p = 0.005) and a highly significant increase in white blood cell count (p < 0.001). Although ferritin levels rose after admission, this change was not statistically significant (Figure 6).
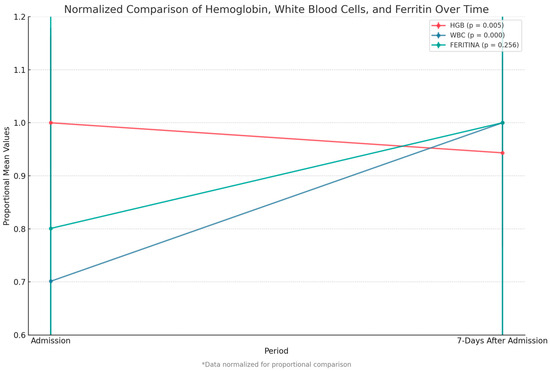
Figure 6.
Comparison of hemoglobin, white blood cell count, and ferritin between admission and 7 days after. Data were normalized to allow proportional comparison between biomarkers.
Patients with superinfections required significantly more hours of mechanical ventilation (246.2 ± 167.0) than those without superinfections (131.4 ± 105.9) (p < 0.001).
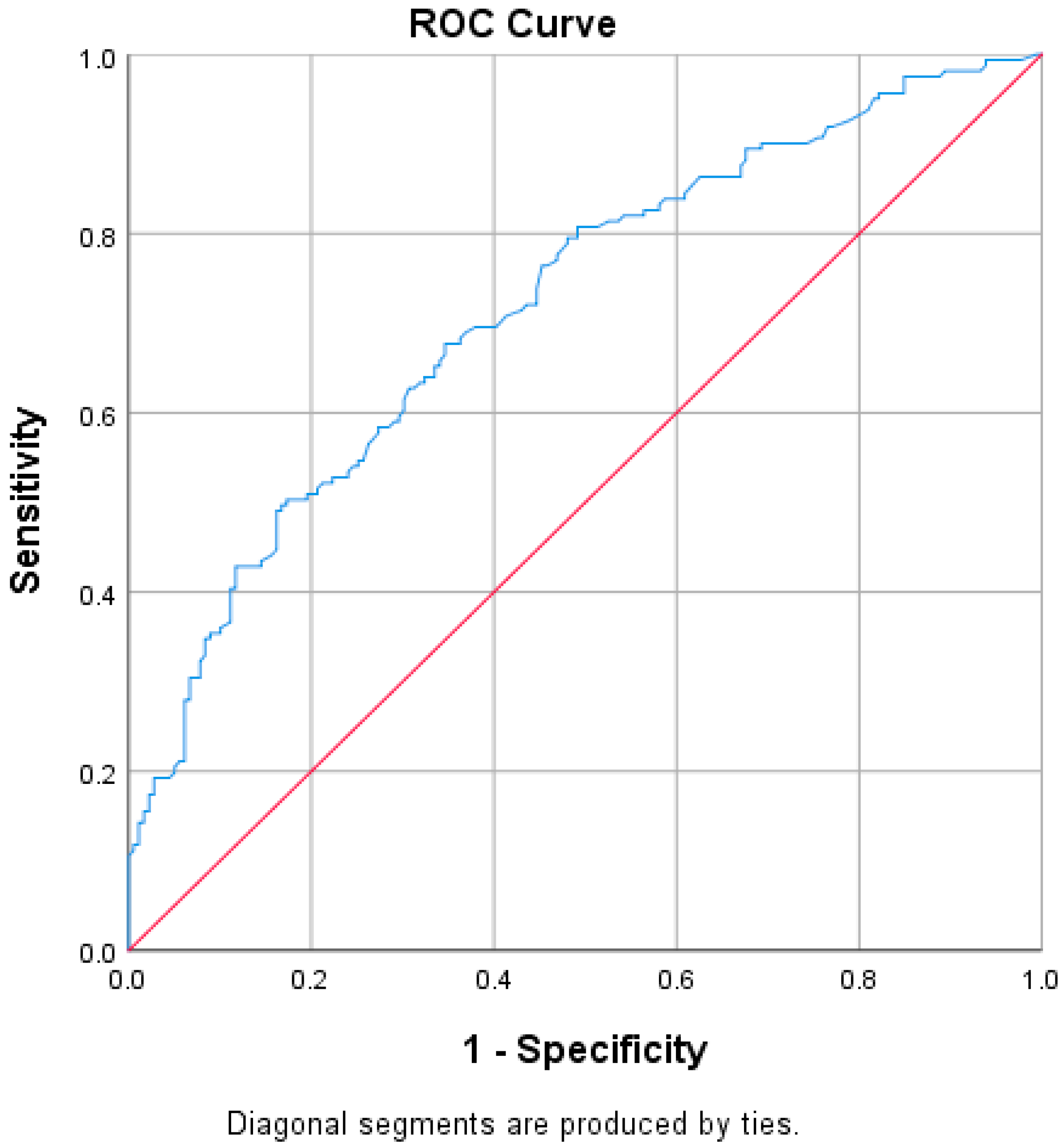
We conducted a receiver operating characteristic (ROC) analysis to assess whether ventilation duration is a reliable predictor of superinfection. The area under the curve was 0.719 (95% CI: 0.666–0.773, p < 0.001), indicating that ventilation hours is a strong and statistically significant predictor. We used the Youden index to identify the optimal cutoff point that balances sensitivity and specificity. The maximum Youden index was 0.826, corresponding to a cutoff of 65.5 h. At this threshold, sensitivity was 89.4% and specificity was 92.0% for predicting the development of a superinfection (Figure 7). However, when comparing ventilation hours between survivors and non-survivors, no statistically significant differences were observed (p = 0.804).
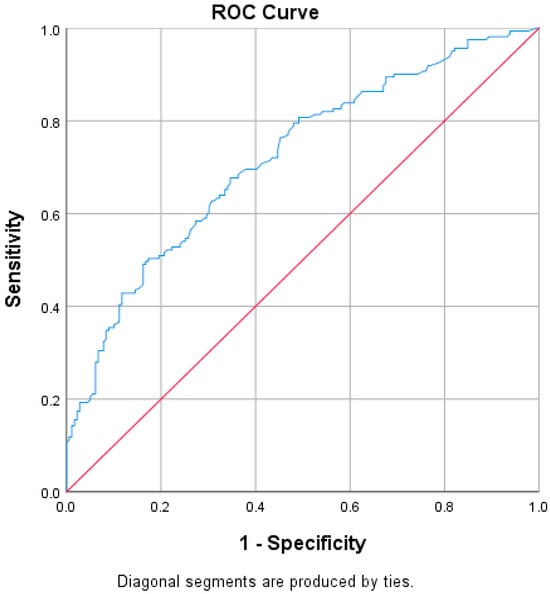
Figure 7.
ROC curve regarding ventilation hours and superinfection. In the ROC curve (Figure 7), the blue line represents the Receiver Operating Characteristic (ROC) curve, which illustrates the performance of the model in distinguishing between the presence and absence of superinfection based on ventilation hours. This curve plots the true positive rate (sensitivity) against the false positive rate (1-specificity) at various threshold settings. The red diagonal line represents the line of no discrimination, corresponding to the performance of a random classifier. A model with predictive ability will produce a ROC curve above this diagonal line, indicating better-than-random classification performance.
4. Discussion
Superimposed bacterial and fungal infections in critically ill patients admitted to the ICU with COVID-19 were associated with worse outcomes. The literature on the incidence of infections and co-infections in such patients is varied, with differing results reported across studies [28,29,30,31]. We examined these infections to fill this knowledge gap, focusing on their prevalence, impact, and associated factors in critically ill patients admitted to our clinic.
At the ICU in MCCH, the incidence of co-infections and superinfections in critically ill patients was 47%, consistent with rates in other studies. For example, Chen reported incidences of superinfections in ICU patients with COVID-19 and influenza ranging from 33.3% to 43.9% and 35.2% to 52.4%, respectively [32]. Conway-Morris et al. found that 54% of ICU patients acquired ICU-associated infections, with bacterial pneumonia diagnosed in 44% and fungal pneumonia in 9%. Additionally, 25% of the identified microorganisms were MDR [30]. These findings underscore the substantial burden of nosocomial infections in ICU settings and highlight the importance of effective infection management to improve patient outcomes.
The microbiology of these infections is often complex and involves a range of pathogens, necessitating rapid diagnosis and targeted therapy. Early identification of infections, co-infections, and antibiotic resistance profiles is essential for managing critically ill patients and optimizing clinical outcomes [31].
The average age of critically ill patients with infections in our study was 72 years (63.5–79), approximately the same as that in the study of Conway-Morris et al. [30]. It is well known that older individuals are more predisposed to sepsis and severe infections, which negatively affect their prognosis by increasing both morbidity and mortality [32,33,34]. Older age is often associated with reduced immune function, making it more difficult for the body to respond effectively to infections [35]. Moreover, age-related comorbidities, including cardiovascular diseases, diabetes, and chronic respiratory conditions, are common among older patients, further increasing their vulnerability to severe infections and complicating treatment outcomes [36].
Regarding gender distribution, we found that although more men were admitted to the ICU with COVID-19, women showed a higher predisposition to superinfections. Specifically, we observed a greater incidence of fungal infections and a higher rate of combined fungal and bacterial infections in women. This finding aligns with studies suggesting gender-based differences in infection susceptibility, with women sometimes showing a stronger immune response but also a higher likelihood of developing certain types of infections, including fungal ones [37,38,39]. However, the current literature contains limited data specifically addressing gender-related susceptibility to superinfection in COVID-19 patients.
The APACHE II and SOFA did not show significant differences between the two groups of patients (those with and without infections), as indicated in Table 1. Patients who died without superinfection had a higher APACHE score (p < 0.001). In patients with superinfection, there were no statistically significant differences between the survivors and deceased regarding APACHE score (p = 0.219). The SOFA score showed statistically significant differences, higher in deceased patients than survivors, regardless of the presence or absence of superinfection. Mehryar HR et al. reported an average APACHE II score of 10.1 ± 6.3, suggesting a low association between mortality and the APACHE II score in COVID-19 patients [40]. Several other studies support the predictive roles of the APACHE II, SOFA, and SAPS scores in superinfections or mortality. These studies suggest that higher severity scores, such as those found in our research, are strong indicators of adverse outcomes, including death. Some authors identify these scores as independent risk factors for infection-related complications [41,42,43].
Our findings also indicate that a longer ICU stay (over 10 days) is significantly associated with superinfections (p < 0.001). Furthermore, our ROC analysis identified 65.5 h of ventilation as a reliable cutoff in predicting superinfection, with a sensitivity of 89.4% and a specificity of 92.0%. This suggests that prolonged ventilation is a key risk indicator and that this threshold could be clinically valuable for early risk stratification and preventive strategies. This is consistent with the results of Schoettler et al., who found that the average time from ICU admission to identification of co-infection or superinfection was significantly shorter in COVID-19 patients than in those with influenza pneumonia (8.9 ± 6.9 days vs. 16.7 ± 13.2 days, p = 0.0028) [44]. Other studies also demonstrate strong associations between mechanical ventilation, ICU stay, and the development of infections and superinfections in critically ill patients, further supporting the relevance of these risk factors [10,43].
In our study, classifying infections by the site of microorganism isolation revealed that VAP was the most common infection, followed by UTIs, BSIs, HAP, and other non-HAP/VAP infections (Table 3). We observed a VAP incidence of 23% among ventilated patients. By comparison, Pickens et al. reported a higher VAP incidence of 44%, likely due to the use of multiplex PCR alongside quantitative cultures from bronchoalveolar lavage, which improves detection sensitivity [10]. Similarly, Grasselli et al. reported a VAP incidence of 50% among COVID-19 ICU patients with superinfections, with BSIs at 34% and catheter-related BSIs at 10% [45]. In terms of pathogen distribution, the most frequently isolated microorganisms in COVID-19 patients with VAP were Acinetobacter spp. (33.73%), Pseudomonas aeruginosa (16.8%), Candida albicans (13.25%), and Candida non-albicans spp. (13.25%). Yoon et al. also reported a high incidence of MDR Klebsiella spp. (38%) in bronchial secretions, blood, and urine [41]. These findings align with those in the literature, which highlights the growing prevalence of MDR pathogens in superimposed infections—an issue of growing concern for public health and infection control strategies [46,47]. Our study supports these observations, particularly highlighting the high prevalence of Acinetobacter spp. in Romania, which may reflect local epidemiological patterns and antimicrobial resistance profiles.
The spectrum of microorganisms involved in HAP in our study closely mirrors that reported in the literature, with non-albicans Candida spp., Acinetobacter spp., Candida albicans, Klebsiella spp., methicillin-resistant Staphylococcus aureus (MRSA), and Pseudomonas aeruginosa being the most frequently isolated pathogens. The increased prevalence of Candida albicans, non-albicans Candida spp., and Aspergillus spp. may be attributed to the immunosuppressive effects of corticosteroid treatment, as well as the use of anakinra and tocilizumab before or during ICU admission. These treatments, particularly for COVID-19, have been associated with an increased risk of fungal infections due to their impact on immune modulation. This is consistent with the results of Chen et al., who identified ICU-acquired superinfections, corticosteroid therapy before ICU admission, and a SOFA score ≥ 7 as independent prognostic factors for adverse outcomes in COVID-19 patients [32].
In our study, the most frequently isolated microorganisms in the urine of COVID-19 patients were Candida albicans, Enterococcus spp., and non-albicans Candida spp. These findings are consistent with those in the literature, although Mancuso et al. identified Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, and Staphylococcus spp. [48]. The higher incidence of UTIs observed in our cohort may be linked to increased exposure to pathogenic microorganisms in the ICU and the disruption of the urinary tract’s natural defense mechanism, often resulting from urethral instrumentation and catheterization [49,50,51].
BSIs were relatively common in our ICU patients, accounting for 24.8% of all infections. The literature emphasizes the high prevalence of BSIs in critically ill patients, particularly those requiring mechanical ventilation or intravenous catheters. Patients with these devices are more susceptible to infections caused by MDR pathogens [52,53,54,55]. Our study supports these findings, reinforcing the importance of careful infection control practices and early detection strategies to reduce the risk of BSIs and associated complications in ICU settings.
The most frequently isolated microorganism among the ICU patients was Acinetobacter spp. (26.4%), followed by Enterococcus faecium (17.8%), Clostridioides difficile (16.89%), Pseudomonas aeruginosa/stutzeri (13.2%), and Klebsiella pneumoniae/oxytoca (9.13%). Fungal infections have also been reported in the literature, with commonly isolated species including Aspergillus spp., Candida spp., Mucorales, Histoplasma spp., Cryptococcus spp., and Pneumocystis jirovecii [56]. The most commonly isolated bacteria are Acinetobacter spp., Corynebacterium striatum, Klebsiella pneumoniae, and Pseudomonas aeruginosa, which are frequently reported in ICU settings [40,57].
In contrast, a study from Romania by Pintea-Simion et al. reported Acinetobacter spp. and Klebsiella pneumoniae as the most prevalent pathogens, with incidences of 31.1% and 18.9%, respectively. It is important to note that these results and ours come from two distinct studies conducted in different healthcare institutions in the same geographical region. This highlights the local variability in ICU microbial profiles, even within a shared epidemiological context [58].
The high prevalence of both Candida albicans and non-albicans Candida species supports the growing concern regarding fungal infections in ICUs, particularly among immunocompromised patients who have received broad-spectrum antibiotics. The antibiotic resistance profiles of the most commonly isolated microorganisms show a high prevalence of MDR strains, particularly among Acinetobacter spp., Klebsiella pneumoniae, and Pseudomonas aeruginosa. These MDR strains present treatment challenges due to mechanisms such as ESBL and plasmid-mediated AmpC β-lactamase and include carbapenem-resistant Enterobacterales, Acinetobacter baumannii, and Pseudomonas aeruginosa, as well as MRSA, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus spp. [59,60]. Acinetobacter spp. are particularly common in ICUs due to their ease of transmission and have been highlighted in numerous studies as among the most problematic microorganisms in these settings worldwide. Notably, 51.3% of isolated strains demonstrate resistance to antimicrobial therapy, contributing to prolonged hospital stays, increased morbidity, and higher costs [61,62].
Organ failure associated with the extrapulmonary manifestations of COVID-19 may be linked to laboratory markers of inflammation, such as elevated C-reactive protein levels [63]. C-reactive protein is an affordable and commonly used marker in low-resource settings to help differentiate bacterial from nonbacterial infections in febrile patients [64]. Our study also found that C-reactive protein levels at ICU admission were significantly higher in patients with bacterial superinfections than in those without these complications.
The inflammatory pathways activated in COVID-19, along with the recruitment of immune cells that produce proinflammatory cytokines, contribute to lesions in alveolar epithelial cells, endothelial cells, and other tissues and organs. Cardiovascular complications, acute liver and kidney failure, rhabdomyolysis, and coagulopathy may accompany organ failure [63,65]. Altered local immune barriers, pre-existing conditions, and organ dysfunction create a favorable environment for bacterial superinfections in COVID-19 patients admitted to the ICU, who are often subjected to multiple invasive procedures (e.g., mechanical ventilation, central venous catheters, and urinary catheters). These infections can exacerbate organ dysfunction and ARDS, prolong the need for mechanical ventilation, or necessitate extracorporeal membrane oxygenation, and may contribute to renal failure, thus requiring replacement therapies [65,66].
The presence of comorbidities is a well-established risk factor for increased susceptibility to infections [67,68,69,70]. However, our study did not find a statistically significant association between comorbidities and the incidence of superinfections (Table 1).
Corticosteroid therapy has been widely used to manage severe COVID-19 cases because of its strong anti-inflammatory effects. A 2022 review concluded that corticosteroids improve outcomes by reducing mortality, the need for mechanical ventilation, and the duration of hospitalization in severe COVID-19 cases [71]. However, concerns have emerged about superinfections, particularly secondary infections caused by corticosteroid use. Invasive fungal infections such as mucormycosis and aspergillosis have been reported in COVID-19 patients treated with corticosteroids [72,73]. The use of corticosteroids and tocilizumab may also promote bacterial superinfections. However, studies have shown that tocilizumab, by inhibiting the action of IL-6, is associated with increased survival in patients severely affected by COVID-19 [74,75]. The RECOVERY study also reported a reduction in 28-day mortality in patients who received dexamethasone treatment [76]. In our study, there was no statistically significant difference in the incidence of bacterial infections in patients who received corticosteroids and those who did not (p = 0.07).
Extensive empirical antibiotic therapy also contributes to rising antimicrobial resistance. Kollef et al. recommended managing bacterial and fungal infections in ICU patients and preventing resistance through antimicrobial de-escalation and careful adjustment of pharmacokinetics [77]. The literature emphasizes that continuous collaboration among ICU teams, microbiology laboratories, and infectious disease departments is essential for stringent control of antibiotic use [71,78,79].
Recent studies have shown a shift toward strategies aimed at reducing infections associated with medical care. Key recommendations include minimizing invasive procedures and device use, strengthening infection control protocols, and employing advanced technologies such as noninvasive monitoring and ventilation. Additionally, it is crucial to monitor the prevalence of antibiotic-resistant pathogens in the ICU [25,78]. Incorporating these strategies into routine clinical practice could reduce hospital-acquired infections, particularly in the ICU.
5. Limitations of This Study
This study was conducted in a single medical unit and included a relatively small number of patients; it was not a multicenter study. Additionally, due to insufficient data, there was no clear distinction between BSIs caused by intravenous catheters and those due to UTIs associated with urinary catheters. Although this study observed an increasing trend in antibiotic resistance, it was not designed to establish general treatment recommendations. The high incidence of superinfections in COVID-19 patients and their associated increased mortality highlight the need for more comprehensive studies or meta-analyses to better understand how to protect critically ill patients with viral infections, including COVID-19.
The microbiological diagnosis was based on bronchial aspirate and sputum, and we were unable to accurately determine how many samples were obtained through bronchoalveolar lavage. Another limitation of this study is the absence of pulmonary biopsies, which would have allowed for clearer differentiation between VAP and Candida spp./Aspergillus colonization in these patients.
The collection of cultures for antibiograms and the pathogen identification was based on clinical judgment, depending on the patient’s symptoms and paraclinical and radiological data. Some infections, including cutaneous ones or those located in other tissues, may have been omitted and underdiagnosed. The use of broad-spectrum antibiotics may also have suppressed these infections.
Another limitation is the retrospective nature of data collection, which may have led to the omission of relevant variables, such as detailed information on patients’ immunological status. It is also important to note that our results may not be generalizable to other ICUs in Romania or internationally. Finally, the absence of a pre-study sample size calculation may affect the reliability of our findings. Although such a calculation was not feasible, we performed a post hoc power analysis, which indicated that the study sample size had sufficient power to detect significant associations.
6. Conclusions
The incidence and variability of bacterial and fungal co-infections and superinfections were significantly higher among critically ill ICU patients. In the context of COVID-19, these infections are closely associated with a poor prognosis. Although more men have been admitted to ICUs with severe SARS-CoV-2 cases, our findings indicate that older individuals, particularly women, are at a higher risk of developing bacterial and fungal superinfections. Prolonged mechanical ventilation and the use of invasive devices increase the likelihood of colonization and infection. Additionally, the high prevalence of MDR pathogens presents significant challenges at both the ICU and public health levels due to their strong association with mortality.
Rigorous screening, early diagnosis, and continuous monitoring are essential. Effective infection control requires coordinated efforts among clinicians, laboratory specialists, and epidemiologists. Carbapenems, glycopeptides, and oxazolidinones have been the primarily used antibiotics, often in combination. To address resistance and enhance antimicrobial stewardship, strict monitoring protocols and policies limiting the use of invasive devices in ICUs are critical.
Standardized, one-size-fits-all approaches may overlook local variations and undermine antimicrobial stewardship efforts. Strict monitoring protocols and policies limiting invasive devices in ICUs are therefore critical to combating resistance and enhancing patient care.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13061333/s1, Table S1: Classification of infections according to the isolation site, Table S2: Antibiotics used in anti-infectious therapy.
Author Contributions
Conceptualization, M.S. and A.S. (Adina Stoian); methodology, M.S.; software, A.S. (Andrei Stîngaciu) and A.M.; validation, M.S., A.S. (Adina Stoian), and A.A.; formal analysis, L.A.; investigation, M.S., A.-M.V., and A.H.; resources, M.S.; data curation, D.D. and S.R.B.; writing—original draft preparation, A.S. (Andrei Stîngaciu), A.-M.V., and A.M.; writing—review and editing, A.A. and A.S. (Adina Stoian); visualization, L.A.; supervision, M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș, Research Grant number 170/1/09.01.2024.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. We received approval from the local ethics committee of Mureș County Clinical Hospital, no. 11567, dated 25 July 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APACHE | Acute Physiology and Chronic Health Evaluation |
| ARF | Acute respiratory failure |
| ARDS | Acute respiratory distress syndrome |
| BSI | Bloodstream infection |
| CAUTI | Catheter-associated infection |
| CDI | Clostridioides difficile infection |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CPE | Carbapenemase-producing enterobacteria |
| ECMO | Extracorporeal membrane oxygenation |
| ESBL | Extended-spectrum beta-lactamase |
| ETT | Endotracheal tube |
| HAP | Hospital-acquired pneumonia |
| ICU | Intensive care unit |
| ICU-AI | Intensive care unit-associated infection |
| IQR | Interquartile range |
| IMV | Invasive mechanical ventilation |
| MODS | Multiple organ dysfunction syndrome |
| NIV | Noninvasive mechanical ventilation |
| MCCH | Mures Clinical County Hospital |
| MDR | Multidrug-resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MLSBi | MRSA with inducible resistance to macrolides |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| OR | Odds ratio |
| ROC | Receiver operating characteristic |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SD | Standard deviation |
| SOFA | Sequential Organ Failure Assessment |
| TS | Tracheostomy |
| UTI | Urinary tract infection |
| VAP | Ventilator-associated pneumonia |
| VRE | Vancomycin-resistant enterococcus |
References
- World Health Organization. Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 24 May 2025).
- De Francesco, M.A.; Signorini, L.; Piva, S.; Pellizzeri, S.; Fumarola, B.; Corbellini, S.; Piccinelli, G.; Simonetti, F.; Carta, V.; Mangeri, L.; et al. Bacterial and Fungal Superinfections Are Detected at Higher Frequency in Critically Ill Patients Affected by SARS CoV-2 Infection than Negative Patients and Are Associated to a Worse Outcome. J. Med. Virol. 2023, 95, e28892. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Roman, A.; Boeriu, A.; Onișor, D.; Bandila, S.R.; Babă, D.F.; Cocuz, I.; Niculescu, R.; Costan, A.; Laszlo, S. Ștefan; et al. Long-Term Radiological Pulmonary Changes in Mechanically Ventilated Patients with Respiratory Failure Due to SARS-CoV-2 Infection. Biomedicines 2023, 11, 2637. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Huang, C.-Y.; Li, L.-F. Prolonged Mechanical Ventilation: Outcomes and Management. J. Clin. Med. 2022, 11, 2451. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Kaimakamis, E.; Voutsas, V.; Bitzani, M. An Observational Study on Factors Associated with ICU Mortality in COVID-19 Patients and Critical Review of the Literature. Sci. Rep. 2023, 13, 7804. [Google Scholar] [CrossRef] [PubMed]
- Kaçmaz, B.; Keske, Ş.; Sişman, U.; Ateş, S.T.; Güldan, M.; Beşli, Y.; Palaoğlu, E.; Çakar, N.; Ergönül, Ö. COVID-19 Associated Bacterial Infections in Intensive Care Unit: A Case Control Study. Sci. Rep. 2023, 13, 13345. [Google Scholar] [CrossRef]
- Novacescu, A.N.; Buzzi, B.; Bedreag, O.; Papurica, M.; Rogobete, A.F.; Sandesc, D.; Sorescu, T.; Baditoiu, L.; Musuroi, C.; Vlad, D.; et al. Bacterial and Fungal Superinfections in COVID-19 Patients Hospitalized in an Intensive Care Unit from Timișoara, Romania. Infect. Drug Resist. 2022, 15, 7001–7014. [Google Scholar] [CrossRef] [PubMed]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary Infections in Patients Hospitalized with COVID-19: Incidence and Predictive Factors. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Sang, L.; Xi, Y.; Lin, Z.; Pan, Y.; Song, B.; Li, C.; Zheng, X.; Zhong, M.; Jiang, L.; Pan, C.; et al. Secondary Infection in Severe and Critical COVID-19 Patients in China: A Multicenter Retrospective Study. Ann. Palliat. Med. 2021, 10, 8557570–8558570. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Rafat, Z.; Ramandi, A.; Khaki, P.A.; Ansari, S.; Ghaderkhani, S.; Haidar, H.; Tajari, F.; Roostaei, D.; Ghazvini, R.D.; Hashemi, S.J.; et al. Fungal and Bacterial Co-Infections of the Respiratory Tract among Patients with COVID-19 Hospitalized in Intensive Care Units. Gene Rep. 2022, 27, 101588. [Google Scholar] [CrossRef]
- Pourajam, S.; Kalantari, E.; Talebzadeh, H.; Mellali, H.; Sami, R.; Soltaninejad, F.; Amra, B.; Sajadi, M.; Alenaseri, M.; Kalantari, F.; et al. Secondary Bacterial Infection and Clinical Characteristics in Patients with COVID-19 Admitted to Two Intensive Care Units of an Academic Hospital in Iran During the First Wave of the Pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 784130. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; de la Court, J.R.; Buis, D.T.P.; Meijboom, L.J.; Schade, R.P.; Visser, C.E.; van Hest, R.; Kuijvenhoven, M.; Prins, J.M.; Nijman, S.F.M.; et al. Low Frequency of Community-Acquired Bacterial Co-Infection in Patients Hospitalized for COVID-19 Based on Clinical, Radiological and Microbiological Criteria: A Retrospective Cohort Study. Antimicrob. Resist. Infect. Control 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnejad, E.; Manshadi, S.A.D.; Mohammadi, M.T.B.; Abdollai, A.; Seifi, A.; Salehi, M.R.; Gheshlagh, R.G. Prevalence of Nosocomial Infections in COVID-19 Patients Admitted to the Intensive Care Unit of Imam Khomeini Complex Hospital in Tehran. Iran. J. Microbiol. 2021, 13, 764–768. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised with COVID-19 during the First Pandemic Wave from the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Paparoupa, M.; Aldemyati, R.; Roggenkamp, H.; Berinson, B.; Nörz, D.; Olearo, F.; Kluge, S.; Roedl, K.; de Heer, G.; Wichmann, D. The Prevalence of Early- and Late-Onset Bacterial, Viral, and Fungal Respiratory Superinfections in Invasively Ventilated COVID-19 Patients. J. Med. Virol. 2022, 94, 1920–1925. [Google Scholar] [CrossRef]
- Ramos, R.; de la Villa, S.; García-Ramos, S.; Padilla, B.; García-Olivares, P.; Piñero, P.; Garrido, A.; Hortal, J.; Muñoz, P.; Caamaño, E.; et al. COVID-19 Associated Infections in the ICU Setting: A Retrospective Analysis in a Tertiary-Care Hospital. Enferm. Infecc. Microbiol. Clin. 2023, 41, 278–283. [Google Scholar] [CrossRef]
- Peghin, M.; Vena, A.; Graziano, E.; Giacobbe, D.R.; Tascini, C.; Bassetti, M. Improving Management and Antimicrobial Stewardship for Bacterial and Fungal Infections in Hospitalized Patients with COVID-19. Ther. Adv. Infect. Dis. 2022, 9, 20499361221095732. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Z.G.; Zewotir, T. Mortality-Related Risk Factors of COVID-19: A Systematic Review and Meta-Analysis of 42 Studies and 423,117 Patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-Directed Immunotherapy of Viral and Bacterial Infections: Past, Present and Future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Marino, A.; Pulvirenti, S.; Coco, V.; Busà, B.; Nunnari, G.; Cacopardo, B.S. Bacterial and Fungal Co-Infections and Superinfections in a Cohort of COVID-19 Patients: Real-Life Data from an Italian Third Level Hospital. Infect. Dis. Rep. 2022, 14, 372–382. [Google Scholar] [CrossRef]
- Kurt, A.F.; Mete, B.; Urkmez, S.; Demirkiran, O.; Dumanli, G.Y.; Bozbay, S.; Dilken, O.; Karaali, R.; Balkan, I.I.; Saltoğlu, N.; et al. Incidence, Risk Factors, and Prognosis of Bloodstream Infections in COVID-19 Patients in Intensive Care: A Single-Center Observational Study. J. Intensive Care Med. 2022, 37, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Akter, S.; Mishu, I.D.; Islam, M.R.; Rahman, M.S.; Akhter, M.; Islam, I.; Hasan, M.M.; Rahaman, M.M.; Sultana, M.; et al. Microbial Co-Infections in COVID-19: Associated Microbiota and Underlying Mechanisms of Pathogenesis. Microb. Pathog. 2021, 156, 104941. [Google Scholar] [CrossRef] [PubMed]
- Brandi, N.; Ciccarese, F.; Balacchi, C.; Rimondi, M.R.; Modolon, C.; Sportoletti, C.; Capozzi, C.; Renzulli, M.; Paccapelo, A.; Castelli, A.; et al. Co-Infections and Superinfections in COVID-19 Critically Ill Patients Are Associated with CT Imaging Abnormalities and the Worst Outcomes. Diagnostics 2022, 12, 1617. [Google Scholar] [CrossRef]
- Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Laszlo, S.Ș.; Lupu, G.; Danielescu, A.; Baba, D.-F.; Văsieșiu, A.M.; Manea, A.; et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J.; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20 (Suppl. S2), 1–26. [Google Scholar] [CrossRef]
- Boeriu, A.; Roman, A.; Dobru, D.; Stoian, M.; Voidăzan, S.; Fofiu, C. The Impact of Clostridioides Difficile Infection in Hospitalized Patients: What Changed during the Pandemic? Diagnostics 2022, 12, 3196. [Google Scholar] [CrossRef]
- Murgia, F.; Fiamma, M.; Serra, S.; Marras, G.; Argiolas, R.; Mattana, C.; Mattu, M.G.; Garau, M.C.; Doneddu, S.; Olla, S.; et al. The Impact of the Secondary Infections in ICU Patients Affected by COVID-19 during Three Different Phases of the SARS-CoV-2 Pandemic. Clin. Exp. Med. 2023, 23, 1251–1263. [Google Scholar] [CrossRef]
- Conway Morris, A.; Kohler, K.; De Corte, T.; Ercole, A.; De Grooth, H.-J.; Elbers, P.W.G.; Povoa, P.; Morais, R.; Koulenti, D.; Jog, S.; et al. Co-Infection and ICU-Acquired Infection in COIVD-19 ICU Patients: A Secondary Analysis of the UNITE-COVID Data Set. Crit. Care 2022, 26, 236. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial Infections Associated to COVID-19 in the Intensive Care Unit: Clinical Characteristics and Outcome. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Chen, Z.; Zhan, Q.; Huang, L.; Wang, C. Coinfection and Superinfection in ICU Critically Ill Patients with Severe COVID-19 Pneumonia and Influenza Pneumonia: Are the Pictures Different? Front. Public Health 2023, 11, 1195048. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Cox, M.C.; Mohr, A.M.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older Sepsis Survivors Suffer Persistent Disability Burden and Poor Long-Term Survival. J. Am. Geriatr. Soc. 2020, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Artero, A.; López-Cruz, I.; Alberola, J.; Eiros, J.M.; Resa, E.; Piles, L.; Madrazo, M. Influence of Sepsis on the Middle-Term Outcomes for Urinary Tract Infections in Elderly People. Microorganisms 2023, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, M.; Haas, L.E.M.; Ceccato, A.; Artigas, A. The Critically Ill Older Patient with Sepsis: A Narrative Review. Ann. Intensive Care 2024, 14, 6. [Google Scholar] [CrossRef]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef]
- Patrascu, R.; Dumitru, C.S.; Laza, R.; Besliu, R.S.; Gug, M.; Zara, F.; Laitin, S.M.D. The Role of Age and Comorbidity Interactions in COVID-19 Mortality: Insights from Cardiac and Pulmonary Conditions. J. Clin. Med. 2024, 13, 7510. [Google Scholar] [CrossRef]
- McClelland, E.E.; Smith, J.M. Gender Specific Differences in the Immune Response to Infection. Arch. Immunol. Ther. Exp. 2011, 59, 203–213. [Google Scholar] [CrossRef]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and Gender Differences in Bacterial Infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef]
- Mehryar, H.R.; Yarahmadi, P.; Anzali, B.C. Mortality Predictive Value of APACHE II Scores in COVID-19 Patients in the Intensive Care Unit: A Cross-Sectional Study. Ann. Med. Surg. 2023, 85, 2464–2468. [Google Scholar] [CrossRef]
- Yoon, S.M.; Lee, J.; Lee, S.-M.; Lee, H.Y. Incidence and Clinical Outcomes of Bacterial Superinfections in Critically Ill Patients with COVID-19. Front. Med. 2023, 10, 1079721. [Google Scholar] [CrossRef]
- Beigmohammadi, M.T.; Amoozadeh, L.; Rezaei Motlagh, F.; Rahimi, M.; Maghsoudloo, M.; Jafarnejad, B.; Eslami, B.; Salehi, M.R.; Zendehdel, K. Mortality Predictive Value of APACHE II and SOFA Scores in COVID-19 Patients in the Intensive Care Unit. Can. Respir. J. 2022, 2022, 5129314. [Google Scholar] [CrossRef] [PubMed]
- Stoian, M.; Andone, A.; Boeriu, A.; Bândilă, S.R.; Dobru, D.; Laszlo, S.Ș.; Corău, D.; Arbănași, E.M.; Russu, E.; Stoian, A. COVID-19 and Clostridioides Difficile Coinfection Analysis in the Intensive Care Unit. Antibiotics 2024, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Schoettler, J.J.; Sandrio, S.; Boesing, C.; Bauer, L.; Miethke, T.; Thiel, M.; Krebs, J. Bacterial Co- or Superinfection in Patients Treated in Intensive Care Unit with COVID-19- and Influenza-Associated Pneumonia. Pathogens 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Akrami, S.; Montazeri, E.A.; Saki, M.; Neisi, N.; Khedri, R.; Dini, S.A.; Motlagh, A.A.; Ahmadi, F. Bacterial Profiles and Their Antibiotic Resistance Background in Superinfections Caused by Multidrug-resistant Bacteria among COVID-19 ICU Patients from Southwest Iran. J. Med. Virol. 2023, 95, e28403. [Google Scholar] [CrossRef]
- Boccabella, L.; Palma, E.G.; Abenavoli, L.; Scarlata, G.G.M.; Boni, M.; Ianiro, G.; Santori, P.; Tack, J.F.; Scarpellini, E. Post-Coronavirus Disease 2019 Pandemic Antimicrobial Resistance. Antibiotics 2024, 13, 233. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Burián, K. Urinary Tract Infections in Elderly Patients: A 10-Year Study on Their Epidemiology and Antibiotic Resistance Based on the WHO Access, Watch, Reserve (AWaRe) Classification. Antibiotics 2021, 10, 1098. [Google Scholar] [CrossRef]
- Dickson, K.; Zhou, J.; Lehmann, C. Lower Urinary Tract Inflammation and Infection: Key Microbiological and Immunological Aspects. J. Clin. Med. 2024, 13, 315. [Google Scholar] [CrossRef]
- Todorov, S.D.; Weeks, R.; Popov, I.; Franco, B.D.G.d.M.; Chikindas, M.L. In Vitro Anti-Candida albicans Mode of Action of Enterococcus mundtii and Enterococcus faecium. Microorganisms 2023, 11, 602. [Google Scholar] [CrossRef]
- Mantzarlis, K.; Deskata, K.; Papaspyrou, D.; Leontopoulou, V.; Tsolaki, V.; Zakynthinos, E.; Makris, D. Incidence and Risk Factors for Blood Stream Infection in Mechanically Ventilated COVID-19 Patients. Antibiotics 2022, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Pozza, G.; Casalini, G.; Ciubotariu, C.L.; Giacomelli, A.; Galimberti, M.; Zacheo, M.; Rabbione, A.; Pieruzzi, M.; Oreni, L.; Galimberti, L.; et al. Bloodstream Infections in Intensive Care Unit during Four Consecutive SARS-CoV-2 Pandemic Waves. Antibiotics 2023, 12, 1448. [Google Scholar] [CrossRef]
- Giannitsioti, E.; Louka, C.; Mamali, V.; Kousouli, E.; Velentza, L.; Papadouli, V.; Loizos, G.; Mavroudis, P.; Kranidiotis, G.; Rekleiti, N.; et al. Bloodstream Infections in a COVID-19 Non-ICU Department: Microbial Epidemiology, Resistance Profiles and Comparative Analysis of Risk Factors and Patients’ Outcome. Microorganisms 2022, 10, 1314. [Google Scholar] [CrossRef]
- Munro, C.; Zilberberg, M.D.; Shorr, A.F. Bloodstream Infection in the Intensive Care Unit: Evolving Epidemiology and Microbiology. Antibiotics 2024, 13, 123. [Google Scholar] [CrossRef]
- Scendoni, R.; Bury, E.; Lima Arrais Ribeiro, I.; Cingolani, M.; Cameriere, R.; De Benedictis, A.; De Micco, F. Leading Pathogens Involved in Co-Infection and Super-Infection with COVID-19: Forensic Medicine Considerations after a Systematic Review and Meta-Analysis. Pathogens 2023, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Ramli, S.R.; Abdul Hadi, F.S.; Nor Amdan, N.A.; Kamaradin, I.H.; Zabari, N.; Maniam, S.; Sulaiman, N.S.; Ghazali, S.; Seman, Z.; Hashim, R.; et al. Secondary and Co-Infections in Hospitalized COVID-19 Patients: A Multicenter Cross-Sectional Study in Malaysia. Antibiotics 2023, 12, 1547. [Google Scholar] [CrossRef]
- Pintea-Simon, I.-A.; Bancu, L.; Mare, A.D.; Ciurea, C.N.; Toma, F.; Brukner, M.C.; Văsieșiu, A.-M.; Man, A. Secondary Bacterial Infections in Critically Ill COVID-19 Patients Admitted in the Intensive Care Unit of a Tertiary Hospital in Romania. J. Clin. Med. 2024, 13, 6201. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, R. Growing Group of Extended-Spectrum Beta-Lactamases: The CTX-M Enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef]
- Bedenić, B.; Bratić, V.; Mihaljević, S.; Lukić, A.; Vidović, K.; Reiner, K.; Schöenthaler, S.; Barišić, I.; Zarfel, G.; Grisold, A. Multidrug-Resistant Bacteria in a COVID-19 Hospital in Zagreb. Pathogens 2023, 12, 117. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Cristian, R.-E.; Dobre, E.-G.; Zenoaga-Barbarosie, C.; Chirea, E.-T.; Crunteanu, I.; Dionisie, M.-V. The Impact of Acinetobacter baumannii Infections in COVID-19 Patients Admitted in Hospital Intensive Care Units. Biol. Life Sci. Forum 2023, 31, 1. [Google Scholar] [CrossRef]
- Granata, G.; Schiavone, F.; Pipitone, G.; Taglietti, F.; Petrosillo, N. Antibiotics Use in COVID-19 Patients: A Systematic Literature Review. J. Clin. Med. 2022, 11, 7207. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Escadafal, C.; Incardona, S.; Fernandez-Carballo, B.L.; Dittrich, S. The Good and the Bad: Using C Reactive Protein to Distinguish Bacterial from Non-Bacterial Infection among Febrile Patients in Low-Resource Settings. BMJ Glob. Health 2020, 5, e002396. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Santana, S.; Mora-Quintero, M.-L.; Saavedra, P.; Montiel-González, R.; Sánchez-Ramírez, C.; Pérez-Acosta, G.; Martín-Velasco, M.; Rodríguez-Mata, C.; Lorenzo-García, J.-M.; Parrilla-Toribio, D.; et al. COVID-19 Secondary Infections in ICU Patients and Prevention Control Measures: A Preliminary Prospective Multicenter Study. Antibiotics 2022, 11, 1016. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Taccone, F.S.; Izzi, A.; Yin, N.; Garufi, A.; Hublet, S.; Njimi, H.; Ego, A.; Gorham, J.; Byl, B.; et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms 2020, 8, 1821. [Google Scholar] [CrossRef]
- Omoush, S.A.; Alzyoud, J.A.M. The Prevalence and Impact of Coinfection and Superinfection on the Severity and Outcome of COVID-19 Infection: An Updated Literature Review. Pathogens 2022, 11, 445. [Google Scholar] [CrossRef]
- Hernández-García, M.; Solito, C.; Pavón Ortiz, A.; Arguedas Casamayor, N.; Melé-Casas, M.; Pons-Tomàs, G.; F. de Sevilla, M.; Pino, R.; Launes, C.; Guitart, C.; et al. Characteristics and Risk Factors Associated with SARS-CoV-2 Pneumonias in Hospitalized Pediatric Patients: A Pilot Study. Children 2023, 10, 1703. [Google Scholar] [CrossRef]
- Greco, R.; Panetta, V.; Della Rocca, M.T.; Durante, A.; Di Caprio, G.; Maggi, P. Profile of Co-Infection Prevalence and Antibiotics Use among COVID-19 Patients. Pathogens 2022, 11, 1250. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Schinas, G.; Bletsa, E.; Bristianou, M.; Lanaras, L.; Michailides, C.; Katsikas, T.; Barkas, F.; Liberopoulos, E.; Kotsis, V.; et al. COVID-19 Outcomes and Diabetes Mellitus: A Comprehensive Multicenter Prospective Cohort Study. Microorganisms 2023, 11, 1416. [Google Scholar] [CrossRef]
- Liu, J.; Dong, J.; Yu, Y.; Yang, X.; Shu, J.; Bao, H. Corticosteroids Showed More Efficacy in Treating Hospitalized Patients with COVID-19 than Standard Care but the Effect Is Minimal: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 847695. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive Fungal Infections Complicating COVID-19: A Narrative Review. J. Fungi 2021, 7, 921. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Parmar, K.; Patel, D.; Patel, S.; Sheth, D.; Beladiya, J.V. Effect of Corticosteroid Therapy on Mortality in COVID-19 Patients-A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2022, 32, e2386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association Between Early Treatment with Tocilizumab and Mortality Among Critically Ill Patients with COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Søvik, S.; Barratt-Due, A.; Kåsine, T.; Olasveengen, T.; Strand, M.W.; Tveita, A.A.; Berdal, J.E.; Lehre, M.A.; Lorentsen, T.; Heggelund, L.; et al. Corticosteroids and Superinfections in COVID-19 Patients on Invasive Mechanical Ventilation. J. Infect. 2022, 85, 57–63. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Kollef, M.H.; Shorr, A.F.; Bassetti, M.; Timsit, J.-F.; Micek, S.T.; Michelson, A.P.; Garnacho-Montero, J. Timing of Antibiotic Therapy in the ICU. Crit. Care 2021, 25, 360. [Google Scholar] [CrossRef]
- Gavi, F.; Fiori, B.; Gandi, C.; Campetella, M.; Bientinesi, R.; Marino, F.; Fettucciari, D.; Rossi, F.; Moretto, S.; Murri, R.; et al. Prevalence and Antimicrobial Resistance Patterns of Hospital Acquired Infections through the COVID-19 Pandemic: Real-Word Data from a Tertiary Urological Centre. J. Clin. Med. 2023, 12, 7278. [Google Scholar] [CrossRef]
- Schinas, G.; Polyzou, E.; Spernovasilis, N.; Gogos, C.; Dimopoulos, G.; Akinosoglou, K. Preventing Multidrug-Resistant Bacterial Transmission in the Intensive Care Unit with a Comprehensive Approach: A Policymaking Manual. Antibiotics 2023, 12, 1255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).