Abstract
Background/Objectives: SARS-CoV-2 has strained healthcare systems, emphasizing the need for biomarkers to predict disease severity. Recent studies suggest that soluble urokinase plasminogen activator receptor (suPAR) is a promising marker for COVID-19 pneumonia, though its utility alongside the CURB-65 score remains unstudied. This study evaluates the prognostic value of suPAR in comparison to leukocyte count and CURB-65, and its potential for enhancing risk stratification in a combined CURB-65 model. Methods: Biomarkers and CURB-65 scores were obtained for 240 immunocompetent patients hospitalised with COVID-19 pneumonia. Intensive care unit admission and in-hospital mortality were assessed using receiver operating characteristic (ROC) curves and Kaplan–Meier analysis. Additionally, a Net Reclassification Improvement (NRI) analysis was performed to evaluate the predictive value of suPAR combined with the CURB-65 score for risk stratification. Results: suPAR demonstrated strong diagnostic accuracy, outperforming lymphocyte count and showing greater precision than the CURB-65 score for ICU admission. Notably, no patient with suPAR < 4 ng/mL experienced the studied outcomes. NRI analysis revealed a significant improvement in risk classification when suPAR was combined with CURB-65. Conclusions: The addition of the suPAR biomarker to the CURB-65 score represents a substantial improvement in the risk classification of patients with COVID-19 pneumonia, with a potential impact on daily clinical practice.
1. Introduction
The identification of a new coronavirus, SARS-CoV-2, as the cause of atypical pneumonia cases has challenged the healthcare systems of many countries. The disease caused by this novel coronavirus was named COVID-19 and was declared a pandemic by the World Health Organization (WHO) on 11 March 2020. The manifestations of COVID-19 infection can range from mild to moderate or severe. Some patients presented asymptomatically; however, others necessitated ICU admission, mechanical ventilation, or experienced rapid clinical deterioration resulting in death [1,2].
Despite extensive research conducted since the pandemic began, high numbers of COVID-19 infections persist, and cases of patients with severe COVID-19 pneumonia continue to appear. Numerous studies have identified pre-existing comorbidities and advanced age as significant factors associated with increased disease severity [3,4]. Nonetheless, severe clinical presentations have also been documented in young patients without underlying comorbidities, leaving unanswered questions regarding the pathophysiology of the disease and the multisystemic inflammation observed.
Considering this, laboratory medicine has played a crucial role in supporting clinical decision-making during the pandemic and beyond, making research on COVID-19 essential to identify biomarkers that can stratify patients according to their risk of adverse outcomes, even in those without pre-existing risk factors.
The literature includes multiple studies evaluating the role of classical inflammatory biomarkers, such as C-reactive protein and procalcitonin, as well as changes in haematological parameters, in relation to the severity of COVID-19 [5]. In reference to the alteration of haematological parameters, different articles have described an increase in leukocytes at the expense of neutrophils, along with lymphopenia in patients with COVID-19, with lymphopenia serving as a marker of disease severity [6,7]. Additional research indicates that biomarkers of endothelial damage may be crucial for identifying patients at elevated risk of complications during SARS-CoV-2 infection, particularly those involving cardiovascular outcomes [8,9,10].
In addition to the aforementioned biomarkers, recent studies have proposed soluble urokinase plasminogen activator receptor (suPAR) as a potential biomarker capable of stratifying patients in different diseases [11,12,13].
SuPAR is the soluble form of the urokinase-type plasminogen activator receptor (uPAR) generated through the proteolytic cleavage and subsequent release of the membrane-bound receptor. It is detectable in various biological fluids, including plasma, urine, blood, serum, and cerebrospinal fluid [14,15,16]. SuPAR plays a role in the immune response and its concentrations in blood have been shown to increase in various pathologies, including infections, cardiovascular disease, renal disease, and others. Elevated levels of suPAR are associated with a higher risk of mortality in both healthy and diseased individuals [11,17]. The use of suPAR has been shown to be effective for general patient triage in the emergency department [18]. Additionally, regarding COVID-19, several studies have explored the correlation between suPAR levels, Anakinra therapy, and the severity of patients’ clinical presentations [19,20,21]. However, there is variability in the outcomes assessed in the existing studies, along with limited literature comparing the utility of suPAR in conjunction with the scales used for the initial evaluation of pneumonia severity [22].
Accordingly, the aim of our study was to evaluate the prognostic value of suPAR in COVID-19 pneumonia compared with leukocyte count and the CURB-65 score, as well as its ability to stratify patients by creating a combined model with the CURB-65 score.
2. Materials and Methods
2.1. Study Design and Participation
We conducted a prospective, observational study in immunocompetent patients admitted for COVID-19 in our hospital, La Fe University and Polytechnic Hospital in Valencia (Spain), from 1 August 2020 to 31 January 2021. The Biomedical Research Ethics Committee Hospital La Fe approved this study (2020-114-1 and 2022-895-1). An informed consent exemption was granted due to the use of remnant samples from the clinical laboratory.
Participants were included if they did not meet immunosuppression criteria, were aged 18 years or over, were not pregnant, and had COVID-19 pneumonia.
The diagnosis of COVID-19 pneumonia required compatible signs and symptoms, along with a positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) test from a nasopharyngeal swab or sputum sample.
The exclusion criteria considered were immunosuppression, aged less than 18 years, pregnant women, previous admission within the last 30 days, and a negative SARS-CoV-2 test at admission.
An immunosuppressed patient was defined as an individual with active onco-haematological diseases, ongoing cancer, HIV infection, primary immunodeficiencies, or undergoing treatment with immunosuppressive drugs. This included chronic corticosteroid use (10 mg or more of prednisone or equivalent), biological therapies, and other immunomodulatory agents that impair immune function.
Demographic data, previous comorbidities, complementary examinations and data on the evolution during admission were recorded using a data collection protocol. Measurement of the initial severity of the pneumonic episode was assessed using the CURB-65 score, stratifying patients into 0–1 (low risk), 2 (intermediate risk or grey zone), and 3–5 (high risk). The CURB-65 score is a validated clinical tool for assessing community-acquired pneumonia (CAP) severity and guiding hospital admission decisions. The acronym CURB-65 encompasses five key clinical parameters: confusion (C), urea > 7 mmol/L (U), respiratory rate ≥ 30 breaths per minute (R), blood pressure (B) (systolic < 90 mmHg or diastolic ≤ 60 mmHg), and age ≥ 65 years (65). Each criterion is assigned one point, with higher scores correlating with increased disease severity and mortality risk [23].
2.2. Blood Samples
Samples were obtained on admission to the emergency department (ED) or within the first 12 h of the patient’s hospitalisation.
Whole blood samples were collected in K2-EDTA tubes via venipuncture. Samples with elevated haemolysis or lipemia were rejected. The K2-EDTA tubes were centrifugated (2400 G) for 10 min to obtain plasma and aliquoted for storage at −80 °C until examination.
2.3. Biomarker Determination
The lymphocyte count was obtained from routine analysis performed in the emergency department using XN-9000 analyzers (Sysmex®, Hamburg, Germany). Determination of the suPAR biomarker was performed in plasma K2-EDTA tubes on the Alinity c analyzers (Abbott Diagnostics®, Abbot Park, Lake County, IL, USA) via immunoassay using the validated reagent kit suPARnostic® TurbiLatex by Virogates (Birkerød, Denmark).
For the purpose of this study, the suPAR biomarker was categorised based on intervals published in previous studies for patient stratification in the emergency department: 0–4 ng/mL (low risk), 4–6 ng/mL (intermediate risk), and >6 ng/mL (high risk) [18,19]. In addition, for lymphocytes, a value of 724 cel/μL was considered for patient stratification, based on previous similar studies in the literature [24,25].
2.4. Clinical Outcomes
The outcomes considered in this study were the need for admission to the ICU, in-hospital mortality, and the composite outcome of in-hospital mortality and/or ICU admission.
2.5. Statistical Analysis
Statistical analyses were performed using IBM SPSS Statics version 26.0 software, RStudio version 4.2.3, and GraphPad Prism 8.0. The data were summarised as median (1st quartile, 3rd quartile) for continuous variables and count (%) for categorical variables.
The normality of the variables was assessed using the Shapiro–Wilk test. To compare biomarker levels according to the presence or absence of the outcome under study, the Mann–Whitney U test was performed.
The predictive potential of lymphocytes, CURB-65, and suPAR was assessed using ROC analysis and survival curves. The ROC curves were generated using the pROC package in R, whereas Kaplan–Meier curves were constructed using the survival and survmisc packages. Graphical representations were created with ggplot2. The differences between survival curves were analysed using the Log-Rank test, and confidence intervals for the AUC were computed to assess the discriminative ability of each biomarker.
A p-value < 0.05 was considered statistically significant.
Based on the results obtained from the ROC curves, the biomarker with superior performance between lymphocytes and suPAR was chosen to determine whether its combination with the CURB-65 score improves reclassification in patient stratification. For this purpose, the Net Reclassification Index (NRI) was calculated using a customised ad hoc script.
3. Results
3.1. Patient Characteristics
This study involved 240 immunocompetent patients with COVID-19 pneumonia who met the inclusion criteria. The demographic characteristics and comorbidities of the patients enrolled are presented in Table 1.

Table 1.
Baseline characteristics, comorbidities, and severity.
The median age of patients diagnosed with COVID-19 pneumonia was 55 years, with 52% being male. Overweight was the most prevalent comorbidity, affecting 47.1% of the cohort. Notably, only 4.2% of patients were classified as high risk according to the CURB-65 score; however, 6.2% died during hospitalisation and 10.8% required admission to the intensive care unit (ICU).
3.2. Biomarkers and Clinical Outcomes
The Mann–Whitney U test was employed to compare biomarker levels between groups based on the presence or absence of the outcome under investigation.
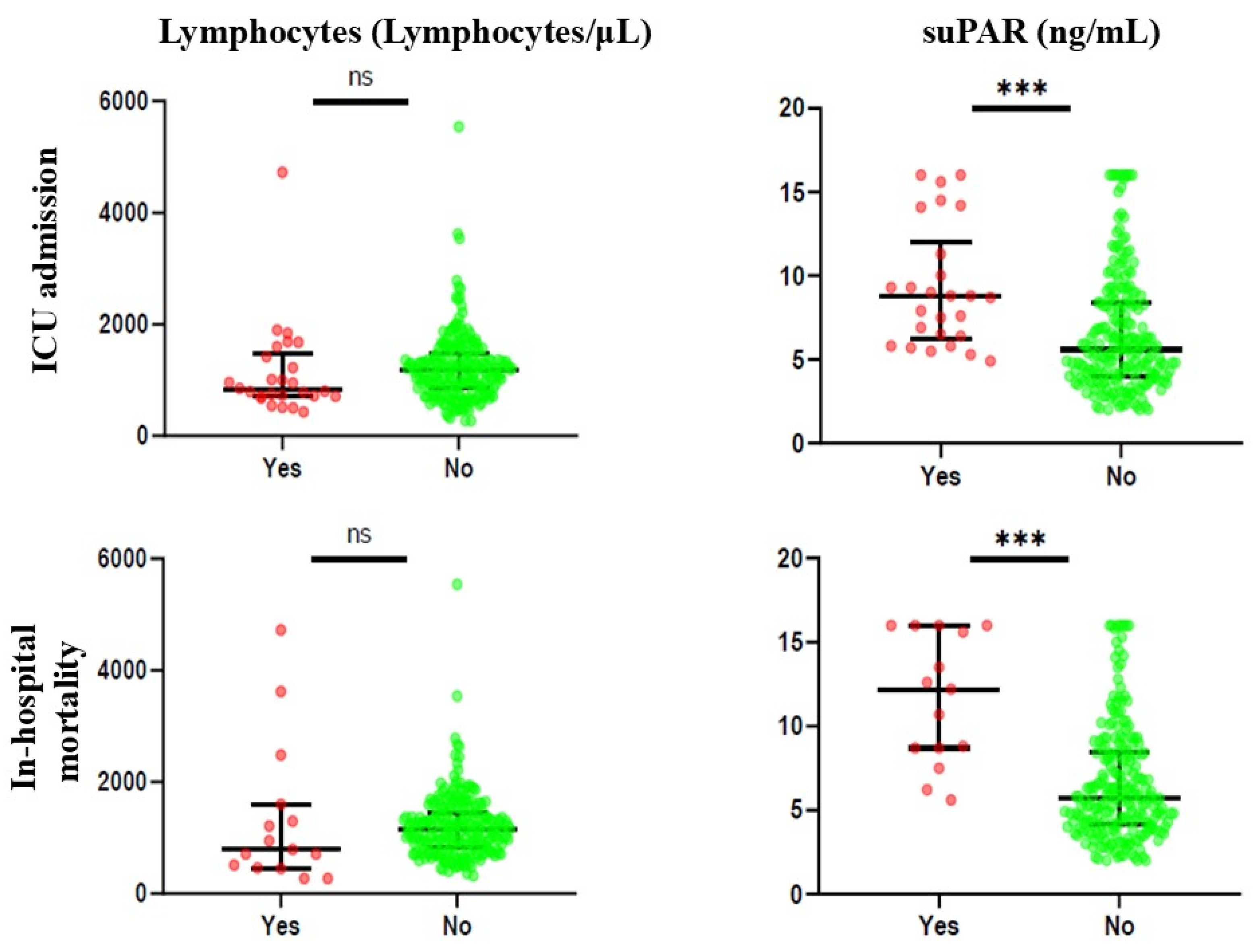
Figure 1 and Supplementary Table S1 illustrate the biomarker levels based on the presence or absence of the outcome considered. For ICU admission, it was noted that only suPAR was statistically significant, with increased values of the biomarker in patients with the presence of the outcome (median 8.75 ng/mL and 5.60 ng/mL, respectively). Similarly, for in-hospital mortality, suPAR levels were significantly higher in patients who died during hospitalisation than in those who did not (median 12.20 ng/mL and 5.70 ng/mL, respectively).
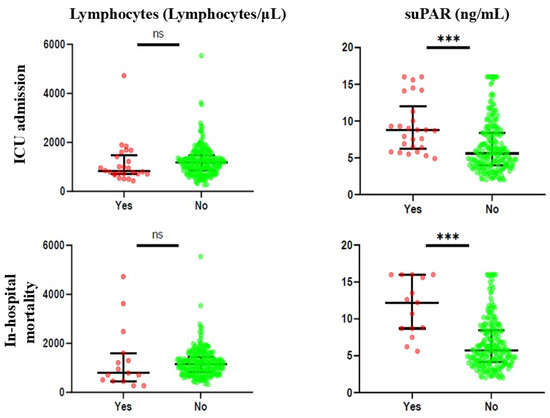
Figure 1.
Differences in biomarker levels between patients with the presence or absence of the outcome considered. ns: not statistically significant; ***: p < 0.001.
In contrast, lymphocyte levels were not statistically significant for any of the outcomes.
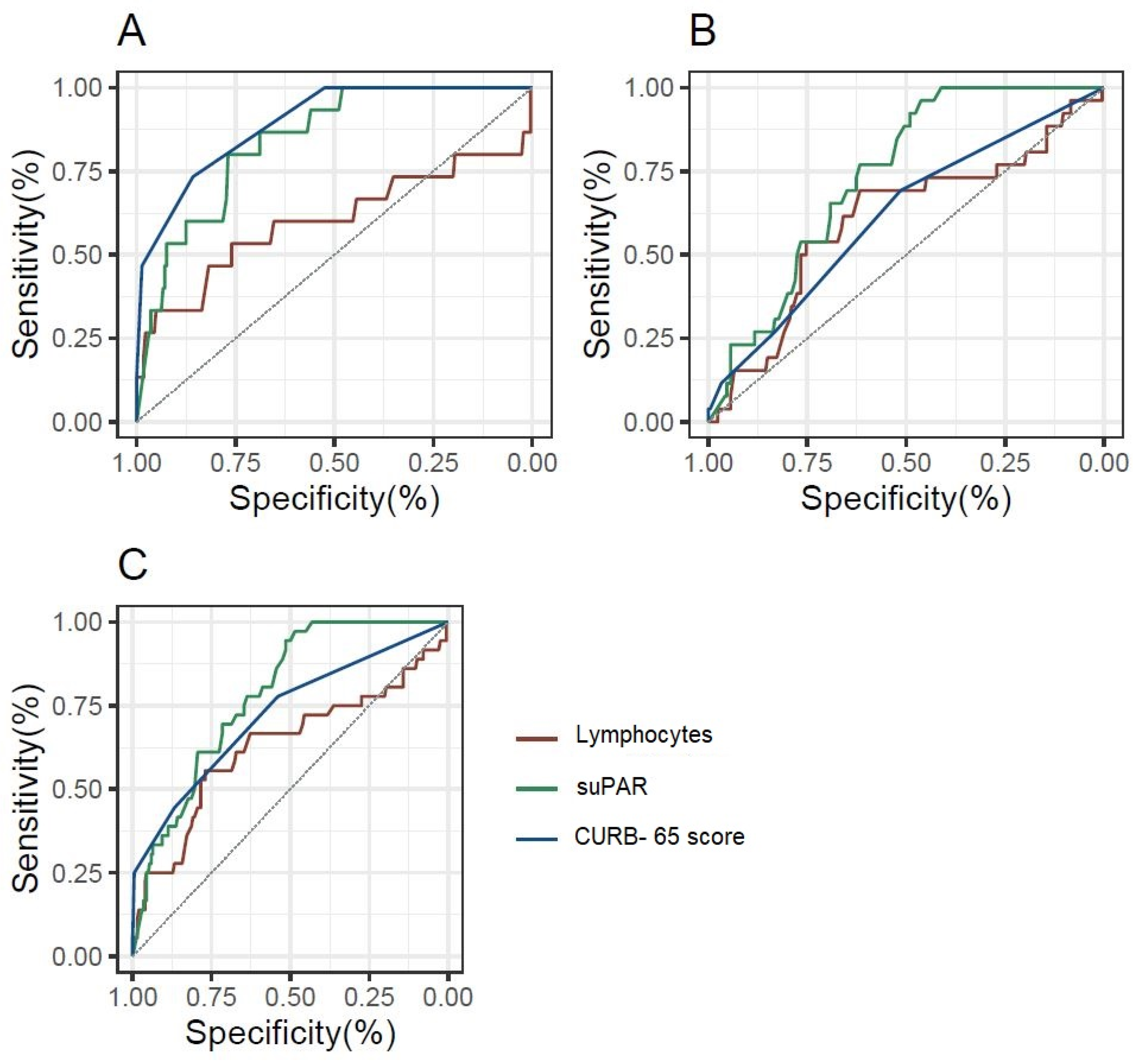
To evaluate the predictive potential of lymphocytes, CURB-65, and suPAR, a Receiver Operating Characteristic (ROC) analysis was conducted. The ROC curve was used to assess the accuracy of each biomarker in discriminating between patients with and without the outcome. Confidence intervals for the AUC were computed to provide a range of values that reflect the uncertainty around the point estimate of the biomarker’s performance, offering a more robust evaluation of discriminative ability.
Figure 2 and Supplementary Table S2 depicts the prognostic value of lymphocytes, suPAR, and CURB-65 score for ICU admission and in-hospital mortality through ROC curve analysis. It is noteworthy that for in-hospital mortality, both the CURB-65 score and the suPAR biomarker have good prognostic value, whereas lymphocytes have slight prognostic capacity without statistical significance. In addition, for ICU admission and the composite outcome of in-hospital mortality and/or ICU admission, suPAR exhibited the greatest diagnostic performance, with its AUC being superior to that of the CURB-65 score.
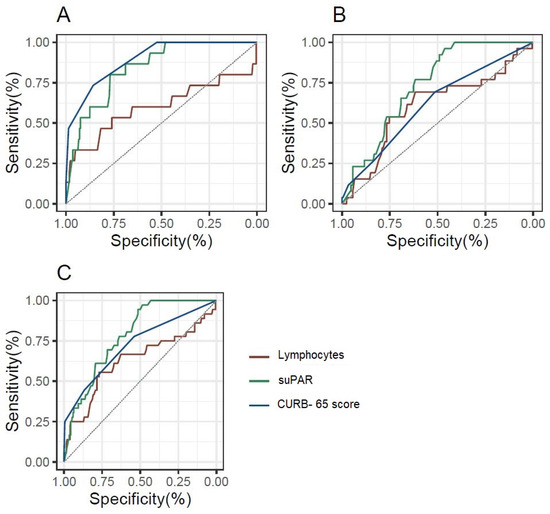
Figure 2.
ROC curves for the diagnostic performance of lymphocytes, suPAR, and the CURB-65 score, depending on the outcome considered. (A): In-hospital mortality; (B): ICU admission; (C): In-hospital mortality and/or ICU admission.
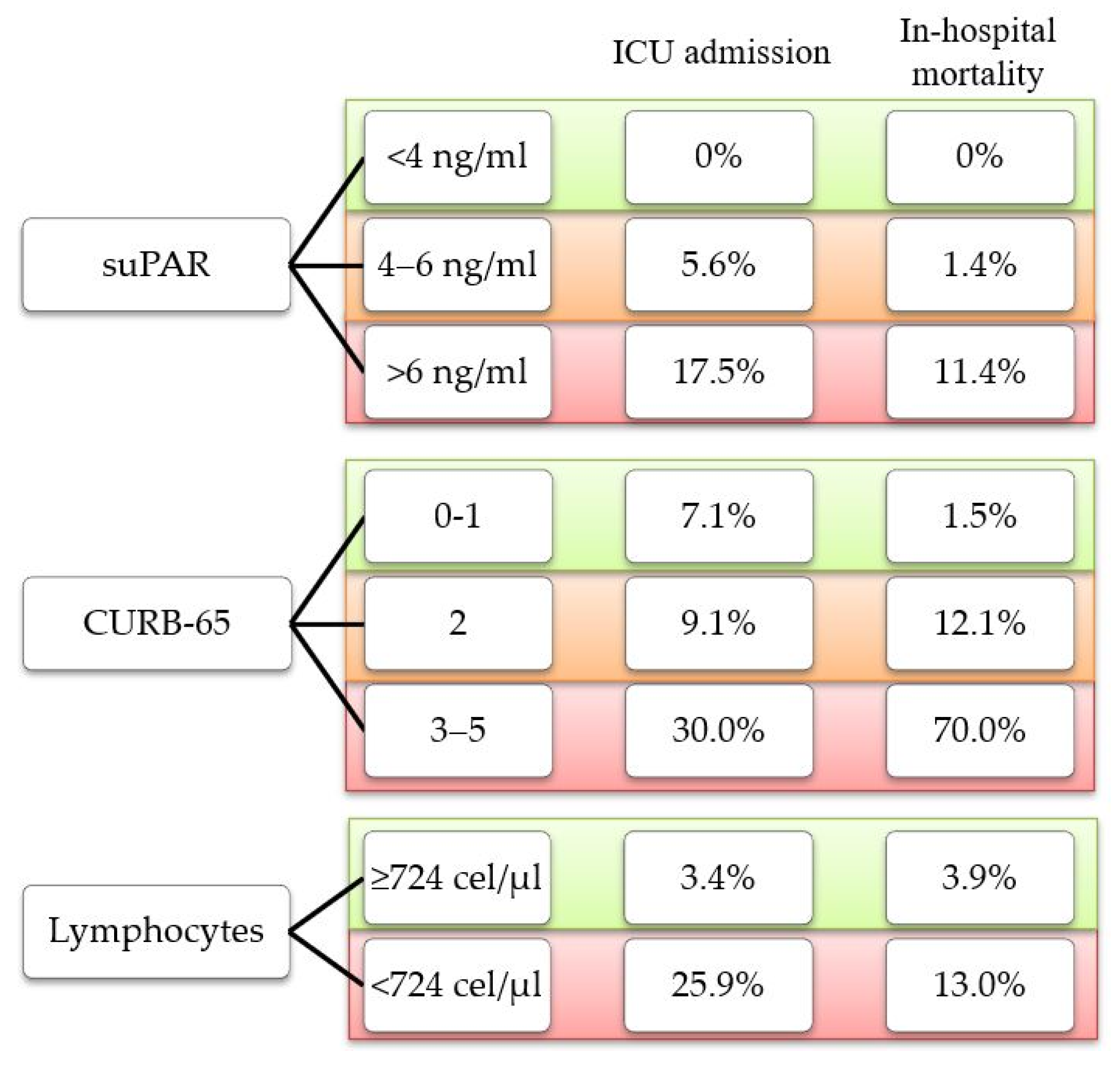
It is noteworthy that in our cohort, none of the outcomes under study were found in patients with low suPAR levels (<4 ng/mL). On the contrary, we found cases of ICU admission and in-hospital mortality among patients classified as low risk according to the CURB-65 score and those with lymphopenia (<724 cells/μL) (Figure 3).
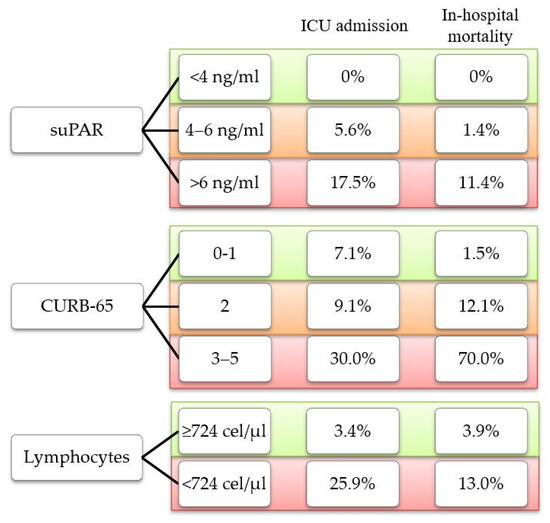
Figure 3.
Percentage of outcome observed according to risk classification for each parameter.
3.3. Survival Analysis
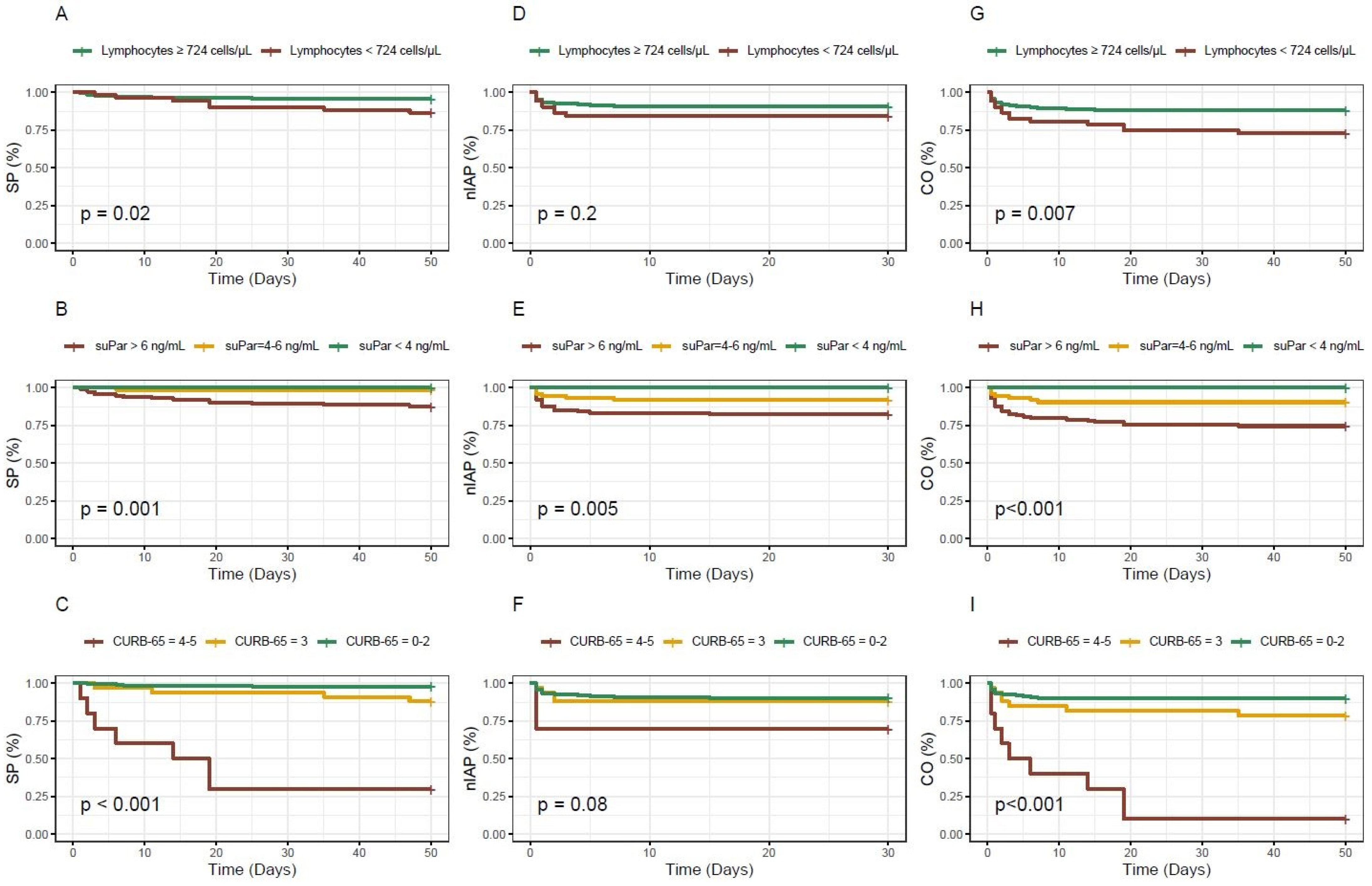
A survival analysis was performed using Kaplan–Meier curves, and the statistical significance of the differences between survival curves was tested using the Log Rank test, evaluating whether there were significant differences in survival distributions between the groups.
Figure 4 displays the survival curves for the different biomarkers and the CURB-65 score based on the outcome. For lymphocytes, patients with levels below the established cut-off exhibited a lower probability of survival or a higher probability of experiencing the combined outcome over time. Nonetheless, no statistically significant association was observed for ICU admission. Concerning the biomarker suPAR, statistical significance was observed for all analysed outcomes and the figure presents how it appropriately stratifies patients according to the three different cut-off points used. Finally, the CURB-65 score exhibits the most notable survival curve for in-hospital mortality; however, for ICU admission, it is remarkable that the curve for patients with intermediate scores is practically indistinguishable from the curve for low-risk patients.
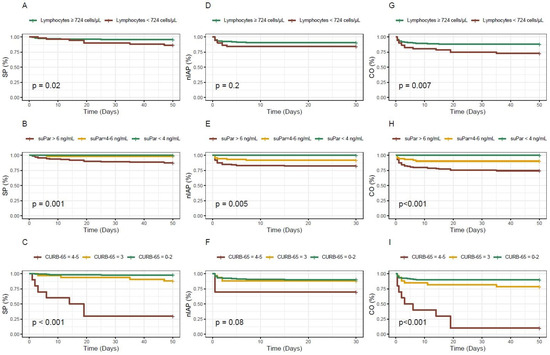
Figure 4.
Kaplan–Meier survival curves for the different biomarkers and the CURB-65 score based on the outcome considered. (A–C): in-hospital mortality; (D–F): ICU admission; (G–I): in-hospital mortality and/or ICU admission; SP: survival probability; nIAP: non ICU admission probability; CO: combined outcome probability.
3.4. Assessment of the Utility of suPAR Combined with the CURB-65 Score
The biomarker demonstrating superior performance (between lymphocytes and suPAR) was chosen to explore whether its combination with the CURB-65 score could enhance patient stratification. The Net Reclassification Index (NRI) was calculated to assess the improvement in risk reclassification when adding the selected biomarker to the CURB-65 score.
Table 2 presents a contingency table showing the different combinations of the risk values obtained using the CURB-65 score and those obtained for the suPAR biomarker in order to perform the Net Reclassification Improvement (NRI) analysis. Note that no patient with low suPAR developed in-hospital mortality or ICU admission outcomes.

Table 2.
Contingency table for the combined CURB-65 score and suPAR model for the different clinical outcomes.
Table 3 shows the results of the NRI analysis for the different clinical outcomes considered in this study and the combined model of the CURB-65 score and the biomarker suPAR. In-hospital mortality showed the most significant results in the reclassification of individuals with the event (NRI for events = 1.33), despite a tendency to increase false positives (NRI for non-events = −0.43). Overall, the combination results in a significant enhancement of patient risk classification (total NRI = 0.90).

Table 3.
Results of the Net Reclassification Improvement (NRI) analysis for the combined CURB-65 and suPAR model.
On the other hand, regarding the outcome of ICU admission, there was also an improvement in the reclassification of patient risk when using the combined model of the score and biomarker (total NRI = 0.25).
Finally, statistically significant findings were also observed for the composite outcome of in-hospital mortality and/or ICU admission, although the magnitude of the association was comparatively lower (total NRI = 0.14).
4. Discussion
The most significant results of our study are as follows: (1) the suPAR biomarker showed high diagnostic value both for ICU admission and in-hospital mortality; (2) no patient with low suPAR (<4 ng/mL) experienced the outcomes studied; (3) the addition of the suPAR biomarker to the CURB-65 score substantially improved patient risk stratification.
The search for biomarkers capable of predicting the severity of COVID-19 infection has been increasing since the beginning of the pandemic. The evaluation of new biomarkers, such as soluble urokinase-type plasminogen activator receptor (suPAR), has acquired importance. Different studies available in the literature have explored the role of the suPAR biomarker in COVID-19 disease, finding a correlation between suPAR levels and the severity of the infection and suggesting possible cut-off points for patient risk stratification [19,26]. Nevertheless, there is no literature available that provides evidence regarding the potential of using current severity assessment scales in patients with pneumonia, such as CURB-65, together with suPAR.
Our study confirms that the most severely ill patients, those who required admission to the ICU or died during their hospital stay, had higher suPAR values than those who did not.
At the same time, our study demonstrates the good prognostic significance of suPAR for both outcomes studied (ICU admission and in-hospital mortality), which is higher than that observed for lymphocytes. As compared to the validated CURB-65 score, suPAR demonstrated better discriminatory ability for ICU admission and similar ability for in-hospital mortality.
In addition, our study found that none of the patients with low suPAR levels (<4 ng/mL) developed the considered outcomes. In the range of 4–6 ng/mL, the percentage of patients exhibiting the studied outcomes was low, suggesting a potential grey zone. Notably, with suPAR levels > 6 ng/mL, the percentage of patients presenting with ICU admission or in-hospital mortality increased substantially, highlighting the clinical relevance of higher values. These findings are consistent with the published literature, which proposes a cutoff value of 4 ng/mL as a criterion supporting patient discharge and levels above 6 ng/mL as indicator of severity [18,19]. Due to the nonspecific nature of the suPAR biomarker, which may be elevated in various pathologies, its primary utility in patients with COVID-19 pneumonia lies in its prognostic role rather than diagnostic role, effectively stratifying patients into survivors, non-survivors, or those requiring ICU admission.
Finally, the addition of the suPAR biomarker to the CURB-65 score demonstrated a considerable improvement in the reassessment of patient risk. For in-hospital mortality, the total NRI score was significantly elevated, indicating a major improvement in the reclassification of patients, either by improving the identification of those at high risk of death or by avoiding the misclassification of low-risk patients. The NRI values obtained for this outcome indicate that the use of the score alongside the biomarker correctly reclassifies 90% of patients compared to using the CURB-65 score alone. This implies that the combined model (CURB-65 score plus suPAR) has high clinical value, as it could potentially improve decision making in the management of patients with pneumonia, optimizing interventions such as hospitalisation, intensive treatment, or early discharge. Regarding ICU admission, a significant improvement in patient risk classification was observed, although the degree of reclassification, as reflected by NRI values, was more moderate than for the previous outcome. Similarly, the combination of the clinical score with the suPAR biomarker resulted in a moderate improvement in risk stratification for the composite outcome of in-hospital mortality and/or ICU admission.
Some limitations of our study must be acknowledged: (1) Our study lacks a cohort of healthy controls for biomarker level comparisons. However, for lymphocyte count, we applied the cut-off established in previous studies on patients with community-acquired pneumonia [24,25], whereas for suPAR, we used the threshold defined in various studies [18,19]. (2) Although this study includes a considerable number of patients, the sample size remains limited in terms of mortality and certain complications. (3) COVID-19 patients were enrolled at the onset of the pandemic; therefore, these findings may not be fully generalisable to patients from subsequent waves, given the impact of vaccination status, the emergence of SARS-CoV-2 variants, and changes in treatment protocols. (4) Slight increases in suPAR levels have been described in the literature depending on different comorbidities, such as diabetes, hypertension, or smoking [27]. To address this limitation, given that the greatest increase has been reported in smokers, the influence of smoking status was analysed in our cohort and no significant differences were obtained for suPAR levels. As other comorbidities were not assessed and baseline values for the patients were unavailable, slight interference of these factors on suPAR levels cannot be excluded.
5. Conclusions
Our findings highlight the clinical utility of the suPAR biomarker and its value in combination with severity assessment scores for pneumonia. Specifically, integrating suPAR with the CURB-65 score enables more precise risk reclassification of patients with COVID-19 pneumonia. This combined approach holds significant clinical relevance, as it may improve decision making in pneumonia management, optimizing key interventions such as hospitalisation, intensive treatment, or early discharge.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13040896/s1. Table S1: Biomarker values expressed as the mean and interquartile range based on the studied outcomes, Table S2: Diagnostic performance based on Area Under the Curve (AUC) of the different biomarkers evaluated and the CURB-65 score for the assessed outcomes.
Author Contributions
Conceptualization: M.P., P.G.-J., R.M. (Rosario Menéndez) and R.M. (Raúl Méndez); methodology: M.P., P.G.-J., A.L., J.T.-C., R.M. (Rosario Menéndez) and R.M. (Raúl Méndez); software: M.P., P.G.-J. and J.T.-C.; formal analysis: M.P., P.G.-J., R.A. and A.L.; data curation: M.P., P.G.-J., A.L., N.M., S.R., I.A.-E., F.S.-H. and R.M. (Raúl Méndez); writing—original draft preparation: M.P., P.G.-J., A.L. and J.T.-C., writing—review and editing: R.M. (Rosario Menéndez) and R.M. (Raúl Méndez); funding acquisition: P.G.-J., M.P. and R.M. (Raúl Méndez). All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Sociedad Española de Neumología y Cirugía Torácica (SEPAR): 1078/2020, Sociedad Valenciana de Neumología (SVN): 2021, and the Instituto de Salud Carlos III (ISCIII) through Project [COV20/00385] (co-funded by the European Regional Development Fund/European Social Fund, “Investing in your future”). We also received a non-conditional grant from Menarini S.A. (2020-282-01), who did not participate in the design, data collection, statistical analysis, or writing of the article. Paula González-Jiménez was the recipient of a Rio Hortega grant supported by the Instituto de Salud Carlos III (CM23/00062). Mónica Piqueras was the recipient of a post-resident research grant supported by the Health Research Institute La Fe (2023-875-1). Raúl Méndez was the recipient of the Juan Rodés grant, supported by the Instituto de Salud Carlos III (ISCIII JR21/00051).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by The Biomedical Research Ethics Committee Hospital La Fe (2020-114-1 and 2022-895-1).
Informed Consent Statement
Patient consent was waived due to the use of remnant samples from the clinical laboratory.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank the Integrate Research Programme (PII) of Respiratory Infections of the Sociedad Española de Neumología y Cirugía Torácica (SEPAR).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar]
- Honardoost, M.; Janani, L.; Aghili, R.; Emami, Z.; Khamseh, M.E. The Association between Presence of Comorbidities and COVID-19 Severity: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2021, 50, 132–140. [Google Scholar] [PubMed]
- Perez-Anibal, E.; Contreras-Arrieta, S.; Rojas-Suarez, J.; Coronell-Rodriguez, W.; Aguilar-Schotborgh, M.; Borre-Naranjo, D.; Almanza-Hurtado, A.; Duenas-Castell, C. Association of Chronic Critical Illness and COVID-19 in Patients Admitted to Intensive Care Units: A Prospective Cohort Study. Arch. Bronconeumol. 2023, 59, 126–128. [Google Scholar] [PubMed]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [PubMed]
- Lopez-Pintor, J.M.; Herraez Carrera, O.; Sanchez-Lopez, J.; Gaitan Pitera, J.; Huertas Vaquero, M.; Tejera-Munoz, A.; Arias-Arias, A.; Asencio Egea, M.A. Evolucion de marcadores de laboratorio en pacientes con deteccion persistente de SARS-CoV-2. Rev. Esp. Salud Publica 2023, 97, e202305039. [Google Scholar]
- Velazquez, S.; Madurga, R.; Castellano, J.M.; Rodriguez-Pascual, J.; de Aguiar Diaz Obregon, S.R.; Jimeno, S.; Montero, J.I.; Wichner, P.S.V.; Lopez-Escobar, A. Hemogram-derived ratios as prognostic markers of ICU admission in COVID-19. BMC Emerg. Med. 2021, 21, 89. [Google Scholar]
- Gonzalez-Jimenez, P.; Mendez, R.; Latorre, A.; Mengot, N.; Piqueras, M.; Reyes, S.; Moscardo, A.; Alonso, R.; Amara-Elori, I.; Menendez, R. Endothelial Damage, Neutrophil Extracellular Traps and Platelet Activation in COVID-19 vs. Community-Acquired Pneumonia: A Case-Control Study. Int. J. Mol. Sci. 2023, 24, 13194. [Google Scholar] [CrossRef]
- Mendez, R.; Gonzalez-Jimenez, P.; Latorre, A.; Piqueras, M.; Bouzas, L.; Yepez, K.; Ferrando, A.; Zaldivar-Olmeda, E.; Moscardo, A.; Alonso, R.; et al. Acute and sustained increase in endothelial biomarkers in COVID-19. Thorax 2022, 77, 400–403. [Google Scholar]
- Vasbinder, A.; Padalia, K.; Pizzo, I.; Machado, K.; Catalan, T.; Presswalla, F.; Anderson, E.; Ismail, A.; Hutten, C.; Huang, Y.; et al. SuPAR, biomarkers of inflammation, and severe outcomes in patients hospitalized for COVID-19: The International Study of Inflammation in COVID-19. J. Med. Virol. 2024, 96, e29389. [Google Scholar]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (uPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.C.; Hatzl, S.; Prattes, J.; Hackl, G.; Schilcher, G.; Eisner, F.; Niedrist, T.; Raggam, R.; Krause, R.; Eller, P. Soluble urokinase plasminogen activator receptor (suPAR) in bronchoalveolar fluid and blood in critically ill patients—A prospective cohort study. Infection 2024, 52, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Ye, L.; Shen, C.; Bai, X.; Li, C. Analysis of the Predictive Efficacy of Serum suPAR Combined with APN and IgE Test and the Relationship of Patients with CHF and Cardiac Function. Altern. Ther. Health Med. 2024, 30, 124–129. [Google Scholar] [PubMed]
- Ostergaard, C.; Benfield, T.; Lundgren, J.D.; Eugen-Olsen, J. Soluble urokinase receptor is elevated in cerebrospinal fluid from patients with purulent meningitis and is associated with fatal outcome. Scand. J. Infect. Dis. 2004, 36, 14–19. [Google Scholar] [CrossRef]
- Stephens, R.W.; Pedersen, A.N.; Nielsen, H.J.; Hamers, M.J.; Hoyer-Hansen, G.; Ronne, E.; Dybkjaer, E.; Dano, K.; Brunner, N. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin. Chem. 1997, 43, 1868–1876. [Google Scholar] [CrossRef]
- Thuno, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef]
- Santeri, S.; Peter, A.A.; Kristiina, N.; Jesper, E.O.; Harri, H. suPAR cut-offs for stratification of low, medium, and high-risk acute medical patients in the emergency department. BMC Emerg. Med. 2021, 21, 149. [Google Scholar] [CrossRef]
- Altintas, I.; Eugen-Olsen, J.; Seppala, S.; Tingleff, J.; Stauning, M.A.; El Caidi, N.O.; Elmajdoubi, S.; Gamst-Jensen, H.; Lindstrom, M.B.; Rasmussen, L.J.H.; et al. suPAR Cut-Offs for Risk Stratification in Patients With Symptoms of COVID-19. Biomark. Insights 2021, 16, 11772719211034685. [Google Scholar]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Akinosoglou, K.; Florou, E.; Kouriannidi, E.; Bogosian, A.; Tsachouridou, O.; Syrigos, K.N.; Gatselis, N.; Milionis, H.; Papanikolaou, I.C.; et al. Anakinra efficacy in COVID-19 pneumonia guided by soluble urokinase plasminogen activator receptor: Association with the inflammatory burden of the host. Int. J. Antimicrob. Agents 2025, 65, 107405. [Google Scholar]
- Ulaj, A.; Ibsen, A.; Azurmendi, L.; Sanchez, J.C.; Prendki, V.; Roux, X. Improving prognostication of pneumonia among elderly patients: Usefulness of suPAR. BMC Geriatr. 2024, 24, 709. [Google Scholar]
- Lim, W.S.; van der Eerden, M.M.; Laing, R.; Boersma, W.G.; Karalus, N.; Town, G.I.; Lewis, S.A.; Macfarlane, J.T. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; Cilloniz, C.; Mendez, R.; Almansa, R.; Gabarrus, A.; Ceccato, A.; Torres, A.; Menendez, R. Lymphopenic Community Acquired Pneumonia (L-CAP), an Immunological Phenotype Associated with Higher Risk of Mortality. EBioMedicine 2017, 24, 231–236. [Google Scholar] [PubMed]
- Mendez, R.; Menendez, R.; Amara-Elori, I.; Feced, L.; Piro, A.; Ramirez, P.; Sempere, A.; Ortega, A.; Bermejo-Martin, J.F.; Torres, A. Lymphopenic community-acquired pneumonia is associated with a dysregulated immune response and increased severity and mortality. J. Infect. 2019, 78, 423–431. [Google Scholar]
- Chalkias, A.; Skoulakis, A.; Papagiannakis, N.; Laou, E.; Tourlakopoulos, K.; Pagonis, A.; Michou, A.; Ntalarizou, N.; Mermiri, M.; Ragias, D.; et al. Circulating suPAR associates with severity and in-hospital progression of COVID-19. Eur. J. Clin. Investig. 2022, 52, e13794. [Google Scholar]
- Haupt, T.H.; Kallemose, T.; Ladelund, S.; Rasmussen, L.J.; Thorball, C.W.; Andersen, O.; Pisinger, C.; Eugen-Olsen, J. Risk factors associated with serum levels of the inflammatory biomarker soluble urokinase plasminogen activator receptor in a general population. Biomark. Insights 2014, 9, 91–100. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).