Diabetes-Driven Retinal Neurodegeneration: Its Role in the Pathogenesis of Diabetic Retinopathy
Abstract
1. Introduction
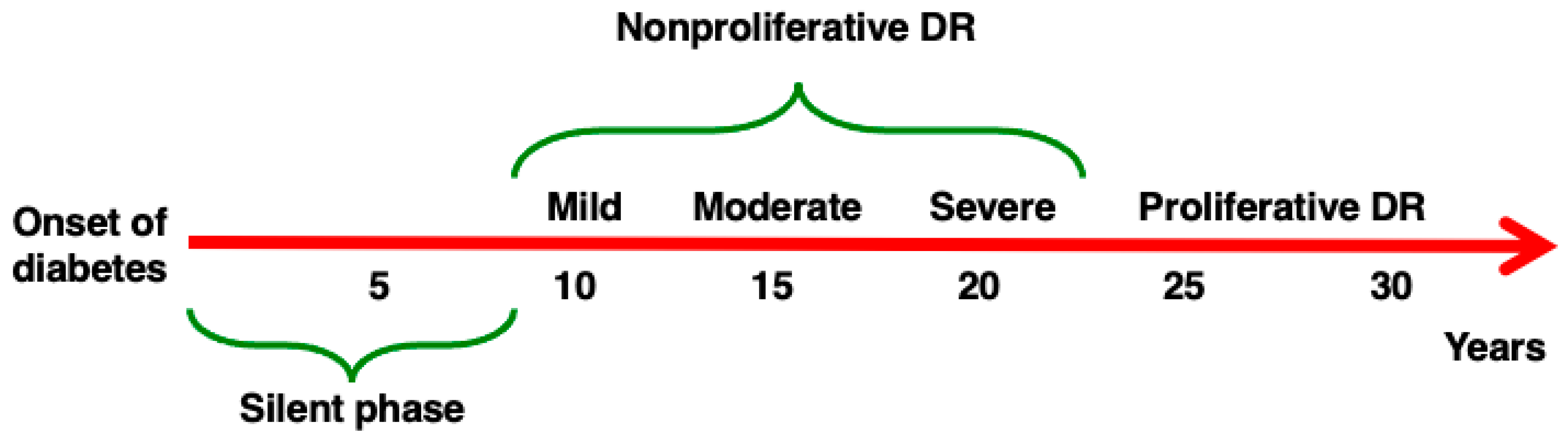
2. Diabetic Retinopathy
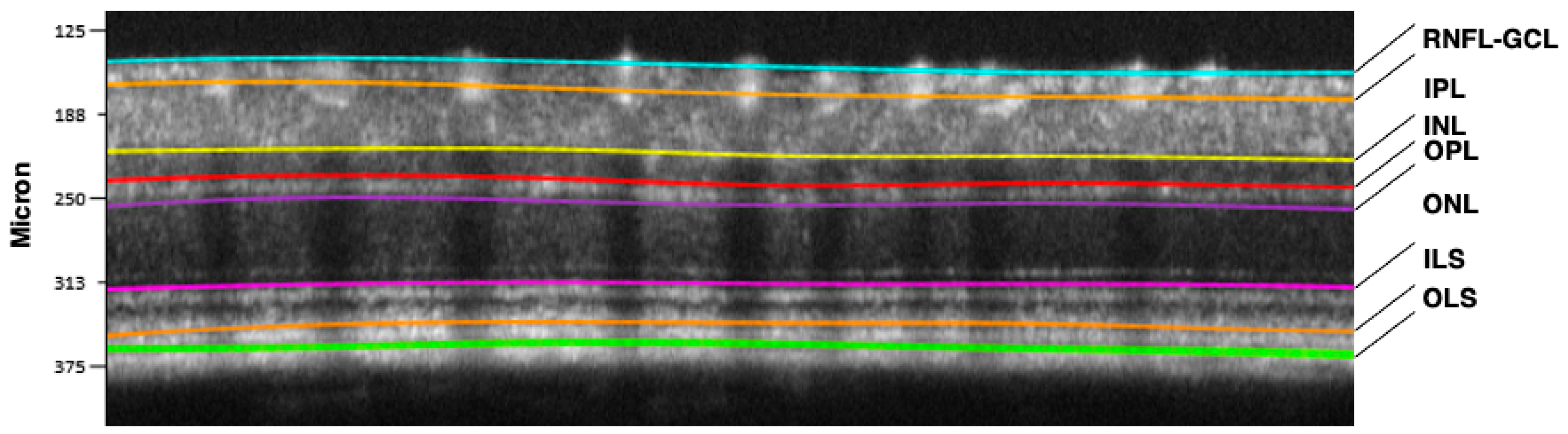
3. Neurovascular Unit
4. Diseases Characterized by Retinal Neurodegeneration
5. Diabetes-Driven Retinal Neurodegeneration
6. Diabetes-Driven Retinal Neurodegeneration as the First Stage of Diabetic Retinopathy
7. Diabetes-Driven Retinal Neurodegeneration as a Pharmacological Target to Prevent the Vascular Stages of Diabetic Retinopathy
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Carr, A.L.J.; Evans-Molina, C.; Oram, R.A. Precision medicine in type 1 diabetes. Diabetologia 2022, 65, 1854–1866. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Schiborn, C.; Schulze, M.B. Precision prognostics for the development of complications in diabetes. Diabetologia 2022, 65, 1867–1882. [Google Scholar] [CrossRef]
- Mishriky, B.M.; Cummings, D.M.; Powell, J.R. Diabetes-Related Microvascular Complications—A Practical Approach. Prim. Care 2022, 49, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Keenan, H.A.; Cavallerano, J.D.; Asztalos, B.F.; Schaefer, E.J.; Sell, D.R.; Strauch, C.M.; Monnier, V.M.; Doria, A.; Aiello, L.P.; et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: The joslin 50-year medalist study. Diabetes Care 2011, 34, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Keenan, H.A.; Costacou, T.; Sun, J.K.; Doria, A.; Cavellerano, J.; Coney, J.; Orchard, T.J.; Aiello, L.P.; King, G.L. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: The 50-year medalist study. Diabetes Care 2007, 30, 1995–1997. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Balistreri, C.R.; Geng, Y.J.; De Caterina, R. Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vasc. Pharmacol. 2017, 90, 1–7. [Google Scholar] [CrossRef]
- Madonna, R.; Giovannelli, G.; Confalone, P.; Renna, F.V.; Geng, Y.J.; De Caterina, R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: Implications for diabetic retinopathy. Cardiovasc. Diabetol. 2016, 15, 18. [Google Scholar] [CrossRef]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef]
- Bandello, F.; Lattanzio, R.; Zucchiatti, I.; Del Turco, C. Pathophysiology and treatment of diabetic retinopathy. Acta Diabetol. 2013, 50, 1–20. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.W.; Bearse, M.A., Jr.; Ng, J.S.; Jewell, N.P.; Barez, S.; Burger, D.; Schneck, M.E.; Adams, A.J. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 772–777. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care 2016, 39, 1643–1649. [Google Scholar] [CrossRef]
- Fenwick, E.; Rees, G.; Pesudovs, K.; Dirani, M.; Kawasaki, R.; Franzco, T.Y.W.; Lamoureux, E. Social and emotional impact of diabetic retinopathy: A review. Clin. Exp. Ophthalmol. 2012, 40, 27–38. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21, S3–S9. [Google Scholar] [CrossRef]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Batista, A.; Guimarães, P.; Martins, J.; Moreira, P.I.; Ambrósio, A.F.; Castelo-Branco, M.; Serranho, P.; Bernardes, R. Normative mice retinal thickness: 16-month longitudinal characterization of wild-type mice and changes in a model of Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1161847. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review. Clin. Ther. 2021, 43, 1792–1818. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Li, P.; Yao, K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int. J. Mol. Sci. 2022, 23, 4883. [Google Scholar] [CrossRef]
- Kamde, S.P.; Anjankar, A. Retinitis Pigmentosa: Pathogenesis, Diagnostic Findings, and Treatment. Cureus 2023, 15, e48006. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011, 54 (Suppl. S1), S204–S217. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.R.; Robinson, E.; Koronyo, Y.; Salobrar-Garcia, E.; Rentsendorj, A.; Gaire, B.P.; Mirzaei, N.; Kayed, R.; Sadun, A.A.; Ljubimov, A.V.; et al. Retinal ganglion cell vulnerability to pathogenic tau in Alzheimer’s disease. Acta Neuropathol. Commun. 2025, 13, 31. [Google Scholar] [CrossRef]
- Poveda, S.; Arellano, X.; Bernal-Pacheco, O.; Valencia López, A. Structural changes in the retina as a potential biomarker in Parkinson’s disease: An approach from optical coherence tomography. Front. Neuroimaging 2024, 3, 1340754. [Google Scholar] [CrossRef]
- Murueta-Goyena, A.; Romero-Bascones, D.; Teijeira-Portas, S.; Urcola, J.A.; Ruiz-Martínez, J.; Del Pino, R.; Acera, M.; Petzold, A.; Wagner, S.K.; Keane, P.A.; et al. Association of retinal neurodegeneration with the progression of cognitive decline in Parkinson’s disease. npj Park. Dis. 2024, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Murueta-Goyena, A.; Teijeira-Portas, S.; Blanco Martín, E.; Vázquez-Picón, R.; Ruiz Bajo, B.; Bocos, J.; Sánchez-Molina, J.; Alves Dias, P.; Croitoru, I.; Rodríguez Agirretxe, I.; et al. Dynamics of retinal changes in early-stage Parkinson’s disease. Acta Neuropathol. Commun. 2025, 13, 20. [Google Scholar] [CrossRef]
- Lorenzi, M.; Cagliero, E.; Toledo, S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes 1985, 34, 621–627. [Google Scholar] [CrossRef]
- Lorenzi, M.; Montisano, D.F.; Toledo, S.; Barrieux, A. High glucose induces DNA damage in cultured human endothelial cells. J. Clin. Investig. 1986, 77, 322–325. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Milstein, J.L.; Ferris, H.A. The brain as an insulin-sensitive metabolic organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef]
- Gralle, M. The neuronal insulin receptor in its environment. J. Neurochem. 2017, 140, 359–367. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Brownlee, M. The missing link: A single unifying mechanism for diabetic complications. Kidney Int. 2000, 58, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Barber, A.J. Retinal ganglion cells in diabetes. J. Physiol. 2008, 15, 4401–4408. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Zheng, L.; Szab, C.; Kern, T.S. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes 2004, 53, 2960–2967. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, V.; Xiong, Y.; Ho, Y.S. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic. Biol. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef]
- Romeo, G.; Liu, W.H.; Asnaghi, V.; Kern, T.S.; Lorenzi, M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes 2002, 51, 2241–2248. [Google Scholar] [CrossRef]
- Zheng, L.; Howell, S.J.; Hatala, D.A.; Huang, K.; Kern, T.S. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 2007, 56, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Harada, T.; Harada, C.; Ohtsuka, K.; Kotake, S.; Ohno, S.; Tanaka, K.; Takeuchi, S.; Wada, K. NF-kappaB in epiretinal membranes after human diabetic retinopathy. Diabetologia 2003, 46, 699–703. [Google Scholar] [CrossRef]
- Zhu, H.; Li, B.; Huang, T.; Wang, B.; Li, S.; Yu, K.; Cai, L.; Ye, Y.; Chen, S.; Zhu, H.; et al. Update in the molecular mechanism and biomarkers of diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167758. [Google Scholar] [CrossRef]
- Ramos, H.; Hernández, C.; Simó, R.; Simó-Servat, O. Inflammation: The Link between Neural and Vascular Impairment in the Diabetic Retina and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 8796. [Google Scholar] [CrossRef]
- Little, K.; Llorián-Salvador, M.; Scullion, S.; Hernández, C.; Simó-Servat, O.; Del Marco, A.; Bosma, E.; Vargas-Soria, M.; Carranza-Naval, M.J.; Van Bergen, T.; et al. Common pathways in dementia and diabetic retinopathy: Understanding the mechanisms of diabetes-related cognitive decline. Trends Endocrinol. Metab. 2022, 33, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Simó-Servat, O.; Porta, M.; Grauslund, J.; Harding, S.P.; Frydkjaer-Olsen, U.; García-Arumí, J.; Ribeiro, L.; Scanlon, P.; Cunha-Vaz, J.; et al. Serum glial fibrillary acidic protein and neurofilament light chain as biomarkers of retinal neurodysfunction in early diabetic retinopathy: Results of the EUROCONDOR study. Acta Diabetol. 2023, 60, 837–844. [Google Scholar] [CrossRef]
- Hajari, J.N.; Ilginis, T.; Pedersen, T.T.; Lønkvist, C.S.; Saunte, J.P.; Hofsli, M.; Schmidt, D.C.; Al-Abaiji, H.A.; Ahmed, Y.; Bach-Holm, D.; et al. Novel Blood-Biomarkers to Detect Retinal Neurodegeneration and Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2025, 26, 2625. [Google Scholar] [CrossRef]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140195. [Google Scholar] [CrossRef]
- Aung, M.H.; Park, H.N.; Han, M.K.; Obertone, T.S.; Abey, J.; Aseem, F.; Thule, P.M.; Iuvone, P.M.; Pardue, M.T. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J. Neurosci. 2014, 34, 726–736. [Google Scholar] [CrossRef]
- Gastinger, M.J.; Kunselman, A.R.; Conboy, E.E.; Bronson, S.K.; Barber, A.J. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2635–2642. [Google Scholar] [CrossRef]
- Fragiotta, S.; Pinazo-Durán, M.D.; Scuderi, G. Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients 2022, 14, 792. [Google Scholar] [CrossRef]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Lieth, E.; LaNoue, K.F.; Antonetti, D.A.; Ratz, M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Exp. Eye Res. 2000, 70, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pannicke, T.; Iandiev, I.; Wurm, A.; Uckermann, O.; vom Hagen, F.; Reichenbach, A.; Wiedemann, P.; Hammes, H.P.; Bringmann, A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes 2006, 55, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Antonetti, D.A.; Gardner, T.W. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3561–3568. [Google Scholar]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Kim, K.; Kim, E.S.; Kim, D.G.; Yu, S.Y. Progressive retinal neurodegeneration and microvascular change in diabetic retinopathy: Longitudinal study using OCT angiography. Acta Diabetol. 2019, 56, 1275–1282. [Google Scholar] [CrossRef]
- Park, J.C.; Chau, F.Y.; Lim, J.I.; McAnany, J.J. Electrophysiological and pupillometric measures of inner retina function in nonproliferative diabetic retinopathy. Doc. Ophthalmol. 2019, 139, 99–111. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Z.; Bao, T.; Wang, S.; Jiang, T.; Zhang, Y.; Li, Q.; Zhu, X. Evaluation of Early Retinal Nerve Injury in Type 2 Diabetes Patients Without Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 475672. [Google Scholar] [CrossRef]
- McAnany, J.J.; Persidina, O.S.; Park, J.C. Clinical electroretinography in diabetic retinopathy: A review. Surv. Ophthalmol. 2022, 67, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, W.; Guo, C.; Guo, C.; Chen, M. Early diagnosis of retinal neurovascular injury in diabetic patients without retinopathy by quantitative analysis of OCT and OCTA. Acta Diabetol. 2023, 60, 1063–1074. [Google Scholar] [CrossRef]
- Tyrberg, M.; Lindblad, U.; Melander, A.; Lövestam-Adrian, M.; Ponjavic, V.; Andréasson, S. Electrophysiological studies in newly onset type 2 diabetes without visible vascular retinopathy. Doc. Ophthalmol. 2011, 123, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.; Cortese, F.; Nilsson, J.; Westall, C. Analysis of multifocal electroretinograms from a population with type 1 diabetes using partial least squares reveals spatial and temporal distribution of changes to retinal function. Doc. Ophthalmol. 2012, 125, 31–42. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, E.E.; Schouten, J.S.; Berendschot, T.T.; Kessels, A.G.; Nuijts, R.M.; Beckers, H.J.; Schram, M.T.; Stehouwer, C.D.; Webers, C.A. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: A systematic review. Lancet Diabetes Endocrinol. 2015, 3, 653–663. [Google Scholar] [CrossRef]
- Shoji, T.; Sakurai, Y.; Sato, H.; Chihara, E.; Takeuchi, M. Do type 2 diabetes patients without diabetic retinopathy or subjects with impaired fasting glucose have impaired colour vision? The Okubo Color Study Report. Diabet. Med. 2011, 28, 865–871. [Google Scholar] [CrossRef]
- Tan, N.C.; Yip, W.F.; Kallakuri, S.; Sankari, U.; Koh, Y.L.E. Factors associated with impaired color vision without retinopathy amongst people with type 2 diabetes mellitus: A cross-sectional study. BMC Endocr. Disord. 2017, 17, 29. [Google Scholar] [CrossRef]
- Safi, H.; Safi, S.; Hafezi-Moghadam, A.; Ahmadieh, H. Early detection of diabetic retinopathy. Surv. Ophthalmol. 2018, 63, 601–608. [Google Scholar] [CrossRef]
- Bloodworth, J.M. Diabetic retinopathy. Diabetes 1962, 11, 1–22. [Google Scholar]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Takano, M.; Sango, K.; Horie, H.; Sato, M.; Iijima, Y.; Ohno, S.; Inoue, S.; Ishikawa, Y. Diabetes alters neurite regeneration from mouse retinal explants in culture. Neurosci. Lett. 1999, 275, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A.; Penn State Retina Research Group. Retinal neurodegeneration: Early pathology in diabetes. Clin. Exp. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Lorenzi, M.; Gerhardinger, C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia 2001, 44, 791–804. [Google Scholar] [CrossRef]
- Fletcher, E.L.; Phipps, J.A.; Wilkinson-Berka, J.L. Dysfunction of retinal neurons and glia during diabetes. Clin. Exp. Optom. 2005, 88, 132–145. [Google Scholar] [CrossRef]
- Asnaghi, V.; Gerhardinger, C.; Hoehn, T.; Adeboje, A.; Lorenzi, M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes 2003, 52, 506–511. [Google Scholar] [CrossRef]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- Oshitari, T.; Yamamoto, S.; Hata, N.; Roy, S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. Br. J. Ophthalmol. 2008, 92, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Gajree, S.; Borooah, S.; Dhillon, B. Imaging in Diabetic Retinopathy: A Review of Current and Future Techniques. Curr. Diabetes Rev. 2017, 13, 26–34. [Google Scholar] [CrossRef]
- Crincoli, E.; Sacconi, R.; Querques, L.; Querques, G. OCT angiography 2023 update: Focus on diabetic retinopathy. Acta Diabetol. 2024, 61, 533–541. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Simão, S.; Costa, M.Â.; Sun, J.K.; Cunha-Vaz, J.; Simó, R.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Development of a Normative Database for Multifocal Electroretinography in the Context of a Multicenter Clinical Trial. Ophthalmic Res. 2017, 57, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Baccouche, B. Neurodegeneration in diabetic retinopathy: Potential for novel therapies. Vision. Res. 2017, 139, 82–92. [Google Scholar] [CrossRef]
- Reste-Ferreira, D.; Marques, I.P.; Santos, T.; Ribeiro, M.L.; Mendes, L.; Santos, A.R.; Lobo, C.; Cunha-Vaz, J. Retinal neurodegeneration in eyes with NPDR risk phenotypes: A two-year longitudinal study. Acta Ophthalmol. 2024, 102, e539–e547. [Google Scholar] [CrossRef]
- Lott, M.E.; Slocomb, J.E.; Shivkumar, V.; Smith, B.; Gabbay, R.A.; Quillen, D.; Gardner, T.W.; Bettermann, K. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol. 2012, 90, e434–e441. [Google Scholar] [CrossRef]
- Tecilazich, F.; Feke, G.T.; Mazzantini, S.; Sobrin, L.; Lorenzi, M. Defective Myogenic Response of Retinal Vessels Is Associated with Accelerated Onset of Retinopathy in Type 1 Diabetic Individuals. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.B.; Frydkjaer-Olsen, U.; Grauslund, J. Vascular Changes and Neurodegeneration in the Early Stages of Diabetic Retinopathy: Which Comes First? Ophthalmic Res. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- Santos, A.R.; Ribeiro, L.; Bandello, F.; Lattanzio, R.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; Gibson, J.; Grauslund, J.; Harding, S.P.; et al. Functional and Structural Findings of Neurodegeneration in Early Stages of Diabetic Retinopathy: Cross-sectional Analyses of Baseline Data of the EUROCONDOR Project. Diabetes 2017, 66, 2503–2510. [Google Scholar] [CrossRef]
- Sacconi, R.; Lamanna, F.; Borrelli, E.; Mulinacci, G.; Casaluci, M.; Gelormini, F.; Carnevali, A.; Querques, L.; Zerbini, G.; Bandello, F.; et al. Morphofunctional analysis of the retina in patients with type 1 diabetes without complications after 30 years of disease. Sci. Rep. 2020, 10, 206. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. New Insights into Treating Early and Advanced Stage Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 23, 8513. [Google Scholar] [CrossRef]
- Sachdeva, M.M. Retinal Neurodegeneration in Diabetes: An Emerging Concept in Diabetic Retinopathy. Curr. Diabetes Rep. 2021, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Klahan, B.; O’Reilly, N.J.; Sigurdsson, H.H.; Chauhan, A.; Mering, S.; Fitzhenry, L. Delivery of Fenofibrate to Ocular Tissues using 2-Hydroxypropyl-beta-cyclodextrin-Based Micelles. Int. J. Pharm. 2025, 673, 125417. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Hernández, C.; Corraliza, L.; Carvalho, A.R.; Simó, R. Effect of fenofibrate on retinal neurodegeneration in an experimental model of type 2 diabetes. Acta Diabetol. 2015, 52, 113–122. [Google Scholar] [CrossRef]
- Preiss, D.; Logue, J.; Sammons, E.; Zayed, M.; Emberson, J.; Wade, R.; Wallendszus, K.; Stevens, W.; Cretney, R.; Harding, S.; et al. Effect of Fenofibrate on Progression of Diabetic Retinopathy. NEJM Evid. 2024, 3, EVIDoa2400179. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. Fenofibrate for Treating Diabetic Eye Disease. Diabetes 2023, 72, 838–840. [Google Scholar] [CrossRef]
- Enright, J.M.; Zhang, S.; Thebeau, C.; Siebert, E.; Jin, A.; Gadiraju, V.; Zhang, X.; Chen, S.; Semenkovich, C.F.; Rajagopal, R. Fenofibrate Reduces the Severity of Neuroretinopathy in a Type 2 Model of Diabetes without Inducing Peroxisome Proliferator-Activated Receptor Alpha-Dependent Retinal Gene Expression. J. Clin. Med. 2020, 10, 126. [Google Scholar] [CrossRef]
- Hanaguri, J.; Nagai, N.; Yokota, H.; Kushiyama, A.; Watanabe, M.; Yamagami, S.; Nagaoka, T. Fenofibrate Nano-Eyedrops Ameliorate Retinal Blood Flow Dysregulation and Neurovascular Coupling in Type 2 Diabetic Mice. Pharmaceutics 2022, 14, 384. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Solà-Adell, C.; Bogdanov, P.; Hernández, C.; Simó, R. Mechanisms of retinal neuroprotection of calcium dobesilate: Therapeutic implications. Neural Regen. Res. 2017, 12, 1620–1622. [Google Scholar] [CrossRef]
- Bogdanov, P.; Solà-Adell, C.; Hernández, C.; García-Ramírez, M.; Sampedro, J.; Simó-Servat, O.; Valeri, M.; Pasquali, C.; Simó, R. Calcium dobesilate prevents the oxidative stress and inflammation induced by diabetes in the retina of db/db mice. J. Diabetes Complicat. 2017, 31, 1481–1490. [Google Scholar] [CrossRef]
- Solà-Adell, C.; Bogdanov, P.; Hernández, C.; Sampedro, J.; Valeri, M.; Garcia-Ramirez, M.; Pasquali, C.; Simó, R. Calcium Dobesilate Prevents Neurodegeneration and Vascular Leakage in Experimental Diabetes. Curr. Eye Res. 2017, 42, 1273–1286. [Google Scholar] [CrossRef]
- Roberts, R.; Luan, H.; Berkowitz, B.A. Blocking ET-1 receptors does not correct subnormal retinal oxygenation response in experimental diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3550–3555. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Simó-Servat, O.; Sampedro, J.; Solà-Adell, C.; Garcia-Ramírez, M.; Ramos, H.; Guerrero, M.; Suñé-Negre, J.M.; Ticó, J.R.; Montoro, B.; et al. Topical Administration of Bosentan Prevents Retinal Neurodegeneration in Experimental Diabetes. Int. J. Mol. Sci. 2018, 19, 3578. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.C.; Rollins, S.D.; Ye, M.; Batlle, D.; Fawzi, A.A. Endothelin receptor-A antagonist attenuates retinal vascular and neuroretinal pathology in diabetic mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2516–2525. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Rojas, M.; Caldwell, R.W.; Caldwell, R.B. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 2011, 3, 609–628. [Google Scholar] [CrossRef]
- Ye, Q.; Lin, Y.N.; Xie, M.S.; Yao, Y.H.; Tang, S.M.; Huang, Y.; Wang, X.H.; Zhu, Y.H. Effects of etanercept on the apoptosis of ganglion cells and expression of Fas, TNF-alpha, caspase-8 in the retina of diabetic rats. Int. J. Ophthalmol. 2019, 12, 1083–1088. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, I.; Das, G.K.; Sahu, P.K.; Rohatgi, J. Commentary: Recent concepts of pathophysiology and advancements in treatment strategies of diabetic retinopathy. Indian. J. Ophthalmol. 2021, 69, 3050–3051. [Google Scholar] [CrossRef]
- Baker, B.J.; Akhtar, L.N.; Benveniste, E.N. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009, 30, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, C.M.; Johnson, H.M.; Lewin, A.S. Corneal application of SOCS1/3 peptides for the treatment of eye diseases mediated by inflammation and oxidative stress. Front. Immunol. 2024, 15, 1416181. [Google Scholar] [CrossRef]
- Ahmed, C.M.; Patel, A.P.; Johnson, H.M.; Ildefonso, C.J.; Lewin, A.S. Suppressor of cytokine signaling 3-derived peptide as a therapeutic for inflammatory and oxidative stress-induced damage to the retina. Mol. Vis. 2023, 29, 338–356. [Google Scholar]
- Hattori, Y.; Hashizume, K.; Nakajima, K.; Nishimura, Y.; Naka, M.; Miyanaga, K. The effect of long-term treatment with sulindac on the progression of diabetic retinopathy. Curr. Med. Res. Opin. 2007, 23, 1913–1917. [Google Scholar] [CrossRef]
- Robles-Rivera, R.R.; Castellanos-González, J.A.; Olvera-Montaño, C.; Flores-Martin, R.A.; López-Contreras, A.K.; Arevalo-Simental, D.E.; Cardona-Muñoz, E.G.; Roman-Pintos, L.M.; Rodríguez-Carrizalez, A.D. Adjuvant Therapies in Diabetic Retinopathy as an Early Approach to Delay Its Progression: The Importance of Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2020, 2020, 3096470. [Google Scholar] [CrossRef] [PubMed]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Hierro-Bujalance, C.; Ortegon, E.; Pickett, E.; Jackson, R.; Hernandez-Pacho, F.; Spires-Jones, T.; Garcia-Alloza, M. Antidiabetic Polypill Improves Central Pathology and Cognitive Impairment in a Mixed Model of Alzheimer’s Disease and Type 2 Diabetes. Mol. Neurobiol. 2018, 55, 6130–6144. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-Vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Haydinger, C.D.; Oliver, G.F.; Ashander, L.M.; Smith, J.R. Oxidative Stress and Its Regulation in Diabetic Retinopathy. Antioxidants 2023, 12, 1649. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Gao, S.; Li, N.; Huang, H.; Liu, X.; Yao, H.; Shen, X. Pigment epithelium-derived factor exerts neuroprotection in oxygen-induced retinopathy by targeting endoplasmic reticulum stress and oxidative stress. Exp. Eye Res. 2024, 249, 110147. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Q.; Li, Y.; Zeng, L.; Liu, J.; Ou, K. On implications of somatostatin in diabetic retinopathy. Neural Regen. Res. 2024, 19, 1984–1990. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef]
- Bogdanov, P.; Ramos, H.; Valeri, M.; Deàs-Just, A.; Huerta, J.; Simó, R.; Hernández, C. Minimum Effective Dose of DPP-4 Inhibitors for Treating Early Stages of Diabetic Retinopathy in an Experimental Model. Biomedicines 2022, 10, 465. [Google Scholar] [CrossRef]
- Ramos, H.; Augustine, J.; Karan, B.M.; Hernández, C.; Stitt, A.W.; Curtis, T.M.; Simó, R. Sitagliptin eye drops prevent the impairment of retinal neurovascular unit in the new Trpv2+/− rat model. J. Neuroinflammation 2024, 21, 312. [Google Scholar] [CrossRef]
- Simó, R.; Simó-Servat, O.; Bogdanov, P.; Hernández, C. Neurovascular Unit: A New Target for Treating Early Stages of Diabetic Retinopathy. Pharmaceutics 2021, 13, 1320. [Google Scholar] [CrossRef]
- Attia, M.S.; Ayman, F.; Attia, M.S.; Yahya, G.; Zahra, M.H.; Khalil, M.M.I.; Diab, A.A.A. Mitigating diabetes-related complications: Empowering metformin with cholecalciferol and taurine supplementation in type 2 diabetic rats. World J. Diabetes 2024, 15, 1778–1792. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M. Diabetic retinopathy and dysregulated innate immunity. Vision. Res. 2017, 139, 39–46. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, G.; Maestroni, S.; Leocani, L.; Mosca, A.; Godi, M.; Paleari, R.; Belvedere, A.; Gabellini, D.; Tirassa, P.; Castoldi, V.; et al. Topical nerve growth factor prevents neurodegenerative and vascular stages of diabetic retinopathy. Front. Pharmacol. 2022, 13, 1015522. [Google Scholar] [CrossRef]
- Tanase, D.M.; Valasciuc, E.; Gosav, E.M.; Floria, M.; Buliga-Finis, O.N.; Ouatu, A.; Cucu, A.I.; Botoc, T.; Costea, C.F. Enhancing Retinal Resilience: The Neuroprotective Promise of BDNF in Diabetic Retinopathy. Life 2025, 15, 263. [Google Scholar] [CrossRef]
- Castoldi, V.; Zerbini, G.; Maestroni, S.; Viganò, I.; Rama, P.; Leocani, L. Topical Nerve Growth Factor (NGF) restores electrophysiological alterations in the Ins2Akita mouse model of diabetic retinopathy. Exp. Eye Res. 2023, 237, 109693. [Google Scholar] [CrossRef]
- Pelusi, L.; Hurst, J.; Detta, N.; Pipino, C.; Lamolinara, A.; Conte, G.; Mastropasqua, R.; Allegretti, M.; Di Pietrantonio, N.; Romeo, T.; et al. Effects of mesenchymal stromal cells and human recombinant Nerve Growth Factor delivered by bioengineered human corneal lenticule on an innovative model of diabetic retinopathy. Front. Endocrinol. 2024, 15, 1462043. [Google Scholar] [CrossRef]
- Fiori, A.; Terlizzi, V.; Kremer, H.; Gebauer, J.; Hammes, H.P.; Harmsen, M.C.; Bieback, K. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology 2018, 223, 729–743. [Google Scholar] [CrossRef]
- Lechner, J.; Medina, R.J.; Lois, N.; Stitt, A.W. Advances in cell therapies using stem cells/progenitors as a novel approach for neurovascular repair of the diabetic retina. Stem Cell Res. Ther. 2022, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Kąpa, M.; Koryciarz, I.; Kustosik, N.; Jurowski, P.; Pniakowska, Z. Future Directions in Diabetic Retinopathy Treatment: Stem Cell Therapy, Nanotechnology, and PPARα Modulation. J. Clin. Med. 2025, 14, 683. [Google Scholar] [CrossRef] [PubMed]

| Name of Drugs | Properties |
|---|---|
| Fenofibrate | Neuroprotective and vasculotropic effect: reduction in inflammation, oxidative stress and anti-apoptotic activity [102,103,104,105,106,107] |
| Calcium dobesilate | Neuroprotective and vasculotropic effect: reduction in inflammation, oxidative stress, anti-apoptotic activity, and blockade of ET-1, ETA-R and ETB-R [108,109,110] |
| Endothelin receptor blockers | Neuroprotective (ETB-R) and vasculotropic (ETA-R) effects [111,112,113] |
| TNF-alpha blockers | Anti-inflammatory and neuroprotective effect [54,114,115,116] |
| SOCS proteins | Neuroprotective and vasculotropic effect: inhibition of neuroinflammation and vascular leakage [117,118,119] |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | Anti-inflammatory and neuroprotective effect [54,120,121,122] |
| Antioxidants | Neuroprotective and vasculoprotective effect [123,124,125] |
| Peptides with neurotrophic and anti-angiogenic properties: PEDF, GLP-1, GLP-1 receptor agonists, DPP-IV inhibitors, somatostatin | Neuroprotective and vasculotropic effect. Activity on synaptic connectivity (DPP-IV inhibitors and GLP-1 receptor agonists) [126,127,128,129,130,131] |
| Taurine and lutein | Improvement of synaptic connections [132,133] |
| Modulators of complement activation | Protect from early synaptic loss and dendritic atrophy induced by complement cascade activation [131,134,135] |
| Neurotrophins, nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) | Neuroprotective, neurotrophic and vasculoprotective effects [136,137,138,139] |
| Stem cell-based treatments | Neuroprotective effect exerted either by the secretion of specific growth factors (FGF, VEGF, IGF, NGF, BDNF) or through the direct integration into the retina [140,141,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viganò, I.; Galbiati, S.; Aragona, E.; Gabellini, D.; Lattanzio, R.; Pedon, V.; Basile, G.; Arrigo, A.; Bandello, F.; Zerbini, G. Diabetes-Driven Retinal Neurodegeneration: Its Role in the Pathogenesis of Diabetic Retinopathy. Biomedicines 2025, 13, 1328. https://doi.org/10.3390/biomedicines13061328
Viganò I, Galbiati S, Aragona E, Gabellini D, Lattanzio R, Pedon V, Basile G, Arrigo A, Bandello F, Zerbini G. Diabetes-Driven Retinal Neurodegeneration: Its Role in the Pathogenesis of Diabetic Retinopathy. Biomedicines. 2025; 13(6):1328. https://doi.org/10.3390/biomedicines13061328
Chicago/Turabian StyleViganò, Ilaria, Silvia Galbiati, Emanuela Aragona, Daniela Gabellini, Rosangela Lattanzio, Vittoria Pedon, Giulia Basile, Alessandro Arrigo, Francesco Bandello, and Gianpaolo Zerbini. 2025. "Diabetes-Driven Retinal Neurodegeneration: Its Role in the Pathogenesis of Diabetic Retinopathy" Biomedicines 13, no. 6: 1328. https://doi.org/10.3390/biomedicines13061328
APA StyleViganò, I., Galbiati, S., Aragona, E., Gabellini, D., Lattanzio, R., Pedon, V., Basile, G., Arrigo, A., Bandello, F., & Zerbini, G. (2025). Diabetes-Driven Retinal Neurodegeneration: Its Role in the Pathogenesis of Diabetic Retinopathy. Biomedicines, 13(6), 1328. https://doi.org/10.3390/biomedicines13061328







