Abstract
Obesity is a major contributor to male infertility, not only exacerbating infertility but also impairing the effectiveness of both surgical interventions and medical treatments. This review examines the complex relationship between obesity, the immune microenvironment, and male infertility, highlighting how obesity-induced changes in immune function lead to testicular dysfunction and impaired spermatogenesis. Key mechanisms include chronic low-grade inflammation, immune cell infiltration, and dysregulated adipokines such as leptin and adiponectin. We also explore current therapeutic strategies aimed at alleviating these effects, including lifestyle interventions, anti-inflammatory treatments, metabolic therapies, and regenerative medicine approaches, such as exosome-based therapies. Despite promising results, substantial research gaps remain, particularly in understanding the molecular mechanisms and identifying novel biomarkers for early diagnosis. Future studies should focus on multi-omics approaches, large-scale cohort studies, the gut–testis axis, and the psychological and social factors influencing male infertility. A deeper understanding of these processes is crucial for developing more effective, targeted therapies for obesity-related male infertility.
1. Introduction
Obesity has become a significant global health issue, and its association with male infertility is garnering increasing attention. Studies have shown that male factors account for approximately 30–50% of infertility cases, with obesity and its related metabolic abnormalities being important contributing factors [1]. The World Health Organization (WHO) estimates that about 10–15% of couples of reproductive age globally face fertility issues, with male factors contributing to approximately 50% of these cases [2,3]. Obesity is associated with a range of reproductive health issues in men, including varicocele (VC), testicular dysfunction, compromised sperm quality, and immune dysregulation, all of which significantly impact male fertility [4,5,6]. Studies indicate that 35–40% of infertile men have VC, with overweight and obese males showing a lower prevalence of left and bilateral VC, but a 63.3% increased prevalence of right VC [4,7]. Obese men have a 42% higher likelihood of having oligospermia and an 81% higher likelihood of having azoospermia compared to men with normal weight [8].
The mechanisms linking obesity to impaired male reproductive function are multifaceted, involving several biological pathways. These include hypogonadism [9], testicular heat stress and hypoxia-induced apoptosis, and endocrine disruption by obesogens [10]. The vicious cycle between obesity and hypogonadism is closely tied to metabolic changes, such as insulin resistance. Leptin’s regulatory role in this process may be compromised by resistance, which in turn affects male reproductive health and testicular function. Abdominal fat accumulation due to obesity can raise intra-abdominal pressure, affecting blood return in the pampiniform venous plexus, leading to venous congestion and dilation [11]. This can also increase scrotal temperature, inducing Deoxyribonucleic Acid (DNA) repair impairment mediated by Heat Shock Factor 1 (HSF1), and exacerbating oxidative stress and apoptosis. VC also leads to local oxidative damage in the testes [12]. The combination of these factors may jointly impair male fertility through chronic inflammation, oxidative stress, and the disruption of spermatogenesis. Moreover, endocrine disruptors in obesity-promoting diets can interfere with the hypothalamic–pituitary–gonadal axis, further impairing reproductive function [13].
Obesity is a metabolic disease caused by genetic and behavioral factors. In addition to being closely associated with various chronic diseases such as metabolic syndrome, hyperlipidemia, type 2 diabetes, and cardiovascular diseases, recent studies have begun to reveal the connection between obesity and immune system dysregulation [14,15,16]. The interaction between metabolism and the immune system is mediated by various factors, including immune cells, adipocytes, and the systemic inflammation induced by obesity [17]. In the context of male infertility, the immune system may mount an immune response against sperm, compromising their normal function. This immune reaction can result in decreased sperm quality, reduced motility, and even sperm death. Autoimmune factors are not uncommon in male infertility, yet the precise mechanisms underlying these immune-mediated processes remain incompletely understood [18]. The immune system’s role extends beyond mere immune responses; it interacts closely with metabolic and endocrine systems, creating a complex interplay that can profoundly affect reproductive health. Obesity-induced changes in the immune microenvironment, such as chronic inflammation and increased levels of cytokines and reactive oxygen species (ROS), can further exacerbate the detrimental effects on sperm production and function.
Although numerous systematic reviews and meta-analyses have established a significant association between obesity and male infertility—primarily highlighting the statistical correlations between increased body mass index (BMI) and impaired semen parameters (such as sperm count, motility, and morphology), as well as the dysregulation of reproductive hormones (e.g., testosterone, estradiol, inhibin B)—most studies remain confined to the epidemiological level. The underlying biological mechanisms, particularly the role of immune-related pathways, have yet to be thoroughly investigated. Based on our team’s long-term experience in clinical research on male infertility, this review focuses on the testicular immune microenvironment as a central theme. For the first time, we systematically propose a mechanistic framework wherein obesity disrupts immune homeostasis, thereby impairing spermatogenesis. We provide an in-depth analysis of how chronic low-grade inflammation—through inflammatory cytokines, adipokines, and oxidative stress—compromises immune tolerance, damages the blood–testis barrier, and inhibits sperm production. Furthermore, we integrate and summarize the regulatory roles of several key signaling pathways involved in this process, including Suppressor of Cytokine Signaling 3/Signal Transducer and Activator of Transcription 3 (SOCS3/STAT3), Focal Adhesion Kinase (FAK), Nuclear Factor Erythroid 2-Related Factor 2/Mitogen-Activated Protein Kinases (NRF2/MAPKs), and Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/AKT), thereby constructing a more focused and explanatory mechanistic model distinct from the generalized listings found in previous narrative reviews. This reflects both academic originality and depth. On the basis of mechanistic insights, we also evaluate the translational potential of various intervention strategies, such as anti-inflammatory therapy, metabolic improvement, and advanced regenerative approaches involving exosomes. Finally, we propose that future studies should emphasize multi-omics integration, large-scale prospective cohort studies, and emerging directions such as the “gut–testis axis”, to support the development and optimization of precision medicine approaches for male infertility.
The structure of this review is as follows: Section 2 introduces the physiological role of the testicular immune microenvironment; Section 3 discusses the specific immune dysregulations induced by obesity; Section 4 integrates basic and clinical evidence to elucidate the causal links among obesity, immune imbalance, and male infertility; and the final section presents therapeutic strategies targeting immune mechanisms and outlines future research directions aimed at improving male reproductive health under the precision medicine paradigm.
2. Immune Microenvironment and Male Reproductive Health
2.1. Normal Immune Microenvironment in the Male Reproductive System
2.1.1. Overview of Immune Cells and Their Functions in the Testes
The testis, as an important organ of the male reproductive system, primarily functions in sperm production and the synthesis of male hormones [19]. Figure 1 illustrates the structure of the testis, including its immune microenvironment. The testis hosts a unique immune microenvironment composed of various immune cells, such as macrophages, dendritic cells (DCs), and T lymphocytes, situated in the interstitial space [20]. These cells play a critical role in maintaining immune tolerance and supporting normal testicular function under both physiological and pathological conditions by sustaining an immunosuppressive environment that prevents autoimmune attacks on germ cells [21].
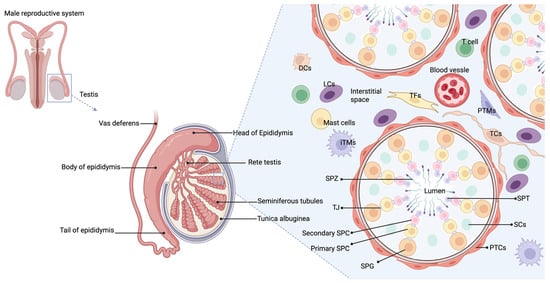
Figure 1.
Structure of the testis.
The testis is composed of seminiferous tubules, interstitial cells, and a complex network of blood vessels and immune cells that support sperm production and hormone synthesis. Immune regulation in the testis is maintained through the production of immunosuppressive factors, which support immune privilege and promote germ cell survival. In rodent testes, resident macrophages, tolerogenic dendritic cells, T cells, and mast cells in the interstitium interact with interstitial cells, Sertoli cells, and peritubular cells to release factors that create a favorable microenvironment. Abbreviations: PTCs (peritubular myoid cells); LCs (Leydig cells); TCs (telocytes); TFs (Testicular fibroblasts); iTMs (Interstitial macrophages); PTMs (Peritubular macrophages); DCs (dendritic cells); SPG (spermatogonia); SPC (spermatocyte); TJ (tight junction); SPT (spermatid); SPZ (spermatozoa); SCs (Sertoli cells); T cell (T lymphocyte).
Macrophages are immune cells with phagocytic characteristics, capable of ingesting foreign substances, pathogens such as bacteria, and clearing cell debris. In the testis, they account for about 20% of the interstitial cells, making them one of the most abundant and heterogeneous immune populations [22]. Under normal conditions, most testicular macrophages (TMs) express core markers such as F4/80, cluster of differentiation (CD)-11b, apoptosis-inducing factor (AIF), and C-X3-C Motif Chemokine Receptor 1 (CX3CR1) [23]. Their heterogeneity is evident from the differential expression of surface markers like CD64 and major histocompatibility complex class II (MHC II) [24]. Two main types of TM exist: interstitial (iTMs) and peritubular (pTMs) macrophages. The former are located in the testicular interstitium, while the latter are near the basal membrane of the seminiferous tubules. iTMs, which are M2-type, express immunosuppressive cytokine Interleukin (IL)-10, whereas pTMs, an M1-type, produce high levels of pro-inflammatory cytokines such as IL-1β [25]. The developmental origins of these TMs are not fully understood: iTMs may derive from embryonic progenitors, while pTMs are thought to originate from bone marrow-derived cells [23]. The testicular microenvironment contains various immune-regulatory molecules, including testosterone, prostaglandins, corticosterone, activin, and 25-hydroxycholesterol (25-HC), which likely influence macrophage phenotype and function [22]. TMs also display immunosuppressive functions, secreting high levels of IL-10 and producing low levels of tumor necrosis factor-alpha (TNF-α) and nitric oxide (NO) upon lipopolysaccharide (LPS) stimulation. However, excessive TNF-α and NO production can compromise the immune privilege of the testis, impairing spermatogenesis and steroidogenesis [26,27]. Additionally, TMs suppress T cell activation and proliferation, promoting the differentiation of naïve T cells into regulatory T cells (Tregs) [28]. Following testicular inflammation (such as after acute LPS stimulation or experimental chronic autoimmune epididymo-orchitis), TM numbers increase, playing a key role in local immune responses and tissue repair [29,30,31].
DCs are professional antigen-presenting cells (APCs) involved in both innate and adaptive immune responses, playing a role in T cell activation and tolerance induction. In the interstitial cells of normal testes, DCs account for about one-tenth of the macrophage population [27]. CD103+ DCs expressing MHCII and co-stimulatory molecules such as CD80 and CD86 have been observed in both normal rat testes and testicular draining lymph nodes (TLNs) [32]. Compared to inflamed testes, DCs in normal testes show lower levels of C-C chemokine receptor (CCR)7 and IL12 subunit p35 messenger RNA (Il12p35 mRNA), indicating an immature state [33]. CCR7 is involved in the migration of DCs to the LNs, while Il12p35 promotes T helper 1 cell (Th1) immune responses. The low expression levels of these molecules in immature DCs suggest they lack the ability to trigger a strong immune response, thereby contributing to the maintenance of testicular immune tolerance and immune privilege. When cultured with Sertoli cells, DCs show reduced expression of surface molecules such as MHCII, CD80, CD86, CCR7, and CD11c, indicating lower immunogenicity [34]. Furthermore, these DCs exhibit enhanced immunoregulatory capabilities, such as inhibiting T cell proliferation and promoting the generation of Foxp3+ Tregs, thereby preventing the immune system from attacking normal testicular tissue [34].
T cells differentiate into effector or regulatory lineages depending on environmental signals, exerting pro-inflammatory or immune-tolerant effects, ultimately leading to either inflammation or immune tolerance [35]. In the testes, CD4+, CD8+ T cells, CD8+ memory T cells, and Foxp3+ Tregs have been identified. Both CD4+ and CD8+ T cells release pro-inflammatory cytokines, such as TNF-α, Interferon gamma (IFN-γ), and Fas Ligand (FasL), with CD8+ T cells being the primary producers of FasL and TNF-α. Under normal conditions, occasional IL-4-expressing T cells are found in the testes [19]. Foxp3+ Tregs are present in normal testes, most of which exhibit a memory phenotype and produce transforming growth factor beta (TGF-β). Tregs are activated by antigens from the seminiferous tubules, triggering proliferative responses and suppressing conventional T cell proliferation, thereby maintaining a tolerogenic environment [36]. Under inflammatory conditions, pathogenic T cells may overwhelm the suppressive function of Tregs, leading to autoimmune responses. Despite the increased suppressive capacity of Tregs from the TLN, they are still unable to prevent germ cell attack [37].
Mast cells (MCs) are primarily localized around the seminiferous tubules in the testis, with an increased number observed under inflammatory conditions, such as spermatogenesis defects, VC, infertility, experimental autoimmune orchitis (EAO), and testicular torsion. The tryptase released by MCs promotes fibroblast proliferation and collagen synthesis, leading to fibrosis of the seminiferous tubules [38].
2.1.2. Blood–Testis Barrier (BTB) and Immune Privilege in Testes
The immune privilege of the testes is maintained by its unique physical structure, systemic immune tolerance, and local immune-suppressive environment, which together prevent immune reactions against germ cells. The BTB, formed by tight junctions between Sertoli cells, divides the seminiferous epithelium into basal and adluminal compartments, and by isolating late-stage germ cells from immune cells, it maintains immune privilege (Figure 2) [39]. The BTB also supports the transition of spermatogonia to sperm and facilitates the movement of pre-leptotene spermatocytes from the basal to the adluminal compartment, a key step in spermatogenesis [40]. Bioactive peptides and molecules secreted by testicular cells regulate the BTB function, further supporting spermatogenesis.
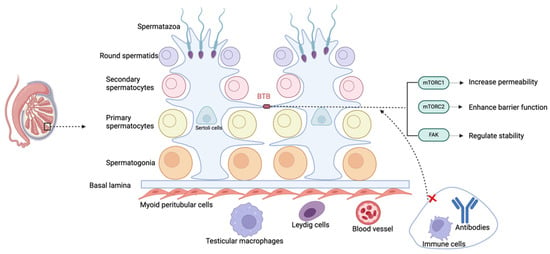
Figure 2.
Schematic representation of the blood–testis barrier (BTB).
Inside the testis, the seminiferous tubules contain spermatogonia and other stages of meiotic cells. Sertoli cells lining these tubules create the BTB by establishing tight junctions between their cytoplasmic extensions, which regulate permeability and help maintain immune privilege. The abbreviations used are as follows: BTB (blood–testis barrier); mTORC1 (Mechanistic Target of Rapamycin Complex 1); mTORC2 (Mechanistic Target of Rapamycin Complex 2); and FAK (Focal Adhesion Kinase).
The BTB limits the entry of immune cells and antibodies into the seminiferous tubules and, through lactate dehydrogenase isoenzyme (LDHc4), reversely crosses the barrier, forming immune complexes to promote immune tolerance [41]. The dynamic regulation of the BTB is controlled by mechanistic Target of Rapamycin (mTOR) and FAK. Mechanistic Target of Rapamycin Complex (mTORC)1 increases its permeability, while mTORC2 strengthens it. Phosphorylation sites of FAK regulate the “leakiness” and stability of the BTB [42,43]. However, environmental pollutants such as molybdenum, cadmium, nickel, arsenic, etc., can damage the BTB through oxidative stress, leading to testicular toxicity and reproductive dysfunction. The BTB also allows for xenografts or allografts in the testicular interstitial space to survive long term, indicating that the testes’ immune privilege is not solely dependent on the structural barrier provided by the BTB [44,45].
2.2. Immune Dysregulation and Male Infertility
2.2.1. Autoimmune Infertility
When the immune system mistakenly identifies sperm as foreign invaders, it produces anti-sperm antibodies (ASAs), triggering an immune response [46]. Under normal conditions, sperm are protected from immune surveillance by the BTB [47]. However, when the BTB is compromised due to injury, surgery, infection, or congenital defects, sperm antigens become exposed, leading to ASA production [48,49,50]. ASAs are an important cause of male immune infertility, with studies showing a prevalence of 3.4% in a large-scale retrospective study [51].
ASAs negatively affect semen parameters, including reduced sperm motility and sperm aggregation. They bind to apoptosis-related proteins such as caspase-3 and hsp70, promoting sperm apoptosis [52]. Additionally, ASAs hinder sperm passage, inhibit fertilization, and affect early embryonic development [53,54,55]. While both IgA- and IgG-type ASAs are commonly present, IgA is more clinically significant, whereas IgG is more effective in activating the complement system, leading to sperm lysis [56]. While not all ASAs affect fertility significantly, their impact varies by semen heterogeneity and gender differences [57]. In addition, when the immune system mistakenly recognizes testicular or epididymal tissue as foreign, especially in cases of genital tract infections, surgical injuries, or trauma, it can lead to chronic inflammation and dysfunction of the testicular or epididymal tissues, causing spermatogenesis disorders, hormonal imbalances, and infertility [58].
2.2.2. Failure of Immune Tolerance
The male reproductive system protects sperm from immune attacks through immune tolerance mechanisms. Under conditions such as trauma, infection, chronic inflammation, autoimmune diseases, and environmental factors, this mechanism may fail, leading to immune cells attacking sperm or reproductive tissues, which can result in infertility. In Treg-depleted mice, mononuclear phagocytes (MPs) project towards and invade the epididymal lumen, leading to a decrease in sperm count and motility, and causing severe fertility defects [59].
2.2.3. Chronic Inflammation
Local inflammation, such as chronic inflammation in the male reproductive system or reproductive tract infections, can lead to an excessive release of inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β), disrupting spermatogenesis and inducing oxidative stress. This, in turn, causes sperm DNA fragmentation (SDF), reduced motility, and even programmed cell death (apoptosis) [60]. Environmental factors, lifestyle habits, and other factors may contribute to the abnormal activation of the immune system, exacerbating this process. Studies have shown that up to 15% of male infertility cases are linked to infections and inflammatory conditions [61]. Additionally, systemic inflammatory response syndrome (SIRS) may exacerbate fertility decline by affecting sperm function. Factors such as obesity, diabetes, and systemic infections can induce a systemic inflammatory state, negatively impacting the reproductive system [62,63]. Studies have shown that increased ROS and oxidative stress are the main causes of male infertility induced by diabetes [64]. Low levels of ROS play a positive regulatory role in sperm function, including promoting spermatogenesis, capacitation, maturation, and fertilization [65]. However, excessive ROS can increase the antioxidant capacity of sperm, leading to oxidative damage. High levels of ROS attack polyunsaturated fatty acids in the sperm cell membrane, causing lipid peroxidation, which disrupts the lipid bilayer of the cell membrane and affects the fluidity of the sperm membrane, material transport, energy metabolism, and immune defense [66]. Oxidative stress damages sperm nuclear and mitochondrial DNA, accelerates sperm apoptosis, and reduces sperm count and semen quality [67]. Clinical evidence shows that the levels of DNA oxidation and apoptosis in sperm from infertile men are significantly higher than in normal men [68]. Hyperglycemia leads to elevated ROS levels, inhibiting the PI3K/AKT and mTOR signaling pathways, which in turn induces abnormal autophagy in testicular cells [64]. The degradation of the autophagy-related protein P62 inhibits the activation of NRF2, weakening the antioxidant capacity of the testis, exacerbating oxidative stress, and creating a vicious cycle [69]. Furthermore, the effects of autophagy on male reproductive function are manifested in several aspects: Leydig cell dysfunction leading to decreased testosterone secretion, inhibited Sertoli cell proliferation affecting spermatogenesis, BTB disruption weakening immune protection, PI3K/AKT/mTOR signaling inhibition resulting in sperm deformities and decreased motility, and impaired spermatogonia proliferation affecting spermatogenesis [69,70,71,72,73].
3. Obesity and Its Impact on the Immune System
3.1. Chronic Low-Grade Inflammation
Chronic low-grade inflammation induced by obesity is a key driver of various metabolic disorders, particularly playing a significant role in insulin resistance and vascular dysfunction. The following discusses how obesity activates inflammatory responses through multiple signaling pathways, leading to the onset of chronic low-grade inflammation.
In obesity, the enlargement of adipose tissue, particularly abdominal fat, is a major contributor to chronic low-grade inflammation. As adipocytes increase in size, cellular metabolic activity intensifies, leading to higher levels of stress, including oxidative stress and endoplasmic reticulum (ER) stress. These stressors activate key inflammatory pathways such as Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) in the liver, promoting the production of pro-inflammatory cytokines like IL-6, IL-1β, and TNF-α, which together foster insulin resistance and low-grade inflammation [74]. Furthermore, both metabolic and ER stress can generate ROS, which further enhance the expression of TNF-α in Tamm-Horsfall Protein-1 (THP-1) cells through ROS/ C/EBP homologous protein (CHOP)/Hypoxia-Inducible Factor 1 alpha (HIF-1α) and MAPK/NF-κB-dependent mechanisms [75]. High-fat diets (HFDs) exacerbate this process by activating the Jun N-terminal kinases (JNK), TNF-α, and NF-κB signaling pathways, thereby amplifying inflammation and insulin resistance. Interestingly, raspberry treatment has been shown to reduce the levels of TNF-α, IL-1β, IL-6, and the phosphorylation of JNK in HFD mice, improving insulin sensitivity in skeletal muscle via AMP-activated protein kinase-α1 activation [76]. Additionally, SOCS proteins, which negatively regulate cytokine signaling in the Janus Kinase (JAK)/STAT pathway, play a significant role in promoting inflammation in obesity. The upregulation of SOCS3 and SOCS1 in tissues like muscle, liver, and adipose further inhibits insulin signaling while exacerbating inflammation by stimulating p38MAPK and STAT3 phosphorylation, without affecting anti-inflammatory cytokines [77]. In Toll-Like Receptor 4 (TLR4) mice, HFD-induced obesity activates phosphorylated Akt (pAkt) in the liver, with TLR4 signaling—via NF-κB activation—leading to palmitic acid-induced vascular inflammation and insulin resistance, disrupting endothelial NO signaling [78]. Lastly, noncanonical Wingless/Int (Wnt) signaling, particularly through Wnt5a, has been linked to increased adipose tissue inflammation and insulin resistance, promoting the release of pro-inflammatory cytokines independent of adipose tissue expansion [79]. In summary, obesity-induced inflammation is a multifaceted process involving the activation of multiple signaling pathways, including NF-κB, JAK/STAT, MAPK, and noncanonical Wnt signaling.
3.2. Adipokines
Adipokines are peptides that convey the functional status of adipose tissue to organs like the brain, liver, pancreas, immune system, blood vessels, and muscles, regulating energy balance, fat metabolism, inflammation, and other physiological processes through interactions with various cells. Their secretion is altered when adipose tissue becomes dysfunctional [80].
Under physiological conditions, circulating leptin levels are positively correlated with white adipose tissue (WAT) mass. In overweight and obese individuals, leptin levels increase significantly due to the expansion of adipose tissue, and it is recognized as a crucial pro-inflammatory adipokine [81]. In the central nervous system, leptin regulates energy homeostasis by inducing anorexigenic factors and inhibiting orexigenic factors, thereby reducing food intake and promoting energy expenditure [82]. Given its dual nature as both a hormone and cytokine, leptin serves as a critical link between the neuroendocrine and immune systems [83]. In the immune system, leptin modulates both innate and adaptive immune responses through multiple mechanisms. It promotes monocyte and macrophage activation, enhances neutrophil chemotaxis, and stimulates natural killer (NK) cell development and pro-inflammatory cytokine release. In adaptive immunity, leptin promotes the proliferation of naïve T and B cells, suppresses Treg cell differentiation, and drives the polarization of TH1 cells toward a pro-inflammatory phenotype while increasing T helper 17 cell (TH17) proliferation and reactivity [84]. Leptin plays a time- and dose-dependent role in regulating immune cell function and metabolism, with its specific mechanisms remaining unclear under different experimental conditions. Resistin and apelin are thought to promote inflammation by inducing the secretion of pro-inflammatory cytokines [85,86].
On the contrary, adiponectin has anti-inflammatory and immune-regulatory effects, but its plasma levels are significantly reduced in obesity, weakening its anti-inflammatory effects and leading to worsened chronic low-grade inflammation and metabolic disorders [87]. Adiponectin also affects the function of myeloid monocytes, which are key regulators of innate immunity, and negatively impacts the phagocytic activity of macrophages [88]. The dysregulation of adipokines, particularly the imbalance between pro-inflammatory and anti-inflammatory adipokines, is a central mechanism underlying obesity.
3.3. Immune Cell Infiltration
Under normal conditions, the immune environment in adipose tissue is maintained by anti-inflammatory cells such as Tregs and M2 macrophages to ensure tissue homeostasis. However, in obesity, the infiltration of immune cells significantly increases, and at the same time, the metabolism of these cells undergoes a shift, driving immune cells to transition from an oxidative phosphorylation-dependent metabolic mode to one that prefers glycolysis. For example, macrophages shift from the anti-inflammatory M2 phenotype to the pro-inflammatory M1 phenotype, secreting more pro-inflammatory cytokines (such as TNF-α and IL-6). This process is partially mediated through the monocyte chemoattractant protein-1 (MCP-1)/ CCR2 pathway derived from hypertrophic adipocytes, which promotes the accumulation of more macrophages in the adipose tissue of obese individuals [89]. Additionally, the increased extracellular lipid concentration in the local environment is a key mechanism leading to macrophage accumulation, inducing lipid metabolism disorders in macrophages and exacerbating inflammatory responses. In the early stages of obesity, neutrophils rapidly infiltrate adipose tissue, induced by IL-8 secreted by adipocytes and macrophages, as well as lipid peroxidation products. They produce ROS and neutrophil elastase (NE), amplifying oxidative stress and sustaining the pro-inflammatory environment [90,91]. The long-term upregulation of high levels of leptin and MHC II molecules promotes the differentiation of CD4+ T cells and CD8+ T cells into pro-inflammatory subtypes (such as Th1 and Th17) [92], which alters the function of APCs and adipose tissue macrophages, enhancing the inflammatory response and inhibiting the function of Tregs, thus leading to immune dysregulation and the suppression of immune defense against infections [93]. Obesity also promotes the infiltration of B cells into visceral adipose tissue and activates T cells through MHC II-mediated antigen presentation, which further enhances the activation of M1 macrophages and the inflammatory response in adipose tissue [94]. Moreover, obesity promotes the formation of a specific NK cell subpopulation through the IL-6/Stat3 signaling pathway, which expresses IL6Ra and Colony Stimulating Factor 1 Receptor (Csf1r), contributing to the onset of obesity, insulin resistance, and associated inflammation [95]. Overall, obesity alters the immune environment through multiple mechanisms, driving the infiltration, metabolic changes, and functional alterations of immune cells. These changes together promote chronic inflammatory responses in adipose tissue.
4. Intersection of Obesity, Immune Microenvironment, and Male Infertility
Obesity can directly or indirectly lead to male reproductive dysfunction by altering the immune microenvironment of the testes. The immune microenvironment of the testes plays a key role in maintaining immune tolerance and tissue homeostasis. However, chronic low-grade inflammation, immune cell imbalance, and metabolic abnormalities induced by obesity disrupt this balance, increasing the risk of male infertility (Table 1).

Table 1.
Immune microenvironment dysregulation in obesity: implications for male testicular function and infertility.
This table summarizes the impact of obesity on testicular function through the modulation of inflammatory cytokines, oxidative stress, hormonal imbalance, and adipokine dysregulation. These mechanisms disrupt the testicular immune microenvironment, impair spermatogenesis, and lead to reduced sperm quality and hormonal dysfunction. Abbreviations: IL-6 (Interleukin-6); TNF-α (Tumor Necrosis Factor-alpha); SOCS3 (Suppressor of Cytokine Signaling 3); STAT3 (Signal Transducer and Activator of Transcription 3); NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells); NLRP3 (NOD-like receptor family, pyrin domain containing 3); BTB (blood–testis barrier); ZO-1 (Zonula Occludens-1); CX43 (Connexin 43); PI3K (Phosphoinositide 3-Kinase); cAMP-PKA (Cyclic Adenosine Monophosphate-Protein Kinase A); RBP4 (Retinol-Binding Protein 4); APCs (Antigen-Presenting Cells); CD4+ (Cluster of Differentiation 4 Positive); VC (Varicocele); NR3C1 (Nuclear Receptor Subfamily 3 Group C Member 1); MTHFR (Methylenetetrahydrofolate Reductase); IL-1 (Interleukin-1); Zn (Zinc); Se (Selenium); Cu (Copper); Fe (Iron); SDF (Sperm DNA Fragmentation); DHT (Dihydrotestosterone); ROS (reactive oxygen species).
4.1. Inflammatory Cytokines in Obesity and Their Role in Male Infertility
With the increase in body fat tissue, chronic low-grade inflammation induced by obesity becomes a major threat to male reproductive health. This alteration of the immune microenvironment, particularly the excessive secretion of pro-inflammatory cytokines, may disrupt the immune tolerance mechanisms of the testes, thereby impairing the function of the reproductive system and leading to male infertility.
Obesity increases the secretion of IL-6 and other pro-inflammatory cytokines from macrophages, Leydig cells, and Sertoli cells in the testis, further exacerbating testicular inflammation [96]. Additionally, the elevated IL-6 induced by obesity activates the SOCS3/STAT3 signaling pathway, inhibiting the expression of Zinc Finger Protein 637 (Zfp637) and subsequently downregulating SRY-box transcription factor 2 (SOX2) expression, thereby disrupting spermatogonial cell differentiation in mice [97]. Obesity increases TNF-α levels in multiple tissues, including the testis, where it plays a crucial role in inflammation [14]. Elevated TNF-α impairs spermatogenesis by inducing germ cell apoptosis, disrupting Sertoli cell junctions, and inhibiting steroidogenesis in Leydig cells [98]. It also disrupts Ca2+ homeostasis, which is essential for sperm function, leading to reduced motility and abnormal morphology [99]. A study of seminal plasma revealed a negative correlation between TNF-α and sperm motility and morphology [100]. TNF-α activates NF-κB, a transcription factor involved in immune response regulation and germ cell apoptosis, contributing to male infertility [101]. Elevated TNF-α and NF-κB expression in obese mice testes correlated with impaired sperm function and lower pregnancy rates in mated females. Semen samples from 272 male donors confirmed a correlation between obesity, sperm quality, and pro-inflammatory cytokine levels, showing elevated IL-6 and TNF-α in the semen of obese men, along with reduced sperm concentration and motility [102].
Chronic inflammation, characterized by elevated TNF-α, IL-1β, and IL-6 levels, activates the IκB Kinase Beta (IKKβ)/NF-κB pathway. This leads to testicular structural damage, reduced testosterone levels, and decreased sperm motility [103]. Studies have shown that a high-fat diet significantly increases the levels of the NLRP3 inflammasome and pro-inflammatory cytokines (such as IL-6 and TNF-α) in the testis, epididymis, prostate, and seminal vesicles, with a corresponding increase in these cytokines in the serum [102]. In rats on a high-fat diet, the elevation of inflammation markers like High Mobility Group Box 1 (HMGB1) and NLRP3 in the testes is closely associated with a reduction in sperm count, a decrease in seminiferous tubule diameter and epithelial height, and a decline in Johnsen score [104]. Additionally, elevated corticosterone and IgG levels in the serum of obese mice indicated that the inflammatory response induced by obesity is closely related to immune regulation. Corticosterone has potent immunosuppressive and anti-inflammatory effects, while IgG plays a role in the inflammatory immune response [105]. Furthermore, obesity exacerbates the pathological effects of VC, a condition that significantly increases levels of ASAs and pro-inflammatory cytokines such as IL-1 and TNF-α. These cytokines activate the NLRP3 inflammasome pathway, disrupting immune tolerance in the testis and aggravating male infertility [4].
The gut microbiota critically regulates host metabolism, immunity, and endocrine function, directly impacting male reproductive health. It synthesizes essential micronutrients (vitamin A, K, folate, calcium) vital for testicular function, spermatogenesis, and BTB integrity [106,107]. HFD-induced gut dysbiosis in obesity disrupts nutrient synthesis and promotes inflammation. Fecal microbiota transplantation (FMT) from HFDs fed to normal mice reproduces gut imbalance, impairing Leydig cells, BTB integrity, and spermatogenesis [108]. Intervention with catalpol, an anti-inflammatory and antioxidant agent, partially restores gut microbiota diversity and improves testicular function, suggesting that gut dysbiosis-mediated oxidative stress and inflammation contribute to reproductive dysfunction [109]. Dysbiosis has been associated with decreased serum testosterone levels, and studies in germ-free mice have shown a downregulation of genes related to steroidogenesis and BTB formation—such as occludin, Zonula Occludens-2 (ZO-2), and E-cadherin—highlighting the essential role of balanced gut microbiota in maintaining endocrine and barrier functions in the testes [110].
In obese men, variations in the NR3C1 gene are associated with a reduction in sperm count. The NR3C1 gene, which is responsible for regulating the body’s response to cortisol, can indirectly affect sperm quality by modulating stress and inflammatory responses [111]. When the MTHFR gene is mutated, it disrupts the one-carbon metabolism process, leading to a decrease in the number of methyl donors. This change alters the DNA methylation status of genes closely related to inflammation, such as TNF-α and IL-6, thereby activating an excessive immune response [112]. Moreover, the mutation of the MTHFR gene increases the level of homocysteine, triggering an oxidative stress response and promoting the release of inflammatory factors, which further exacerbates the imbalance of the immune microenvironment [113]. Under the combined action of factors like obesity, chronic low-grade inflammation is further aggravated, which inhibits sperm production and reduces sperm quality, ultimately leading to male infertility.
4.2. Oxidative Stress, Hormonal Imbalance, and Testicular Dysfunction Induced by Obesity
Chronic low-grade inflammation induced by obesity leads to the excessive generation of ROS, which damage Leydig cells, impair testosterone bioavailability, and promote germ cell apoptosis [114]. Additionally, oxidative stress disrupts the BTB by reducing the expression of key proteins such as zonula occludens-1 (ZO-1), osteocalcin (OCN), CX43, N-Cadherin (N-CAD), and Vang-like 2 (VANGL2), impairing the transport of spermatogonia and spermatogenesis [115]. Moreover, excessive ROS damage sperm membranes and DNA, triggering lipid peroxidation and exacerbating SDF [116]. In the obese state, the antioxidant system in the testes becomes weakened [117], unable to effectively neutralize excess ROS. This overwhelms the antioxidant defense system in the genital tract, leading to oxidative stress, which negatively affects sperm concentration, motility, and progressive motility [118].
Furthermore, obesity triggers inflammation in adipose tissue, which increases the expression of aromatase in adipocytes through Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) and pro-inflammatory factors. This leads to the excessive conversion of androgens into estrogens, disrupting the hormonal balance in the male body and impairing the spermatogenic function of the testis [119]. Finally, FAK interferes with the dynamic regulation of the BTB through Proto-oncogene tyrosine-protein kinase Src (Src) kinase phosphorylation, while the dysregulation of the NRF2/MAPKs signaling pathway exacerbates BTB disruption and germ cell apoptosis, further impairing the immune-privileged microenvironment and ultimately affecting normal sperm production [120].
Trace elements such as zinc (Zn), copper (Cu), iron (Fe), and selenium (Se) serve as essential cofactors for key antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPx). These enzymes play a pivotal role in maintaining redox homeostasis and scavenging ROS, thereby protecting spermatozoa from oxidative damage. However, under obese conditions, deficiencies in trace elements—particularly Zn and Se—can impair antioxidant enzyme activity, weaken the oxidative defense system, and contribute to a vicious cycle of oxidative stress and inflammation [121,122,123]. Obesity-induced dysfunction or altered expression of zinc transporters (ZIPs), such as ZIP family members (e.g., ZIP12), may disturb both systemic and testicular zinc levels, compromising sperm quality and fertilization capacity [124]. Zinc, as a critical cofactor of zinc-α2-glycoprotein (ZAG), is indispensable for ZAG’s anti-inflammatory and lipolytic functions [125]. Deficiency of Zn impairs these activities, contributing to metabolic dysregulation. Additionally, trace elements such as Zn, nickel (Ni), and cobalt (Co) can modulate the expression of immune-related cytokines in adipocytes, thereby attenuating obesity-induced inflammation [126]. Moreover, the impact of trace elements on reproductive function exhibits a clear dose-dependent effect. For instance, molybdenum (Mo) at an appropriate concentration (e.g., 25 mg/L) may improve sperm quality, whereas excessive exposure (≥100 mg/L) exacerbates oxidative damage [127].
4.3. The Role of Adipokines in Testicular Function
Adipokines are crucial mediators between obesity and the testicular immune microenvironment, and their abnormal secretion significantly impacts testicular function. Obesity-induced hyperleptinemia binds to receptors on testicular seminiferous tubule cells, activates the PI3K pathway, increases free radical production, and inhibits antioxidant enzyme activity, leading to oxidative stress. This damages seminiferous tubule cells and sperm DNA, resulting in cell apoptosis, DNA fragmentation, reduced sperm count, abnormal morphology, and seminiferous tubule atrophy [128]. Moreover, hyperleptinemia upregulates SOCS3, inhibiting JAK2 phosphorylation and blocking leptin signaling, impairing testicular function [129]. Leptin regulates testicular function via the JAK2/STAT3 pathway, promoting the transcription of steroidogenic genes in Leydig cells. However, the upregulation of SOCS3 inhibits this process, leading to decreased testosterone levels [130].
Adiponectin and its receptors (AdipoR1 and AdipoR2), which are expressed in Leydig cells and seminiferous tubules, suggest its involvement in testicular function [131]. Adiponectin promotes testosterone synthesis through the cAMP-PKA pathway and has anti-inflammatory and metabolic regulatory effects. In obesity, decreased adiponectin levels reduce its protective role, increasing the risk of inflammation and fibrosis in the testes. Restoring adiponectin levels can improve testicular inflammation, oxidative stress, and steroidogenesis. Similar results were observed in diabetic mice, where adiponectin replacement therapy improved testicular function, including steroidogenesis, energy substrate transport, and reduced oxidative stress markers [132].
Retinol-binding protein 4 (RBP4) is a multifunctional protein that serves both as a transporter of retinol and as an adipokine. Under obese conditions, elevated RBP4 secretion from adipose tissue activates APCs and macrophages, promoting the release of pro-inflammatory cytokines and driving CD4+ T cell activation. This chronic low-grade inflammation not only exacerbates systemic insulin resistance, but may also impair testicular immune homeostasis by disrupting the BTB or interfering with Sertoli cell function [133]. Experimental studies further demonstrate that excessive RBP4 directly suppresses the transcription and translation of key steroidogenic enzymes (3β-hydroxysteroid dehydrogenase (3β-HSD) and steroid 5 alpha-reductase type 1 (SRD5A1)) in Sertoli cells, leading to reduced testosterone and dihydrotestosterone (DHT) synthesis, along with downregulated androgen receptor (AR) expression. Conversely, RBP4 knockdown produces the opposite effects, significantly enhancing steroidogenic enzyme activity and androgen secretion [134]. Thus, obesity-associated RBP4 elevation contributes to male reproductive dysfunction through dual mechanisms: indirectly via systemic inflammation and directly by inhibiting steroidogenesis and AR signaling pathways. These findings establish RBP4 as a critical pathological mediator linking metabolic disorders to male infertility. Figure 3 illustrates the relationship between obesity, immune microenvironment dysregulation, and male infertility.
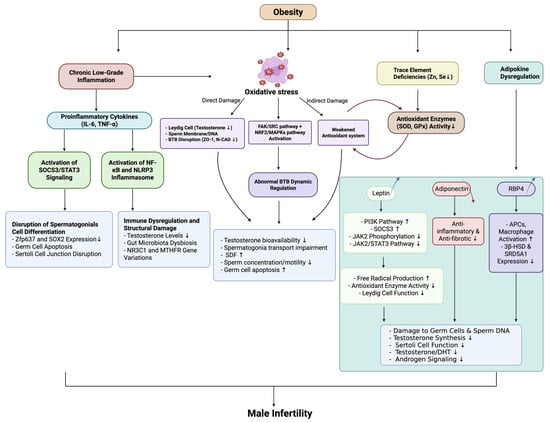
Figure 3.
The intersection of obesity, immune microenvironment disruption, and male infertility. Abbreviations: IL-6 (Interleukin-6); TNF-α (Tumor Necrosis Factor-alpha); SOCS3 (Suppressor of Cytokine Signaling 3); STAT3 (Signal Transducer and Activator of Transcription 3); NF-κB (Nuclear Factor kappa-light-chain-enhancer of Activated B Cells); NLRP3 (NLR Family Pyrin Domain Containing 3); Zfp637 (Zinc Finger Protein 637); SOX2 (Sex-Determining Region Y-Box 2); NR3C1 (Nuclear Receptor Subfamily 3 Group C Member 1); MTHFR (Methylenetetrahydrofolate Reductase); BTB (blood–testis barrier); ZO-1 (Zona Occludens-1); N-CAD (Neural Cadherin); FAK (Focal Adhesion Kinase); SRC (Src Family Kinase); SDF (sperm DNA fragmentation); Zn (Zinc); Se (Selenium); SOD (Superoxide Dismutase); GPx (Glutathione Peroxidase); PI3K (Phosphatidylinositol 3-Kinase); JAK2 (Janus Kinase 2); APCs (Antigen-Presenting Cells); 3β-HSD (3β-Hydroxysteroid Dehydrogenase); SRD5A1 (Steroid 5α-Reductase Type 1); DHT (Dihydrotestosterone).
5. Obesity and Male Infertility: Immune Microenvironment Intervention and Therapeutic Strategies
5.1. Lifestyle Interventions
Lifestyle modification constitutes the cornerstone of managing obesity-related male infertility. Weight reduction through structured exercise and dietary adjustment has been consistently associated with significant improvements in sperm parameters, including concentration, motility, and morphology in obese men [135,136]. Nutritional strategies emphasizing polyunsaturated fatty acids, dietary fiber, and antioxidants exert anti-inflammatory effects and contribute to the restoration of endocrine homeostasis, thereby enhancing reproductive function [137,138]. These non-pharmacologic approaches provide a foundation upon which more targeted therapeutic interventions can be developed.
5.2. Integrated Anti-Inflammatory and Metabolic Therapies: From Pharmacological Agents to Microbiota-Derived Metabolites
Pharmacological strategies complement lifestyle modifications by targeting the chronic inflammation and metabolic dysregulation inherent in obesity. Anti-inflammatory agents, including TNF-α inhibitors and IL-6 antagonists—originally developed for autoimmune and inflammatory disorders—have demonstrated efficacy in preclinical models in attenuating testicular inflammation and improving spermatogenic function [139,140].
Metabolic regulators such as metformin and glucagon-like peptide-1 (GLP-1) receptor agonists offer additional benefits. Metformin enhances insulin sensitivity, reduces testicular inflammation, increases testosterone production, and improves sperm motility [141,142]. GLP-1 receptor agonists, such as semaglutide, have been shown to restore redox homeostasis and mitigate oxidative injury in testicular tissues, thereby contributing to the recovery of reproductive function [143,144]. Furthermore, therapeutic strategies aimed at increasing adiponectin levels may offer dual anti-inflammatory and metabolic benefits, given its crucial role in energy homeostasis and reproductive physiology [145,146].
Elevated oxidative stress in obese individuals is associated with decreased activity of antioxidant enzymes, impaired mitochondrial membrane potential in spermatozoa, and compromised sperm quality [147]. While supplementation with antioxidants such as coenzyme Q10 and omega-3 fatty acids has been reported to reduce DNA fragmentation index (DFI) [148,149], its effects on seminal oxidative-reduction potential (sORP) remain limited [150]. These findings suggest that antioxidant therapy alone may be insufficient to fully reverse redox imbalance in the male reproductive tract.
Recent advances highlight the pivotal role of the gut–testis axis in regulating male fertility under metabolic stress. Short-chain fatty acids (SCFAs), particularly acetate and butyrate—produced by gut microbiota—exert anti-inflammatory and antioxidative effects. Acetate has been shown to activate the NRF2/peroxisome proliferator-activated receptor gamma (PPAR-γ) signaling cascade, thereby attenuating oxidative stress and inflammation in obese rodents [151]. Butyrate restores key metabolic pathways associated with steroidogenesis, contributing to improved sperm quality in animal models [152]. Future drug development may focus on several key areas, including SCFA-targeted delivery systems, NRF2/PPAR-γ pathway-specific agonists, and metabolic reprogramming regulators, employing multi-target intervention strategies to address obesity-related male infertility. However, clinical translation still requires overcoming critical challenges such as optimizing drug delivery efficiency and personalizing treatment regimens.
Natural bioactive compounds such as resveratrol (RSV) and icariin (ICA) have also demonstrated therapeutic potential. Both compounds modulate the gut microbiota, suppress the activation of the NLRP3 inflammasome, and restore testicular steroidogenesis, providing multi-targeted benefits for reproductive and metabolic health [153,154]. Their synergistic effects highlight the promise of microbiota-targeted nutraceuticals in the management of obesity-induced male infertility.
Additionally, bacterial contamination of semen—commonly involving Escherichia coli, Staphylococcus haemolyticus, and Bacteroides ureolyticus—can induce leukocyte activation and excessive production of reactive oxygen intermediates (ROIs). This promotes lipid peroxidation of sperm membranes and contributes to reduced sperm motility and function [155]. Therapeutic strategies aimed at limiting leukocyte-driven ROS generation may serve as adjunctive measures in reducing oxidative sperm damage.
5.3. Regenerative Medicine and Biologic Therapies
In the field of regenerative medicine and biologic therapies, exosomes derived from mesenchymal stem cells have drawn much attention for their immunomodulatory and anti-inflammatory properties, among other properties, and lack of risk of stem cell transplantation. Exosomes and mesenchymal stem cells are potential therapies for improving testicular function. They can regulate the local immune microenvironment of the testes, repair testicular inflammation and damage caused by obesity, promote spermatogenesis, restore normal testicular function, and offer new treatment ideas for male infertility related to obesity [156].
6. Future Directions and Research Gaps
Although significant progress has been made in elucidating the relationship between obesity, the immune microenvironment, and male infertility, many critical questions remain unanswered. These knowledge gaps hinder the development of effective diagnostic tools and therapeutic interventions. Future research should prioritize the following directions, all centered on deepening our understanding of immune dysregulation in obesity-related male infertility.
6.1. Mechanistic Insights and Biomarker Discovery
Currently, the mechanisms by which obesity alters the testicular immune microenvironment remain incompletely understood. Future research leveraging multi-omics approaches, including transcriptomics, metabolomics, and proteomics, are promising tools to reveal how obesity-induced metabolic stress reprograms immune signaling networks within the testis [157]. A particular focus should be placed on identifying key cytokines, exosomal proteins, and metabolic mediators in blood and semen that reflect testicular immune homeostasis [158,159]. These biomarkers could serve not only for early diagnosis but also for the real-time monitoring of immune status before and after interventions, such as metabolic surgery or pharmacologic therapy. Furthermore, non-invasive diagnostic platforms based on these biomarkers would significantly improve clinical management by enabling the dynamic assessment of immune-related fertility impairments.
6.2. Longitudinal and Population-Based Studies of Immune Signatures
The majority of existing studies examining the link between obesity and male infertility are cross-sectional, which limits causal inference, particularly regarding immune disturbances. Future research should focus on large-scale, long-term prospective cohort studies to track how chronic obesity-induced immune dysregulation evolves over time and contributes to testicular dysfunction [160]. These studies should incorporate serial sampling of immune markers, such as inflammatory cytokines, leukocyte profiles, and oxidative stress indicators, to clarify temporal relationships. Given that most current studies predominantly involve Western populations, there is a pressing need to expand research to include diverse populations, particularly those from Asia and Africa, which would help to explore genetic or lifestyle modifiers of immune responses to obesity, thereby informing ethnically tailored therapeutic approaches.
6.3. Immunomodulatory Role of the Gut–Testis Axis
The gut microbiota play a pivotal role in regulating systemic and local immune homeostasis. Dysbiosis in obesity is known to amplify systemic inflammation and may contribute to immune alterations in distant organs, including the testes. Future studies should explore the gut–testis axis, focusing on how microbial metabolites—such as short-chain fatty acids—modulate testicular immune cell populations and barrier integrity [161]. Additionally, evaluating the therapeutic potential of probiotics or prebiotics in reversing dysbiosis-driven immune activation may yield novel strategies to restore immune balance and improve male reproductive outcomes.
6.4. Psychological and Social Determinants of Immune Dysfunction
Emerging evidence suggests that chronic psychological stress can alter immune function via neuroendocrine-immune pathways, thereby exacerbating obesity-related inflammation and potentially impairing testicular immunity [162]. Future research should examine how anxiety, depression, and social isolation influence the immune microenvironment in obese infertile men. Psychosocial interventions that reduce stress-induced immune activation may serve as adjunctive strategies to support fertility. Moreover, assessing the impact of social support and lifestyle interventions on immunological markers could help guide holistic, patient-centered treatment frameworks.
7. Conclusions
Obesity, along with the immune microenvironment, is closely linked to male infertility, and treatments such as interventional surgery and medical therapies, as obesity-induced changes in the immune microenvironment, significantly impact male reproductive health. The underlying mechanisms are complex, involving chronic low-grade inflammation, immune cell infiltration, and the dysregulation of adipokines such as leptin and adiponectin. These changes disrupt testicular function, impair spermatogenesis, and contribute to hormonal imbalances. A deeper understanding of how obesity influences the immune landscape within the testes and its subsequent impact on male fertility is crucial for developing more effective therapeutic strategies. Future research should prioritize investigating the specific immune pathways involved in obesity-related male infertility, with a focus on identifying novel molecular targets and exploring potential therapies to correct these immune dysregulations.
Author Contributions
R.F. and D.C. for Data curation and Formal analysis; R.F., D.C., W.Z. and J.Z. for Investigation; R.F. for Writing—original draft; S.C. and Y.X. for Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Medical and Health Science and Technology Project of Zhejiang Province (No. C-2024-w1178).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, O.; Wszołek, K.; Pięt, M.; Wilczak, M.; Chmaj-Wierzchowska, K. Women’s Awareness of Reproductive Health. Medicina 2024, 60, 158. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef]
- Fang, Y.; Su, Y.; Xu, J.; Hu, Z.; Zhao, K.; Liu, C.; Zhang, H. Varicocele-Mediated Male Infertility: From the Perspective of Testicular Immunity and Inflammation. Front. Immunol. 2021, 12, 729539. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, X.; Liu, Y.F.; Song, B.; Sun, Z.; Zhao, S. Immunoregulation and male reproductive function: Impacts and mechanistic insights into inflammation. Andrology 2024. [Google Scholar] [CrossRef]
- Hu, X.; Yang, X.; Zhao, J.; Guan, T.; Dai, Q.; Yang, J.; Zhang, H.; Zhang, D.; Zhang, Y.; Shang, L.; et al. Association between body mass index and varicocele among 211 989 Chinese reproductive-age males. Int. J. Urol. 2022, 29, 853–859. [Google Scholar] [CrossRef]
- Sermondade, N.; Faure, C.; Fezeu, L.; Lévy, R.; Czernichow, S. Obesity and increased risk for oligozoospermia and azoospermia. Arch. Intern. Med. 2012, 172, 440–442. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Oliveira, P.F.; Alves, M.G.; Monteiro, M.P. Obesity and male hypogonadism: Tales of a vicious cycle. Obes. Rev. 2019, 20, 1148–1158. [Google Scholar] [CrossRef]
- Phillips, K.P.; Tanphaichitr, N. Mechanisms of obesity-induced male infertility. Expert. Rev. Endocrinol. Metab. 2010, 5, 229–251. [Google Scholar] [CrossRef]
- Handel, L.N.; Shetty, R.; Sigman, M. The relationship between varicoceles and obesity. J. Urol. 2006, 176, 2138–2140, discussion 2140. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Ramezani Tehrani, F. Potential Adverse Effects of Female and Male Obesity on Fertility: A Narrative Review. Int. J. Endocrinol. Metab. 2020, 18, e101776. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Chen, M.; Xu, K.; Chen, B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell. Mol. Life Sci. 2021, 78, 7275–7288. [Google Scholar] [CrossRef]
- Xu, K.; Fu, A.; Li, Z.; Miao, L.; Lou, Z.; Jiang, K.; Lau, C.; Su, T.; Tong, T.; Bao, J.; et al. Elevated extracellular matrix protein 1 in circulating extracellular vesicles supports breast cancer progression under obesity conditions. Nat. Commun. 2024, 15, 1685. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Warren, B.D.; Ahn, S.H.; Brittain, K.S.; Nanjappa, M.K.; Wang, H.; Wang, J.; Blanco, G.; Sanchez, G.; Fan, Y.; Petroff, B.K.; et al. Multiple Lesions Contribute to Infertility in Males Lacking Autoimmune Regulator. Am. J. Pathol. 2021, 191, 1592–1609. [Google Scholar] [CrossRef]
- Bhushan, S.; Theas, M.S.; Guazzone, V.A.; Jacobo, P.; Wang, M.; Fijak, M.; Meinhardt, A.; Lustig, L. Immune Cell Subtypes and Their Function in the Testis. Front. Immunol. 2020, 11, 583304. [Google Scholar] [CrossRef]
- Li, S.Y.; Kumar, S.; Gu, X.; DeFalco, T. Testicular immunity. Mol. Asp. Med. 2024, 100, 101323. [Google Scholar] [CrossRef]
- Gong, M.; Han, D. Immunologic Environment of the Testis. Adv. Exp. Med. Biol. 2021, 1288, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fijak, M.; Hossain, H.; Markmann, M.; Nüsing, R.M.; Lochnit, G.; Hartmann, M.F.; Wudy, S.A.; Zhang, L.; Gu, H.; et al. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J. Immunol. 2017, 198, 4327–4340. [Google Scholar] [CrossRef] [PubMed]
- Mossadegh-Keller, N.; Gentek, R.; Gimenez, G.; Bigot, S.; Mailfert, S.; Sieweke, M.H. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 2017, 214, 2829–2841. [Google Scholar] [CrossRef]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef]
- Gu, X.; Heinrich, A.; Li, S.Y.; DeFalco, T. Testicular macrophages are recruited during a narrow fetal time window and promote organ-specific developmental functions. Nat. Commun. 2023, 14, 1439. [Google Scholar] [CrossRef]
- Bhushan, S.; Tchatalbachev, S.; Lu, Y.; Fröhlich, S.; Fijak, M.; Vijayan, V.; Chakraborty, T.; Meinhardt, A. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol. 2015, 194, 5455–5464. [Google Scholar] [CrossRef]
- Xia, W.; Wong, E.W.; Mruk, D.D.; Cheng, C.Y. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009, 327, 48–61. [Google Scholar] [CrossRef]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J. Leukoc. Biol. 2011, 90, 133–143. [Google Scholar] [CrossRef]
- Rival, C.; Theas, M.S.; Suescun, M.O.; Jacobo, P.; Guazzone, V.; van Rooijen, N.; Lustig, L. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J. Pathol. 2008, 215, 108–117. [Google Scholar] [CrossRef]
- Klein, B.; Bhushan, S.; Günther, S.; Middendorff, R.; Loveland, K.L.; Hedger, M.P.; Meinhardt, A. Differential tissue-specific damage caused by bacterial epididymo-orchitis in the mouse. Mol. Hum. Reprod. 2020, 26, 215–227. [Google Scholar] [CrossRef]
- Rival, C.; Lustig, L.; Iosub, R.; Guazzone, V.A.; Schneider, E.; Meinhardt, A.; Fijak, M. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006, 324, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Guazzone, V.A.; Hollwegs, S.; Mardirosian, M.; Jacobo, P.; Hackstein, H.; Wygrecka, M.; Schneider, E.; Meinhardt, A.; Lustig, L.; Fijak, M. Characterization of dendritic cells in testicular draining lymph nodes in a rat model of experimental autoimmune orchitis. Int. J. Androl. 2011, 34, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Rival, C.; Guazzone, V.A.; von Wulffen, W.; Hackstein, H.; Schneider, E.; Lustig, L.; Meinhardt, A.; Fijak, M. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol. Hum. Reprod. 2007, 13, 853–861. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Wang, Y.; Han, F.; Cai, W.; Zhao, B.; Li, Y.; Han, S.; Wu, X.; Hu, D. Murine Sertoli cells promote the development of tolerogenic dendritic cells: A pivotal role of galectin-1. Immunology 2016, 148, 253–265. [Google Scholar] [CrossRef]
- Khan, U.; Ghazanfar, H. T Lymphocytes and Autoimmunity. Int. Rev. Cell Mol. Biol. 2018, 341, 125–168. [Google Scholar] [CrossRef]
- Jacobo, P.; Guazzone, V.A.; Pérez, C.V.; Lustig, L. CD4+ Foxp3+ regulatory T cells in autoimmune orchitis: Phenotypic and functional characterization. Am. J. Reprod. Immunol. 2015, 73, 109–125. [Google Scholar] [CrossRef]
- Tung, K.S.; Harakal, J.; Qiao, H.; Rival, C.; Li, J.C.; Paul, A.G.; Wheeler, K.; Pramoonjago, P.; Grafer, C.M.; Sun, W.; et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Investig. 2017, 127, 1046–1060. [Google Scholar] [CrossRef]
- Moreno, D.; Sobarzo, C.M.; Lustig, L.; Rodríguez Peña, M.G.; Guazzone, V.A. Effect of ketotifen fumarate on experimental autoimmune orchitis and torsion of the spermatic cord. Asian J. Androl. 2020, 22, 112–117. [Google Scholar] [CrossRef]
- Wanjari, U.R.; Gopalakrishnan, A.V. Blood-testis barrier: A review on regulators in maintaining cell junction integrity between Sertoli cells. Cell Tissue Res. 2024, 396, 157–175. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The biology of spermatogenesis: The past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1459–1463. [Google Scholar] [CrossRef]
- Dufour, J.M.; Rajotte, R.V.; Seeberger, K.; Kin, T.; Korbutt, G.S. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation 2003, 10, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wei, Y.; Zhou, Y.; Wu, H.; Hong, Y.; Long, C.; Wang, J.; Wu, Y.; Wu, S.; Shen, L.; et al. Wnt5a Regulates Junctional Function of Sertoli cells Through PCP-mediated Effects on mTORC1 and mTORC2. Endocrinology 2021, 162, bqab149. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, M.; Li, H.; Wu, S.; Ge, R.; Wong, C.K.C.; Silvestrini, B.; Sun, F.; Cheng, C.Y. The Non-hormonal Male Contraceptive Adjudin Exerts its Effects via MAPs and Signaling Proteins mTORC1/rpS6 and FAK-Y407. Endocrinology 2021, 162, bqaa196. [Google Scholar] [CrossRef]
- Anyanwu, B.O.; Orisakwe, O.E. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. J. Environ. Sci. Health Part C Toxicol. Carcinog. 2020, 38, 204–244. [Google Scholar] [CrossRef]
- Cao, X.N.; Shen, L.J.; Wu, S.D.; Yan, C.; Zhou, Y.; Xiong, G.; Wang, Y.C.; Liu, Y.; Liu, B.; Tang, X.L.; et al. Urban fine particulate matter exposure causes male reproductive injury through destroying blood-testis barrier (BTB) integrity. Toxicol. Lett. 2017, 266, 1–12. [Google Scholar] [CrossRef]
- Vazquez-Levin, M.H.; Marín-Briggiler, C.I.; Veaute, C. Antisperm antibodies: Invaluable tools toward the identification of sperm proteins involved in fertilization. Am. J. Reprod. Immunol. 2014, 72, 206–218. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef]
- Negri, L.; Romano, M.; Cirillo, F.; Grilli, L.; Morenghi, E.; Romualdi, D.; Albani, E.; Setti, P.E.L. Influence of inguinal hernia repair on sperm autoimmunity: The largest single center experience. Andrology 2022, 10, 105–110. [Google Scholar] [CrossRef]
- Chereshnev, V.A.; Pichugova, S.V.; Beikin, Y.B.; Chereshneva, M.V.; Iukhta, A.I.; Stroev, Y.I.; Churilov, L.P. Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation. Pathophysiology 2021, 28, 471–488. [Google Scholar] [CrossRef]
- Barbonetti, A.; Castellini, C.; D’Andrea, S.; Cordeschi, G.; Santucci, R.; Francavilla, S.; Francavilla, F. Prevalence of anti-sperm antibodies and relationship of degree of sperm auto-immunization to semen parameters and post-coital test outcome: A retrospective analysis of over 10,000 men. Hum. Reprod. 2019, 34, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Bohring, C.; Krause, E.; Habermann, B.; Krause, W. Isolation and identification of sperm membrane antigens recognized by antisperm antibodies, and their possible role in immunological infertility disease. Mol. Hum. Reprod. 2001, 7, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Verón, G.L.; Molina, R.I.; Tissera, A.D.; Estofan, G.M.; Marín-Briggiler, C.I.; Vazquez-Levin, M.H. Incidence of Sperm Surface Autoantibodies and Relationship with Routine Semen Parameters and Sperm Kinematics. Am. J. Reprod. Immunol. 2016, 76, 59–69. [Google Scholar] [CrossRef]
- Yoshitake, H.; Oda, R.; Yanagida, M.; Kawasaki, Y.; Sakuraba, M.; Takamori, K.; Hasegawa, A.; Fujiwara, H.; Araki, Y. Identification of an anti-sperm auto-monoclonal antibody (Ts4)-recognized molecule in the mouse sperm acrosomal region and its inhibitory effect on fertilization in vitro. J. Reprod. Immunol. 2016, 115, 6–13. [Google Scholar] [CrossRef]
- Chen, Y.; Hasegawa, A.; Honda, H.; Wakimoto, Y.; Shibahara, H. Characterization of a spontaneously occurring self-reactive antibody against sperm in mice. J. Reprod. Immunol. 2023, 157, 103930. [Google Scholar] [CrossRef]
- Chiu, W.W.; Chamley, L.W. Clinical associations and mechanisms of action of antisperm antibodies. Fertil. Steril. 2004, 82, 529–535. [Google Scholar] [CrossRef]
- Shibahara, H.; Chen, Y.; Honda, H.; Wakimoto, Y.; Fukui, A.; Hasegawa, A. Sex difference in anti-sperm antibodies. Reprod. Med. Biol. 2022, 21, e12477. [Google Scholar] [CrossRef]
- Jacobo, P.; Guazzone, V.A.; Theas, M.S.; Lustig, L. Testicular autoimmunity. Autoimmun. Rev. 2011, 10, 201–204. [Google Scholar] [CrossRef]
- Barrachina, F.; Ottino, K.; Elizagaray, M.L.; Gervasi, M.G.; Tu, L.J.; Markoulaki, S.; Spallanzani, R.G.; Capen, D.; Brown, D.; Battistone, M.A. Regulatory T cells play a crucial role in maintaining sperm tolerance and male fertility. Proc. Natl. Acad. Sci. USA 2023, 120, e2306797120. [Google Scholar] [CrossRef]
- Attia, H.; Finocchi, F.; Orciani, M.; Mehdi, M.; Zidi Jrah, I.; Lazzarini, R.; Balercia, G.; Mattioli Belmonte, M. Pro-inflammatory cytokines and microRNAs in male infertility. Mol. Biol. Rep. 2021, 48, 5935–5942. [Google Scholar] [CrossRef]
- Rivero, M.J.; Kulkarni, N.; Thirumavalavan, N.; Ramasamy, R. Evaluation and management of male genital tract infections in the setting of male infertility: An updated review. Curr. Opin. Urol. 2023, 33, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Linn, T.; Drlica, K.; Shi, L. Diabetes as a potential compounding factor in COVID-19-mediated male subfertility. Cell Biosci. 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Portela, J.M.D.; Sousa, M.I.; Mota, P.C.; Ramalho-Santos, J.; Amaral, S. High glucose levels affect spermatogenesis: An in vitro approach. Reprod. Fertil. Dev. 2017, 29, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chen, T.L.; Tseng, Y.Y.; Wu, G.J.; Hsieh, M.H.; Lin, Y.W.; Chen, R.M. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol. Appl. Pharmacol. 2016, 304, 59–69. [Google Scholar] [CrossRef]
- Ford, W.C. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Zini, A.; San Gabriel, M.; Baazeem, A. Antioxidants and sperm DNA damage: A clinical perspective. J. Assist. Reprod. Genet. 2009, 26, 427–432. [Google Scholar] [CrossRef]
- Tian, Y.; Song, W.; Xu, D.; Chen, X.; Li, X.; Zhao, Y. Autophagy Induced by ROS Aggravates Testis Oxidative Damage in Diabetes via Breaking the Feedforward Loop Linking p62 and Nrf2. Oxid. Med. Cell. Longev. 2020, 2020, 7156579. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, W.; Wu, J.; Zhang, D.; Abou-Shakra, A.; Di, L.; Wang, Z.; Wang, L.; Yang, F.; Qiao, Z. Nicotine induced autophagy of Leydig cells rather than apoptosis is the major reason of the decrease of serum testosterone. Int. J. Biochem. Cell Biol. 2018, 100, 30–41. [Google Scholar] [CrossRef]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Zhou, W.; Yuan, M.; Shi, Y.; Yang, K. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: Involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology 2016, 341–343, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.E.I.; Xiang-Liang, T.; Yu, Z.; Bin, L.; Lian-Ju, S.; Chun-Lan, L.; Tao, L.I.N.; Da-Wei, H.E.; Sheng-de, W.U.; Guang-Hui, W.E.I. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes. Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, L.; Chen, X.; Ding, Y.; He, J.; Zhu, J.; Wang, Y.; Liu, X. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reprod. Biomed. Online 2016, 32, 207–217. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Arefanian, H.; Thomas, R.; Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. Endoplasmic Reticulum Stress Promotes the Expression of TNF-α in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1α and MAPK/NF-κB Pathways. Int. J. Mol. Sci. 2023, 24, 15186. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, T.; Gomez, N.A.; Wang, B.; Zhu, M.J.; Du, M. Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) α1. Nutr. Diabetes 2018, 8, 39. [Google Scholar] [CrossRef]
- Chaves de Souza, J.A.; Nogueira, A.V.; Chaves de Souza, P.P.; Kim, Y.J.; Silva Lobo, C.; Pimentel Lopes de Oliveira, G.J.; Cirelli, J.A.; Garlet, G.P.; Rossa, C., Jr. SOCS3 expression correlates with severity of inflammation, expression of proinflammatory cytokines, and activation of STAT3 and p38 MAPK in LPS-induced inflammation in vivo. Mediat. Inflamm. 2013, 2013, 650812. [Google Scholar] [CrossRef]
- Kim, F.; Pham, M.; Luttrell, I.; Bannerman, D.D.; Tupper, J.; Thaler, J.; Hawn, T.R.; Raines, E.W.; Schwartz, M.W. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 2007, 100, 1589–1596. [Google Scholar] [CrossRef]
- Fuster, J.J.; Zuriaga, M.A.; Ngo, D.T.; Farb, M.G.; Aprahamian, T.; Yamaguchi, T.P.; Gokce, N.; Walsh, K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 2015, 64, 1235–1248. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Lago, F.; Gómez, R.; Gómez-Reino, J.J.; Dieguez, C.; Gualillo, O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 2009, 34, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Leibel, R.L. 20 years of leptin: Role of leptin in energy homeostasis in humans. J. Endocrinol. 2014, 223, T83–T96. [Google Scholar] [CrossRef] [PubMed]
- Carlton, E.D.; Demas, G.E.; French, S.S. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm. Behav. 2012, 62, 272–279. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Li, C.; Cheng, H.; Adhikari, B.K.; Wang, S.; Yang, N.; Liu, W.; Sun, J.; Wang, Y. The Role of Apelin-APJ System in Diabetes and Obesity. Front. Endocrinol. 2022, 13, 820002. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, E.; Deng, L.; Zhu, Y.; Lu, X.; Li, X.; Li, F.; Yan, Y.; Han, J.Y.; Li, Y.; et al. Immunological roles for resistin and related adipokines in obesity-associated tumors. Int. Immunopharmacol. 2024, 142 Pt A, 112911. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Yokota, T.; Oritani, K.; Takahashi, I.; Ishikawa, J.; Matsuyama, A.; Ouchi, N.; Kihara, S.; Funahashi, T.; Tenner, A.J.; Tomiyama, Y.; et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000, 96, 1723–1732. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [CrossRef]
- Ali, M.; Jasmin, S.; Fariduddin, M.; Alam, S.M.K.; Arslan, M.I.; Biswas, S.K. Neutrophil elastase and myeloperoxidase mRNA expression in overweight and obese subjects. Mol. Biol. Rep. 2018, 45, 1245–1252. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils Actively Contribute to Obesity-Associated Inflammation and Pathological Complications. Cells 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Green, W.D.; Beck, M.A. Obesity altered T cell metabolism and the response to infection. Curr. Opin. Immunol. 2017, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Cho, K.W.; Delproposto, J.L.; Oatmen, K.E.; Geletka, L.M.; Martinez-Santibanez, G.; Singer, K.; Lumeng, C.N. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013, 62, 2762–2772. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Haas, K.M.; Beck, M.A.; Teague, H. The effects of diet-induced obesity on B cell function. Clin. Exp. Immunol. 2015, 179, 90–99. [Google Scholar] [CrossRef]
- Theurich, S.; Tsaousidou, E.; Hanssen, R.; Lempradl, A.M.; Mauer, J.; Timper, K.; Schilbach, K.; Folz-Donahue, K.; Heilinger, C.; Sexl, V.; et al. IL-6/Stat3-Dependent Induction of a Distinct, Obesity-Associated NK Cell Subpopulation Deteriorates Energy and Glucose Homeostasis. Cell Metab. 2017, 26, 171–184.e176. [Google Scholar] [CrossRef]
- Maegawa, M.; Kamada, M.; Irahara, M.; Yamamoto, S.; Yoshikawa, S.; Kasai, Y.; Ohmoto, Y.; Gima, H.; Thaler, C.J.; Aono, T. A repertoire of cytokines in human seminal plasma. J. Reprod. Immunol. 2002, 54, 33–42. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, M.; Zhang, J.; Li, J.; Gong, D.; Li, Y.; Zhang, J.; Lin, P.; Huang, L. IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway. Sci. Rep. 2016, 6, 28012. [Google Scholar] [CrossRef]
- Lysiak, J.J. The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod. Biol. Endocrinol. 2004, 2, 9. [Google Scholar] [CrossRef]
- Publicover, S.; Harper, C.V.; Barratt, C. [Ca2+]i signalling in sperm--making the most of what you’ve got. Nat. Cell Biol. 2007, 9, 235–242. [Google Scholar] [CrossRef]
- Koçak, I.; Yenisey, C.; Dündar, M.; Okyay, P.; Serter, M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. [Google Scholar] [CrossRef]
- Pentikäinen, V.; Suomalainen, L.; Erkkilä, K.; Martelin, E.; Parvinen, M.; Pentikäinen, M.O.; Dunkel, L. Nuclear factor-kappa B activation in human testicular apoptosis. Am. J. Pathol. 2002, 160, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y.; Liu, Y.; Zhang, Z.; Lu, L.; Ding, Z. Obesity or Overweight, a Chronic Inflammatory Status in Male Reproductive System, Leads to Mice and Human Subfertility. Front. Physiol. 2017, 8, 1117. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Shang, Y.; Wang, Y.; Chen, Y.; Mu, B.; Zheng, Q.; Liu, H. 1-DNJ Alleviates Obesity-Induced Testicular Inflammation in Mice Model by Inhibiting IKKβ/ NF-kB Pathway. Reprod. Sci. 2024, 31, 2103–2113. [Google Scholar] [CrossRef]
- Elmorsy, E.H.; Aly, R.G.; Badae, N.M.; Aboghazala, M.M.; Omar, S.S. Zinc alleviates high fat diet-induced spermatogenic dysfunction in Wistar rats: Role of oxidative stress, HMGB1 and inflammasome. Rev. Int. Androl. 2024, 22, 44–52. [Google Scholar] [CrossRef]
- Hueston, C.M.; Deak, T. The inflamed axis: The interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol. Behav. 2014, 124, 77–91. [Google Scholar] [CrossRef]
- Shiba, S.; Ikeda, K.; Horie-Inoue, K.; Azuma, K.; Hasegawa, T.; Amizuka, N.; Tanaka, T.; Takeiwa, T.; Shibata, Y.; Koji, T.; et al. Vitamin K-Dependent γ-Glutamyl Carboxylase in Sertoli Cells Is Essential for Male Fertility in Mice. Mol. Cell. Biol. 2021, 41, e00404-20. [Google Scholar] [CrossRef]
- Takumi, N.; Shirakawa, H.; Ohsaki, Y.; Ito, A.; Watanabe, T.; Giriwono, P.E.; Sato, T.; Komai, M. Dietary vitamin K alleviates the reduction in testosterone production induced by lipopolysaccharide administration in rat testis. Food Funct. 2011, 2, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Stukenborg, J.B.; Reda, A.; Anuar, F.; Strand, M.L.; Hedin, L.; Pettersson, S.; Söder, O. The gut microbiota and developmental programming of the testis in mice. PLoS ONE 2014, 9, e103809. [Google Scholar] [CrossRef]
- Chandra Sekar, P.K.; Veerabathiran, R. Genes linked to obesity-related infertility: Bridging the knowledge gap. Reprod. Dev. Med. 2024, 8, 121–129. [Google Scholar] [CrossRef]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Falvo, S.; Minucci, S.; Santillo, A.; Senese, R.; Chieffi Baccari, G.; Venditti, M. A short-term high-fat diet alters rat testicular activity and blood-testis barrier integrity through the SIRT1/NRF2/MAPKs signaling pathways. Front. Endocrinol. 2023, 14, 1274035. [Google Scholar] [CrossRef]
- Elmas, M.A.; Ozakpinar, O.B.; Kolgazi, M.; Sener, G.; Arbak, S.; Ercan, F. Exercise improves testicular morphology and oxidative stress parameters in rats with testicular damage induced by a high-fat diet. Andrologia 2022, 54, e14600. [Google Scholar] [CrossRef]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Giacone, F.; Mongioi, L.M.; La Vignera, S. Non-hormonal treatment for male infertility: The potential role of Serenoa repens, selenium and lycopene. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3112–3120. [Google Scholar] [CrossRef]
- Yi, X.; Tang, D.; Cao, S.; Li, T.; Gao, H.; Ma, T.; Yao, T.; Li, J.; Chang, B. Effect of Different Exercise Loads on Testicular Oxidative Stress and Reproductive Function in Obese Male Mice. Oxid. Med. Cell. Longev. 2020, 2020, 3071658. [Google Scholar] [CrossRef]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Yuxin, L.; Chen, L.; Xiaoxia, L.; Yue, L.; Junjie, L.; Youzhu, L.; Huiliang, Z.; Qicai, L. Research Progress on the Relationship between Obesity-Inflammation-Aromatase Axis and Male Infertility. Oxid. Med. Cell. Longev. 2021, 2021, 6612796. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Yang, J.M.; Ye, R.J.; Liu, N.; Zhang, W.J.; Ma, L.; Zheng, P.; Niu, J.G.; Liu, P.; Yu, J.Q. Protective effects of betaine on diabetic induced disruption of the male mice blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Biomed. Pharmacother. 2019, 120, 109474. [Google Scholar] [CrossRef]
- Calderón, B.; Gómez-Martín, J.M.; Cuadrado-Ayuso, M.; Cobeta, P.; Vega-Piñero, B.; Mateo, R.; Galindo, J.; Botella-Carretero, J.I. Circulating Zinc and Copper Levels are Associated with Sperm Quality in Obese Men after Metabolic Surgery: A Pilot Study. Nutrients 2020, 12, 3354. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Domínguez-Riscart, J.; Millán-Martínez, M.; Mateos-Bernal, R.M.; Lechuga-Sancho, A.M.; González-Domínguez, R. Trace elements as potential modulators of puberty-induced amelioration of oxidative stress and inflammation in childhood obesity. Biofactors 2023, 49, 820–830. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Millán-Martínez, M.; Domínguez-Riscart, J.; Lechuga-Sancho, A.M.; González-Domínguez, R. Metal Homeostasis and Exposure in Distinct Phenotypic Subtypes of Insulin Resistance among Children with Obesity. Nutrients 2023, 15, 2347. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, C.; Wu, W.; Shi, L.; Jiang, C.; Wang, L.; Ding, Z.; Liu, Y. Zinc transporter ZIP12 maintains zinc homeostasis and protects spermatogonia from oxidative stress during spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 17. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; Beserra, J.B.; Dos Santos, L.R.; de Sousa Melo, S.R.; de Sousa, G.S.; de Matos Neto, E.M.; Henriques, G.S.; do Nascimento Marreiro, D. Role of Zinc in Zinc-α2-Glycoprotein Metabolism in Obesity: A Review of Literature. Biol. Trace Elem. Res. 2020, 193, 81–88. [Google Scholar] [CrossRef]
- Romier, B.; Tourniaire, F.; Marcotorchino, J.; Gouranton, E.; Astier, J.; Malezet, C.; Blouin, E.; Landrier, J.F. Bioeffects of a combination of trace elements on adipocyte biology. Metallomics 2013, 5, 524–531. [Google Scholar] [CrossRef]
- Zhai, X.W.; Zhang, Y.L.; Qi, Q.; Bai, Y.; Chen, X.L.; Jin, L.J.; Ma, X.G.; Shu, R.Z.; Yang, Z.J.; Liu, F.J. Effects of molybdenum on sperm quality and testis oxidative stress. Syst. Biol. Reprod. Med. 2013, 59, 251–255. [Google Scholar] [CrossRef]
- Almabhouh, F.A.; Singh, H.J. The impact of leptin on sperm. Reprod. Fertil. Dev. 2023, 35, 459–468. [Google Scholar] [CrossRef]
- Bjorbak, C.; Lavery, H.J.; Bates, S.H.; Olson, R.K.; Davis, S.M.; Flier, J.S.; Myers, M.G., Jr. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000, 275, 40649–40657. [Google Scholar] [CrossRef]
- Landry, D.A.; Sormany, F.; Haché, J.; Roumaud, P.; Martin, L.J. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol. Cell. Biochem. 2017, 433, 79–95. [Google Scholar] [CrossRef]
- Nogueiras, R.; Barreiro, M.L.; Caminos, J.E.; Gaytán, F.; Suominen, J.S.; Navarro, V.M.; Casanueva, F.F.; Aguilar, E.; Toppari, J.; Diéguez, C.; et al. Novel expression of resistin in rat testis: Functional role and regulation by nutritional status and hormonal factors. J. Cell Sci. 2004, 117 Pt 15, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Krishna, A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie 2020, 168, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Vieira, P.M.; Yore, M.M.; Dwyer, P.M.; Syed, I.; Aryal, P.; Kahn, B.B. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014, 19, 512–526. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Li, Y.; Zhao, X.; Zhang, Y. RBP4 regulates androgen receptor expression and steroid synthesis in Sertoli cells from Bactrian camels. Reprod. Domest. Anim. 2022, 57, 429–437. [Google Scholar] [CrossRef]
- Andersen, E.; Juhl, C.R.; Kjøller, E.T.; Lundgren, J.R.; Janus, C.; Dehestani, Y.; Saupstad, M.; Ingerslev, L.R.; Duun, O.M.; Jensen, S.B.K.; et al. Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: A randomized controlled trial. Hum. Reprod. 2022, 37, 1414–1422. [Google Scholar] [CrossRef]
- Lee, G.; Choi, H.Y.; Yang, S.J. Effects of Dietary and Physical Activity Interventions on Metabolic Syndrome: A Meta-analysis. J. Korean Acad. Nurs. 2015, 45, 483–494. [Google Scholar] [CrossRef]
- Rodak, K.; Kratz, E.M. PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders. Pharmaceuticals 2023, 16, 723. [Google Scholar] [CrossRef]
- Ghewade, P.; Vagha, S.; Ghewade, B.; Gadkari, P. Role of Dietary Antioxidant Supplements in Male Infertility: A Review. Cureus 2024, 16, e61951. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, N.; Diao, Z.; Chen, Y.; Zhang, Y. Therapeutic potential of TNFα inhibitors in chronic inflammatory disorders: Past and future. Genes. Dis. 2021, 8, 38–47. [Google Scholar] [CrossRef]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chang, T.C.; Lin, S.H.; Wu, S.T.; Cha, T.L.; Tsao, C.W. Metformin Ameliorates Testicular Function and Spermatogenesis in Male Mice with High-Fat and High-Cholesterol Diet-Induced Obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Lane, M. Metformin treatment of high-fat diet-fed obese male mice restores sperm function and fetal growth, without requiring weight loss. Asian J. Androl. 2020, 22, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, D.M.; Alsemeh, A.E.; Khamis, T. Semaglutide early intervention attenuated testicular dysfunction by targeting the GLP-1-PPAR-α-Kisspeptin-Steroidogenesis signaling pathway in a testicular ischemia-reperfusion rat model. Peptides 2022, 149, 170711. [Google Scholar] [CrossRef]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef]
- Padmalayam, I.; Suto, M. Role of adiponectin in the metabolic syndrome: Current perspectives on its modulation as a treatment strategy. Curr. Pharm. Des. 2013, 19, 5755–5763. [Google Scholar] [CrossRef]
- Lee, S.; Kwak, H.B. Role of adiponectin in metabolic and cardiovascular disease. J. Exerc. Rehabil. 2014, 10, 54–59. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Bio 2023, 13, 763–778. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.A.; Malmir, M.; Naderi Noreini, S.; Faraji, T.; Ebrahimi, Z. The effect of vitamin E on sperm motility and viability in asthenoteratozoospermic men: In vitro study. Andrologia 2021, 53, e13891. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Humaidan, P.; Haahr, T.; Povlsen, B.B.; Kofod, L.; Laursen, R.J.; Alsbjerg, B.; Elbaek, H.O.; Esteves, S.C. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: A pilot study. Int. Braz. J. Urol. 2022, 48, 131–156. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Akintayo, C.O.; Oniyide, A.A.; Omoaghe, A.O.; Oyeleke, M.B.; Fafure, A.A. Acetate supplementation restores testicular function by modulating Nrf2/PPAR-γ in high fat diet-induced obesity in Wistar rats. J. Diabetes Metab. Disord. 2021, 20, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, Y.; Zhu, T.; Ding, X.; Zhou, D.; Han, C. Butyrate improves testicular spermatogenic dysfunction induced by a high-fat diet. Transl. Androl. Urol. 2025, 14, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Chiang, Y.F.; Huang, K.C.; Chen, H.Y.; Ali, M.; Hsia, S.M. Alleviating 3-MCPD-induced male reproductive toxicity: Mechanistic insights and resveratrol intervention. Ecotoxicol. Environ. Saf. 2024, 271, 115978. [Google Scholar] [CrossRef]
- Wei, Y.; Tu, J.; Ji, L.; Wang, R.; Zhou, R.; Lei, X.; Hu, L.; Huang, H. Icariin inhibition of NLRP3 mediated Leydig cell pyroptosis and insulin resistance ameliorates spermatogenesis disorders in obese mice. Int. Immunopharmacol. 2025, 151, 114280. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil. Steril. 2007, 88 (Suppl. S4), 1076–1085. [Google Scholar] [CrossRef]
- Izadi, M.; Dehghan Marvast, L.; Rezvani, M.E.; Zohrabi, M.; Aliabadi, A.; Mousavi, S.A.; Aflatoonian, B. Mesenchymal Stem-Cell Derived Exosome Therapy as a Potential Future Approach for Treatment of Male Infertility Caused by Chlamydia Infection. Front. Microbiol. 2021, 12, 785622. [Google Scholar] [CrossRef]
- Fernie, A.R.; Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: Coping with increasing complexity in logic, chemistry, and network interactions scientific correspondence. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Ribas-Maynou, J.; Salas-Huetos, A.; Yeste, M. A systematic review identifying fertility biomarkers in semen: A clinical approach through Omics to diagnose male infertility. Fertil. Steril. 2022, 118, 291–313. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Santacroce, L.; Imbimbo, C.; Ballini, A.; Crocetto, F.; Scacco, S.; Cantore, S.; Di Zazzo, E.; Colella, M.; Jirillo, E. Testicular Immunity and Its Connection with the Microbiota. Physiological and Clinical Implications in the Light of Personalized Medicine. J. Pers. Med. 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Kocełak, P.; Chudek, J.; Naworska, B.; Bąk-Sosnowska, M.; Kotlarz, B.; Mazurek, M.; Madej, P.; Skrzypulec-Plinta, V.; Skałba, P.; Olszanecka-Glinianowicz, M. Psychological disturbances and quality of life in obese and infertile women and men. Int. J. Endocrinol. 2012, 2012, 236217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).