Immune Microenvironment Dysregulation: A Contributing Factor to Obesity-Associated Male Infertility
Abstract
1. Introduction
2. Immune Microenvironment and Male Reproductive Health
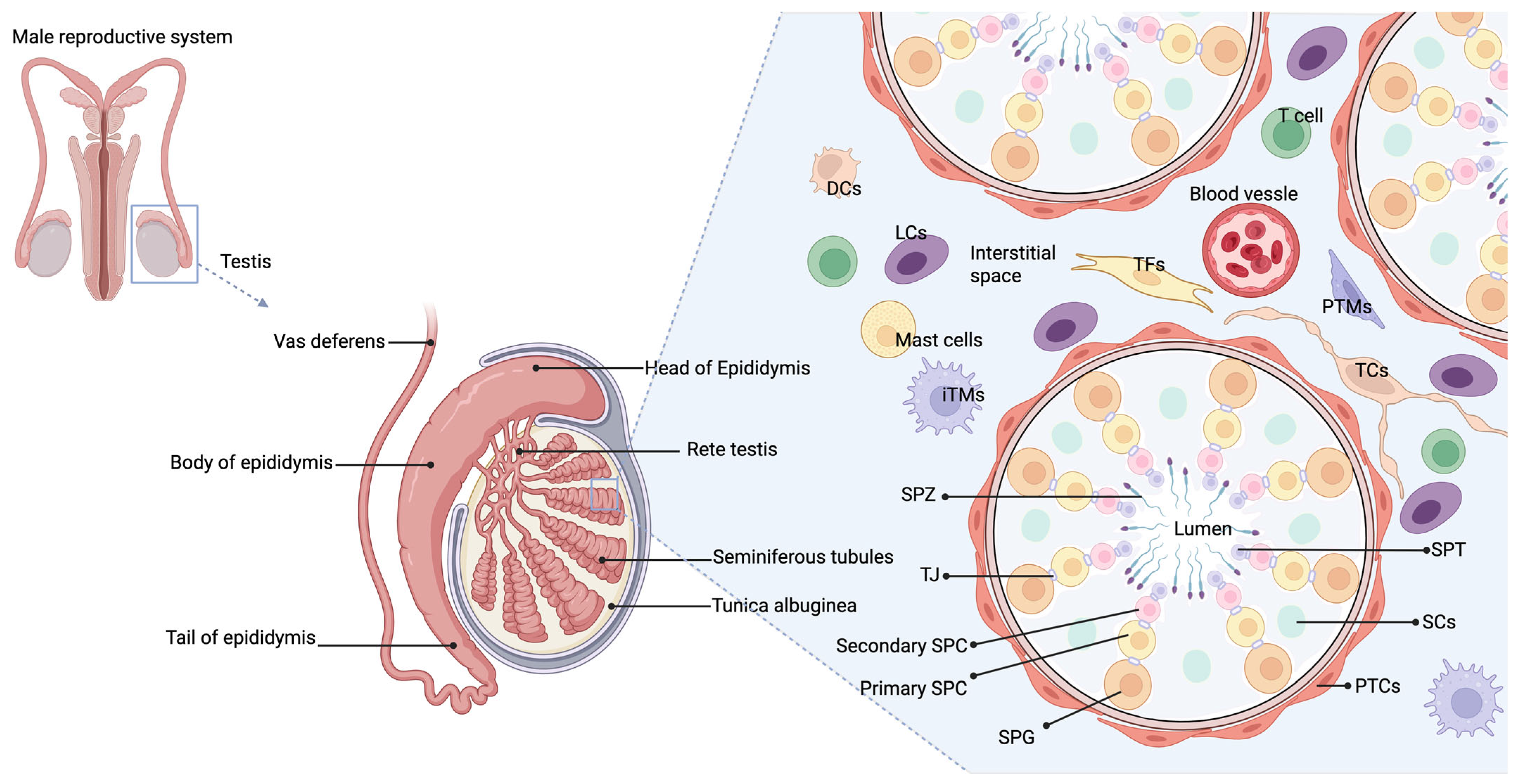
2.1. Normal Immune Microenvironment in the Male Reproductive System
2.1.1. Overview of Immune Cells and Their Functions in the Testes
2.1.2. Blood–Testis Barrier (BTB) and Immune Privilege in Testes
2.2. Immune Dysregulation and Male Infertility
2.2.1. Autoimmune Infertility
2.2.2. Failure of Immune Tolerance
2.2.3. Chronic Inflammation
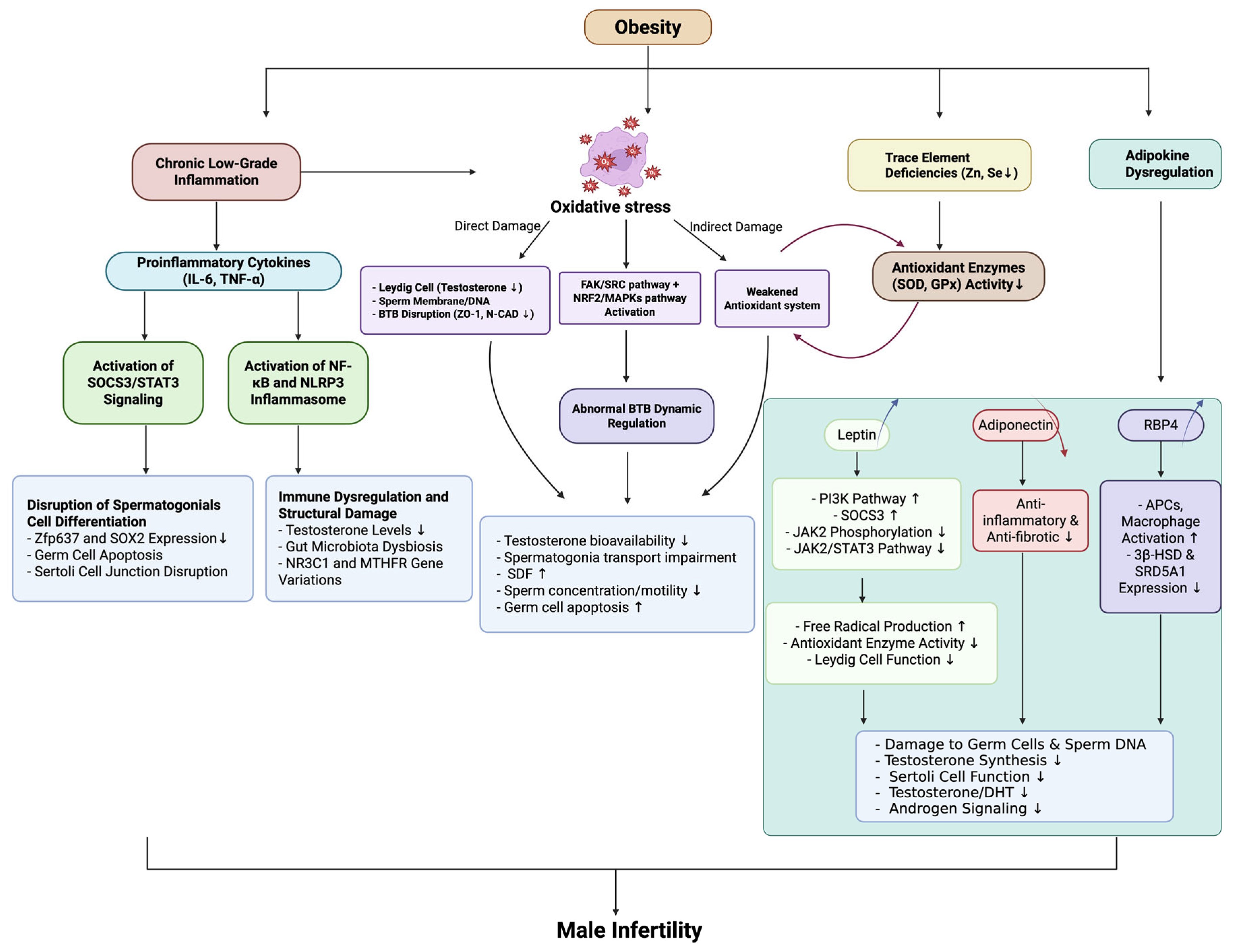
3. Obesity and Its Impact on the Immune System
3.1. Chronic Low-Grade Inflammation
3.2. Adipokines
3.3. Immune Cell Infiltration
4. Intersection of Obesity, Immune Microenvironment, and Male Infertility
4.1. Inflammatory Cytokines in Obesity and Their Role in Male Infertility
4.2. Oxidative Stress, Hormonal Imbalance, and Testicular Dysfunction Induced by Obesity
4.3. The Role of Adipokines in Testicular Function
5. Obesity and Male Infertility: Immune Microenvironment Intervention and Therapeutic Strategies
5.1. Lifestyle Interventions
5.2. Integrated Anti-Inflammatory and Metabolic Therapies: From Pharmacological Agents to Microbiota-Derived Metabolites
5.3. Regenerative Medicine and Biologic Therapies
6. Future Directions and Research Gaps
6.1. Mechanistic Insights and Biomarker Discovery
6.2. Longitudinal and Population-Based Studies of Immune Signatures
6.3. Immunomodulatory Role of the Gut–Testis Axis
6.4. Psychological and Social Determinants of Immune Dysfunction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, O.; Wszołek, K.; Pięt, M.; Wilczak, M.; Chmaj-Wierzchowska, K. Women’s Awareness of Reproductive Health. Medicina 2024, 60, 158. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef]
- Fang, Y.; Su, Y.; Xu, J.; Hu, Z.; Zhao, K.; Liu, C.; Zhang, H. Varicocele-Mediated Male Infertility: From the Perspective of Testicular Immunity and Inflammation. Front. Immunol. 2021, 12, 729539. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, X.; Liu, Y.F.; Song, B.; Sun, Z.; Zhao, S. Immunoregulation and male reproductive function: Impacts and mechanistic insights into inflammation. Andrology 2024. [Google Scholar] [CrossRef]
- Hu, X.; Yang, X.; Zhao, J.; Guan, T.; Dai, Q.; Yang, J.; Zhang, H.; Zhang, D.; Zhang, Y.; Shang, L.; et al. Association between body mass index and varicocele among 211 989 Chinese reproductive-age males. Int. J. Urol. 2022, 29, 853–859. [Google Scholar] [CrossRef]
- Sermondade, N.; Faure, C.; Fezeu, L.; Lévy, R.; Czernichow, S. Obesity and increased risk for oligozoospermia and azoospermia. Arch. Intern. Med. 2012, 172, 440–442. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Oliveira, P.F.; Alves, M.G.; Monteiro, M.P. Obesity and male hypogonadism: Tales of a vicious cycle. Obes. Rev. 2019, 20, 1148–1158. [Google Scholar] [CrossRef]
- Phillips, K.P.; Tanphaichitr, N. Mechanisms of obesity-induced male infertility. Expert. Rev. Endocrinol. Metab. 2010, 5, 229–251. [Google Scholar] [CrossRef]
- Handel, L.N.; Shetty, R.; Sigman, M. The relationship between varicoceles and obesity. J. Urol. 2006, 176, 2138–2140, discussion 2140. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Ramezani Tehrani, F. Potential Adverse Effects of Female and Male Obesity on Fertility: A Narrative Review. Int. J. Endocrinol. Metab. 2020, 18, e101776. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Chen, M.; Xu, K.; Chen, B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell. Mol. Life Sci. 2021, 78, 7275–7288. [Google Scholar] [CrossRef]
- Xu, K.; Fu, A.; Li, Z.; Miao, L.; Lou, Z.; Jiang, K.; Lau, C.; Su, T.; Tong, T.; Bao, J.; et al. Elevated extracellular matrix protein 1 in circulating extracellular vesicles supports breast cancer progression under obesity conditions. Nat. Commun. 2024, 15, 1685. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Warren, B.D.; Ahn, S.H.; Brittain, K.S.; Nanjappa, M.K.; Wang, H.; Wang, J.; Blanco, G.; Sanchez, G.; Fan, Y.; Petroff, B.K.; et al. Multiple Lesions Contribute to Infertility in Males Lacking Autoimmune Regulator. Am. J. Pathol. 2021, 191, 1592–1609. [Google Scholar] [CrossRef]
- Bhushan, S.; Theas, M.S.; Guazzone, V.A.; Jacobo, P.; Wang, M.; Fijak, M.; Meinhardt, A.; Lustig, L. Immune Cell Subtypes and Their Function in the Testis. Front. Immunol. 2020, 11, 583304. [Google Scholar] [CrossRef]
- Li, S.Y.; Kumar, S.; Gu, X.; DeFalco, T. Testicular immunity. Mol. Asp. Med. 2024, 100, 101323. [Google Scholar] [CrossRef]
- Gong, M.; Han, D. Immunologic Environment of the Testis. Adv. Exp. Med. Biol. 2021, 1288, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fijak, M.; Hossain, H.; Markmann, M.; Nüsing, R.M.; Lochnit, G.; Hartmann, M.F.; Wudy, S.A.; Zhang, L.; Gu, H.; et al. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J. Immunol. 2017, 198, 4327–4340. [Google Scholar] [CrossRef] [PubMed]
- Mossadegh-Keller, N.; Gentek, R.; Gimenez, G.; Bigot, S.; Mailfert, S.; Sieweke, M.H. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 2017, 214, 2829–2841. [Google Scholar] [CrossRef]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef]
- Gu, X.; Heinrich, A.; Li, S.Y.; DeFalco, T. Testicular macrophages are recruited during a narrow fetal time window and promote organ-specific developmental functions. Nat. Commun. 2023, 14, 1439. [Google Scholar] [CrossRef]
- Bhushan, S.; Tchatalbachev, S.; Lu, Y.; Fröhlich, S.; Fijak, M.; Vijayan, V.; Chakraborty, T.; Meinhardt, A. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol. 2015, 194, 5455–5464. [Google Scholar] [CrossRef]
- Xia, W.; Wong, E.W.; Mruk, D.D.; Cheng, C.Y. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009, 327, 48–61. [Google Scholar] [CrossRef]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J. Leukoc. Biol. 2011, 90, 133–143. [Google Scholar] [CrossRef]
- Rival, C.; Theas, M.S.; Suescun, M.O.; Jacobo, P.; Guazzone, V.; van Rooijen, N.; Lustig, L. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J. Pathol. 2008, 215, 108–117. [Google Scholar] [CrossRef]
- Klein, B.; Bhushan, S.; Günther, S.; Middendorff, R.; Loveland, K.L.; Hedger, M.P.; Meinhardt, A. Differential tissue-specific damage caused by bacterial epididymo-orchitis in the mouse. Mol. Hum. Reprod. 2020, 26, 215–227. [Google Scholar] [CrossRef]
- Rival, C.; Lustig, L.; Iosub, R.; Guazzone, V.A.; Schneider, E.; Meinhardt, A.; Fijak, M. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006, 324, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Guazzone, V.A.; Hollwegs, S.; Mardirosian, M.; Jacobo, P.; Hackstein, H.; Wygrecka, M.; Schneider, E.; Meinhardt, A.; Lustig, L.; Fijak, M. Characterization of dendritic cells in testicular draining lymph nodes in a rat model of experimental autoimmune orchitis. Int. J. Androl. 2011, 34, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Rival, C.; Guazzone, V.A.; von Wulffen, W.; Hackstein, H.; Schneider, E.; Lustig, L.; Meinhardt, A.; Fijak, M. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol. Hum. Reprod. 2007, 13, 853–861. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Wang, Y.; Han, F.; Cai, W.; Zhao, B.; Li, Y.; Han, S.; Wu, X.; Hu, D. Murine Sertoli cells promote the development of tolerogenic dendritic cells: A pivotal role of galectin-1. Immunology 2016, 148, 253–265. [Google Scholar] [CrossRef]
- Khan, U.; Ghazanfar, H. T Lymphocytes and Autoimmunity. Int. Rev. Cell Mol. Biol. 2018, 341, 125–168. [Google Scholar] [CrossRef]
- Jacobo, P.; Guazzone, V.A.; Pérez, C.V.; Lustig, L. CD4+ Foxp3+ regulatory T cells in autoimmune orchitis: Phenotypic and functional characterization. Am. J. Reprod. Immunol. 2015, 73, 109–125. [Google Scholar] [CrossRef]
- Tung, K.S.; Harakal, J.; Qiao, H.; Rival, C.; Li, J.C.; Paul, A.G.; Wheeler, K.; Pramoonjago, P.; Grafer, C.M.; Sun, W.; et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Investig. 2017, 127, 1046–1060. [Google Scholar] [CrossRef]
- Moreno, D.; Sobarzo, C.M.; Lustig, L.; Rodríguez Peña, M.G.; Guazzone, V.A. Effect of ketotifen fumarate on experimental autoimmune orchitis and torsion of the spermatic cord. Asian J. Androl. 2020, 22, 112–117. [Google Scholar] [CrossRef]
- Wanjari, U.R.; Gopalakrishnan, A.V. Blood-testis barrier: A review on regulators in maintaining cell junction integrity between Sertoli cells. Cell Tissue Res. 2024, 396, 157–175. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The biology of spermatogenesis: The past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1459–1463. [Google Scholar] [CrossRef]
- Dufour, J.M.; Rajotte, R.V.; Seeberger, K.; Kin, T.; Korbutt, G.S. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation 2003, 10, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wei, Y.; Zhou, Y.; Wu, H.; Hong, Y.; Long, C.; Wang, J.; Wu, Y.; Wu, S.; Shen, L.; et al. Wnt5a Regulates Junctional Function of Sertoli cells Through PCP-mediated Effects on mTORC1 and mTORC2. Endocrinology 2021, 162, bqab149. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, M.; Li, H.; Wu, S.; Ge, R.; Wong, C.K.C.; Silvestrini, B.; Sun, F.; Cheng, C.Y. The Non-hormonal Male Contraceptive Adjudin Exerts its Effects via MAPs and Signaling Proteins mTORC1/rpS6 and FAK-Y407. Endocrinology 2021, 162, bqaa196. [Google Scholar] [CrossRef]
- Anyanwu, B.O.; Orisakwe, O.E. Current mechanistic perspectives on male reproductive toxicity induced by heavy metals. J. Environ. Sci. Health Part C Toxicol. Carcinog. 2020, 38, 204–244. [Google Scholar] [CrossRef]
- Cao, X.N.; Shen, L.J.; Wu, S.D.; Yan, C.; Zhou, Y.; Xiong, G.; Wang, Y.C.; Liu, Y.; Liu, B.; Tang, X.L.; et al. Urban fine particulate matter exposure causes male reproductive injury through destroying blood-testis barrier (BTB) integrity. Toxicol. Lett. 2017, 266, 1–12. [Google Scholar] [CrossRef]
- Vazquez-Levin, M.H.; Marín-Briggiler, C.I.; Veaute, C. Antisperm antibodies: Invaluable tools toward the identification of sperm proteins involved in fertilization. Am. J. Reprod. Immunol. 2014, 72, 206–218. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef]
- Negri, L.; Romano, M.; Cirillo, F.; Grilli, L.; Morenghi, E.; Romualdi, D.; Albani, E.; Setti, P.E.L. Influence of inguinal hernia repair on sperm autoimmunity: The largest single center experience. Andrology 2022, 10, 105–110. [Google Scholar] [CrossRef]
- Chereshnev, V.A.; Pichugova, S.V.; Beikin, Y.B.; Chereshneva, M.V.; Iukhta, A.I.; Stroev, Y.I.; Churilov, L.P. Pathogenesis of Autoimmune Male Infertility: Juxtacrine, Paracrine, and Endocrine Dysregulation. Pathophysiology 2021, 28, 471–488. [Google Scholar] [CrossRef]
- Barbonetti, A.; Castellini, C.; D’Andrea, S.; Cordeschi, G.; Santucci, R.; Francavilla, S.; Francavilla, F. Prevalence of anti-sperm antibodies and relationship of degree of sperm auto-immunization to semen parameters and post-coital test outcome: A retrospective analysis of over 10,000 men. Hum. Reprod. 2019, 34, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Bohring, C.; Krause, E.; Habermann, B.; Krause, W. Isolation and identification of sperm membrane antigens recognized by antisperm antibodies, and their possible role in immunological infertility disease. Mol. Hum. Reprod. 2001, 7, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Verón, G.L.; Molina, R.I.; Tissera, A.D.; Estofan, G.M.; Marín-Briggiler, C.I.; Vazquez-Levin, M.H. Incidence of Sperm Surface Autoantibodies and Relationship with Routine Semen Parameters and Sperm Kinematics. Am. J. Reprod. Immunol. 2016, 76, 59–69. [Google Scholar] [CrossRef]
- Yoshitake, H.; Oda, R.; Yanagida, M.; Kawasaki, Y.; Sakuraba, M.; Takamori, K.; Hasegawa, A.; Fujiwara, H.; Araki, Y. Identification of an anti-sperm auto-monoclonal antibody (Ts4)-recognized molecule in the mouse sperm acrosomal region and its inhibitory effect on fertilization in vitro. J. Reprod. Immunol. 2016, 115, 6–13. [Google Scholar] [CrossRef]
- Chen, Y.; Hasegawa, A.; Honda, H.; Wakimoto, Y.; Shibahara, H. Characterization of a spontaneously occurring self-reactive antibody against sperm in mice. J. Reprod. Immunol. 2023, 157, 103930. [Google Scholar] [CrossRef]
- Chiu, W.W.; Chamley, L.W. Clinical associations and mechanisms of action of antisperm antibodies. Fertil. Steril. 2004, 82, 529–535. [Google Scholar] [CrossRef]
- Shibahara, H.; Chen, Y.; Honda, H.; Wakimoto, Y.; Fukui, A.; Hasegawa, A. Sex difference in anti-sperm antibodies. Reprod. Med. Biol. 2022, 21, e12477. [Google Scholar] [CrossRef]
- Jacobo, P.; Guazzone, V.A.; Theas, M.S.; Lustig, L. Testicular autoimmunity. Autoimmun. Rev. 2011, 10, 201–204. [Google Scholar] [CrossRef]
- Barrachina, F.; Ottino, K.; Elizagaray, M.L.; Gervasi, M.G.; Tu, L.J.; Markoulaki, S.; Spallanzani, R.G.; Capen, D.; Brown, D.; Battistone, M.A. Regulatory T cells play a crucial role in maintaining sperm tolerance and male fertility. Proc. Natl. Acad. Sci. USA 2023, 120, e2306797120. [Google Scholar] [CrossRef]
- Attia, H.; Finocchi, F.; Orciani, M.; Mehdi, M.; Zidi Jrah, I.; Lazzarini, R.; Balercia, G.; Mattioli Belmonte, M. Pro-inflammatory cytokines and microRNAs in male infertility. Mol. Biol. Rep. 2021, 48, 5935–5942. [Google Scholar] [CrossRef]
- Rivero, M.J.; Kulkarni, N.; Thirumavalavan, N.; Ramasamy, R. Evaluation and management of male genital tract infections in the setting of male infertility: An updated review. Curr. Opin. Urol. 2023, 33, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Linn, T.; Drlica, K.; Shi, L. Diabetes as a potential compounding factor in COVID-19-mediated male subfertility. Cell Biosci. 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Portela, J.M.D.; Sousa, M.I.; Mota, P.C.; Ramalho-Santos, J.; Amaral, S. High glucose levels affect spermatogenesis: An in vitro approach. Reprod. Fertil. Dev. 2017, 29, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chen, T.L.; Tseng, Y.Y.; Wu, G.J.; Hsieh, M.H.; Lin, Y.W.; Chen, R.M. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol. Appl. Pharmacol. 2016, 304, 59–69. [Google Scholar] [CrossRef]
- Ford, W.C. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Zini, A.; San Gabriel, M.; Baazeem, A. Antioxidants and sperm DNA damage: A clinical perspective. J. Assist. Reprod. Genet. 2009, 26, 427–432. [Google Scholar] [CrossRef]
- Tian, Y.; Song, W.; Xu, D.; Chen, X.; Li, X.; Zhao, Y. Autophagy Induced by ROS Aggravates Testis Oxidative Damage in Diabetes via Breaking the Feedforward Loop Linking p62 and Nrf2. Oxid. Med. Cell. Longev. 2020, 2020, 7156579. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, W.; Wu, J.; Zhang, D.; Abou-Shakra, A.; Di, L.; Wang, Z.; Wang, L.; Yang, F.; Qiao, Z. Nicotine induced autophagy of Leydig cells rather than apoptosis is the major reason of the decrease of serum testosterone. Int. J. Biochem. Cell Biol. 2018, 100, 30–41. [Google Scholar] [CrossRef]
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Zhou, W.; Yuan, M.; Shi, Y.; Yang, K. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: Involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology 2016, 341–343, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.E.I.; Xiang-Liang, T.; Yu, Z.; Bin, L.; Lian-Ju, S.; Chun-Lan, L.; Tao, L.I.N.; Da-Wei, H.E.; Sheng-de, W.U.; Guang-Hui, W.E.I. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes. Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, L.; Chen, X.; Ding, Y.; He, J.; Zhu, J.; Wang, Y.; Liu, X. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reprod. Biomed. Online 2016, 32, 207–217. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Arefanian, H.; Thomas, R.; Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. Endoplasmic Reticulum Stress Promotes the Expression of TNF-α in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1α and MAPK/NF-κB Pathways. Int. J. Mol. Sci. 2023, 24, 15186. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, T.; Gomez, N.A.; Wang, B.; Zhu, M.J.; Du, M. Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) α1. Nutr. Diabetes 2018, 8, 39. [Google Scholar] [CrossRef]
- Chaves de Souza, J.A.; Nogueira, A.V.; Chaves de Souza, P.P.; Kim, Y.J.; Silva Lobo, C.; Pimentel Lopes de Oliveira, G.J.; Cirelli, J.A.; Garlet, G.P.; Rossa, C., Jr. SOCS3 expression correlates with severity of inflammation, expression of proinflammatory cytokines, and activation of STAT3 and p38 MAPK in LPS-induced inflammation in vivo. Mediat. Inflamm. 2013, 2013, 650812. [Google Scholar] [CrossRef]
- Kim, F.; Pham, M.; Luttrell, I.; Bannerman, D.D.; Tupper, J.; Thaler, J.; Hawn, T.R.; Raines, E.W.; Schwartz, M.W. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ. Res. 2007, 100, 1589–1596. [Google Scholar] [CrossRef]
- Fuster, J.J.; Zuriaga, M.A.; Ngo, D.T.; Farb, M.G.; Aprahamian, T.; Yamaguchi, T.P.; Gokce, N.; Walsh, K. Noncanonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes 2015, 64, 1235–1248. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Lago, F.; Gómez, R.; Gómez-Reino, J.J.; Dieguez, C.; Gualillo, O. Adipokines as novel modulators of lipid metabolism. Trends Biochem. Sci. 2009, 34, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Leibel, R.L. 20 years of leptin: Role of leptin in energy homeostasis in humans. J. Endocrinol. 2014, 223, T83–T96. [Google Scholar] [CrossRef] [PubMed]
- Carlton, E.D.; Demas, G.E.; French, S.S. Leptin, a neuroendocrine mediator of immune responses, inflammation, and sickness behaviors. Horm. Behav. 2012, 62, 272–279. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Li, C.; Cheng, H.; Adhikari, B.K.; Wang, S.; Yang, N.; Liu, W.; Sun, J.; Wang, Y. The Role of Apelin-APJ System in Diabetes and Obesity. Front. Endocrinol. 2022, 13, 820002. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, E.; Deng, L.; Zhu, Y.; Lu, X.; Li, X.; Li, F.; Yan, Y.; Han, J.Y.; Li, Y.; et al. Immunological roles for resistin and related adipokines in obesity-associated tumors. Int. Immunopharmacol. 2024, 142 Pt A, 112911. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed. Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Yokota, T.; Oritani, K.; Takahashi, I.; Ishikawa, J.; Matsuyama, A.; Ouchi, N.; Kihara, S.; Funahashi, T.; Tenner, A.J.; Tomiyama, Y.; et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000, 96, 1723–1732. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [CrossRef]
- Ali, M.; Jasmin, S.; Fariduddin, M.; Alam, S.M.K.; Arslan, M.I.; Biswas, S.K. Neutrophil elastase and myeloperoxidase mRNA expression in overweight and obese subjects. Mol. Biol. Rep. 2018, 45, 1245–1252. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils Actively Contribute to Obesity-Associated Inflammation and Pathological Complications. Cells 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Green, W.D.; Beck, M.A. Obesity altered T cell metabolism and the response to infection. Curr. Opin. Immunol. 2017, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Cho, K.W.; Delproposto, J.L.; Oatmen, K.E.; Geletka, L.M.; Martinez-Santibanez, G.; Singer, K.; Lumeng, C.N. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013, 62, 2762–2772. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Haas, K.M.; Beck, M.A.; Teague, H. The effects of diet-induced obesity on B cell function. Clin. Exp. Immunol. 2015, 179, 90–99. [Google Scholar] [CrossRef]
- Theurich, S.; Tsaousidou, E.; Hanssen, R.; Lempradl, A.M.; Mauer, J.; Timper, K.; Schilbach, K.; Folz-Donahue, K.; Heilinger, C.; Sexl, V.; et al. IL-6/Stat3-Dependent Induction of a Distinct, Obesity-Associated NK Cell Subpopulation Deteriorates Energy and Glucose Homeostasis. Cell Metab. 2017, 26, 171–184.e176. [Google Scholar] [CrossRef]
- Maegawa, M.; Kamada, M.; Irahara, M.; Yamamoto, S.; Yoshikawa, S.; Kasai, Y.; Ohmoto, Y.; Gima, H.; Thaler, C.J.; Aono, T. A repertoire of cytokines in human seminal plasma. J. Reprod. Immunol. 2002, 54, 33–42. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, M.; Zhang, J.; Li, J.; Gong, D.; Li, Y.; Zhang, J.; Lin, P.; Huang, L. IL-6 mediates differentiation disorder during spermatogenesis in obesity-associated inflammation by affecting the expression of Zfp637 through the SOCS3/STAT3 pathway. Sci. Rep. 2016, 6, 28012. [Google Scholar] [CrossRef]
- Lysiak, J.J. The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod. Biol. Endocrinol. 2004, 2, 9. [Google Scholar] [CrossRef]
- Publicover, S.; Harper, C.V.; Barratt, C. [Ca2+]i signalling in sperm--making the most of what you’ve got. Nat. Cell Biol. 2007, 9, 235–242. [Google Scholar] [CrossRef]
- Koçak, I.; Yenisey, C.; Dündar, M.; Okyay, P.; Serter, M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. [Google Scholar] [CrossRef]
- Pentikäinen, V.; Suomalainen, L.; Erkkilä, K.; Martelin, E.; Parvinen, M.; Pentikäinen, M.O.; Dunkel, L. Nuclear factor-kappa B activation in human testicular apoptosis. Am. J. Pathol. 2002, 160, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y.; Liu, Y.; Zhang, Z.; Lu, L.; Ding, Z. Obesity or Overweight, a Chronic Inflammatory Status in Male Reproductive System, Leads to Mice and Human Subfertility. Front. Physiol. 2017, 8, 1117. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Shang, Y.; Wang, Y.; Chen, Y.; Mu, B.; Zheng, Q.; Liu, H. 1-DNJ Alleviates Obesity-Induced Testicular Inflammation in Mice Model by Inhibiting IKKβ/ NF-kB Pathway. Reprod. Sci. 2024, 31, 2103–2113. [Google Scholar] [CrossRef]
- Elmorsy, E.H.; Aly, R.G.; Badae, N.M.; Aboghazala, M.M.; Omar, S.S. Zinc alleviates high fat diet-induced spermatogenic dysfunction in Wistar rats: Role of oxidative stress, HMGB1 and inflammasome. Rev. Int. Androl. 2024, 22, 44–52. [Google Scholar] [CrossRef]
- Hueston, C.M.; Deak, T. The inflamed axis: The interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol. Behav. 2014, 124, 77–91. [Google Scholar] [CrossRef]
- Shiba, S.; Ikeda, K.; Horie-Inoue, K.; Azuma, K.; Hasegawa, T.; Amizuka, N.; Tanaka, T.; Takeiwa, T.; Shibata, Y.; Koji, T.; et al. Vitamin K-Dependent γ-Glutamyl Carboxylase in Sertoli Cells Is Essential for Male Fertility in Mice. Mol. Cell. Biol. 2021, 41, e00404-20. [Google Scholar] [CrossRef]
- Takumi, N.; Shirakawa, H.; Ohsaki, Y.; Ito, A.; Watanabe, T.; Giriwono, P.E.; Sato, T.; Komai, M. Dietary vitamin K alleviates the reduction in testosterone production induced by lipopolysaccharide administration in rat testis. Food Funct. 2011, 2, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Sun, H. Catalpol ameliorates hepatic insulin resistance in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway. Pharmacol. Res. 2018, 130, 466–480. [Google Scholar] [CrossRef]
- Al-Asmakh, M.; Stukenborg, J.B.; Reda, A.; Anuar, F.; Strand, M.L.; Hedin, L.; Pettersson, S.; Söder, O. The gut microbiota and developmental programming of the testis in mice. PLoS ONE 2014, 9, e103809. [Google Scholar] [CrossRef]
- Chandra Sekar, P.K.; Veerabathiran, R. Genes linked to obesity-related infertility: Bridging the knowledge gap. Reprod. Dev. Med. 2024, 8, 121–129. [Google Scholar] [CrossRef]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Falvo, S.; Minucci, S.; Santillo, A.; Senese, R.; Chieffi Baccari, G.; Venditti, M. A short-term high-fat diet alters rat testicular activity and blood-testis barrier integrity through the SIRT1/NRF2/MAPKs signaling pathways. Front. Endocrinol. 2023, 14, 1274035. [Google Scholar] [CrossRef]
- Elmas, M.A.; Ozakpinar, O.B.; Kolgazi, M.; Sener, G.; Arbak, S.; Ercan, F. Exercise improves testicular morphology and oxidative stress parameters in rats with testicular damage induced by a high-fat diet. Andrologia 2022, 54, e14600. [Google Scholar] [CrossRef]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Giacone, F.; Mongioi, L.M.; La Vignera, S. Non-hormonal treatment for male infertility: The potential role of Serenoa repens, selenium and lycopene. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3112–3120. [Google Scholar] [CrossRef]
- Yi, X.; Tang, D.; Cao, S.; Li, T.; Gao, H.; Ma, T.; Yao, T.; Li, J.; Chang, B. Effect of Different Exercise Loads on Testicular Oxidative Stress and Reproductive Function in Obese Male Mice. Oxid. Med. Cell. Longev. 2020, 2020, 3071658. [Google Scholar] [CrossRef]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Yuxin, L.; Chen, L.; Xiaoxia, L.; Yue, L.; Junjie, L.; Youzhu, L.; Huiliang, Z.; Qicai, L. Research Progress on the Relationship between Obesity-Inflammation-Aromatase Axis and Male Infertility. Oxid. Med. Cell. Longev. 2021, 2021, 6612796. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Yang, J.M.; Ye, R.J.; Liu, N.; Zhang, W.J.; Ma, L.; Zheng, P.; Niu, J.G.; Liu, P.; Yu, J.Q. Protective effects of betaine on diabetic induced disruption of the male mice blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Biomed. Pharmacother. 2019, 120, 109474. [Google Scholar] [CrossRef]
- Calderón, B.; Gómez-Martín, J.M.; Cuadrado-Ayuso, M.; Cobeta, P.; Vega-Piñero, B.; Mateo, R.; Galindo, J.; Botella-Carretero, J.I. Circulating Zinc and Copper Levels are Associated with Sperm Quality in Obese Men after Metabolic Surgery: A Pilot Study. Nutrients 2020, 12, 3354. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Domínguez-Riscart, J.; Millán-Martínez, M.; Mateos-Bernal, R.M.; Lechuga-Sancho, A.M.; González-Domínguez, R. Trace elements as potential modulators of puberty-induced amelioration of oxidative stress and inflammation in childhood obesity. Biofactors 2023, 49, 820–830. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, Á.; Millán-Martínez, M.; Domínguez-Riscart, J.; Lechuga-Sancho, A.M.; González-Domínguez, R. Metal Homeostasis and Exposure in Distinct Phenotypic Subtypes of Insulin Resistance among Children with Obesity. Nutrients 2023, 15, 2347. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, C.; Wu, W.; Shi, L.; Jiang, C.; Wang, L.; Ding, Z.; Liu, Y. Zinc transporter ZIP12 maintains zinc homeostasis and protects spermatogonia from oxidative stress during spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 17. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; Beserra, J.B.; Dos Santos, L.R.; de Sousa Melo, S.R.; de Sousa, G.S.; de Matos Neto, E.M.; Henriques, G.S.; do Nascimento Marreiro, D. Role of Zinc in Zinc-α2-Glycoprotein Metabolism in Obesity: A Review of Literature. Biol. Trace Elem. Res. 2020, 193, 81–88. [Google Scholar] [CrossRef]
- Romier, B.; Tourniaire, F.; Marcotorchino, J.; Gouranton, E.; Astier, J.; Malezet, C.; Blouin, E.; Landrier, J.F. Bioeffects of a combination of trace elements on adipocyte biology. Metallomics 2013, 5, 524–531. [Google Scholar] [CrossRef]
- Zhai, X.W.; Zhang, Y.L.; Qi, Q.; Bai, Y.; Chen, X.L.; Jin, L.J.; Ma, X.G.; Shu, R.Z.; Yang, Z.J.; Liu, F.J. Effects of molybdenum on sperm quality and testis oxidative stress. Syst. Biol. Reprod. Med. 2013, 59, 251–255. [Google Scholar] [CrossRef]
- Almabhouh, F.A.; Singh, H.J. The impact of leptin on sperm. Reprod. Fertil. Dev. 2023, 35, 459–468. [Google Scholar] [CrossRef]
- Bjorbak, C.; Lavery, H.J.; Bates, S.H.; Olson, R.K.; Davis, S.M.; Flier, J.S.; Myers, M.G., Jr. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000, 275, 40649–40657. [Google Scholar] [CrossRef]
- Landry, D.A.; Sormany, F.; Haché, J.; Roumaud, P.; Martin, L.J. Steroidogenic genes expressions are repressed by high levels of leptin and the JAK/STAT signaling pathway in MA-10 Leydig cells. Mol. Cell. Biochem. 2017, 433, 79–95. [Google Scholar] [CrossRef]
- Nogueiras, R.; Barreiro, M.L.; Caminos, J.E.; Gaytán, F.; Suominen, J.S.; Navarro, V.M.; Casanueva, F.F.; Aguilar, E.; Toppari, J.; Diéguez, C.; et al. Novel expression of resistin in rat testis: Functional role and regulation by nutritional status and hormonal factors. J. Cell Sci. 2004, 117 Pt 15, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Krishna, A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. Biochimie 2020, 168, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Vieira, P.M.; Yore, M.M.; Dwyer, P.M.; Syed, I.; Aryal, P.; Kahn, B.B. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab. 2014, 19, 512–526. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Li, Y.; Zhao, X.; Zhang, Y. RBP4 regulates androgen receptor expression and steroid synthesis in Sertoli cells from Bactrian camels. Reprod. Domest. Anim. 2022, 57, 429–437. [Google Scholar] [CrossRef]
- Andersen, E.; Juhl, C.R.; Kjøller, E.T.; Lundgren, J.R.; Janus, C.; Dehestani, Y.; Saupstad, M.; Ingerslev, L.R.; Duun, O.M.; Jensen, S.B.K.; et al. Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: A randomized controlled trial. Hum. Reprod. 2022, 37, 1414–1422. [Google Scholar] [CrossRef]
- Lee, G.; Choi, H.Y.; Yang, S.J. Effects of Dietary and Physical Activity Interventions on Metabolic Syndrome: A Meta-analysis. J. Korean Acad. Nurs. 2015, 45, 483–494. [Google Scholar] [CrossRef]
- Rodak, K.; Kratz, E.M. PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders. Pharmaceuticals 2023, 16, 723. [Google Scholar] [CrossRef]
- Ghewade, P.; Vagha, S.; Ghewade, B.; Gadkari, P. Role of Dietary Antioxidant Supplements in Male Infertility: A Review. Cureus 2024, 16, e61951. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, N.; Diao, Z.; Chen, Y.; Zhang, Y. Therapeutic potential of TNFα inhibitors in chronic inflammatory disorders: Past and future. Genes. Dis. 2021, 8, 38–47. [Google Scholar] [CrossRef]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chang, T.C.; Lin, S.H.; Wu, S.T.; Cha, T.L.; Tsao, C.W. Metformin Ameliorates Testicular Function and Spermatogenesis in Male Mice with High-Fat and High-Cholesterol Diet-Induced Obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef] [PubMed]
- McPherson, N.O.; Lane, M. Metformin treatment of high-fat diet-fed obese male mice restores sperm function and fetal growth, without requiring weight loss. Asian J. Androl. 2020, 22, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, D.M.; Alsemeh, A.E.; Khamis, T. Semaglutide early intervention attenuated testicular dysfunction by targeting the GLP-1-PPAR-α-Kisspeptin-Steroidogenesis signaling pathway in a testicular ischemia-reperfusion rat model. Peptides 2022, 149, 170711. [Google Scholar] [CrossRef]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef]
- Padmalayam, I.; Suto, M. Role of adiponectin in the metabolic syndrome: Current perspectives on its modulation as a treatment strategy. Curr. Pharm. Des. 2013, 19, 5755–5763. [Google Scholar] [CrossRef]
- Lee, S.; Kwak, H.B. Role of adiponectin in metabolic and cardiovascular disease. J. Exerc. Rehabil. 2014, 10, 54–59. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Bio 2023, 13, 763–778. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.A.; Malmir, M.; Naderi Noreini, S.; Faraji, T.; Ebrahimi, Z. The effect of vitamin E on sperm motility and viability in asthenoteratozoospermic men: In vitro study. Andrologia 2021, 53, e13891. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Humaidan, P.; Haahr, T.; Povlsen, B.B.; Kofod, L.; Laursen, R.J.; Alsbjerg, B.; Elbaek, H.O.; Esteves, S.C. The combined effect of lifestyle intervention and antioxidant therapy on sperm DNA fragmentation and seminal oxidative stress in IVF patients: A pilot study. Int. Braz. J. Urol. 2022, 48, 131–156. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Akintayo, C.O.; Oniyide, A.A.; Omoaghe, A.O.; Oyeleke, M.B.; Fafure, A.A. Acetate supplementation restores testicular function by modulating Nrf2/PPAR-γ in high fat diet-induced obesity in Wistar rats. J. Diabetes Metab. Disord. 2021, 20, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, Y.; Zhu, T.; Ding, X.; Zhou, D.; Han, C. Butyrate improves testicular spermatogenic dysfunction induced by a high-fat diet. Transl. Androl. Urol. 2025, 14, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Chiang, Y.F.; Huang, K.C.; Chen, H.Y.; Ali, M.; Hsia, S.M. Alleviating 3-MCPD-induced male reproductive toxicity: Mechanistic insights and resveratrol intervention. Ecotoxicol. Environ. Saf. 2024, 271, 115978. [Google Scholar] [CrossRef]
- Wei, Y.; Tu, J.; Ji, L.; Wang, R.; Zhou, R.; Lei, X.; Hu, L.; Huang, H. Icariin inhibition of NLRP3 mediated Leydig cell pyroptosis and insulin resistance ameliorates spermatogenesis disorders in obese mice. Int. Immunopharmacol. 2025, 151, 114280. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil. Steril. 2007, 88 (Suppl. S4), 1076–1085. [Google Scholar] [CrossRef]
- Izadi, M.; Dehghan Marvast, L.; Rezvani, M.E.; Zohrabi, M.; Aliabadi, A.; Mousavi, S.A.; Aflatoonian, B. Mesenchymal Stem-Cell Derived Exosome Therapy as a Potential Future Approach for Treatment of Male Infertility Caused by Chlamydia Infection. Front. Microbiol. 2021, 12, 785622. [Google Scholar] [CrossRef]
- Fernie, A.R.; Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: Coping with increasing complexity in logic, chemistry, and network interactions scientific correspondence. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Ribas-Maynou, J.; Salas-Huetos, A.; Yeste, M. A systematic review identifying fertility biomarkers in semen: A clinical approach through Omics to diagnose male infertility. Fertil. Steril. 2022, 118, 291–313. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Santacroce, L.; Imbimbo, C.; Ballini, A.; Crocetto, F.; Scacco, S.; Cantore, S.; Di Zazzo, E.; Colella, M.; Jirillo, E. Testicular Immunity and Its Connection with the Microbiota. Physiological and Clinical Implications in the Light of Personalized Medicine. J. Pers. Med. 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Kocełak, P.; Chudek, J.; Naworska, B.; Bąk-Sosnowska, M.; Kotlarz, B.; Mazurek, M.; Madej, P.; Skrzypulec-Plinta, V.; Skałba, P.; Olszanecka-Glinianowicz, M. Psychological disturbances and quality of life in obese and infertile women and men. Int. J. Endocrinol. 2012, 2012, 236217. [Google Scholar] [CrossRef] [PubMed]

| Category | Mechanism | Impact on Male Reproductive Health |
|---|---|---|
| Chronic Inflammation |
|
|
| Oxidative Stress |
|
|
| Hormonal Imbalance |
|
|
| Adipokine Dysregulation |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, R.; Cheng, D.; Zhang, W.; Zhang, J.; Chen, S.; Xia, Y. Immune Microenvironment Dysregulation: A Contributing Factor to Obesity-Associated Male Infertility. Biomedicines 2025, 13, 1314. https://doi.org/10.3390/biomedicines13061314
Feng R, Cheng D, Zhang W, Zhang J, Chen S, Xia Y. Immune Microenvironment Dysregulation: A Contributing Factor to Obesity-Associated Male Infertility. Biomedicines. 2025; 13(6):1314. https://doi.org/10.3390/biomedicines13061314
Chicago/Turabian StyleFeng, Rui, Dexin Cheng, Wei Zhang, Jiayun Zhang, Sixiang Chen, and Yan Xia. 2025. "Immune Microenvironment Dysregulation: A Contributing Factor to Obesity-Associated Male Infertility" Biomedicines 13, no. 6: 1314. https://doi.org/10.3390/biomedicines13061314
APA StyleFeng, R., Cheng, D., Zhang, W., Zhang, J., Chen, S., & Xia, Y. (2025). Immune Microenvironment Dysregulation: A Contributing Factor to Obesity-Associated Male Infertility. Biomedicines, 13(6), 1314. https://doi.org/10.3390/biomedicines13061314





