Abstract
The whey protein (WP) fraction represents 18–20% of the total milk nitrogen content. It was originally considered a dairy industry waste, but upon its chemical characterization, it was found to be a precious source of bioactive components, growing in popularity as nutritional and functional food ingredients. This has generated a remarkable increase in interest in applications in the different sectors of nutrition, food industry, and pharmaceutics. WPs comprise immunoglobulins and proteins rich in branched and essential amino acids, and peptides endowed with several biological activities (antimicrobial, antihypertensive, antithrombotic, anticancer, antioxidant, opioid, immunomodulatory, and gut microbiota regulation) and technological properties (gelling, water binding, emulsification, and foaming ability). Currently, various process technologies and biotechnological methods are available to recover WPs and convert them into BioActive Peptides (BAPs) for commercial use. Additionally, in silico approaches could have a significant impact on the development of novel foods and/or ingredients and therapeutic agents. This review provides an overview of current and emerging methods for the production, selection, and application of whey peptides, offering insights into bioactivity profiling and potential therapeutic targets. Recent updates in legislation related to commercialized WPs-based products are also presented.
1. Introduction
Whey is a byproduct of dairy industries, which is formed during the milk coagulation process in the manufacture of dairy products. Considering that approximately 9 kg of whey is produced for 1 kg of cheese, a production of 187–206 million tons of whey was reported for 2023, and 203–241 million tons is estimated by 2030 [1]. In the past, whey was considered a waste, and there was no sustainable management of its disposal, which consisted of discharge into wastewater, into water without pretreatment or distributed onto the land [2]. This procedure is highly risky for the environment due to the high polluting power of whey linked to the high Biological and Chemical Oxygen Demand (BOD and COD) of 20–60 g/L and 50–102 g/L, respectively [3]. Although approximately 40% of whey is still discarded globally [4], the implementation of waste regulations and greater attention to the environment have encouraged the valorization of whey in a circular economy perspective.
Whey represents, in fact, a very rich source of nutrients, such as soluble proteins, lactose, vitamins, minerals, and fat [5,6], endowed with nutritional [1,7,8], techno-functional [9,10], and bioactive properties [3,11,12,13,14,15].
Whey proteins (WPs) constitute around 20% of the total protein content in milk [16] and include β-lactoglobulin (β-LG), α-lactalbumin (α-LA), immunoglobulins (IGs), bovine serum albumin (BSA), lactoferrin (LF), and lactoperoxidase (LP), together with other minor components [17]. Except for some minor species, the nutrient contents in whey from several mammal species are quite similar [18]. In general, WPs from alternative milk sources (e.g., buffalo, sheep, goat, camel, horse, yak, and donkey) are less commonly utilized, mainly due to limited awareness and availability; however, increasing interest in their properties and their potential application in dairy industries has recently been documented [19,20,21].
WPs are renowned for their high-quality protein content and excellent amino acid profile, which ensures good digestibility and provides essential amino acids that are readily absorbed and metabolized by the body [4,9]. For instance, sulfur amino acids play a role as antioxidants and as precursors of the powerful intracellular antioxidant glutathione [22]; amino acids such as isoleucine, leucine, and valine are critical as regulators of various metabolic functions and blood glucose homeostasis [8,22]. A recent narrative review explored the health implications of WPs supplementation, highlighting both benefits and potential risks [23]. WP dietary supplementation is avoided in subjects with hepatic and renal compromised functions, as well as in acne susceptibility; beneficial effects can be found in the intestinal microbiota, emotional and behavioral responses, and bone and muscle mass in elderly [23]. This study underscores the need for balanced WP consumption and further research to clarify its long-term health impacts, urging health professionals and consumers to make informed decisions [23].
Both WPs and their peptides have been widely studied for their ability to elicit positive physiological effects, suggesting food and pharmaceutical applications [5,15].
BioActive Peptides (BAPs) are generally released upon enzymatic breakdown during gastrointestinal digestion by proteinases (e.g., pepsin, chymotrypsin, and trypsin) and peptidases (e.g., carboxypeptidase, enteropeptidase, aminopeptidase, and dipeptidyl-peptidase IV) or during food processes. BAPs from whey proteins exhibit various biological activities, including antimicrobial, antihypertensive, antithrombotic, anticancer, antioxidant, opioid, immunomodulatory, and gut microbiota regulation [15].
In general, WPs are more rapidly digested than caseins in the gastrointestinal tract (2 vs. 7 h, respectively; [17]); however, excessive heating can potentially reduce WPs digestibility by modifying the structure of native proteins, which in turn limit cleavage site accessibility to enzymes [24]. Moderate heating during processing typically has a minimal impact on WPs’ gastroenteric digestibility [25,26].
In the dairy industry, milk processing promotes the release of BAPs of different molecular weights [27]; various proteinases are naturally present in milk, generally classified as cysteine- (cathepsin B), serine- (plasmin), metalloproteinase- (thermolysin), and aspartic-proteinase (cathepsin D). Among these proteinases, the most important is surely plasmin, the active form of plasminogen released from casein micelles following their destabilization under appropriate pH, temperature, and ionic conditions [28,29]. Besides rennet enzymes (chimosin, pepsin), non-dairy related proteases, including plant cardosin, bromelain, papain, actinidain, ficin, fungal flavorzyme, and bacterial subtilisin, have also been used to obtain functional foods [30,31] or to produce hydrolysates enriched in antimicrobial and antioxidant peptides; the latter have been successfully exploited both to extend the shelf-life and enhance the safety of refrigerated minimally processed foods [12,32,33,34,35]. Moreover, flavorzyme produced by Aspergillus oryzae is used to enhance flavor in various food products by hydrolyzing proteins, releasing amino acids that contribute to savory tastes, and debittering [36]. Figure 1 provides an overview of the impact of whey recovery and its transformation into high-value products.
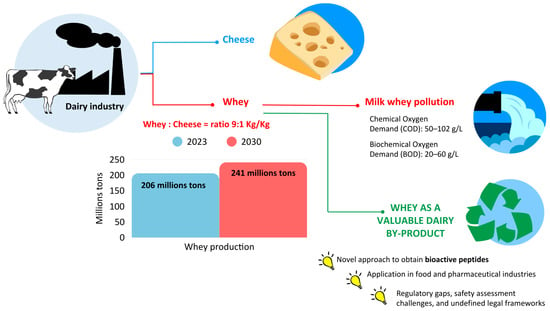
Figure 1.
Whey: from waste to precious resource. The data regarding whey production and pollution were reported by Buchanan et al. [1].
Current trends focus on the mass production of BAPs. Besides conventional protocols, novel green technologies (e.g., ultrasound, microwave, hydrostatic pressure, pulsed electric field, and subcritical water) have made significant progress and have been applied to improve the yield and peptide stability; innovative membrane reactor for enzymatic hydrolysis, immobilized enzymes, and genetic and combinatorial approaches are also being developed for increased hydrolysis rates and greater production [37,38,39]. More targeted approaches have recently been described for the release of specific BAPs in a more predictable and efficient manner. These targeted approaches are mostly based on in silico QSAR models, molecular docking, and molecular dynamics simulation; taken together, these methods can significantly accelerate the identification of new BAPs and improve bioactivity and pharmaceutical properties [40]. Although the peptide therapeutics market is expected to expand over the coming years [41], the list of commercial products containing BAPs is still limited [15,42], and further research to transfer BAPs from laboratory- to large-scale production is still needed. One possible reason for this limitation is that the safety assessment of BAPs is still under investigation [15,21,43]. Chalamaiah et al. [44] recently discussed the lack of scientific validation of the safety and efficacy of many products already on the market that are based on BAPs, including those derived from WPs. This is frequently in contrast to the strict regulations on health claims that are in place in many countries. It is imperative that this scientific gap be addressed as soon as possible by validating precise clinical investigations, evaluating their safety, and determining the intended customer base for goods containing BAPs.
In this fascinating scenario, this review primarily focuses on the description of major and minor WPs and their related BAPs, highlighting the benefits associated with their applications in the food and pharmaceutical sectors. A comprehensive analysis of emerging trends in the production of BAPs from WPs is presented, with an emphasis on innovative extraction methods that enhance yield, efficiency, and functionality compared to conventional techniques.
Additionally, this study delves into the advanced exploration of novel and breakthrough techniques, predicting their biological profile. By leveraging state-of-the-art technologies and computational approaches, this study offers a comprehensive perspective on the potential of whey-derived BAPs to advance nutrition, drive innovation in functional foods, and support therapeutic applications. Notably, it also addresses the regulatory frameworks, existing gaps, and safety assessment challenges related to these compounds; the latter aspect is still unexplored in the current scientific literature.
2. Major and Minor WPs
WPs include five major protein fractions: beta-Lactoglobulin (β-LG), alpha-Lactalbumin (α-LA), bovine serum albumin (BSA), immunoglobulins (IGs), and Glycomacropeptide (GMP). In addition, minor protein fractions such as lactoferrin (LF), lactoperoxidase (LP), and lysozyme (LYZ) contribute to its overall composition. A positive impact on human health linked to the consumption of WPs has been widely reported [5,15,21,23,45,46,47,48].
Bovine WPs have been extensively utilized as a substrate for peptide release. Thus, recent advances in the field include WPs from minor species [21,49,50,51]. Table 1 compares minor and major dairy mammal species in terms of their WP composition: minor species like horse (7.4–9.1 g/L) and donkey (4.9–8 g/L) milk have a relatively high whey protein content, comparable to major species like sheep (10.2–11 g/L) but higher than cow (5.5–7 g/L) and goat (3.7–7 g/L) milk. This makes minor species’ milk a potential alternative for protein-rich diets. Camel milk lacks β-LG, while other species, such as buffalo (3.9 g/L) and yak (3.4–10.1 g/L), have significant levels. β-LG is crucial in milk allergy studies, and camel milk’s absence of this protein is a unique feature [52]. Compared with bovine milk, equine milk contains less β-LG and more α-LA and IGs.
The principal anti-microbial agent in milk is LYZ, and, to a lesser extent, LF, which predominates in horse, donkey, yak, and camel milk, which can contain up to 7.28 g/L [20,51,52]. Both LF and LYZ are very low in bovine milk, in which IGs form the main defense against microbes [53]. Together, IgA, IgG, IgM, LF, and LYZ provide the neonate with immune and non-immune protection against infection [54,55].
Minor dairy mammals, such as donkeys and camels, are notable for their unique protein compositions. Donkey milk, for instance, is exceptionally rich in LYZ, a protein with strong antimicrobial properties, while camel milk stands out for its high LF content, which is known for its antimicrobial and anti-inflammatory benefits [56,57]. These characteristics make milk from minor species particularly appealing to hypoallergenic and therapeutic applications, offering promising potential in specialized nutrition. On the other hand, major dairy mammals like sheep and buffalo provide milk with a rich protein profile, especially in WPs and immunoglobulins [58]. This makes their milk an excellent choice for developing high-protein dairy products, catering to the nutritional needs of a wide range of consumers. Together, these differences highlight the unique advantages of both minor and major dairy species in supporting human health and nutrition.
For further information, a brief description of each WP related to molecular structure and healthy effects is reported below.

Table 1.
Content of WPs in whey from major and minor mammal species.
Table 1.
Content of WPs in whey from major and minor mammal species.
| Proteins (g/L) | Minor Dairy Mammal Species | Major Dairy Mammal Species | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Horse | Donkey | Camel | Yak | Cow | Goat | Sheep | Buffalo | [20,51,57,58] | |
| Total WPs | 7.4–9.1 | 4.9–8 | 5.9–8.1 | 10 | 5.5–7 | 3.7–7 | 10.2–11 | 6 | |
| β-lactoglobulin (β-LG) | 2.55 | 3.3 | N/A * | 3.4–10.1 | 3.2–3.3 | 1.5–5.0 | 6.5–8.5 | 3.9 | |
| α-lactoalbumin (α-LA) | 2.37 | 1.9 | 0.8–3.5 | 0.2–1.7 | 1.2–1.3 | 0.7–2.3 | 1–1.9 | 1.4 | |
| Serum Albumin | 0.37 | 0.4 | 7–11.9 | 0.2–3.1 | 0.3–0.4 | 0.25–1.1 | 0.4–0.6 | 0.29 | |
| Immunoglobulins (IGs) | 1.63 | 1.30 | 1.5–19.6 | 0.1–0.4 | 0.5–1.0 | 0.1–0.5 | 0.7 | 10.66 | |
| IgG | 0.38 | N/A * | 0.72–2.23 | N/A * | 0.15–0.8 | 0.1–0.4 | N/A * | 0.37–1.34 | |
| IgA | 0.96 | N/A * | N/A * | N/A * | 0.05–0.14 | 0.03–0.08 | N/A * | 0.01–0.04 | |
| IgM | 0.02 | N/A * | N/A * | N/A * | 0.04–0.1 | 0.01–0.04 | N/A * | 0.04–1.91 | |
| Lactoferrin (LF) | 0.1–2.0 | 0.07–0.37 | 0.02–7.28 | 0.1–0.7 | 0.02–0.5 | 0.02–0.2 | 0.8 | 0.03–3.4 | |
| Lysozyme (LYZ) | 0.5–1.33 | 1.00–1.43 | 0.000060–0.00135 | N/A * | 0.000070–0.00060 | 0.000250 | 0.000100 | 0.000120–0.000152 | |
| Proteose–peptone (Pp) | N/A * | N/A * | N/A * | N/A * | 0.8–1.2 | N/A * | N/A * | 3.31 | |
* N/A (not available).
2.1. β-Lactoglobulin (β-LG)
β-LG is the major WP in the milk of almost all mammalian species, including cow, sheep, goat, donkey, and mare [59]; β-LG is a water-soluble globular protein that constitutes approximately 50% of total whey proteins, with a chain of 162 amino acids and a molecular weight of around 18.4 kDa [59]. This protein is an important source of cysteine, which is fundamental for the synthesis of glutathione (GSH), a well-known intracellular antioxidant involved in immune regulation, cancer prevention, and the improvement in liver function [60]. Beyond their well-documented nutritional and functional roles, the structural characteristics of globular whey proteins, such as β-lactoglobulin, also possess antioxidant properties that are increasingly relevant in food science. These proteins can act as antioxidants and free radical scavengers, a property linked to their structural characteristics, including disulfide bonds and hydrophobic interactions. Such antioxidant activity contributes to the oxidative stability of food products, thereby extending shelf life and enhancing quality. In this regard, incorporating these WPs in formulations could therefore serve a dual role, offering both functional performance and bioactive protection.
Furthermore, one of the most important physiological functions is the stimulation of lipase activity [59]. β-LG is also an excellent source of essential amino acids such as leucine, which triggers the anabolic properties of muscles, leading to a reduction in the risk of sarcopenia in older adults [61]. β-LG may also be involved in fatty acid metabolism due to a ligand-binding site that tends to bind to hydrophobic molecules such as fat-soluble vitamins as well as lipids [62]. All these aspects make this protein an attractive compound for the formulation of nutraceutical foods and can help to avoid diseases and health issues.
Dairy industries have shown increasing interest in the reuse of whey due to its techno-functional properties bestowed mostly by β-LG, such as the ability to form gels and aggregates and the possibility of using this protein as an emulsifier in various food formulations [63]. A recent study highlights the successful gelation of β-LG, a whey protein, using heat treatment and transglutaminase-catalyzed crosslinking. Optimized conditions (pH 7.5, 5% protein concentration, 80 °C for 30 min) yielded soft, nutritious gels ideal for texture-modified diets, particularly for individuals with dysphagia or the elderly [64]. These gels offer an alternative to agar, improving dietary management and quality of life.
However, β-LG is an allergenic protein in bovine milk intended for human infants. It is known that β-LG increases in antigenicity and allergenicity when subjected to temperatures ranging from 50 to 90 °C; on the contrary, a decrease was observed after 90 °C [65]. For this reason, the development of foods with a reduced content of β-LG has generated much interest [66,67].
In addition to its usefulness in the food industry, the literature has reported interesting and innovative applications of β-LG in the pharmaceutical field, emerging as a promising excipient for amorphous solid dispersions to enhance the oral bioavailability of poorly water-soluble drugs [68].
2.2. α-LActoalbumin (α-LA)
α-LA is the second most abundant WP, accounting for approximately 20% of the total whey protein content. It consists of an amino acid chain with a molecular weight of approximately 14.4 kDa [69].
α-LA contains a high percentage of essential amino acids (63.2% of the total amino acid content) [69], more specifically, lysine, cysteine, and tryptophan [70]. Due to its high tryptophan content as a precursor to serotonin or melatonin, α-LA can help improve mood, sleep, and congestive performance [71,72]. This protein has also been shown to possess anti-tumor activity, as it can selectively induce apoptosis in tumor cells [73]. Additionally, α-LA can form a complex with oleic acid at low pH values, imparting anticancer properties [73].
Human and bovine α-LA share similar amino acid compositions, having 74% sequence homology and similar bioactivities; in fact, it is mostly used in infant formula [74]. It is also involved in the regulation of lactose synthesis, contributing to lactose synthetase, the enzyme that catalyses the final step in the biosynthesis of lactose [75]. α-LA is an important transporter of calcium (Ca2+) and can bind two molecules, thanks to a pocket containing four aspartic acid (Asp) residues. However, at pH < 5, Asp becomes protonated and loses its ability to bind Ca2+. The calcium-containing protein denatures reversibly at relatively low temperatures but renatures in the absence of other WPs and is therefore considered the most heat-stable of the major WPs [66]. In camel whey, α-LA is the major WP of camel milk because of the absence of β-LG; camel α-LA presents differences in its structural characteristics compared with its bovine counterpart, leading to diverse therapeutic properties, biological activities, and techno-functional properties [59,76].
Among the applications, Lu et al. [77] synthesized a dihydromyricetin/α-LA covalent complex with strong antioxidant and glucosidase inhibitory activities. The authors proposed its use as an emulsifier to stabilize β-carotene-loaded nano-emulsions, highlighting its potential for innovative food delivery systems.
α-LA has a wide range of potential applications in food technology, pharmaceutics, and nanotechnology. As a source of BAPs with anticancer, antimicrobial, antiviral, antihypertensive, immunomodulating, opioid, mineral-binding, and antioxidant properties, it plays a key role in the formulation of functional foods. Additionally, nanotubes and nanoparticles derived from this protein serve as effective carriers for transporting active compounds, positioning α-LA as a valuable tool for both the food and pharmaceutical industries [69].
2.3. Albumin of the Bovine Serum (BSA)
BSA amounts to approximately 8% of total WPs, with a chain of 582 amino acids and a molecular weight of 69 kDa. BSA is not synthesized in the mammary gland but appears instead in milk following passive leakage from the bloodstream [66]. Because of its size and higher levels of structure, BSA can bind to free fatty acids and other lipids, as well as flavor compounds [78]. Very recently, BSA has been used as a promising anticancer therapeutic strategy: using Escherichia coli surface-modified by BSA resulted in stronger adhesion and targeting effects on bladder cancer cells, paving the way for future studies exploring the combination of BSA combined with live bacteria for cancer therapy [79]. Additionally, BSA is a precursor for the synthesis of glutathione, a peptide associated with the immunostimulatory effect of whey proteins [80].
The effect of BSA or whey protein isolate (WPI) at various concentrations (0.5%, 1.5%, and 3%; w/v) on the properties of shrimp oil-in-water emulsion was investigated in a recent study [81]. BSA–Chitosan-stabilized emulsions showed greater stability, with a smaller droplet size enlargement during storage and shear-thinning, non-Newtonian behavior. They also optimally retained prawn oil pigment stability when compared to WPI– Chitosan-stabilized emulsions [81].
In a recent study, whey protein–polysaccharide nanotubes, such as α-LA–chitosan and bovine serum albumin–κ-carrageenan ones, were used to encapsulate curcumin [82]. It was reported that the encapsulation efficiency of curcumin ranged between 40% and 45%, and the curcumin-loaded nanotubes exhibited some anticancer effects when using a cell culture model.
2.4. Immunoglobulins (IGs)
IGs exert important immunological protection for neonates, combat infections, and enhance gut health. IGs are globular glycosylated proteins with a molecular weight between 22 and 69 kDa and various forms and structures. They amount to around 10% of total WPs, and their content is mainly high in colostrum and drops significantly during lactation. Horse, buffalo, and camel whey contain higher IG levels compared to bovine, sheep, goat, and human milk, with variations in Ig ratios across species [83]. IgG predominates in horse colostrum, as well as in bovine milk and colostrum, whereas IgA is the major immunoglobulin in mature horse milk and human milk and colostrum [84]. Immune milk was also suggested to lower blood pressure [85].
In a double-blind clinical study, the effects on the reduction in plasma cholesterol and blood pressure were evaluated by involving hypercholesterolemic human subjects. Patients consumed 90 g per day of immune milk produced from dairy cows previously hyperimmunized with a multivalent bacterial vaccine, which was found to be a useful adjuvant in the dietary management of hypercholesterolemia [85].
Furthermore, several studies have shown that milk IGs are effective against rotavirus and other infections [86].
2.5. Glycomacropeptide (GMP)
GMP, also called caseinomacropeptide, is a phosphorylated and glycosylated milk-derived bioactive peptide that is released from κ-casein via enzymatic digestion, either physiologically or in industry during the cheese-making process. GMP (12%) is characterized by an imbalanced amino acid profile. It lacks essential aromatic amino acids such as tryptophan, phenylalanine, and tyrosine, as well as cysteine, but is notably rich in threonine, proline, glutamine, and serine [87].
In addition to its nutritional value, GMP has demonstrated beneficial effects on satiety and the management of phenylketonuria [88]. It exhibits several other bioactive functions that may have therapeutic potential, including the modulation of digestion and metabolism and antibacterial, prebiotic, remineralizing, anti-tumoral, and immuno-modulation activities. In vitro and in vivo evidence suggests the activity may at least in part be associated with post-translational glycosylation of the κ-casein protein [88].
Regarding antibacterial activity, GMP has demonstrated significant inhibitory effects on the adhesion of pathogenic bacteria to target cells, mediated by interactions involving sialic acid or other carbohydrate structures [89,90]. The inhibitory effect of GMP was observed in the adhesion of verotoxigenic and enteropathogenic E. coli to HT-29 human colon adenocarcinoma epithelial cells [91]; E. coli, S. typhimurium, and Shigella flexneri to Caco-2 cells [92]; and enterotoxigenic E. coli K88 to piglet ileal mucosa cells [90]. Notably, this activity was enhanced when GMP underwent protease digestion, and it was demonstrated that GMP interacts directly with the bacteria rather than with their target cells [90]. While substantial in vitro and in vivo evidence supports GMP’s protective effects against enteropathogenic bacterial infections, further research is needed to clarify its mechanisms of action and rule out potential dysbiosis. GMP has shown remineralizing effects, promoted mineral recovery in demineralized bovine enamel [93], improved zinc absorption in infant rhesus monkeys [94], and mitigated bone damage in mice [95]. In male mice on a low-calcium diet, GMP increased the femoral calcium concentration, particularly during growth phases [96], while in female mice, GMP supplementation enhanced bone quality under both high- and low-fat diets [97]. These benefits may be linked to GMP’s prebiotic activity and its role in increasing the cecal concentration of short-chain fatty acids, though further research is needed to confirm this mechanism.
Furthermore, GMP may have a role as a prebiotic in promoting the growth of healthy gut microbiota, particularly Bifidobacterium and lactic acid bacteria, which may prevent or attenuate the growth of pathogenic bacteria [98].
GMP shows promise in combating metabolic syndrome by targeting key risk factors such as inflammation, oxidative stress, and endoplasmic reticulum stress, independent of intestinal microbiota. In high-fat, high-fructose-diet-fed mice, GMP administration for 12 weeks reduced obesity, hyperglycemia, and hyperinsulinemia, improving insulin resistance [99]. Additionally, in the same study, GMP decreased systemic inflammation, enhanced insulin sensitivity, and restored intestinal and hepatic homeostasis by mitigating diet-induced tissue stress and inflammation. Studies on the immunomodulatory activity of GMP, including cellular, animal, and human trials, suggest that GMP primarily downregulates inflammatory pathways, reducing cytokines like TNF-α. However, occasional up-regulation of inflammatory markers has also been observed, indicating a context-dependent response [100].
2.6. Lactoferrin (LF)
LF is an iron-binding glycoprotein, with a MW of 80 kDa, and amounts to around 1–3% of total WPs [26]. LF exhibits multiple biological functions, such as enhanced iron bioavailability, antimicrobial and antibiofilm properties, antioxidant, antiviral, anti-inflammatory, immunomodulatory, and anticancer effects [12,13,101]. Owing to its diverse benefits, bovine LF is extensively employed in both human medicine and the food industry [101]. Compared to bovine milk, camel whey has higher concentrations of LF, which exhibit notable antibacterial, antiviral properties, hypoglycemic, antidiabetic, anti-inflammatory, and immunomodulatory effects [102,103].
Extensive literature and animal model studies highlight the antimicrobial efficacy of LF. This activity is mediated through two mechanisms: the sequestration of free iron, which inhibits bacterial growth by depriving bacteria of an essential nutrient (bacteriostatic effect), and direct interaction with pathogens. LF binds to bacterial wall lipopolysaccharides and may cause membrane damage through peroxide formation catalyzed by LF-bound iron, ultimately leading to bacterial cell lysis [104].
It can protect the mammary gland and gastrointestinal tract against infections; provides and is a rich source of amino acids; and promotes division, differentiation, and growth of the immune system and mucosa intestinal cells. Human milk contains a very high level of LF (~20% of the total nitrogen), and therefore, there is interest in fortifying bovine milk-based infant formulas with this protein [66]. LF is widely incorporated into a variety of foods, including probiotic foods that promote beneficial gut flora and functional foods designed to enhance iron absorption [105,106]. Bovine LF supplementation has demonstrated significant positive effects across various preclinical and clinical models, improving not only the composition of the gut microbiota but also crucial physiological responses such as inflammation, cognitive health, and therapy response.
In mouse models of colitis induced by dextran sulfate sodium salt, oral supplementation with bovine LF (100 mg/kg) reduced inflammation and improved the structure of the colon barrier, demonstrating how LF can alleviate colitis symptoms by modulating the gut microbiota [107]. Additionally, in high-fat diet-induced obese mice, supplementation with bovine lactoferrin resulted in a significant alteration of the microbiota, increasing Bifidobacterium and decreasing Enterobacteriales and Bacteroidetes. These effects were accompanied by reductions in cholesterol and glucose levels, suggesting an improvement in diet-induced intestinal dysbiosis [108]. In the case of C57BL/6J mice fed a Western diet, lactoferrin supplementation (50 mg/kg BW) for 16 weeks led to an increase in Bacteroidetes (e.g., Roseburia), suggesting a potential beneficial effect on the gut–brain microbiota axis with possible implications for cognitive health [109]. In weaning piglets (1–3 g/kg), lactoferrin supplementation enhanced growth performance and reduced diarrhea rates by improving gut barrier function and balancing the intestinal microbiota, increasing Lactobacillus and Bifidobacterium [110].
In pediatric patients undergoing chemotherapy, supplementation with bovine lactoferrin (200 mg/day) for two months promoted gut microbiota eubiosis by controlling the growth of pathobionts such as Enterococcus and modulating the abundance of beneficial taxa like Akkermansia. This approach helped counteract dysbiosis onset, improving overall health and therapy response [111]. Finally, in healthy elderly women, lactoferrin supplementation (1 g/day), alone or in combination with galacto-oligosaccharides and vitamin D, increased Holdemanella and Bifidobacterium in the fecal microbiota, though health effects have yet to be assessed [112].
Additionally, LF plays a role in food preservation by delaying lipid oxidation and inhibiting microbial growth. Its use spans industries including meat, wine, fat processing, and dairy [42]. The increasing demand for natural foods has further elevated the importance of natural inhibitors like LF. It is also used in skin care products for its antioxidant properties [113], in oral care products for oral hygiene [114], and as a nutraceutical to boost the immune system and reduce inflammation [115].
2.7. Proteose–Peptone (Pp)
Pp, also called lactophorin, is a milk protein fraction known for its heat stability and acid solubility, and it is a mixture of phospho- and glycoprotein and peptides. It is a heat-stable and acid-soluble protein fraction in milk. Pp was indigenous in fresh milk and does not result from heat-induced hydrolysis of the major milk proteins [116]. It is obtained by subjecting milk to heat treatment and adjusting its pH to 4.6, which allows its separation from other milk proteins. Pp has been shown to act as an effective emulsifier in oil-in-water emulsion systems by rapidly reducing interfacial tension. This property makes Pp a functional ingredient in products like ice cream, which rely on stable emulsions or foams [117]. A recent study highlights the species-specific bioactive properties of Pp fractions and their potential applications [118].
This study explored the bioactivity of bovine and ovine proteose–peptone fractions, focusing on proteose–peptone component 3 (Pp3) and its enzymatic hydrolysate, with particular attention to antioxidant, antibacterial, and antiviral properties. Antioxidant activity differed significantly between species: bovine Pp exhibited a reduction after fractionation, while ovine Pp showed an increase, consistently displaying superior activity.
Regarding antibacterial effects, hydrolyzed bovine Pp3 demonstrated a strong, concentration-dependent action against Cronobacter sakazakii, achieving a maximum reduction of 6.82 logarithmic cycles at 4 mg/mL after 24 h, whereas ovine Pp3, whether intact or hydrolyzed, was ineffective. In terms of antiviral activity, both bovine and ovine Pp3 neutralized rotavirus in a dose-dependent manner; however, hydrolysis enhanced the antiviral properties of bovine Pp3 while nearly eliminating the activity of ovine Pp3. These findings underscore significant interspecies variations in the bioactivity of Pp fractions [118].
2.8. Lactoperoxidase (LP)
LP accounts for 0.25–0.5% of whey proteins and has a molecular weight of 78 kDa [119]. It consists of a single polypeptide chain with 612 amino acid residues, including 15 cysteines, one heme group, and approximately 10% w/w carbohydrate moieties. The enzyme’s activity is temperature-dependent, with inactivation occurring at 62.5 °C after 30 min, 70 °C after 15 min, or 85 °C after 15 s [120,121]. LP exhibits broad antibacterial activity, making it a promising natural preservative for various food products [122,123]. However, its direct application can be limited due to its diffusion within the food matrix and interactions with food components. To overcome these challenges, LP can be incorporated into packaging materials, particularly edible films and coatings, to retain its antimicrobial properties, thereby enhancing food safety and extending shelf life. This study reviews antimicrobial enzymes, emphasizing LP’s properties and its potential role in combination with edible films for food preservation [122]. Min et al. [124] evaluated the antimicrobial effects of WPs films containing LP against Salmonella enterica and E. coli O157:H7, achieving a 4 log CFU/cm2 reduction. Similarly, WPI films with LPOS inhibited L. monocytogenes on agar media by 4.2 log CFU/cm2. When applied as a coating on cold-smoked salmon, LP-whey protein coatings (40 mg LP/g) reduced L. monocytogenes and total aerobic microorganisms by approximately 3 and 1 log CFU/g, respectively, with inhibition maintained for 35 days at 4 °C and 14 days at 10 °C. The reduced LP efficiency in smoked salmon, compared to agar media, was linked to its higher SH compound content and lower water activity, which hinders the diffusion of oxidizing agents [124].
Saravani et al. [125] evaluated whey protein coatings with LP and Bunium persicum essential oil on Gouda cheese and chicken fillets. The coatings stabilized bacterial counts in cheese and reduced lipid oxidation, with stronger antibacterial effects observed against Gram-negative bacteria. In chicken, coatings with LP and 1% essential oil significantly inhibited L. monocytogenes. LP’s antibacterial activity varies by bacterial type, often requiring combination with other preservation methods for broader efficacy.
2.9. Lysozyme (LYZ)
LYZ is a protein consisting of 129 amino acids and stabilized by four disulfide bonds, although only two of these are essential for its hydrolytic activity [120]. It has a molecular mass of 14,300–14,600 Da and an isoelectric point between 9.5 and 9.7. Similarly to LF, LYZ’s antibacterial properties contribute to simplifying the gut microbiota, boosting resistance to intestinal colonization by certain pathogens, while promoting the growth of beneficial bacteria and aiding recovery from various gastrointestinal disorders [126]. LYZ exhibits antimicrobial properties against Salmonella typhimurium and Escherichia coli [124]. However, its effectiveness against gram-negative bacteria is limited due to the presence of a lipopolysaccharide layer in their outer membrane, which acts as a barrier preventing LYZ from accessing the cell interior. Recently, the inclusion of antimicrobial enzymes, particularly LP and LYZ, in active packaging materials such as cellulose, gelatin, and chitosan edible coatings and films has emerged as a promising approach to preserve the enzymes’ catalytic activity and prevent food spoilage microorganisms [127,128]. Notably, a few antimicrobial enzymes, including LYZ and LP, are classified as GRAS (Generally Recognized As Safe) and have been approved for use in food products [129].
Major characteristics and biological effects above described are summarized in Table 2.

Table 2.
Major and minor whey proteins (WPs) in bovine milk and related properties.
3. Conventional vs. Novel Approaches for the Release of BAPs from WPs
BAPs are specific protein fragments which, in addition to having a purely nutritional value, exert biological activity on the organism. Being often latent or encrypted within parent proteins, they can be released through enzymatic actions during gastrointestinal digestion or food processing. The size of BAPs is usually 2–20 amino acids long, and their primary sequence defines their function [134]. To date, the development of novel technologies—coupled or not with conventional methods and able to generate high yields of BAPs from WPs quickly and at a low cost—is ongoing [135]. Emerging processing technologies, such as ultrasound-assisted processing, microwave-assisted processing, high-pressure processing, pulsed electric field processing, high hydrostatic pressure, subcritical water hydrolysis, and ohmic heating, utilize physical methods to enhance the degree of hydrolysis in the production of BAPs, as reported in Figure 2 [135,136,137]. These novel techniques are increasingly used in the dairy industry to prepare BAPs, offering potential improvements in efficiency and functionality. Although generally less efficient as standalone methods compared to traditional techniques, these novel approaches are often coupled with fermentation or enzymatic hydrolysis to enhance peptide production and improve the release of bioactive compounds [138,139,140].
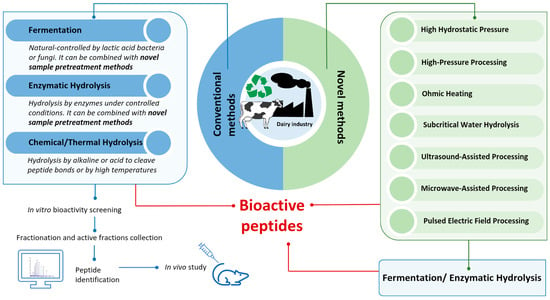
Figure 2.
Classical and novel processing techniques to discover and obtain bioactive peptides (BAPs) from whey.
Classical approaches (microbial fermentation, enzymatic hydrolysis, and thermal and chemical hydrolysis) and recent advances in this field (Figure 2) will be discussed in the following paragraphs. Advantages and disadvantages in industrial application are also summarized in Table 3.

Table 3.
Benefits and limitations of conventional and novel technologies in industrial production of bioactive peptides (BAPs) from whey proteins (WPs).
3.1. Microbial Fermentation (MF)
MF is a widely used, cost-effective, and efficient technique for producing peptides (Table 3) [141]. This method plays a crucial role in the dairy industry, particularly in enhancing the functional properties of milk products and byproducts [141,142]. MF is generally more cost-effective than enzymatic methods for producing BAPs. However, it presents certain industrial limitations, such as low peptide yields and a lack of specificity in peptide generation [143]. On the other hand, using commercial enzymes to apply them in BAPs production processes could become a serious problem, since the enzyme market is already under an oligopoly [144]. Therefore, solid-state fermentation (SSF) based on the use of selected microorganisms (fungi and bacteria) capable of biosynthesizing mixtures of biopeptides even starting from food residues is becoming increasingly popular because of its ability to reduce environmental pollution, adding value to these residues and lower costs in the process. SSF also allows the simultaneous production of proteases and BAPs, optimizing the process and reducing the compounds [145]. Therefore, SSF could become a preferred route to develop BAP-enriched ingredients or foods without resorting to laborious extraction and purification procedures.
SSF has been widely used to produce enzymes and other bio compounds due to advantages such as simplicity, the possibility of using residues as substrates, and enabling a reduction in environmental pollution, adding value to these residues and lower costs in the process. SSF also allows the simultaneous production of proteases and BAPs, optimizing the process and reducing antinutritional compounds, such as allergens. This work aimed to review the literature on the production of proteases and BAPs by solid-state fermentation, including the simultaneous production of these compounds, since the fermented medium can benefit from the hydrolysis capacity of enzymes produced by microorganisms. Usually, the obtaining of these compounds has been studied in isolation and not simultaneously in a single bioprocess, this being the main difference of our review.
Lactic acid bacteria (LABs) and yeast strains are frequently utilized for whey fermentation due to their robust proteolytic activity and capacity to thrive in acidic environments [146]. LAB cell-envelope proteinases hydrolyze whey proteins to produce extracellular oligopeptides with potential bioactivity. These oligopeptides are subsequently transported into LAB cells via active transporters and further broken down into smaller peptides and free amino acids by intracellular endopeptidases [147].
Daliri et al. [142] demonstrated that various LAB families can hydrolyze WPs to generate BAPs with antihypertensive effects. After culturing 34 fermenting bacteria for 48 h at 37 °C, seven strains—including Pediococcus acidilactici SDL1414, Lactobacillus plantarum JDFM44, Enterococcus faecium SC54, P. acidilactici DM9, Lactobacillus brevis SDL1411, Pediococcus pentosaceus SDL1409, and Lactobacillus rhamnosus JDFM6—produced peptides (<7 kDa) with strong ACE-inhibitory activity.
P. acidilactici SDL1414, which exhibited the lowest half-maximal inhibitory concentration (IC50) value of 19.78 ± 1.73 μg/mL, produced 29 compounds with antihypertensive effects. These compounds were identified using Liquid Chromatography–Electrospray Ionization–Quantitative Time-of-Flight Tandem Mass Spectrometry (LC-ESI-TOF–MS/MS). This underscores the potential of P. acidilactici SDL1414 as a source of bioactive compounds with functional properties [142]. Antimicrobial peptides were released after the fermentation of goat whey with Lactobacillus spp. [148]; BAPs were also released from WP concentrate (WPC) with Streptococcus thermophilus [149], Enterococcus faecalis [150], Saccharomyces spp. [151], and natural whey microbial starters [152].
3.2. Enzymatic Hydrolysis
Besides fermentation, hydrolysis with purified proteases (or peptidases) is the most common method for producing BAPs [153]; however, despite their wide usage, current enzymatic treatments often face limitations such as low yields and high costs [153]. Many food-grade proteases from animal, plant, and microbial sources with high substrate specificity are already available on the market (Table 3) [154,155]; not surprisingly, the global hydrolyzed WP market was estimated at EUR 1.86 Billion in 2023 and is projected to reach EUR 3.0 Billion by 2030.
Kheroufi et al. [156] studied the biological (antioxidant) and technological properties (solubility, emulsifying activity index, and foaming activity) of WP hydrolysates (WPHs) obtained using chicken pepsin extract in comparison with porcine pepsin. Bromelain, papain, neutrase, and cardosin are other examples of enzymes usually used to obtain WPHs endowed with biological activities [157,158,159,160].
A recent study focused on hydrolysates from WPC, produced from Colombian cheese whey via ultrafiltration, followed by enzymatic hydrolysis with alcalase, chymotrypsin, and flavorzyme [161]; in particular, the alcalase hydrolysate exhibited higher ABTS radical scavenging capacity, while alcalase and chymotrypsin hydrolysates showed over 85% angiotensin-converting enzyme (ACE) inhibition. After simulated gastrointestinal digestion, changes in biological activities were observed: ABTS scavenging ability increased, while ACE inhibition significantly decreased. The alcalase hydrolysate demonstrated strong antioxidant and ACE-inhibitory properties and better peptide stability post-digestion [161]. Alcalase is known for its high efficiency in hydrolyzing various proteins to produce BAPs; additionally, immobilized alcalase has been proposed based on the affinity of different metal ions, enhancing its stability and catalytic activity for specific applications [162,163]. Immobilized alcalase can effectively hydrolyze whey protein isolate to produce small peptides (<500 Da). Immobilization improves the enzyme’s catalytic performance, particularly for proteins with low solubility. Alcalase@HMSS-3N–Mn+ prevents the enzyme from self-digestion and demonstrates excellent thermal stability, supporting the use of metal ion affinity chromatography for immobilizing enzymes and producing BAPs using various metal ions [162].
A promising method for generating BAPs involves the use of alcalase immobilized on glyoxyl-corn-cob-powder [163]. This method hydrolyzes cheese whey proteins while promoting sustainability by utilizing whey and corn cob, producing hydrolysates with strong bioactive properties suitable for food and pharmaceutical applications. Specifically, WP hydrolysates exhibited antioxidant activity, high iron-chelating capacity (76.2%), and antimicrobial effects (87.75–100%) against E. coli and Listeria monocytogenes and were effective against Candida albicans (MIC = 10 mg/mL) [163].
3.3. Chemical Hydrolysis and Heat Treatment
Chemical hydrolysis is a simple and cost-effective method for producing peptides and amino acids using acids or alkalis [164]. However, it suffers from issues like process control difficulties and variability in chemical compositions, as well as negative impacts on nutritional quality and functionality due to extreme temperature and pH conditions [165] (Table 3).
The chemical hydrolysis of WPs involves the disruption of sulfide bonds within protein structures, enhancing their function and properties. Lowering the pH with succinic acid or affecting disulfide bonds through sulfitolysis can improve protein properties [166]. For example, adjusting whey pH to 3.0–4.6 with citric acid yields high levels of soluble β-LG [167].
The heat treatment of WPs releases free thiol groups, causing denaturation and thiol-disulfide bond formation, with calcium aiding in thermal precipitation. Typically, dehydrated whey is denatured at 90 °C and precipitated at pH 4.4–5.0 for low-solubility protein with good functionality. The process involves acidification (pH 2.5–3.5) followed by heat. Iron addition improves solubility. Heat treatment of β-LG with lysine and varying calcium concentrations yielded seventeen peptides [168]. In addition, β-lactoglobulin denatured by thermal treatment (50–95 °C) provided the most effective protection against lipid oxidation in oil-in-water emulsions when used as a stabilizer [169].
3.4. Ultrasound Treatment
Ultrasound-assisted processing is an eco-friendly, non-thermal technology that employs ultrasonic waves (frequencies > 20 kHz) to enhance peptide production. The mechanical and cavitation effects generated by these waves efficiently modify protein structures, increasing enzymatic activity and accelerating hydrolysis processes [135]. A recent study highlighted that ultrasound treatment of WPI solutions is influenced by factors such as acoustic amplitude, treatment duration, and protein concentration [138]. Pretreatment with ultrasound before enzymatic hydrolysis using bromelain (a plant-derived protease) significantly modified the peptide profile of WPI hydrolysates. This process released peptides with enhanced ACE-inhibitory activity, particularly in the 1–5 kDa range, which notably remained stable after simulated gastric and intestinal digestion [138]. Ultrasound pretreatment enhances enzyme accessibility to peptide bonds, increasing the release of BAPs. This process generates acoustic forces that reduce the fat globule size, which are typically coated with whey proteins and casein micelles, thereby increasing the surface area available for proteolytic enzymes to interact with proteins. As a result, ultrasound improves enzymatic hydrolysis efficiency [170]. Wu et al. [171] demonstrated that ultrasonic pretreatment of WP prior to hydrolysis with alcalase significantly enhanced ACE-inhibitory and immunomodulatory activities. Ultrasound-induced protein unfolding increased free sulfhydryl content and promoted conformational changes, notably the formation of β-sheets and β-turns, which contributed to improved bioactivity of the hydrolysates [171]. Recent studies confirm the utility of ultrasound as a sample pretreatment technique, demonstrating its effectiveness not only in increasing the yield of BAPs from WPs but also in significantly reducing allergenicity [172]. This advancement highlights the potential of ultrasound-assisted methods for the development of hypoallergenic food products [173,174], offering promising applications in the food industry.
Studies have shown that ultrasound-assisted pretreatment, combined with low-purity enzymes, enhances the hydrolysis rate of proteins. This effect is attributed to structural modifications such as changes in free sulfhydryl groups, disulfide bonds, increased hydrophobic protein content, and surface hydrophobicity [42]. Lorenzetti et al. [139] further demonstrated that ultrasound pretreatment of WPI before hydrolysis could facilitate the development of cost-effective ingredients for the dairy industry, highlighting its economic and industrial relevance. Ultrasound-assisted processing systems are energy-efficient (up to 85%), require minimal maintenance, and involve moderate installation costs ranging from EUR 10,000 to EUR 200,000 [175]. Its numerous advantages, including faster processing, selective extraction, precise control, reduced temperature requirements, and efficient mass and energy transfer, make ultrasound-assisted processing a preferred technique for producing BAPs [176] (Table 3).
3.5. Microwave Treatment
Microwave-assisted processing employs electromagnetic waves with wavelengths ranging from 0.001 to 1 m and frequencies between 300 MHz and 300 GHz [177]. This technique enhances food shelf life and functional properties [135] while improving taste and overall quality [177]. Key parameters such as power, frequency, time, and temperature play a crucial role in the effectiveness of microwave-assisted processing [177].
Microwave energy induces molecular interactions through ionic conduction and dipolar rotation mechanisms, generating heat at the molecular level that leads to structural modifications in proteins [135]. Microwave-assisted extraction can operate through thermal or non-thermal mechanisms using nonionizing radiation. Thermal effects result from localized heat generated by water molecule friction, while nonthermal effects involve protein unfolding and rearrangement rates [135]. By breaking disulfide bonds, microwaves facilitate protein opening, enhancing enzymatic hydrolysis, like ultrasound-assisted extraction [177]. Specifically, applying a microwave-assisted protocol (532 W, 40–50 °C, 5 min) to bovine serum albumin concentrates prior to proteolysis with enzymes like pronase, chymotrypsin, papain, or alcalase has been shown to significantly increase hydrolysis efficiency [135].
According to a recent study, microwave-assisted proteolysis of WPs produced extensively hydrolyzed protein hydrolysates that did not trigger allergic reactions, unlike those generated through conventional heating methods [140]. From an industrial perspective, microwave processing offers significant advantages for liquid food products, including enhanced system flexibility, reduced thermal inertia, and potential energy savings compared to traditional heating techniques. This makes microwave-assisted proteolysis a promising and scalable approach for industrial applications in the dairy sector [140] (Table 3).
3.6. Pulsed Electric Field (PEF) Technology
Another example of physical hydrolysis is PEF processing, an innovative technology that enables the non-thermal hydrolysis of food proteins. In this method, a high-intensity electric field is applied (≈50 kV/cm), causing structural changes in proteins and enhancing enzymatic reactions without the need for excessive heat. Although the effects of PEF on WPs are known to vary with electric field intensity, there are contradictions in the literature. Some studies have suggested that PEF treatment has no significant impact on proteins [178]. However, optimizing the electric field density and processing time can enhance the functional and structural properties of peptides in WPs.
Additionally, thermal pretreatment combined with PEF may promote protein unfolding [179]. In industrial applications, PEF’s advantages include its non-thermal nature, reduced processing time, and improved yield of BAPs, making it an efficient method for producing functional food ingredients, particularly for dairy products like whey [180]. However, optimizing the PEF process, including electrode design and pulse parameters, is crucial for maximizing its benefits while avoiding potential negative effects like electrolysis [181]. This technique holds promise for scaling up BAPs production in the dairy industry (Table 3).
3.7. High-Pressure Processing (HPP)
High-pressure processing (HPP) is an innovative, eco-friendly, and non-thermal method that involves the application of pressures ranging from 100 to 1000 MPa, with or without additional heat treatment [135,182]. High-pressure processing denatures proteins by applying pressure, leading to biochemical changes as the volume decreases. The denaturation and/or aggregation of proteins depends on the pressure, temperature, time, and protein characteristics (e.g., pH and composition) [7,130]. At high pressures, β-LG aggregates via disulfide bond exchanges, leading to increased particle size and conformational changes [130]. In contrast, low pressures (<120 MPa) cause partial unfolding of β-LG, resulting in particle shrinkage and an increase in sulfhydryl groups [130]. When used as a pretreatment, HPP enhances enzymatic hydrolysis by destabilizing whey proteins, facilitating active site formation and chemical interactions. For instance, hydrolysis with chymotrypsin, pepsin, and trypsin produces more hydrophobic peptides when β-LG is pretreated with high-pressure processing compared to untreated proteins [183]. The efficiency of hydrolysis is influenced by factors such as pressure, temperature, time, pH, ionic strength, substrate, and enzyme type [135,183]. To prevent reduced susceptibility to proteolysis, hydrolysis should begin immediately after high-pressure processing. When HPP is applied during hydrolysis, protein agglomeration is avoided, ensuring more efficient peptide production [183]. HPP enhances foaming properties by increasing protein adsorption and intermolecular interactions at a neutral pH. However, pressures above 300 MPa reduce foam stability by causing protein opening [7]. At pressures over 100 MPa, HHP induces denaturation of β-LG and α-LA, complicating fractionation. The effects on β-LG are reversible at pressures above 300 MPa [166]. In a study applying batch HPP (0.1, 400, and 600 MPa for 10 min at room temperature) to bovine whey, peptides QEAKDAFLGSF and WENGECAQKK were obtained from β-LG through tryptic hydrolysis, with the highest yield at 400 MPa. However, 600 MPa pressure decreased peptide yield. Additionally, HHP (400 MPa) resulted in longer and more hydrophobic peptides during chymotrypsin hydrolysis of β-LG [135]. The application of HPP has some limitations, including batch operation and high infrastructure costs, ranging from 0.6 to 4 million USD, which account for 75–80% of the initial investment [184]. HPP alone has limited effects on breaking covalent bonds and producing BAPs. Therefore, it is typically combined with enzymatic hydrolysis to denature proteins and enhance enzyme access to cleavage sites, improving the efficiency and yield of BAPs production [137] (Table 3).
3.8. Subcritical Water
Subcritical water, also known as pressurized hot water extraction, superheated water extraction, or pressurized liquid extraction [137], has been positioned as an efficient and sustainable alternative to obtain peptides from various protein sources. Subcritical water has gained attention due to its non-toxic, non-flammable nature and the absence of solvent residues after extraction (Table 3). The use of subcritical water hydrolysis on WPI has been documented in the literature. A hydrolysis degree of 12% was achieved after 17 min at 298 °C, with the highest amino acid production (57.4 mg/g) occurring at 300 °C for 40 min [185]. Unfortunately, no information about biological activities was provided.
3.9. Ohmic Heating
Ohmic heating (also known as joules heating, electrical resistance heating, direct resistance heating, and electro-conductive heating) is the heating technique where heat is produced internally in the food being processed owing to its natural electrical resistance. This technology offers faster, more uniform heating, preserving the nutritional value of food better than conventional methods [186] (Table 3). Alizadeh and Aliakbarlu [187] investigated the antioxidant capacity of whey concentrate fractions subjected to ohmic heating (50 Hz, 0–240 V, 5–15 s), ultrasound-assisted extraction (24 kHz, 400 W, 25 mm titanium probe, 5–15 min), and enzymatic hydrolysis (pepsin). Both pretreatments increased the degree of hydrolysis (except for the combination of 5 min ultrasound-assisted extraction and proteolysis), with the highest hydrolysis achieved with a 15 min ultrasound-assisted extraction and 15 s ohmic heating treatment. This result was attributed to the breaking of covalent bonds and protein denaturation by heat. Both ultrasound-assisted extraction and ohmic heating alone showed similar effects [187]. The effect of ohmic heating on the production of BAPs is still not fully understood, with limited studies available. Sweet whey processed at low electric field intensities (2–4 V cm−1) showed higher ACE-inhibitory activity compared to pasteurization (72–75 °C), but activity decreased at 5 V cm−1 [186]. A similar trend was observed for antioxidant activity, likely due to the release of bioactive compounds during ohmic heating, although peptide formation was not characterized.
4. Prediction of Biological Activities of WP-Derived BAPs by In Silico Approaches
In the past, the study of BAPs derived from WPs was carried out based on traditional drug discovery approaches. Unlike drugs, BAPs are contained in complex food matrices, and identifying them among hundreds of peptides is very challenging. In this respect, the use of in silico methods has been gaining fertile ground to perform rational prioritization studies before in vitro screening and to enhance the success rate of the discovery of BAPs for food uses and possible medical applications by saving time and costs [31,187,188,189,190,191]. To this end, innovative in silico approaches are nowadays available, as depicted in Figure 3 and summarized in Table 4.
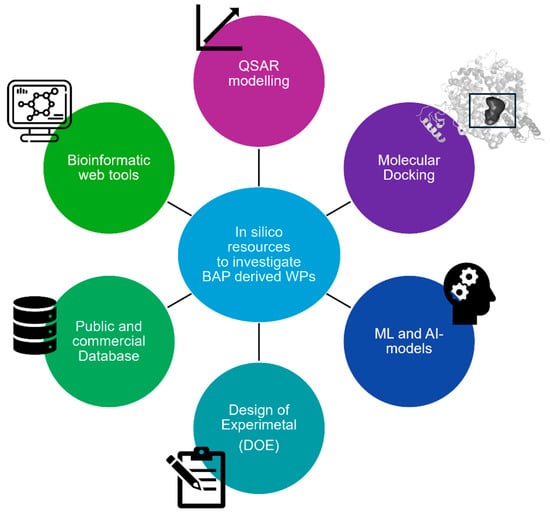
Figure 3.
In silico tools to investigate BAPs derived from WP.

Table 4.
Comparative overview of in silico techniques for WP-derived BAP discovery.
The discovery of BAPs can start by querying public and commercial databases of (i) WP-derived bioactive peptides (e.g., BIOPEP [192], AHTPDB [193], and milk bioactive peptide database (MBPDB) [194], just to mention a few), or (ii) pharmacological targets, such as metabolic enzymes (i.e., ACE, dipeptidyl peptidase-IV (DPP-IV), α-glucosidase), or (iii) markers of health and chronic diseases (i.e., cytokine levels, blood sugar, cholesterolemia, and sarcopenia). In this regard, a large number of collections dedicated to BAPs derived from WPs are nowadays freely available, as detailed in [31,195]. Notably, it emerges that a significant number of BAP-derived WPs are capable of exhibiting multitarget activities [31,190].
Nowadays, several web accessible platforms gather high-quality data from multiple publicly available sources in order to streamline the research of BAP discovery processes. For instance, experimental data are shared in the Open Pharmacological Concepts Triple Store (Open PHACTS) API (www.openphacts.org) based on a partnership involving academia, publishers, enterprises, and pharmaceutical companies with the aim of making drug discovery cheaper and faster. Similarly, bioinformatic platforms, such as ELIXIR, can be used for proteomic and peptidomic studies to predict the bioactivity, safety, and efficacy of BAPs, making research and data analysis more comprehensive [196]. Several works combining an in silico predictive tool for the enzymatic release of potent milk BAPs and in vitro studies [197,198,199,200] are worthy of mention.
A key role has been played by Quantitative Structure-Activity Relationship (QSAR) approaches. QSAR-based investigations have been carried out for the identification of WP-derived BAPs, which were successfully validated with experimental tests [191,201,202]. QSAR relies on mathematical models that correlate molecular and biological properties. Molecular descriptors particularly effective in elucidating the structure–activity relationship of BAPs are related to the global amino acid composition, as suggested by Dubchak et al. in 1995 [203]. The authors proposed as features the frequency of the property changes along the protein sequence and the distribution pattern of the property along the peptide sequence [20]. Next, the Amino Acid Index (AAI), Dipeptide Composition (DPC), tripeptide composition (TPC), and Grouped Dipeptide Composition (GDPC), as well as the structural features (e.g., β-sheet) and physicochemical peptide properties (e.g., hydrophobicity), are also considered as representative descriptors [195,204,205,206]. A QSAR study on whey-derived BAPs reported a correlation between hydrophobicity and positively charged amino acids in the C-terminal position and potent ACE-inhibitory activity. The correlation decreases for peptides with a length equal to or higher than six residues, thus highlighting the relevance of steric properties on ACE-inhibitory activity [207].
In the field of BAPs, molecular docking is a widely used in silico technique to predict the positioning and scoring of putative BAPs within the binding site of target proteins and to address the rational design of newer and more potent candidates [31,207,208,209].
For instance, molecular docking was successfully employed to identify bioactive oligopeptides effective toward ACE and to shed light on the rationale behind their binding [210,211]. In this respect, Norris et al. [210] discovered two new dipeptide stretches (i.e., Asp-Trp and Trp-Pro) with potent in vitro ACE-inhibitory properties. Furthermore, a recent docking study showed that 14 dipeptides from whey and other proteins inhibit ACE by engaging key binding site residues such as His353, Lys511, and Tyr520 through the formation of hydrogen bonds [212].
By exploiting docking simulations, Tondo et al. [157] assessed the activity of β-lactoglobulin-identified BAPs for hypertensive properties based on ACE inhibition. In a parallel investigation, Gambacorta et al. [213] proved that some of these peptides (i.e., IIAEKTK, IPAVF, and MHIRL) could be effective in interfering with SARS-CoV-2 spike main proteases. More specifically, the docking study revealed the potential affinity of these β-lactoglobulin-derived peptides toward the Human Rhinovirus 3C protease of SARS-CoV-2. These three peptides confirmed the importance of the hydrogen bond network within the binding sites, involving residues Q189 and N142, promoting conformational changes for a more effective binding.
A molecular docking approach can also help to unveil latent similarities among peptides and protein targets apparently uncorrelated. For instance, ACE and DPP-IV share similar mechanisms of inhibition and common structural features: both enzymes are able to hydrolyze terminal dipeptides based on their catalytic activity, and their cleaving actions are preferably addressed toward proline residues. Based on this, some BAPs showed their ability to inhibit ACE and DPP-IV in a similar way, as reported in a recent work by Gu et al. [214]. Several whey-derived peptides, such as RGP, FPK, KFTW, and KPW, have been reported to inhibit ACE and DPP-IV. Notably, RGP and KPW form hydrogen bonds with key ACE residues (i.e., Ala354, His383, Gln281, Glu384, and Glu162); otherwise, RGP, FPK, KFTW, and KPW engage a critical hydrogen bond with Arg358 of DPP-IV. Additionally, three out of these peptides (FPK, KFTW, and KPW) engage with only a hydrophobic residue in DPP-IV—that is, Phe357 [214]. As DPP-IV cleaves dipeptides with a proline residue at the penultimate position, DPP-IV inhibitory activity for peptides is expected with Xaa-Pro or Xaa-Pro-Yaa motif [215]. The simulated DPP-IV hydrolysates of KPW and FPK are KP and FP, respectively, reported to exhibit strong DPP-IV-inhibitory activity [214].
One of the major challenges with WPs is flocculation and precipitation. Complexes with sodium alginate can help mitigate these critical issues and stabilize WPI. Molecular docking integrated with deep learning models highlighted specific intermolecular interactions between whey peptides and sodium alginate [216].
The use of Artificial Intelligence (AI) and machine learning (ML) significantly changed the perspective for the rational design and optimization of BAP, including those deriving from [195,217]. ML models have been proven to identify common features and biological functions from large datasets of BAPs generated through enzymatic hydrolysis. Among the ML algorithms, Random Forest (RF), Support Vector Machines (SVMs), K-Nearest Neighbor (KNN), Naive Bayes (NB), Artificial Neural Networks (ANNs), and Logistic Regression (LR) have been exploited to identify and characterize whey-derived BAPs, as well as to profile their functions [195]. For instance, Morales García et al. [218] identified 2572 peptides under different conditions and with various types of enzymes by using different ML algorithms. Among these, 499 peptides were found to have potential antioxidant activity.
Moreover, ML can be applied in various ways to optimize whey management by enhancing the efficiency of whey separation from the curd. For instance, a recent approach suggested by Jox et al. [219] exploits both time-series data (e.g., fermentation and storage parameters) and non-time-series data (e.g., documentation and turbidity measurements) to train eleven models based on different ML algorithms. This protocol utilized five out of six steps of the CRISP-DM (CRoss-Industry Standard Process for Data Mining) methodology, which was specifically adapted to address whey management. BAPs obtained after hydrolysis needed to be separated using ElectroDialysis with Filtration Membranes (EDFM), which operate based on molecular mass exclusion and charge selectivity. Sanchez-Reinoso et al. [220] derived ML models based on DT and Binary Greedy Networks to estimate the peptide migration rate in EDFM by incorporating membrane fraction characteristics and peptide features.
AI-based algorithms are also widely implemented in several bioinformatic tools aiming to predict secondary and tertiary peptide structures from amino acid sequences (e.g., PEP-FOLD PepLook or AlphaFold 2) or function (e.g., APPTEST). Jia et al. exploited a peptide sequence-based multi-label deep learning approach to discover novel whey-derived ACE inhibitors [221]. On the other hand, SPRINT-STR uses RF algorithms to predict various protein-peptide complexes [222]. AI- and ML-based tools could also be exploited for the prediction and characterization of peptide binding sites [223,224,225]. An example is given by Baba et colleagues by using PepSite2 to predict peptide-binding sites on human ACE and renin based on the ACE inhibition of 185 peptides derived from 27 hydrolysates [212]. Additionally, the integration of ML models with docking represents a further option to consolidate the goodness of results. For instance, Petra et al. recently presented a novel pipeline for the screening of proteomes representative of complex protein-rich foods, including WPs. In this work, ML models classified the peptide functional groups from the whey proteome of colostrum from indigenous Greek goats, while molecular docking was employed to assess the binding affinity of the proposed ACE and DPP-IV inhibitory peptides [226].
Last but not least, the Design Of Experiments (DOE) in combination with Response Surface Methodology (RSM) represents an in silico workflow currently employed to aid the optimization of BAPs release during enzymatic hydrolysis and the fermentation of WPs [227]. The aim is to find the optimal number of experimental tests returning the maximum information content.
All DOE protocols consider hierarchical steps as follows: (i) definition of the scope/aims to be achieved from given experiments; (ii) define the biological effect/activity output to be optimize (e.g., antioxidant effects or DPP-IV or ACE potential activity); (iii) appropriate tuning of parameters (e.g., temperature, incubation time, and pH, just to mention a few) influencing the output of interest; (iv) selection of the experimental design, taking into account the boundaries of the study (e.g., the range of pH or temperature in which the enzyme is active). By considering the DOE protocol, the data obtained by experimental test(s) will be used to develop a mathematical model by using the Multiple Linear Regression (MLR) algorithm, returning a solid relationship between the effect of the parameters and the biological effect or activity to be optimized. Finally, the RSM analysis consists of a collection of mathematical and statistical techniques based on the fit of a polynomial equation to experimental data [227,228]. In dairy peptide research, DOE has been employed to optimize the release of BAPs with biological activity [227,229,230,231]. Table 4 provides a comparative overview of in silico techniques used for the discovery of bioactive peptides derived from whey proteins (WP-derived BAPs).
5. Regulatory Frameworks and Safety Assessments for WP-Derived BAPs: Global Perspectives and Challenges
WPs and WPHs serve as a valuable source of BAPs [15,232] and, due to their technological properties, are widely used as ingredients or in the production of functional foods with specific characteristics, providing efficient solutions to meet protein and nutritional needs while supporting overall health [46]. Over the years, various products have indeed been marketed, such as baked goods, extruded products, dairy products, beverages, and others [232]. The use of WPs in food products is subject to regulatory requirements and labeling regulations: while whey-fortified food products offer several nutritional benefits, there are also challenges and limitations, such as the regulation and approval of protein fractions or peptides, whose benefits require further confirmation.
Some WPIs have been granted Generally Recognized As Safe (GRAS) status and are authorized for marketing in the United States [233,234].
Several bovine LF (bLF) products have received GRAS status from the US FDA [235,236,237,238,239,240], and bLF is permitted for use in infant formulae and conventional foods in Japan, China, Korea, and Taiwan. EFSA assessed the safety of bLF in 2012 for various food categories, concluding that the proposed intake levels were safe. In contrast, EFSA has not previously evaluated the safety of bovine LP (bLP), which is accepted in dietary supplements in the USA and Japan [241] and as a processing aid in New Zealand [242].
In Europe, the regulatory framework governing the labeling of WPs is primarily defined by Regulation (EU) No. 1169/2011 on food information to consumers, along with specific provisions under food safety and novel food regulations such as Regulation (EU) 2015/2283.
In 2018, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) concluded that whey basic protein isolate from skimmed cow’s milk is safe for human consumption under the proposed conditions of use as infant and follow-on formula, meal replacement beverages, foods for special medical purposes, and food supplements [243].
The Novel Food (NF) consists of basic WPs obtained via ion exchange chromatography from skimmed cow’s milk. Its main components—bLF, LP, and transforming growth factor b2 (TGF b2)—share 70%, 83%, and 99% amino acid sequence homology, respectively, with their human milk counterparts. The highest estimated intake is 24.8 mg/kg bw/day in infants and 27.8 mg/kg bw/day in toddlers [242]. The composition, production process, and stability data raise no safety concerns, and the NF is not nutritionally disadvantageous at the proposed intake levels. There are no concerns regarding genotoxicity, with a No Observed Adverse Effect Level (NOAEL) of 2000 mg/kg bw/day in a subchronic rat study (highest dose tested) [244,245,246]. The margin of exposure (MOE) is 154 for adults, while for infants and toddlers, it is 81 and 72, respectively. Given that bLF, bLP, and TGF-β2 are already present in bovine-based infant formula, their increased intake from the NF is not considered a risk for the EFSA NDA Panel [247].
In 2019, following a request from the European Commission (EC), the EFSA NDA Panel was asked to deliver a scientific opinion on whey basic protein isolate for extended uses in foods for special medical purposes and food supplements for infants as a novel food (NF) pursuant to Regulation (EU) 2015/2283 [246]. The applicant requested an extension of the conditions of use to include infant formula (30 mg/100 g in powder form and 3.9 mg/100 mL when reconstituted) and follow-on formula (30 mg/100 g in powder form and 4.2 mg/100 mL when reconstituted) as food for special medical purposes, as well as its inclusion in food supplements for infants (25 mg/day). The Panel considered that the proposed extended uses would not lead to an increased intake of the novel food compared to the levels assessed in its 2018 opinion. Consequently, the Panel concluded that whey basic protein isolate is safe for the proposed extended uses, including its incorporation into infant and follow-on formulae, total diet replacements, foods for special medical purposes, and food supplements [246].
In recent years, the interest of the EC has been increasingly shifting toward hydrolysates derived from sources of skimmed cow’s milk, and since 2020, several scientific opinions on the topic have been requested from the EFSA [248,249,250,251,252].
Overall, whey-peptide-based products addressing consumption must meet the requirements of the EC 1924/2006 regulation with health claims [253]. The incorporation of WPHs in infant formula formulations is regulated by [254,255].
According to the scientific opinion published in 2025, to assess the nutritional safety and suitability of a protein hydrolysate derived from skimmed cow’s milk [248], the EFSA found the hydrolysate to be sufficiently characterized and evaluated an intervention study where an infant formula containing 2.3 g/100 kcal of this protein hydrolysate, consumed exclusively for three months, supported similar growth to a formula based on intact cow’s milk protein with 1.9 g/100 kcal. No concerns were raised regarding adverse events or formula tolerance. The panel determined that the protein hydrolysate is a nutritionally safe and suitable protein source for both infant and follow-on formula, provided the formula contains at least 2.3 g/100 kcal of protein and complies with Regulation (EU) 2016/127 and the amino acid profile specified in Annex IIIA [252].
The Codex Alimentarius provides a standard for whey powders, specifically outlined in CXS 289-1995 (formerly CODEX STAN A-15-1995) [256]. This standard covers whey powder and acid whey powder, detailing their composition, permissible additives, hygiene requirements, and labeling provisions. It is applicable to products intended for direct consumption or further processing. However, whey protein hydrolysates are not specifically addressed within the current Codex standards. This absence indicates a regulatory gap concerning the classification, safety assessment, and labeling of these hydrolyzed products. Recognizing this gap, AOAC International has initiated efforts to develop voluntary consensus standards for methods used to characterize WPHs. These standards aim to support the development and validation of official analytical methods, which could potentially be adopted by Codex Alimentarius in the future.
6. Conclusions and Future Perspectives
WPs have a high biological value largely determined by their content of essential amino acids and BAPs. The research of BAPs pushed WPs to the forefront of the functional food sector by making the development of novel technology of WPs recovery and transformation into BAPs an urgent challenge to be addressed; although several efforts have been undertaken in this direction, WPs processing and emergent derivatives remain crucial due to the low yields and time cost and environmental impacts.
Green technologies such as HPP, microwave, ultrasound, and PEF, coupled with enzymatic hydrolysis, seem to overcome these limitations; these technologies have been found to reduce the time and costs of processing and improve the yield of BAPs. In addition, the achievement of WP hydrolysis in reactors should contribute to increased efficiency and production yields and improve production stability between batches; the employment of membranes or immobilized enzyme reactors would save costs associated with enzymes by reducing processing costs. Although this review highlights multiple lines of evidence, significant progress is still needed in this field.
These techniques, indeed, selected for their sustainability and industrial scalability, will increasingly need to be implemented by high-throughput screening, AI-driven bioinformatics, and multi-omics (proteomics and metabolomics) that can improve the identification and characterization of BAPs.
By saving time and reducing costs, in silico approaches can accelerate the identification of BAPs from complex mixtures and facilitate the study of protein–peptide interactions by predicting their biological affinity; these computational methods have been found to be effective in safeguarding the sustainability of the discovery process. This approach can be used to improve the delivery of active peptides through more appropriate encapsulation methodologies, leading to tailored nutritional solutions for different populations, such as athletes, elderly individuals, or people with metabolic disorders. It is therefore evident that there is a strong interest in developing new algorithms and mathematical models to address these concerns. However, here too, there are several limitations; among the latter, the validation studies represent the bottleneck for consolidating strategies that improve peptide manufacturing processes.
With growing consumer demand for high-protein and functional foods, the WP and BAP market is projected to expand significantly in the near future. The growing interest in biologically active whey-derived peptides calls for clearer regulations regarding their use in the food sector, particularly in terms of safety and risk assessment. Future research should also focus on standardization studies and methods to strengthen the stability of the WPs derivatives. An improvement in shelf life can be ensured through the implementation of good manufacturing practices and rigorous quality control. Recent legislative updates acknowledge the high nutritional value of WPs-based products and encourage manufacturers to pursue this direction.
Author Contributions
Conceptualization, L.M. and L.Q.; writing—original draft preparation, A.L., L.Q., D.T. and W.M.S.; writing—review and editing, L.C., L.S., O.N. and L.M.; visualization: A.L. and D.T.; supervision, L.Q., O.N. and L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Nutrizione, Alimentazione & Invecchiamento Attivo” NUTRAGE cod. DBA.AD005.225, DM MUR n. 844 del 16/07/2021 funded by the National Research Council of Italy (CNR).
Conflicts of Interest
Leonardo Smiriglia works for the company Parafarmacia Smiriglia Leonardo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Nicolás, J.L.; González-Olivares, L.G.; Vázquez-Rodríguez, G.A.; Lucho-Constatino, C.A.; Castañeda-Ovando, A.; Cruz-Guerrero, A.E. Valorization of whey using a biorefinery. Biofuels Bioprod. Biorefin. 2020, 14, 1010–1027. [Google Scholar] [CrossRef]
- Soumati, B.; Atmani, M.; Benabderrahmane, A.; Benjelloun, M. Whey Valorization—Innovative Strategies for Sustainable Development and Value-Added Product Creation. J. Ecol. Eng. 2023, 24, 86–104. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Whey proteins and peptides in health-promoting function—A review. Int. Dairy J. 2022, 126, 105269. [Google Scholar] [CrossRef]
- Batista, M.A.; Campos, N.C.A.; Silvestre, M.P.C. Whey and protein derivatives: Applications in food products development, technological properties and functional effects on child health. Cogent Food Agric. 2018, 4, 1509687. [Google Scholar] [CrossRef]
- Mangano, K.M.; Bao, Y.; Zhao, C. Nutritional Properties of Whey Proteins. In Whey Protein Production, Chemistry, Functionality, and Applications; Guo, M., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; Volume 2, pp. 103–140. ISBN 9781119256052. [Google Scholar]
- Yiğit, A.; Bielska, P.; Cais-Sokolińska, D.; Samur, G. Whey proteins as a functional food: Health effects, functional properties, and applications in food. J. Am. Nutr. Assoc. 2023, 42, 758–768. [Google Scholar] [CrossRef] [PubMed]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. Structural and colloidal properties of whey protein aggregates produced by indirect tubular heating and direct steam injection. Food Struct. 2023, 35, 100301. [Google Scholar] [CrossRef]
- Baruzzi, F.; de Candia, S.; Quintieri, L.; Caputo, L.; De Leo, F. Development of a synbiotic beverage enriched with bifidobacteria strains and fortified with whey proteins. Front. Microbiol. 2017, 8, 640. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Monaci, L.; Cavalluzzi, M.M.; Denora, N. Lactoferrin-derived peptides as a control strategy against skinborne staphylococcal biofilms. Biomedicines 2020, 8, 323. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Monaci, L.; Deserio, D.; Morea, M.; Baruzzi, F. Antimicrobial efficacy of pepsin-digested bovine lactoferrin on spoilage bacteria contaminating traditional Mozzarella cheese. Food Microbiol. 2012, 31, 64–71. [Google Scholar] [CrossRef]
- Price, D.; Jackson, K.G.; Lovegrove, J.A.; Givens, D.I. The effects of whey proteins, their peptides and amino acids on vascular function. Nutr. Bull. 2022, 47, 9–26. [Google Scholar] [CrossRef]
- Quintieri, L.; Luparelli, A.; Caputo, L.; Schirinzi, W.; De Bellis, F.; Smiriglia, L.; Monaci, L. Unraveling the Biological Properties of Whey Peptides and Their Role as Emerging Therapeutics in Immune Tolerance. Nutrients 2025, 17, 938. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.K.; Rankin, S.A.; Lee, M.R.; Lee, W.J. Development and characterization of whey protein-based nano-delivery systems: A review. Molecules 2019, 24, 3254. [Google Scholar] [CrossRef]
- Dhar, H.; Verma, S.; Dogra, S.; Katoch, S.; Vij, R.; Singh, G.; Sharma, M. Functional attributes of bioactive peptides of bovine milk origin and application of in silico approaches for peptide prediction and functional annotations. Crit. Rev. Food Sci. Nutr. 2023, 64, 9432–9454. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Ma, Y.; Hou, Y.; Xie, K.; Zhong, J.; Zhou, P. Quantitative Phosphoproteome of Infant Formula: New Insights into the Difference of Phosphorylation in Milk Proteins between Bovine and Goat Species. J. Agric. Food Chem. 2023, 71, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Arora, S.; Sarkar, M. Yak milk and milk products: Functional, bioactive constituents and therapeutic potential. Int. Dairy J. 2023, 142, 105637. [Google Scholar] [CrossRef]
- Nayak, C.M.; Ramachandra, C.T.; Kumar, G.M. A Comprehensive Review on Composition of Donkey Milk in Comparison to Human, Cow, Buffalo, Sheep, Goat, Camel and Horse Milk. Mysore J. Agric. Sci. 2020, 54, 42–50. [Google Scholar]
- Quintieri, L.; Fanelli, F.; Monaci, L.; Fusco, V. Milk and Its Derivatives as Sources of Components and Microorganisms with Health-Promoting Properties: Probiotics and Bioactive Peptides. Foods 2024, 13, 601. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Padua, E.; Campaci, D.; Bernardi, M.; Muthanna, F.M.S.; Caprio, M.; Lombardo, M. Investigating the Health Implications of Whey Protein Consumption: A Narrative Review of Risks, Adverse Effects, and Associated Health Issues. Healthcare 2024, 12, 246. [Google Scholar] [CrossRef]
- Pinto, M.S.; Léonil, J.; Henry, G.; Cauty, C.; Carvalho, A.Ô.F.; Bouhallab, S. Heating and glycation of β-lactoglobulin and β-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef]
- Li, S.; Ye, A.; Singh, H. Impacts of heat-induced changes on milk protein digestibility: A review. Int. Dairy J. 2021, 123, 105160. [Google Scholar] [CrossRef]
- Upadhyay, V.K.; McSweeney, P.L.H.; Magboul, A.A.A.; Fox, P.F. Proteolysis in cheese during ripening. In Cheese: Chemistry, Physics and Microbiology; Academic Press: Cambridge, MA, USA, 2004; Volume 1, pp. 391–433. ISBN 012263652X. [Google Scholar]
- Quintieri, L.; Caputo, L.; De Angelis, M.; Fanelli, F. Genomic Analysis of Three Cheese-Borne Pseudomonas lactis with Biofilm and Spoilage-Associated Behavior. Microorganisms 2020, 8, 1208. [Google Scholar] [CrossRef]
- D’Incecco, P.; Rosi, V.; Fortina, M.G.; Sindaco, M.; Ricci, G.; Pellegrino, L. Biochemical, microbiological, and structural evaluations to early detect age gelation of milk caused by proteolytic activity of Pseudomonas fluorescens. Eur. Food Res. Technol. 2022, 248, 2097–2107. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the discovery, identification and validation of milk protein-derived bioactive peptides. Trends Food Sci. Technol. 2016, 50, 26–43. [Google Scholar] [CrossRef]
- Saubenova, M.; Oleinikova, Y.; Rapoport, A.; Maksimovich, S.; Yermekbay, Z.; Khamedova, E. Bioactive Peptides Derived from Whey Proteins for Health and Functional Beverages. Fermentation 2024, 10, 359. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Brasca, M.; Fanelli, F. Recent advances in the mechanisms and regulation of qs in dairy spoilage by pseudomonas spp. Foods 2021, 10, 3088. [Google Scholar] [CrossRef] [PubMed]
- Baruzzi, F.; Pinto, L.; Quintieri, L.; Carito, A.; Calabrese, N.; Caputo, L. Efficacy of lactoferricin B in controlling ready-to-eat vegetable spoilage caused by Pseudomonas spp. Int. J. Food Microbiol. 2015, 215, 179–186. [Google Scholar] [CrossRef]
- Quintieri, L.; Zühlke, D.; Fanelli, F.; Caputo, L.; Liuzzi, V.C.; Logrieco, A.F.; Hirschfeld, C.; Becher, D.; Riedel, K. Proteomic analysis of the food spoiler Pseudomonas fluorescens ITEM 17298 reveals the antibiofilm activity of the pepsin-digested bovine lactoferrin. Food Microbiol. 2019, 82, 177–193. [Google Scholar] [CrossRef]
- Xiang, Q.; Xia, Y.; Fang, S.; Zhong, F. Enzymatic debittering of cheese flavoring and bitterness characterization of peptide mixture using sensory and peptidomics approach. Food Chem. 2024, 440, 138229. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Romero-Luna, H.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Peredo-Lovillo, A. Bioactive peptides produced by engineered probiotics and other food-grade bacteria: A review. Food Chem. 2022, 13, 100196. [Google Scholar] [CrossRef]
- Losurdo, L.; Quintieri, L.; Caputo, L.; Gallerani, R.; Mayo, B.; De Leo, F. Cloning and expression of synthetic genes encoding angiotensin-I converting enzyme (ACE)-inhibitory bioactive peptides in Bifidobacterium pseudocatenulatum. FEMS Microbiol. Lett. 2013, 340, 24–32. [Google Scholar] [CrossRef]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics approaches to discovering food-derived bioactive peptides: Reviews and perspectives. TrAC Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Cardioprotective Peptides from Milk Processing and Dairy Products: From Bioactivity to Final Products Including Commercialization and Legislation. Foods 2022, 11, 1270. [Google Scholar] [CrossRef]
- Quintieri, L.; Nitride, C.; De Angelis, E.; Lamonaca, A.; Pilolli, R.; Russo, F.; Monaci, L. Alternative Protein Sources and Novel Foods: Benefits, Food Applications and Safety Issues. Nutrients 2023, 15, 1509. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Keskin Ulug, S.; Hong, H.; Wu, J. Regulatory requirements of bioactive peptides (protein hydrolysates) from food proteins. J. Funct. Foods 2019, 58, 123–129. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Zhao, J.; Ren, C.; Yan, W. Oral Yak Whey Protein Can Alleviate UV-Induced Skin Photoaging and Modulate Gut Microbiota Composition. Foods 2024, 13, 2621. [Google Scholar] [CrossRef]
- El-Aidie, S.A.M.; Khalifa, G.S.A. Innovative applications of whey protein for sustainable dairy industry: Environmental and technological perspectives—A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13319. [Google Scholar] [CrossRef] [PubMed]
- Lesgards, J. Benefits of Whey Proteins on Type 2 Diabetes Mellitus. Nutrients 2023, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Galland, F.; de Espindola, J.S.; Sacilotto, E.S.; Almeida, L.G.V.C.; Morari, J.; Velloso, L.A.; dos Santos, L.D.; Rossini, B.C.; Bertoldo Pacheco, M.T. Digestion of whey peptide induces antioxidant and anti-inflammatory bioactivity on glial cells: Sequences identification and structural activity analysis. Food Res. Int. 2024, 188, 114433. [Google Scholar] [CrossRef]
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Prod. Process. Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- Ji, Z.; Dong, R.; Du, Q.; Jiang, H.; Fan, R.; Bu, D.; Wang, J.; Yu, Z.; Han, R.; Yang, Y. Insight into differences in whey proteome from human and eight dairy animal species for formula humanization. Food Chem. 2024, 430, 137076. [Google Scholar] [CrossRef]
- Ali, A.H.; Li, S.; Liu, S.-Q.; Gan, R.-Y.; Li, H.-B.; Kamal-Eldin, A.; Ayyash, M. Invited review: Camel milk and gut health—Understanding digestibility and the effect on gut microbiota. J. Dairy Sci. 2024, 107, 2573–2585. [Google Scholar] [CrossRef]
- Ereifej, K.I.; Alu’datt, M.H.; Alkhalidy, H.A.; Alli, I.; Rababah, T. Comparison and characterisation of fat and protein composition for camel milk from eight Jordanian locations. Food Chem. 2011, 127, 282–289. [Google Scholar] [CrossRef]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Ghafoori, E.M.; Narmuratova, M.; Mohammadi, M.H.; Narmuratova, Z. A Comprehensive Review of General Characteristics of Peptides of Serum Immunoglobulins and Their Health Benefits. Eur. J. Theor. Appl. Sci. 2024, 2, 659–671. [Google Scholar] [CrossRef]
- Baldi, A.; Ioannis, P.; Chiara, P.; Eleonora, F.; Roubini, C.; Vittorio, D. Biological effects of milk proteins and their peptides with emphasis on those related to the gastrointestinal ecosystem. J. Dairy Res. 2005, 72, 66–72. [Google Scholar] [CrossRef]
- Derdak, R.; Sakoui, S.; Pop, O.L.; Muresan, C.I.; Vodnar, D.C.; Addoum, B.; Vulturar, R.; Chis, A.; Suharoschi, R.; Soukri, A.; et al. Insights on health and food applications of equus asinus (Donkey) milk bioactive proteins and peptides—An overview. Foods 2020, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Pang, K.; Zhang, X.Y.; Zhao, L.; Chen, S.W.; Dong, M.L.; Ren, F.Z. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 2007, 90, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Emakpor, O.L.; Edo, G.I.; Jikah, A.N.; Ikpekoro, V.O.; Agbo, J.J.; Ainyanbhor, I.E.; Essaghah, A.E.A.; Ekokotu, H.A.; Oghroro, E.E.A.; Akpoghelie, P.O. Buffalo milk: An essential natural adjuvant. Discov. Food 2024, 4, 38. [Google Scholar] [CrossRef]
- Lajnaf, R.; Attia, H.; Ayadi, M.A. Technological properties and biological activities of camel α-lactalbumin—A review. Int. Dairy J. 2023, 139, 105563. [Google Scholar] [CrossRef]
- Kelly, A.L.; Fox, P.F. Advanced Dairy Chemistry: Volume 1B: Proteins: Applied Aspects, 4th ed.; Springer: New York, NY, USA, 2016; Volume 1, ISBN 9781493928002. [Google Scholar]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef]
- Kong, F.; Kang, S.; Zhang, J.; Jiang, L.; Liu, Y.; Yang, M.; Cao, X.; Zheng, Y.; Shao, J.; Yue, X. The non-covalent interactions between whey protein and various food functional ingredients. Food Chem. 2022, 394, 133455. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; He, J.; Tang, W.; Liu, J. Innovative multistep modifications of β-Lactoglobulin for enhanced emulsifying and antioxidant activities. Food Hydrocoll. 2024, 148, 109465. [Google Scholar] [CrossRef]
- Liu, H.; Nardin, C.; Zhang, Y. Novel soft food gels using beta-lactoglobulin via enzymatic crosslinking as agar gel alternatives. Food Hydrocoll. 2024, 155, 110213. [Google Scholar] [CrossRef]
- Bavaro, S.L.; De Angelis, E.; Barni, S.; Pilolli, R.; Mori, F.; Novembre, E.M.; Monaci, L. Modulation of milk allergenicity by baking milk in foods: A proteomic investigation. Nutrients 2019, 11, 1536. [Google Scholar] [CrossRef] [PubMed]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Milk proteins: An overview. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–98. ISBN 9780128152515. [Google Scholar]
- Quintieri, L.; Monaci, L.; Baruzzi, F.; Giuffrida, M.G.; de Candia, S.; Caputo, L. Reduction of whey protein concentrate antigenicity by using a combined enzymatic digestion and ultrafiltration approach. J. Food Sci. Technol. 2017, 54, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Jasiukenaite, I.; Löbmann, K. β-Lactoglobulin-based amorphous solid dispersions: A graphical review on the state-of-the-art. Eur. J. Pharm. Biopharm. 2024, 202, 114396. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Gómez, A.P.; Arroyo-Maya, I.J.; Hernández-Sánchez, H. Use of α-Lactalbumin [α-La] from Whey as a Vehicle for Bioactive Compounds in Food Technology and Pharmaceutics: A Review. Recent Prog. Mater. 2021, 3, 027. [Google Scholar] [CrossRef]
- Barone, G.; Moloney, C.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemical composition, protein profile and physicochemical properties of whey protein concentrate ingredients enriched in α-lactalbumin. J. Food Compos. Anal. 2020, 92, 103546. [Google Scholar] [CrossRef]
- Mackay-Phillips, K.; Orssatto, L.B.R.; Polman, R.; Van der Pols, J.C.; Trajano, G.S. Effects of α-lactalbumin on strength, fatigue and psychological parameters: A randomised double-blind cross-over study. Eur. J. Appl. Physiol. 2023, 123, 381–393. [Google Scholar] [CrossRef]
- Miles, K.H.; Clark, B.; Fowler, P.M.; Gratwicke, M.J.; Martin, K.; Welvaert, M.; Miller, J.; Pumpa, K.L. α-Lactalbumin Improves Sleep and Recovery after Simulated Evening Competition in Female Athletes. Med. Sci. Sports Exerc. 2021, 53, 2618–2627. [Google Scholar] [CrossRef]
- Chiou, J.T.; Shi, Y.J.; Lee, Y.C.; Wang, L.J.; Chen, Y.J.; Chang, L. Sen Carboxyl group-modified α-lactalbumin induces TNF-α-mediated apoptosis in leukemia and breast cancer cells through the NOX4/p38 MAPK/PP2A axis. Int. J. Biol. Macromol. 2021, 187, 513–527. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Rong, Y.; Liu, Z.; Yang, S.; Zhang, W.; Li, J.; Cai, Y. A novel SNPs in alpha-lactalbumin gene effects on lactation traits in Chinese holstein dairy cows. Animals 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhang, J.; Regenstein, J.M.; Liu, D.; Huang, Y.; Qiao, Y.; Zhou, P. α-Lactalbumin: Functional properties and potential health benefits. Food Biosci. 2024, 60, 104371. [Google Scholar] [CrossRef]
- Lu, N.; Wu, L.; Zhen, S.; Liu, B. Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions. Foods 2023, 12, 2783. [Google Scholar] [CrossRef]
- Huang, B.X.; Kim, H.Y.; Dass, C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1237–1247. [Google Scholar] [CrossRef]
- He, Z.; Chen, K.; An, Y.; He, J.; Zhang, X.; Tang, L.; Sun, F.; Jiang, K. BSA modification of bacterial surface: A promising anti-cancer therapeutic strategy. BMC Microbiol. 2023, 23, 105. [Google Scholar] [CrossRef]
- Masotti, F.; Cattaneo, S.; Stuknytė, M.; De Noni, I. Technological tools to include whey proteins in cheese: Current status and perspectives. Trends Food Sci. Technol. 2017, 64, 102–114. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Singh, A.; Benjakul, S. Combined effect of chitosan and bovine serum albumin/whey protein isolate on the characteristics and stability of shrimp oil-in-water emulsion. J. Food Sci. 2022, 87, 2879–2893. [Google Scholar] [CrossRef]
- Maldonado, L.; Chough, S.; Bonilla, J.; Kim, K.H.; Kokini, J. Mechanism of fabrication and nano-mechanical properties of α-lactalbumin/chitosan and BSA/κ-carrageenan nanotubes through layer-by-layer assembly for curcumin encapsulation and determination of in vitro cytotoxicity. Food Hydrocoll. 2019, 93, 293–307. [Google Scholar] [CrossRef]
- Claeys, W.L.; Verraes, C.; Cardoen, S.; De Block, J.; Huyghebaert, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of raw or heated milk from different species: An evaluation of the nutritional and potential health benefits. Food Control. 2014, 42, 188–201. [Google Scholar] [CrossRef]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, growth factors and growth hormone in bovine colostrum and the effects of processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Sharpe, S.; Gamble, G.; Sharpe, D.N. Cholesterol-lowering of immune blood of immune milk. Am. J. Clin. Nutr. 1994, 59, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, L.; Tan, W.; Zhao, W. Clinical efficacy of oral immunoglobulin Y in infant rotavirus enteritis: Systematic review and meta-analysis. Medicine 2019, 98, e16100. [Google Scholar] [CrossRef]
- Córdova-Dávalos, L.E.; Jiménez, M.; Salinas, E. Glycomacropeptide bioactivity and health: A review highlighting action mechanisms and signaling pathways. Nutrients 2019, 11, 598. [Google Scholar] [CrossRef]
- Neelima; Sharma, R.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [CrossRef] [PubMed]
- Nakajima, K.; Tamura, N.; Kobayashi-Hattori, K.; Yoshida, T.; Hara-Kudo, Y.; Ikedo, M.; Sugita-Konishi, Y.; Hattori, M. Prevention of intestinal infection by glycomacropeptide. Biosci. Biotechnol. Biochem. 2005, 69, 2294–2301. [Google Scholar] [CrossRef]
- Feeney, S.; Ryan, J.T.; Kilcoyne, M.; Joshi, L.; Hickey, R. Glycomacropeptide reduces intestinal epithelial cell barrier dysfunction and adhesion of entero-hemorrhagic and entero-pathogenic Escherichia coli in vitro. Foods 2017, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.R.; Gibson, G.R.; Formentin, K.; Beer, M.; Greenberg, N.; Rastall, R.A. Caseinoglycomacropeptide inhibits adhesion of pathogenic Escherichia coli strains to human cells in culture. J. Dairy Sci. 2005, 88, 3455–3459. [Google Scholar] [CrossRef]
- Brück, W.M.; Kelleher, S.L.; Gibson, G.R.; Graverholt, G.; Lönnerdal, B.L. The effects of α-lactalbumin and glycomacropeptide on the association of CaCo-2 cells by enteropathogenic Escherichia coli, Salmonella typhimurium and Shigella flexneri. FEMS Microbiol. Lett. 2006, 259, 158–162. [Google Scholar] [CrossRef]
- Li, A.; Ma, Y.; Cui, N.; Zhang, X.; Zheng, Q.; Du, P.; Sun, M. Research progress of milk and dairy products to prevent caries. J. Funct. Foods 2023, 110, 105837. [Google Scholar] [CrossRef]
- Kelleher, S.L.; Chatterton, D.; Nielsen, K.; Lönnerdal, B. Glycomacropeptide and α-lactalbumin supplementation of infant formula affects growth and nutritional status in infant rhesus monkeys. Am. J. Clin. Nutr. 2003, 77, 1261–1268. [Google Scholar] [CrossRef]
- Solverson, P.; Murali, S.G.; Litscher, S.J.; Blank, R.D.; Ney, D.M. Low Bone Strength Is a Manifestation of Phenylketonuria in Mice and Is Attenuated by a Glycomacropeptide Diet. PLoS ONE 2012, 7, e45165. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.; Binetti, A.; Torti, P.; Kulozik, U.; Forzani, L.; Renzulli, P.; Vinderola, G.; Reinheimer, J. Administration of caseinomacropeptide-enriched extract to mice enhances the calcium content of femur in a low-calcium diet. Int. Dairy J. 2015, 44, 15–20. [Google Scholar] [CrossRef]
- Sawin, E.A.; Stroup, B.M.; Murali, S.G.; O’Neill, L.M.; Ntambi, J.M.; Ney, D.M. Differential effects of dietary fat content and protein source on bone phenotypeand fatty acid oxidation in female C57Bl/6 mice. PLoS ONE 2016, 11, e0163234. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef]
- Sauvé, M.F.; Feldman, F.; Sané, A.T.; Koudoufio, M.; Patey, N.; Spahis, S.; Butcher, J.; Duan, H.; Figeys, D.; Marcil, V.; et al. Glycomacropeptide as an Efficient Agent to Fight Pathophysiological Mechanisms of Metabolic Syndrome. Nutrients 2024, 16, 871. [Google Scholar] [CrossRef]
- Adler, S.M.; Paluska, M.R.; Svoboda, K.R.; Dallas, D.C. Immunomodulatory bioactivities of glycomacropeptide. J. Funct. Foods 2024, 115, 106084. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from bovine milk: A protective companion for life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Mahala, N.; Mittal, A.; Dubey, U.S. Medicinal Potential of Camel Milk Lactoferrin. In Current Issues and Advances in the Dairy Industry; Salam, A.I., Ed.; IntechOpen: London, UK, 2022; Volume 11, pp. 1–17. [Google Scholar]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic efficacy of lactoferrin in type 2 diabetic pediatrics; Controlling impact on PPAR-γ, SIRT-1, and TLR4 downstream signaling pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef]
- Zarzosa-Moreno, D.; Avalos-Gómez, C.; Ramírez-Texcalco, L.S.; Torres-López, E.; Ramírez-Mondragón, R.; Hernández-Ramírez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and its derived peptides: An alternative for combating virulence mechanisms developed by pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Zhang, F.; Liu, X.; Zhang, Y.; Peng, W.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; et al. Interaction between Dietary Lactoferrin and Gut Microbiota in Host Health. J. Agric. Food Chem. 2024, 72, 7596–7606. [Google Scholar] [CrossRef]
- Griffin, I.J. The Effects of Different Forms of Lactoferrin on Iron Absorption. J. Nutr. 2020, 150, 3053–3054. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, J.; Xiao, D.; Shu, G.; Gu, L. Bovine Lactoferrin Protects Dextran Sulfate Sodium Salt Mice Against Inflammation and Impairment of Colonic Epithelial Barrier by Regulating Gut Microbial Structure and Metabolites. Front. Nutr. 2021, 8, 660598. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, C.; Hurilebagen; Yuan, H.; Hu, R.; Wang, W. Weilisi Effects of lactoferrin on intestinal flora of metabolic disorder mice. BMC Microbiol. 2022, 22, 181. [CrossRef]
- He, Q.; Zhang, L.L.; Li, D.; Wu, J.; Guo, Y.X.; Fan, J.; Wu, Q.; Wang, H.P.; Wan, Z.; Xu, J.Y.; et al. Lactoferrin alleviates Western diet-induced cognitive impairment through the microbiome-gut-brain axis. Curr. Res. Food Sci. 2023, 7, 100533. [Google Scholar] [CrossRef]
- Ma, X.; Hao, Y.; Mao, R.; Yang, N.; Zheng, X.; Li, B.; Wang, Z.; Zhang, Q.; Teng, D.; Wang, J. Effects of dietary supplementation of bovine lactoferrin on growth performance, immune function and intestinal health in weaning piglets. BioMetals 2023, 36, 587–601. [Google Scholar] [CrossRef]
- D’Amico, F.; Decembrino, N.; Muratore, E.; Turroni, S.; Muggeo, P.; Mura, R.; Perruccio, K.; Vitale, V.; Zecca, M.; Prete, A.; et al. Oral Lactoferrin Supplementation during Induction Chemotherapy Promotes Gut Microbiome Eubiosis in Pediatric Patients with Hematologic Malignancies. Pharmaceutics 2022, 14, 1705. [Google Scholar] [CrossRef]
- Konstanti, P.; van Splunter, M.; van den Brink, E.; Belzer, C.; Nauta, A.; van Neerven, R.J.J.; Smidt, H. The Effect of Nutritional Intervention with Lactoferrin, Galactooligosacharides and Vitamin D on the Gut Microbiota Composition of Healthy Elderly Women. Nutrients 2022, 14, 2468. [Google Scholar] [CrossRef]
- Xie, T.; Qiao, W.; Jia, T.; Kaku, K. Skin Care Function of Lactoferrin Was Characterized Using Recombinant Human Epidermal Model. Cosmetics 2024, 11, 98. [Google Scholar] [CrossRef]
- Krupińska, A.M.; Bogucki, Z. Clinical aspects of the use of lactoferrin in dentistry. J. Oral Biosci. 2021, 63, 129–133. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Jiang, P.; Stensballe, A.; Bendixen, E.; Sangild, P.T.; Chatterton, D.E.W. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J. Proteom. 2016, 139, 95–102. [Google Scholar] [CrossRef]
- Saadi, S.; Makhlouf, C.; Nacer, N.E.; Halima, B.; Faiza, A.; Kahina, H.; Wahiba, F.; Afaf, K.; Rabah, K.; Saoudi, Z. Whey proteins as multifunctional food materials: Recent advancements in hydrolysis, separation, and peptidomimetic approaches. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13288. [Google Scholar] [CrossRef] [PubMed]
- Innocente, N.; Comparin, D.; Corradini, C. Proteose-peptone whey fraction as emulsifier in ice-cream preparation. Int. Dairy J. 2002, 12, 69–74. [Google Scholar] [CrossRef]
- Graikini, D.; Hadidian, M.; Abad, I.; Parrón, J.A.; Ripollés, D.; Pérez, M.D.; Calvo, M.; Sánchez, L. Comparative study on the biological activity of bovine and ovine PP3 and of their hydrolysates. Int. Dairy J. 2024, 159, 106058. [Google Scholar] [CrossRef]
- Santa, D.; Srbinovska, S. Whey Valorization. In Whey Valorization—Innovations, Technological Advancements and Sustainable Exploitation; Poonia, A., Trajkovska Petkoska, A., Eds.; Springer: Singapore, 2023; pp. 239–258. ISBN 978-981-99-5458-2. [Google Scholar]
- Modler, W. Pioneer paper: Value-added components derived from whey. Am. Dairy Sci. Assoc. 2009, 1, 1–33. [Google Scholar]
- Mollea, C.; Marmo, L.; Bosco, F. Valorisation of Cheese Whey, a By-Product from the Dairy Industry. In Food Industry; IntechOpen: London, UK, 2013; Volume 11, pp. 549–588. [Google Scholar]
- Yousefi, M.; Nematollahi, A.; Shadnoush, M.; Mortazavian, A.M.; Khorshidian, N. Antimicrobial Activity of Films and Coatings Containing Lactoperoxidase System: A Review. Front. Nutr. 2022, 9, 828065. [Google Scholar] [CrossRef]
- Farshidi, M.; Ehsani, A. The combined effects of lactoperoxidase system and whey protein coating on microbial, chemical, textural, and sensory quality of shrimp (Penaeus merguiensis) during cold storage. Food Sci. Nutr. 2018, 6, 1378–1386. [Google Scholar] [CrossRef]
- Min, S.; Harris, L.J.; Krochta, J.M. Antimicrobial effects of lactoferrin, lysozyme, and the lactoperoxidase system and edible whey protein films incorporating the lactoperoxidase system against Salmonella enterica and Escherichia coli O157: H7. J. Food Sci. 2005, 70, m332–m338. [Google Scholar] [CrossRef]
- Saravani, M.; Ehsani, A.; Aliakbarlu, J.; Ghasempour, Z. Gouda cheese spoilage prevention: Biodegradable coating induced by Bunium persicum essential oil and lactoperoxidase system. Food Sci. Nutr. 2019, 7, 959–968. [Google Scholar] [CrossRef]
- Larsen, I.S.; Jensen, B.A.H.; Bonazzi, E.; Béatrice, S.Y.; Kristensen, N.N.; Gjerløff, E.; Schmidt, W.; Morin, L.; Olsen, P.B.; Skov, L.B.; et al. Fungal lysozyme leverages the gut microbiota to curb DSS-induced colitis. Gut Microbes 2021, 13, 1988836. [Google Scholar] [CrossRef]
- Alonso-González, M.; Corral-González, A.; Felix, M.; Romero, A.; Martin-Alfonso, J.E. Developing active poly(vinyl alcohol)-based membranes with encapsulated antimicrobial enzymes via electrospinning for food packaging. Int. J. Biol. Macromol. 2020, 162, 913–921. [Google Scholar] [CrossRef]
- Anastas, P.T.; Rodriguez, A.; de Winter, T.M.; Coish, P.; Zimmerman, J.B. A review of immobilization techniques to improve the stability and bioactivity8 of lysozyme. Green Chem. Lett. Rev. 2021, 14, 302–338. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef]
- Huppertz, T.; Vasiljevic, T.; Zisu, B.; Deeth, H. Novel Processing Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128121245. [Google Scholar]
- Sousa, A.; Passarinha, L.A.; Rodrigues, L.R.; Teixeira, J.A.; Mendonça, A.; Queiroz, J.A. Separation of different forms of proteose peptone 3 by hydrophobic interaction chromatography with a dual salt system. Biomed. Chromatogr. 2008, 22, 447–449. [Google Scholar] [CrossRef]
- Reza Aghamaali, M.; Borjian-Boroujeni, M.; Nayeri, H.; Beheshti-Maal, K. The Efficacy of Stabilizers on Lactoperoxidase System’s Antibacterial Activity and Stability: A Review. Microbiol. Metab. Biotechnol. 2023, 6, 17–36. [Google Scholar] [CrossRef]
- Pan, M.; Shen, S.; Chen, L.; Dai, B.; Xu, L.; Yun, J.; Yao, K.; Lin, D.Q.; Yao, S.J. Separation of lactoperoxidase from bovine whey milk by cation exchange composite cryogel embedded macroporous cellulose beads. Sep. Purif. Technol. 2015, 147, 132–138. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review. Probiotics Antimicrob. Proteins 2019, 11, 348–369. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.A.; Irfan, S.; Hafiz, I.; Ranjha, M.M.A.N.; Rahaman, A.; Murtaza, M.S.; Ibrahim, S.A.; Siddiqui, S.A. Conventional and Novel Technologies in the Production of Dairy Bioactive Peptides. Front. Nutr. 2022, 9, 1–19. [Google Scholar] [CrossRef]
- Bamdad, F.; Bark, S.; Kwon, C.H.; Suh, J.W.; Sunwoo, H. Anti-inflammatory and antioxidant properties of peptides released from β-lactoglobulin by high hydrostatic pressure-assisted enzymatic hydrolysis. Molecules 2017, 22, 949. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Abadía-García, L.; Castaño-Tostado, E.; Cardador-Martínez, A.; Martín-Del-campo, S.T.; Amaya-Llano, S.L. Production of ACE inhibitory peptides from whey proteins modified by high intensity ultrasound using bromelain. Foods 2021, 10, 2099. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Penha, F.M.; Cunha Petrus, J.C.; Rezzadori, K. Low purity enzymes and ultrasound pretreatment applied to partially hydrolyze whey protein. Food Biosci. 2020, 38, 100784. [Google Scholar] [CrossRef]
- El Mecherfi, K.E.; Curet, S.; Lupi, R.; Larré, C.; Rouaud, O.; Choiset, Y.; Rabesona, H.; Haertlé, T. Combined microwave processing and enzymatic proteolysis of bovine whey proteins: The impact on bovine β-lactoglobulin allergenicity. J. Food Sci. Technol. 2019, 56, 177–186. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H.; Park, B.J.; Kim, S.H.; Oh, D.H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Sci. Biotechnol. 2018, 27, 1781–1789. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Sahu, S.; Agrawal, S.; Sahu, A. Regulatory policies on use of food enzymes. In Enzymes Beyond Traditional Applications in Dairy Science and Technology; Academic Press: Cambridge, MA, USA, 2023; pp. 519–535. ISBN 9780323960106. [Google Scholar]
- Manfredini, P.G.; Cavanhi, V.A.F.; Costa, J.A.V.; Colla, L.M. Bioactive peptides and proteases: Characteristics, applications and the simultaneous production in solid-state fermentation. Biocatal. Biotransform. 2021, 39, 360–377. [Google Scholar] [CrossRef]
- Kadyan, S.; Rashmi, H.M.; Pradhan, D.; Kumari, A.; Chaudhari, A.; Deshwal, G.K. Effect of lactic acid bacteria and yeast fermentation on antimicrobial, antioxidative and metabolomic profile of naturally carbonated probiotic whey drink. LWT 2021, 142, 111059. [Google Scholar] [CrossRef]
- Chourasia, R.; Abedin, M.M.; Chiring Phukon, L.; Sahoo, D.; Singh, S.P.; Rai, A.K. Biotechnological approaches for the production of designer cheese with improved functionality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 960–979. [Google Scholar] [CrossRef]
- Biadała, A.; Szablewski, T.; Cegielska-radziejewska, R.; Lasik-kurdy, M. The Evaluation of Activity of Selected Lactic Acid Bacteria for Bioconversion of Milk and Whey from Goat Milk to Release Biomolecules with Antibacterial Activity. Molecules 2023, 28, 3696. [Google Scholar] [CrossRef]
- Solieri, L.; Valentini, M.; Cattivelli, A.; Sola, L.; Helal, A.; Martini, S.; Tagliazucchi, D. Fermentation of whey protein concentrate by Streptococcus thermophilus strains releases peptides with biological activities. Process Biochem. 2022, 121, 590–600. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Białas, W.; Grajek, W. Integrated approach for obtaining bioactive peptides from whey proteins hydrolysed using a new proteolytic lactic acid bacteria. Food Chem. 2020, 312, 126035. [Google Scholar] [CrossRef]
- Dineshbhai, C.K.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Patil, G.B.; Mankad, M.; Liu, Z.; Hati, S. Exploring the potential of Lactobacillus and Saccharomyces for biofunctionalities and the release of bioactive peptides from whey protein fermentate. Food Biosci. 2022, 48, 101758. [Google Scholar] [CrossRef]
- Helal, A.; Nasuti, C.; Sola, L.; Sassi, G.; Tagliazucchi, D.; Solieri, L. Impact of Spontaneous Fermentation and Inoculum with Natural Whey Starter on Peptidomic Profile and Biological Activities of Cheese Whey: A Comparative Study. Fermentation 2023, 9, 270. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Ahmad, T.; Singh, R.S.; Gupta, G.; Sharma, A.; Kaur, B. Metagenomics in the Search for Industrial Enzymes; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444641144. [Google Scholar]
- Irazoqui, J.M.; Santiago, G.M.; Mainez, M.E.; Amadio, A.F.; Eberhardt, M.F. Enzymes for production of whey protein hydrolysates and other value-added products. Appl. Microbiol. Biotechnol. 2024, 108, 354. [Google Scholar] [CrossRef] [PubMed]
- Kheroufi, A.; Brassesco, M.E.; Campos, D.A.; Mouzai, A.; Boughellouta, H.; Pintado, M.E. Whey protein-derived peptides: The impact of chicken pepsin hydrolysis upon whey proteins concentrate on their biological and technological properties. Int. Dairy J. 2022, 134, 105442. [Google Scholar] [CrossRef]
- Tondo, A.R.; Caputo, L.; Mangiatordi, G.F.; Monaci, L.; Lentini, G.; Logrieco, A.F.; Montaruli, M.; Nicolotti, O.; Quintieri, L. Structure-Based Identification and Design of Angiotensin Converting Enzyme-Inhibitory Peptides from Whey Proteins. J. Agric. Food Chem. 2020, 68, 541–548. [Google Scholar] [CrossRef]
- Osman, A.; El-Hadary, A.; Korish, A.A.; AlNafea, H.M.; Alhakbany, M.A.; Awad, A.A.; Abdel-Hamid, M. Angiotensin-I converting enzyme inhibition and antioxidant activity of papain-hydrolyzed camel whey protein and its hepato-renal protective effects in thioacetamide-induced toxicity. Foods 2021, 10, 468. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Wang, X.; Wang, H. Characterization of structure, physicochemical properties, and hypoglycemic activity of goat milk whey protein hydrolysate processed with different proteases. LWT 2022, 159, 113257. [Google Scholar] [CrossRef]
- Quintieri, L.; Carito, A.; Pinto, L.; Calabrese, N.; Baruzzi, F.; Caputo, L. Application of lactoferricin B to control microbial spoilage in cold stored fresh foods. In Multidisciplinary Approach for Studying and Combating Microbial Pathogens; Mendèz-Vilas, A., Ed.; BrownWalker Press: Boca Raton, FL, USA, 2015; pp. 58–62. [Google Scholar]
- Bustamante, S.Z.; González, J.G.; Sforza, S.; Tedeschi, T. Bioactivity and peptide profile of whey protein hydrolysates obtained from Colombian double-cream cheese production and their products after gastrointestinal digestion. LWT 2021, 145, 111334. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Yang, T.; Zheng, D.; Liu, X.; Zhang, J.; Zheng, M. Preparation of immobilized Alcalase based on metal affinity for efficient production of bioactive peptides. LWT 2022, 162, 113505. [Google Scholar] [CrossRef]
- da Cruz, C.Z.P.; de Mendonça, R.J.; Guimaraes, L.H.S.; dos Santos Ramos, M.A.; Garrido, S.S.; de Paula, A.V.; Monti, R.; Massolini, G. Assessment of the Bioactive Potential of Cheese Whey Protein Hydrolysates Using Immobilized Alcalase. Food Bioprocess Technol. 2020, 13, 2120–2130. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Characterization, Preparation, and Purification of Marine Bioactive Peptides. BioMed Res. Int. 2017, 2017, 9746720. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Huth, P.J. Whey Processing, Functionality and Health Benefits; Onwulata, C.I., Huth, P.J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780813809038. [Google Scholar]
- Wang, G.; Guo, M. Manufacturing technologies of whey protein products. In Whey Protein Production, Chemistry, Functionality, and Applications; Guo, M., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; Volume 2, pp. 13–37. ISBN 9781119256052. [Google Scholar]
- Dufour, E.; Dalgalarrondo, M.; Haertlé, T. Limited proteolysis of β-lactoglobulin using thermolysin. Effects of calcium on the outcome of proteolysis. Int. J. Biol. Macromol. 1994, 16, 37–41. [Google Scholar] [CrossRef]
- Kiokias, S.; Gordon, M.H.; Oreopoulou, V. Effects of composition and processing variables on the oxidative stability of protein-based and oil-in-water food emulsions. Crit. Rev. Food Sci. Nutr. 2017, 57, 549–558. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Walter, V.; Gamlath, C.J.; Yang, M.; Martin, G.J.O.; Ashokkumar, M. Functionalised dairy streams: Tailoring protein functionality using sonication and heating. Ultrason. Sonochem. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Jia, J.; Kuang, C.; Yang, H. Effect of ultrasonic pretreatment on whey protein hydrolysis by alcalase: Thermodynamic parameters, physicochemical properties and bioactivities. Process. Biochem. 2018, 67, 46–54. [Google Scholar] [CrossRef]
- Pang, L.; Liu, M.; Chen, C.; Huang, Z.; Liu, S.; Man, C.; Jiang, Y.; Zhang, W.; Yang, X. Effects of ultrasound pretreatment on the structure, IgE binding capacity, functional properties and bioactivity of whey protein hydrolysates via multispectroscopy and peptidomics revealed. Ultrason. Sonochem. 2024, 110, 107025. [Google Scholar] [CrossRef] [PubMed]
- Monaci, L.; Lamonaca, A.; Luparelli, A.; Pilolli, R.; De Angelis, E. (Bio)technological Approaches for Reducing Allergenicity of Food Ingredients. In Sustainable Food Science: A Comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 86–102. ISBN 9780128239605. [Google Scholar]
- Monaci, L.; Pilolli, R.; Quintieri, L.; Caputo, L.; Luparelli, A.; De Angelis, E. Casein: Allergenicity and molecular properties. In Casein-Structural Properties, Uses, Health Benefits and Nutraceutical Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 363–377. ISBN 9780443158360. [Google Scholar]
- Patist, A.; Bates, D. Ultrasound Technologies for Food and Bioprocessing. In Ultrasound Technologies for Food and Bioprocessing—Food Engineering Series; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer: New York, NY, 2011; Volume 53, pp. 599–616. ISBN 978-1-4419-7471-6. [Google Scholar]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, H.; Hu, Y.; Tu, Z. Insight into the effects of pulsed electric field on the structure, aggregation characteristics and functional properties of whey proteins. Food Hydrocoll. 2024, 154, 110111. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M.V. Pulsed Electric Field-Induced Structural Modification of Whey Protein Isolate. Food Bioprocess Technol. 2008, 4, 1341–1348. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Bala Krishnan, S. Pulsed Electric Field Treatment in Extracting Proteins from Legumes: A Review. Processes 2024, 12, 2667. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D.; Pouliot, Y.; Doyen, A. Effects of High Hydrostatic Pressure Processing on Hen Egg Compounds and Egg Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 707–720. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Yamamoto, K. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef]
- Espinoza, A.D.; Morawicki, R.O.; Hager, T. Hydrolysis of whey protein isolate using subcritical water. J. Food Sci. 2012, 77, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.R.; Cappato, L.P.; Pereira, M.V.S.; Pires, R.P.S.; Moraes, J.; Esmerino, E.A.; Silva, R.; Neto, R.P.C.; Tavares, M.I.B.; Freitas, M.Q.; et al. Ohmic Heating: A potential technology for sweet whey processing. Food Res. Int. 2018, 106, 771–779. [Google Scholar] [CrossRef]
- Alizadeh, O.; Aliakbarlu, J. Effects of ultrasound and ohmic heating pretreatments on hydrolysis, antioxidant and antibacterial activities of whey protein concentrate and its fractions. LWT 2020, 131, 109913. [Google Scholar] [CrossRef]
- Palladino, C.; Breiteneder, H. Peanut allergens. Mol. Immunol. 2018, 100, 58–70. [Google Scholar] [CrossRef]
- Kashung, P.; Karuthapandian, D. Milk-derived bioactive peptides. Food Prod. Process. Nutr. 2025, 7, 6. [Google Scholar] [CrossRef]
- Parastouei, K.; Jabbari, M.; Javanmardi, F.; Barati, M.; Mahmoudi, Y. Estimation of bioactive peptide content of milk from different species using an in silico method. Amino Acids 2023, 55, 1261–1278. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. Artic. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, S.; Singh, S.; Gautam, A.; Raghava, G.P.S. AHTPDB: A comprehensive platform for analysis and presentation of antihypertensive peptides. Food Chem. 2015, 43, 956–962. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, X.; Zhu, Y.; Dai, Z.; Shen, Q.; Xue, Y. Advances in machine learning screening of food bioactive compounds. Trends Food Sci. Technol. 2024, 150, 104578. [Google Scholar] [CrossRef]
- Balech, B.; Brennan, L.; Carrillo de Santa Pau, E.; Cavalieri, D.; Coort, S.; D’Elia, D.; Dragsted, L.O.; Eftimov, T.; Evelo, C.T.; Ferk, P.; et al. The future of food and nutrition in ELIXIR. F1000Research 2022, 11, 978. [Google Scholar] [CrossRef]
- Tulipano, G.; Faggi, L.; Nardone, A.; Cocchi, D.; Maria, A. Characterisation of the potential of b -lactoglobulin and a -lactalbumin as sources of bioactive peptides affecting incretin function: In silico and in vitro comparative studies. Int. Dairy J. 2015, 48, 66–72. [Google Scholar] [CrossRef]
- Norris, R.; Poyarkov, A.; Keeffe, M.B.O.; Fitzgerald, R.J. Characterisation of the hydrolytic specificity of Aspergillus niger derived prolyl endoproteinase on bovine b -casein and determination of ACE inhibitory activity. Food Chem. 2014, 156, 29–36. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Fitzgerald, R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Elisha, C.; Bhagwat, P.; Pillai, S. In silico and in vitro analysis of dipeptidyl peptidase-IV and angiotensin-converting enzyme inhibitory peptides derived from milk lactoferrin. Int. Dairy J. 2025, 160, 106092. [Google Scholar] [CrossRef]
- Jing, P.; Qian, B.; He, Y.; Zhao, X.; Zhang, J.; Zhao, D.; Lv, Y.; Deng, Y. Screening milk-derived antihypertensive peptides using quantitative structure activity relationship (QSAR) modelling and in vitro / in vivo studies on their bioactivity. Int. Dairy J. 2014, 35, 95–101. [Google Scholar] [CrossRef]
- He, R.; Ma, H.; Zhao, W.; Qu, W.; Zhao, J.; Luo, L.; Zhu, W. Modeling the QSAR of ACE-Inhibitory Peptides with ANN and Its Applied Illustration. Int. J. Pept. 2012, 2012, 620609. [Google Scholar] [CrossRef]
- Dubchak, I.; Muchnikt, I.; Holbrook, S.R.; Kim, S. Prediction of protein folding class using global description of amino acid sequence. Proc. Natl. Acad. Sci. USA 1995, 92, 8700–8704. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Hwan Shin, T.; Lee, G. Machine Intelligence in Peptide Therapeutics: A next-Generation Tool for Rapid Disease Screening. Med. Res. Rev. 2020, 40, 1276–1314. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Wong, F.; Collins, J.J.; de la Fuente-Nunez, C. Machine Learning for Antimicrobial Peptide Identification and Design. Nat. Rev. Bioeng. 2024, 2, 392–407. [Google Scholar] [CrossRef]
- Wang, Y.T.; Russo, D.P.; Liu, C.; Zhou, Q.; Zhu, H.; Zhang, Y.H. Predictive Modeling of Angiotensin I-Converting Enzyme Inhibitory Peptides Using Various Machine Learning Approaches. J. Agric. Food Chem. 2020, 28, 12132–12140. [Google Scholar] [CrossRef] [PubMed]
- Pripp, A.H.; Isaksson, T.; Stepaniak, L.; Sørhaug, T. Quantitative structure-activity relationship modelling of ACE-inhibitory peptides derived from milk proteins. Eur. Food Res. Technol. 2004, 219, 579–583. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of Molecular Docking Analysis and Molecular Dynamics Simulations for Studying Food Proteins and Bioactive Peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef]
- Pripp, A.H. Docking and virtual screening of ACE inhibitory dipeptides. Eur. Food Res. Technol. 2007, 225, 589–592. [Google Scholar] [CrossRef]
- Norris, R.; Casey, F.; Fitzgerald, R.J.; Shields, D.; Mooney, C. Predictive modelling of angiotensin converting enzyme inhibitory dipeptides. Food Chem. 2012, 133, 1349–1354. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; Fitzgerald, R.J. Inhibition of dipeptidyl peptidase IV and xanthine oxidase by amino acids and dipeptides. Food Chem. 2013, 141, 644–653. [Google Scholar] [CrossRef]
- Baba, W.N.; Baby, B.; Mudgil, P.; Gan, C.Y.; Vijayan, R.; Maqsood, S. Pepsin generated camel whey protein hydrolysates with potential antihypertensive properties: Identification and molecular docking of antihypertensive peptides. LWT 2021, 143, 111135. [Google Scholar] [CrossRef]
- Gambacorta, N.; Caputo, L.; Quintieri, L.; Monaci, L.; Ciriaco, F.; Nicolotti, O. Rational Discovery of Antiviral Whey Protein-Derived Small Peptides Targeting the SARS-CoV-2 Main Protease. Biomedicines 2022, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, X.; Qi, X.; Ma, Y.; Chan, E.C.Y. In silico identification of novel ACE and DPP-IV inhibitory peptides derived from buffalo milk proteins and evaluation of their inhibitory mechanisms. Amino Acids 2023, 55, 161–171. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef]
- Liu, X.; Qin, X.; Wang, Y.; Zhong, J. Physicochemical properties and formation mechanism of whey protein isolate-sodium alginate complexes: Experimental and computational study. Food Hydrocoll. 2022, 131, 107786. [Google Scholar] [CrossRef]
- Isaac Sharon, K.; Combe, M.; Potter, G.; Sokolenko, S. Machine learning tools for peptide bioactivity evaluation—Implications for cell culture media optimization and the broader cultivated meat industry. Curr. Res. Food Sci. 2024, 9, 100842. [Google Scholar] [CrossRef] [PubMed]
- Morales García, J.; Udenigwe, C.C.; Duitama, J.; Barrios, A.F.G. Peptidomic analysis of whey protein hydrolysates and prediction of their antioxidant peptides. Food Sci. Hum. Wellness 2022, 11, 349–355. [Google Scholar] [CrossRef]
- Jox, D.; Borsum, C.; Hummel, D.; Hinrichs, J.; Krupitzer, C. Enhancing dairy processing with machine learning and domain knowledge: A combined analysis of offline and time series data. J. Food Eng. 2025, 391, 112423. [Google Scholar] [CrossRef]
- Sanchez-Reinoso, Z.; Bazinet, M.; Leblanc, B.; Cl, J.; Germain, P.; Bazinet, L. Application of machine learning tools to study the synergistic impact of physicochemical properties of peptides and filtration membranes on peptide migration during electrodialysis with filtration membranes. Sep. Purif. Technol. 2025, 360, 130877. [Google Scholar] [CrossRef]
- Jia, W.; Peng, J.; Zhang, Y.; Zhu, J.; Qiang, X.; Zhang, R.; Shi, L. Exploring novel ANGICon-EIPs through ameliorated peptidomics techniques: Can deep learning strategies as a core breakthrough in peptide structure and function prediction? Food Res. Int. 2023, 174, 113640. [Google Scholar] [CrossRef]
- Taherzadeh, G.; Zhou, Y.; Liew, A.W.-C.; Yang, Y. Structure-based prediction of protein– peptide binding regions using Random Forest. Bioinformatics 2018, 34, 477–484. [Google Scholar] [CrossRef]
- Johansson-Åkhe, I.; Mirabello, C.; Wallner, B. Predicting protein-peptide interaction sites using distant protein complexes as structural templates. Sci. Rep. 2019, 9, 4267. [Google Scholar] [CrossRef]
- Trisciuzzi, D.; Siragusa, L.; Baroni, M.; Cruciani, G.; Nicolotti, O. An Integrated Machine Learning Model to Spot Peptide Binding Pockets in 3D Protein Screening. J. Chem. Inf. Model. 2022, 62, 6812–6824. [Google Scholar] [CrossRef]
- Trisciuzzi, D.; Siragusa, L.; Baroni, M.; Autiero, I.; Nicolotti, O.; Cruciani, G. Getting Insights into Structural and Energetic Properties of Reciprocal Peptide-Protein Interactions. J. Chem. Inf. Model. 2022, 62, 1113–1125. [Google Scholar] [CrossRef]
- Petre, M.L.; Nefeli, A.; Pertesi, K.; Boulioglou, O.E.; Sarantidi, E.; Korovesi, A.G.; Kozei, A.; Katsafadou, A.I.; Tsangaris, G.T.; Trichopoulou, A.; et al. Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism. Foods 2024, 13, 3949. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Cermeño, M.; Khalesi, M.; Kleekayai, T.; Amigo-Benavent, M. Application of in silico approaches for the generation of milk protein-derived bioactive peptides. J. Funct. Foods 2020, 64, 103636. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Hernández-Ledesma, B.; Amigo, L.; Martín-Álvarez, P.J.; Recio, I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: Optimization by response surface methodology. LWT—Food Sci. Technol. 2011, 44, 9–15. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Enhancing bioactive peptide release and identification using targeted enzymatic hydrolysis of milk proteins. Anal. Bioanal. Chem. 2018, 410, 3407–3423. [Google Scholar] [CrossRef]
- Kheto, A.; Adhikary, U.; Dhua, S.; Sarkar, A.; Kumar, Y.; Vashishth, R.; Shrestha, B.B.; Saxena, D.C. A review on advancements in emerging processing of whey protein: Enhancing functional and nutritional properties for functional food applications. Food Saf. Health 2025, 3, 23–45. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods—Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000037 Whey protein isolate and dairy product solids. In GRAS Notices; American Dairy Products Institute: Chicago, IL, USA; Office of Premarket Approval: Silver Spring, MD, USA, 2000. [Google Scholar]
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000196 Bovine milk basic protein fraction. In GRAS Notices; Snow Brand Milk Products Co., Ltd. and IAS Co., Ltd.: Tokyo, Japan; Office of Food Additive Safety: Silver Spring, MD, USA, 2006. [Google Scholar]
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000067 Milk-derived lactoferrin. In GRAS Notices; Farmland National Packaging Company, L.P.: Liberal, KS, USA; Office of Food Additive Safety: Silver Spring, MD, USA, 2000. [Google Scholar]
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000077 Milk-derived lactoferrin. In GRAS Notices; DMV International: Fraser, NY, USA; Office of Food Additive Safety: Silver Spring, MD, USA, 2001. [Google Scholar]
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000130 Bovine milk-derived lactoferrin. In GRAS Notices; aLF Ventures LLC: Salt Lake City, UT, USA; Office of Food Additive Safety: Silver Spring, MD, USA, 2003. [Google Scholar]
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000464 Cow’s milk-derived lactoferrin. In GRAS Notices; Morinaga Milk Industry Co., Ltd.: Tokyo, Japan; Office of Food Additive Safety: Silver Spring, MD, USA, 2014. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=grasnotices&id=464 (accessed on 10 March 2025).
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000465 Cow’s milk-derived lactoferrin. In GRAS Notices; Morinaga Milk Industry Co., Ltd.: Tokyo, Japan; Office of Food Additive Safety: Silver Spring, MD, USA, 2014. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=465&sort=GRN_No&order=DESC&startrow=1&type=basic&search=465 (accessed on 10 March 2025).
- US FDA Food and Drug Administration; Center for Food Safety & Applied Nutrition (CFSAN). GRN 000669 Bovine Milk-derived Lactoferrin. In GRAS Notices; Synlait Milk Ltd.: Rakaia, New Zealand; Office of Food Additive Safety: Silver Spring, MD, USA, 2017. [Google Scholar]
- MHLW. List of Existing Food Additives—Complied and published by the Ministry of Health and Welfare on April 16, 1996; Japan Food Chemical Research Foundation (JFCRF): Tokyo, Japan, 2014; Available online: https://www.ffcr.or.jp/en/index.html (accessed on 15 March 2025).
- FSANZ. Lactoperoxidase System (Application A404—Final Assessment Report); Food Standards Australia New Zealand (FSANZ): Canberra, Australia, 2002. Available online: https://www.foodstandards.gov.au/food-standards-code/applications/applicationa404thelactoperoxidasesystem/applicationa404lacto1862 (accessed on 15 March 2025).
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Whey basic protein isolates as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2018, 16, e05360. [Google Scholar] [CrossRef]
- Kruger, C.L.; Marano, K.M.; Morita, Y.; Takada, Y.; Kawakami, H.; Kobayashi, T.; Sunaga, M.; Furukawa, M.; Kawamura, K. Safety evaluation of a milk basic protein fraction. Food Chem. Toxicol. 2007, 45, 1301–1307. [Google Scholar] [CrossRef]
- Forster, R.; Bourtourault, M.; Chung, Y.J.; Silvano, J.; Sire, G.; Spezia, F.; Puel, C.; Descotes, J.; Mikogami, T. Safety evaluation of a whey protein fraction containing a concentrated amount of naturally occurring TGF-β2. Regul. Toxicol. Pharmacol. 2014, 69, 398–407. [Google Scholar] [CrossRef]
- Dyer, A.R.; Burdock, G.A.; Carabin, I.G.; Haas, M.C.; Boyce, J.; Alsaker, R.; Read, L.C. In vitro and in vivo safety studies of a proprietary whey extract. Food Chem. Toxicol. 2008, 46, 1659–1665. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of whey basic protein isolate for extended uses in foods for special medical purposes and food supplements for infants pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05659. [Google Scholar] [CrossRef]
- Castenmiller, J.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; Pentieva, K.; et al. Efficacy of an infant formula manufactured from a specific protein hydrolysate derived from whey protein isolate and concentrate produced by Société des Produits Nestlé S.A. in reducing the risk of developing atopic dermatitis. EFSA J. 2021, 19, e06603. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Cámara, M.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Jos, A.; Maciuk, A.; Mangelsdorf, I.; McNulty, B.; Naska, A.; et al. Nutritional safety and suitability of a specific protein hydrolysate manufactured by Fonterra Co-operative Group Ltd. derived from a whey protein concentrate and used in infant formula and follow-on formula. EFSA J. 2025, 23, e9160. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Nutritional safety and suitability of a specific protein hydrolysate derived from whey protein concentrate and used in an infant and follow-on formula manufactured from hydrolysed protein by Danone Trading ELN B.V. EFSA J. 2020, 18, e06304. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K.; et al. Nutritional safety and suitability of a specific protein hydrolysate derived from a whey protein concentrate and used in an infant formula and follow-on formula manufactured from hydrolysed protein by FrieslandCampina Nederland B.V. EFSA J. 2023, 21, e08063. [Google Scholar] [CrossRef]
- Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Katrine Knutsen, H.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Nutritional safety and suitability of a specific protein hydrolysate derived from whey protein concentrate and used in an infant and follow-on formula manufactured from hydrolysed protein by HIPP-Werk Georg Hipp OHG (dossier submitted by meyer.science Gmb. EFSA J. 2022, 20, e07141. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2006/141/EC of 22 December 2006 on Infant Formulae and Follow-on Formulae and Amending Directive 1999/21/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32006L0141&qid=1748261499340 (accessed on 19 March 2025).
- Commission Regulation (EC) No 1609/2006 of 27 October 2006 Authorising the Placing on the Market of Infant Formulae Based on Hydrolysates of Whey Protein Derived From Cows’ Milk Protein for a Two-Year Period. Available online: https://eur-lex.europa.eu/eli/reg/2006/1609/oj/eng (accessed on 19 March 2025).
- Li, J.; Zhu, F. Whey protein hydrolysates and infant formulas: Effects on physicochemical and biological properties. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13337. [Google Scholar] [CrossRef]
- CXS 289-1995; Codex Alimentarius, International Food Standards: Standard for Whey Powders Formerly CODEX STAN A-15-1995, Adopted in 1995, Revised in 2003. Amended in 2006. Codex Alimentarius: Rome, Italy, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).