Long-Term Outcomes After High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy in Very High-Risk Prostate Cancer: A 24-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Treatment Protocol
2.2.1. External Beam Radiation Therapy
2.2.2. High-Dose-Rate Brachytherapy
2.2.3. Androgen-Deprivation Therapy
2.3. Follow-Up
2.4. Statistical Analysis
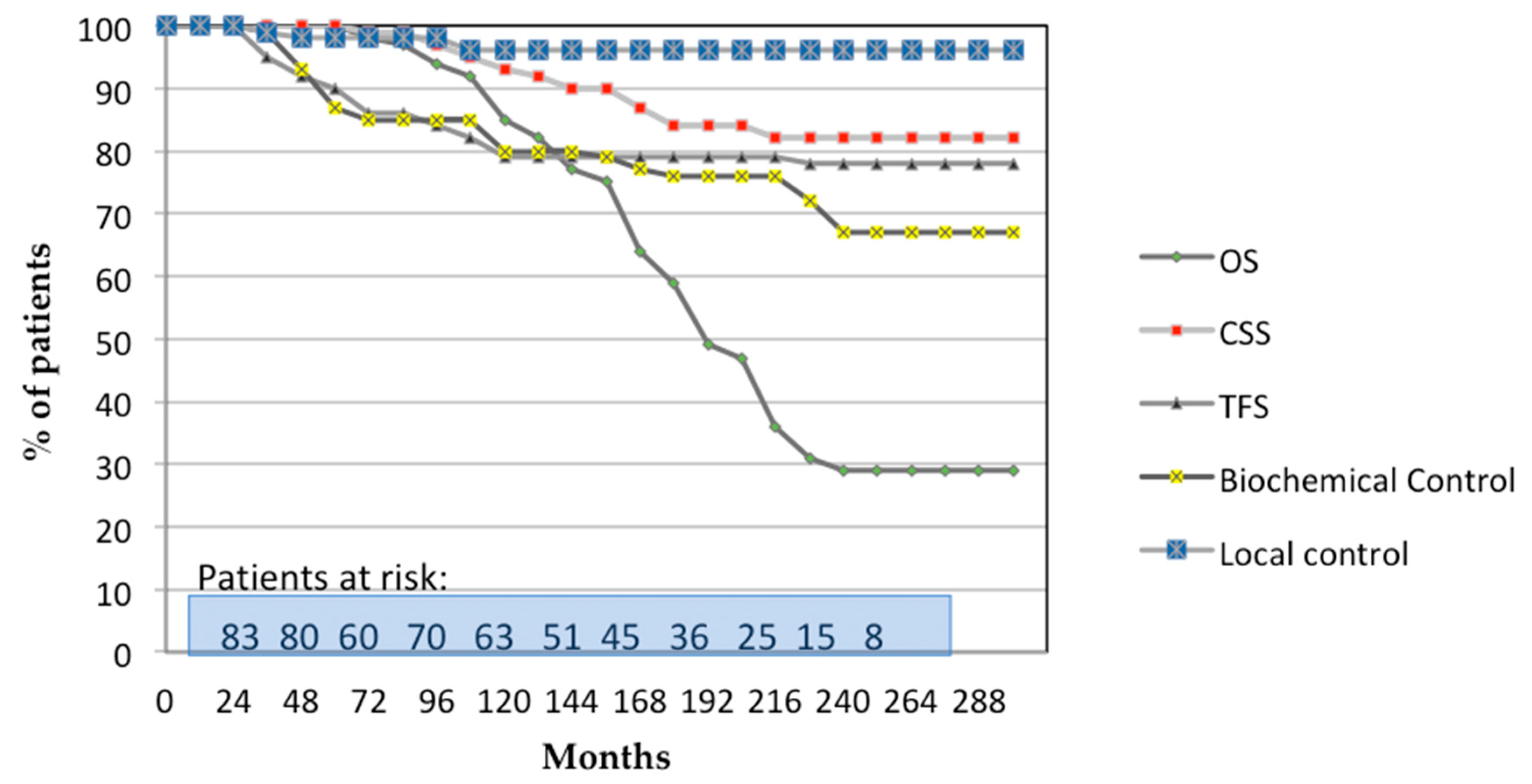
3. Results
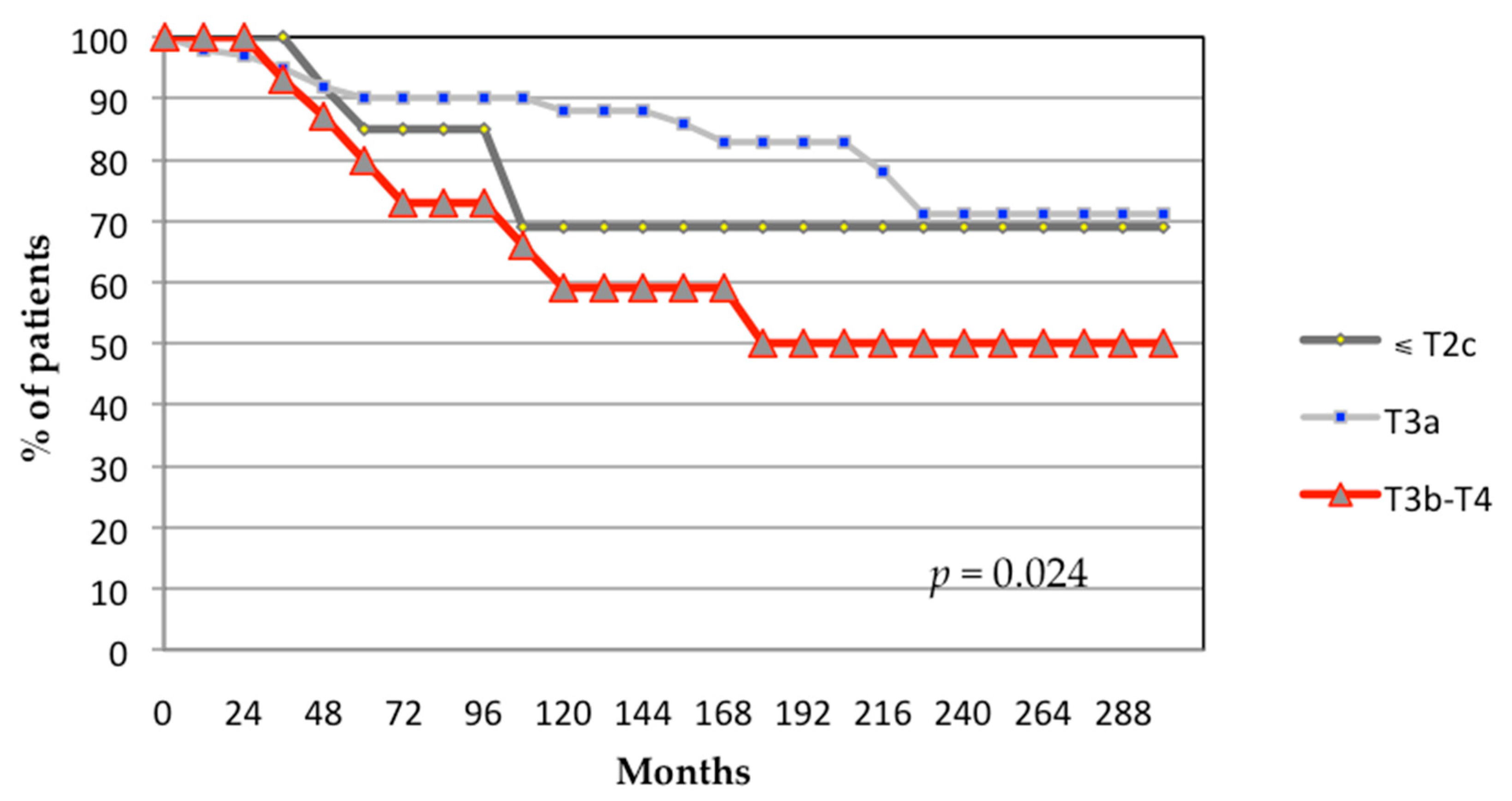
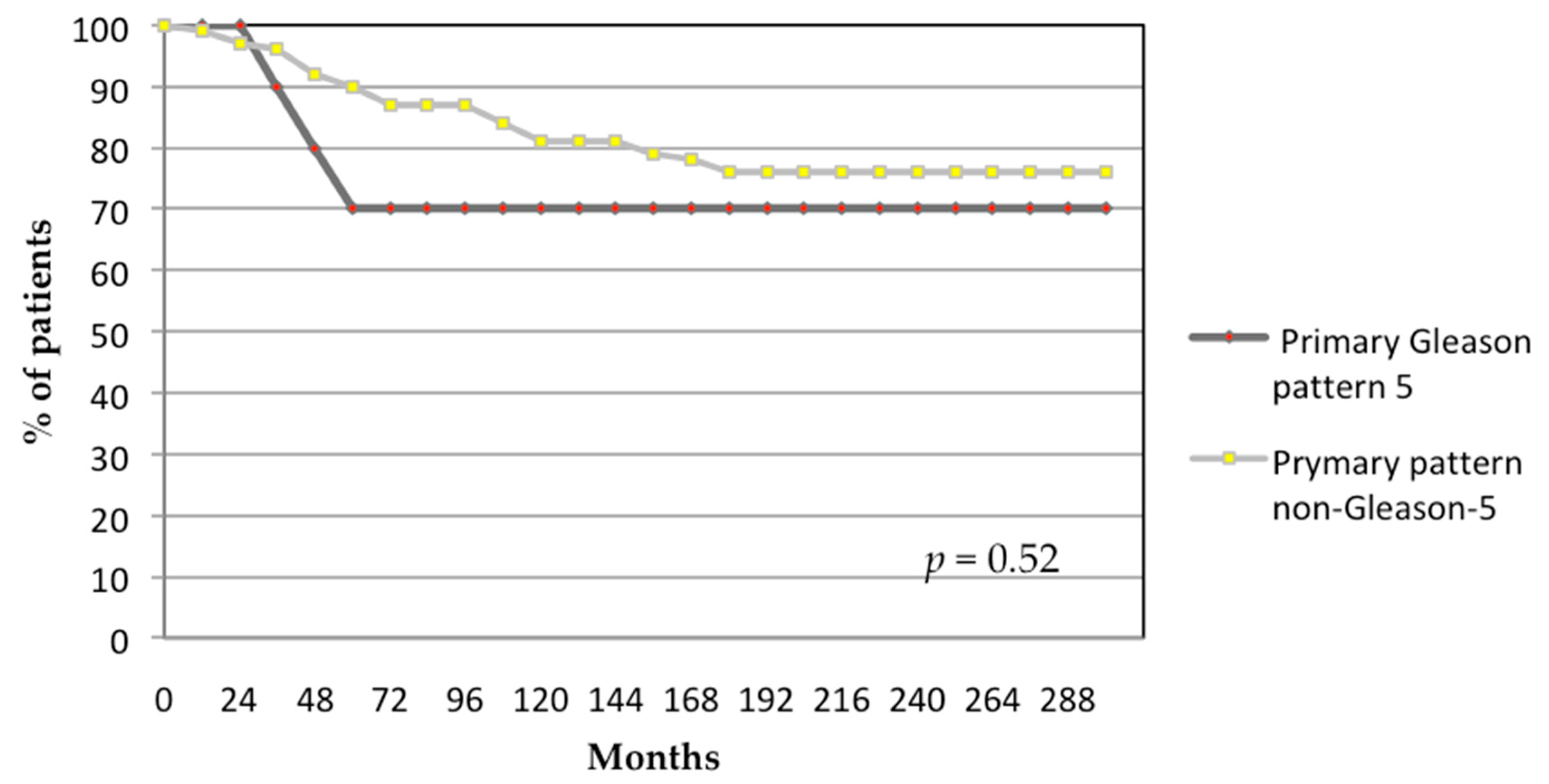
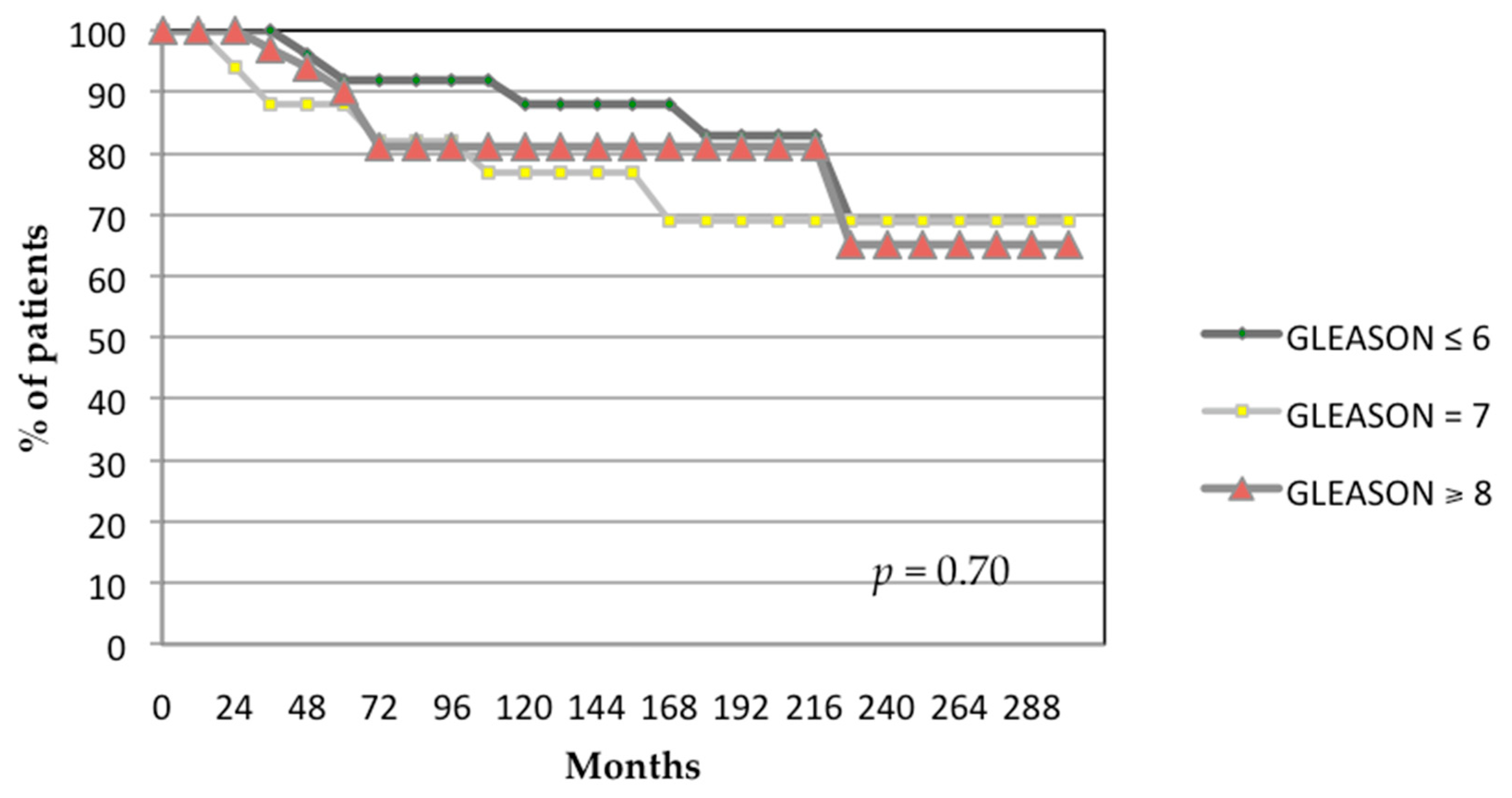
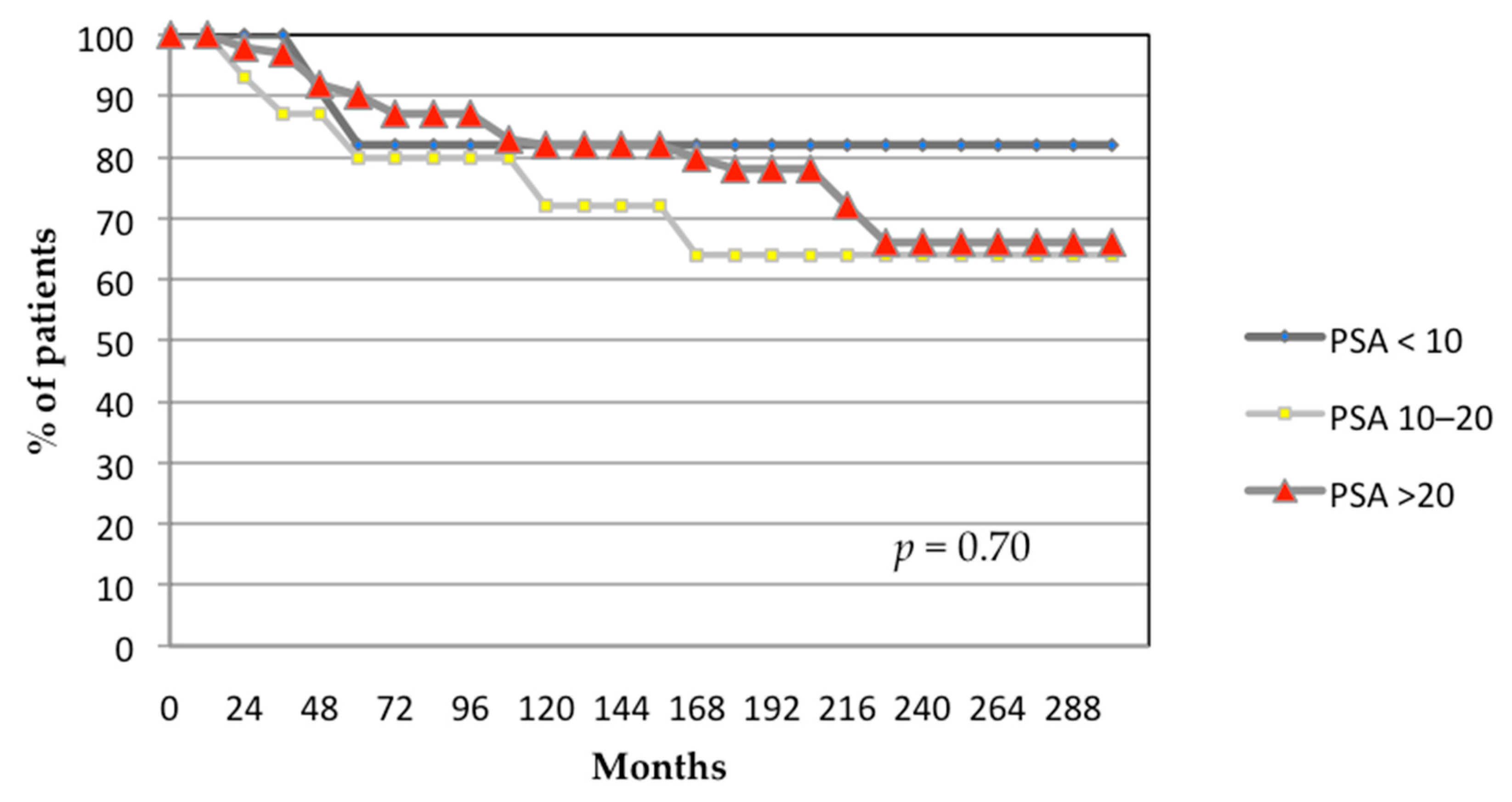
3.1. Oncological Outcomes
3.1.1. Toxicity
Acute Toxicity
Late Toxicity
Sexual Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-EBRT | Three-dimensional external beam radiation therapy |
| ADT | Androgen deprivation therapy |
| AJCC | American Joint Committee on Cancer |
| BED | Biologically effective dose |
| bPFS | Biochemical progression-free survival |
| BT | Brachytherapy |
| CSS | Cancer-specific survival |
| CT | Computed tomography |
| CTCAE | Common terminology criteria for adverse events |
| DRE | Digital rectal examination |
| D90% | The dose that covers 90% of PTV volume. |
| EBRT | External beam radiation therapy |
| ED | Erectile dysfunction |
| GI | Gastrointestinal |
| GU | Genitourinary |
| HDR-BT | High-dose-rate brachytherapy |
| HR | Hazard ratio |
| HR PCa | High-risk prostate cancer |
| IGRT | Image-guided radiation therapy |
| IIEF | International index of erectile function |
| IMRT | Intensity-modulated radiation therapy |
| IPSS | International prostate symptom score |
| LDR-BT | Low-dose-rate brachytherapy |
| MRI | Magnetic resonance imaging |
| MV | Megavoltage |
| NCCN | National Comprehensive Cancer Network |
| OS | Overall survival |
| PCa | Prostate cancer |
| PSA | Prostate-specific antigen |
| PTV | Planning target volume |
| RP | Radical prostatectomy |
| SD | Standard deviation |
| TFS | Tumor-free survival |
| TRUS | Transrectal ultrasound |
| VHR PCa | Very high-risk prostate cancer |
| V40–45 Gy | % or volume (cc) of PTV receiving 40 Gy and 45 Gy, respectively |
| V90–100% | % of PTV volume receiving 90% and 100% of the prescribed dose, respectively |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, A.; Baboudjian, M.; Zapała, P.; Yanagisawa, T.; Miszczyk, M.; Chlosta, M.; Krumpoeck, P.; Moschini, M.; Gandaglia, G.; Ploussard, G.; et al. Survival outcomes of patients treated with local therapy for nonmetastatic prostate cancer with high prostate-specific antigen concentrations. Prostate 2023, 83, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Bologna, E.; Ditonno, F.; Licari, L.C.; Franco, A.; Manfredi, C.; Mossack, S.; Pandolfo, S.D.; De Nunzio, C.; Simone, G.; Leonardo, C.; et al. Tissue-Based Genomic Testing in Prostate Cancer: 10-Year Analysis of National Trends on the Use of Prolaris, Decipher, ProMark, and Oncotype DX. Clin. Pract. 2024, 14, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Sundi, D.; Wang, V.M.; Pierorazio, P.M.; Han, M.; Bivalacqua, T.J.; Ball, M.W.; Antonarakis, E.S.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E. Very-high-risk localized prostate cancer: Definition and outcomes. Prostate Cancer Prostatic Dis. 2014, 17, 57–63. [Google Scholar] [CrossRef]
- Narang, A.K.; Gergis, C.; Robertson, S.P.; He, P.; Ram, A.N.; McNutt, T.R.; Griffith, E.; DeWeese, T.A.; Honig, S.; Singh, H.; et al. Very high-risk localized prostate cancer: Outcomes following definitive radiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 254–262. [Google Scholar] [CrossRef]
- Kadena, S.; Urabe, F.; Iwatani, K.; Suzuki, H.; Imai, Y.; Tashiro, K.; Tsuzuki, S.; Honda, M.; Koike, Y.; Shimomura, T.; et al. The prognostic significance of the clinical T stage and Grade Group in patients with locally advanced prostate cancer treated via high-dose-rate brachytherapy and external beam radiation. Int. J. Clin. Oncol. 2023, 28, 1092–1100. [Google Scholar] [CrossRef]
- Beckendorf, V.; Guerif, S.; Le Prisé, E.; Cosset, J.M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.M.; et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1056–1063. [Google Scholar] [CrossRef]
- Kuban, D.A.; Tucker, S.L.; Dong, L.; Starkschall, G.; Huang, E.H.; Cheung, M.R.; Lee, A.K.; Pollack, A. Long-term results of the MD Anderson randomized dose-escalation trial for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 67–74. [Google Scholar] [CrossRef]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar] [CrossRef]
- Al-Mamgani, A.; van Putten, W.L.; Heemsbergen, W.D.; van Leenders, G.J.; Slot, A.; Dielwart, M.F.; Incrocci, L.; Lebesque, J.V. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 980–988. [Google Scholar] [CrossRef]
- Heemsbergen, W.D.; Al-Mamgani, A.; Slot, A.; Dielwart, M.F.; Lebesque, J.V. Long-term results of the Dutch randomized prostate cancer trial: Impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother. Oncol. 2014, 110, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.T.; Heemsbergen, W.D.; Koper, P.C.; van Putten, W.L.; Slot, A.; Dielwart, M.F.; Bonfrer, J.M.; Incrocci, L.; Lebesque, J.V. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J. Clin. Oncol. 2006, 24, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Yan, Y.; Watkins-Bruner, D.; Bosch, W.R.; Winter, K.; Galvin, J.M.; Bahary, J.P.; Morton, G.C.; Parliament, M.B.; Sandler, H.M. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 932–938. [Google Scholar] [CrossRef]
- Pasalic, D.; Kuban, D.A.; Allen, P.K.; Tang, C.; Mesko, S.M.; Grant, S.R.; Augustyn, A.A.; Frank, S.J.; Choi, S.; Hoffman, K.E.; et al. Dose Escalation for Prostate Adenocarcinoma: A Long-Term Update on the Outcomes of a Phase 3, Single Institution Randomized Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 790–797. [Google Scholar] [CrossRef]
- Kalbasi, A.; Li, J.; Berman, A.; Swisher-McClure, S.; Smaldone, M.; Uzzo, R.G.; Small, D.S.; Mitra, N.; Bekelman, J.E. Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol. 2015, 1, 897–906. [Google Scholar] [CrossRef]
- Hiratsuka, J.; Jo, Y.; Yoshida, K.; Nagase, N.; Fujisawa, M.; Imajo, Y. Clinical results of combined treatment conformal high-dose-rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 684–690. [Google Scholar] [CrossRef]
- Martinez, A.A.; Gonzalez, J.; Ye, H.; Ghilezan, M.; Shetty, S.; Kernen, K.; Gustafson, G.; Krauss, D.; Vicini, F.; Kestin, L. Dose escalation improves cancer–related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihi, O.; Mitra, A.; Payne, H. Challenge of dose escalation in locally advanced unfavourable prostate cancer using HDR brachytherapy. Prostate Cancer Prostatic Dis. 2006, 9, 370–373. [Google Scholar] [CrossRef]
- Fang, F.M.; Wang, Y.M.; Wang, C.J.; Huang, H.Y.; Chiang, P.H. Comparison of the outcome and morbidity for localized or locally advanced prostate cancer treated by high-dose-rate brachytherapy plus external beam radiotherapy (EBRT) versus EBRT alone. Jpn. J. Clin. Oncol. 2008, 38, 474–479. [Google Scholar] [CrossRef]
- Soumarova, R.; Homola, L.; Perkova, H.; Stursa, M. Three-dimensional conformal external beam radiotherapy versus the combination of external radiotherapy with high-dose rate brachytherapy in localized carcinoma of the prostate: Comparison of acute toxicity. Tumori 2007, 93, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wedde, T.B.; Småstuen, M.C.; Brabrand, S.; Fosså, S.D.; Kaasa, S.; Tafjord, G.; Russnes, K.M.; Hellebust, T.P.; Lilleby, W. Ten-year survival after High-Dose-Rate Brachytherapy combined with External Beam Radiation Therapy in high-risk prostate cancer: A comparison with the Norwegian SPCG-7 cohort. Radiother. Oncol. 2019, 132, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef]
- Mendez, L.C.; Morton, G.C. High dose-rate brachytherapy in the treatment of prostate cancer. Transl. Androl. Urol. 2018, 7, 357–370. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. Part III: Principles of Radiation and Future Directions. J. Urol. 2022, 208, 26–33. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J. Natl. Compr. Cancer Netw. 2022, 20, 1288–1298. [Google Scholar]
- Ishiyama, H.; Sato, T.; Kitano, M.; Tabata, K.; Komori, S.; Ikeda, M.; Soda, I.; Kurosaka, S.; Sekiguchi, A.; Kimura, M.; et al. High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term hormonal therapy for high-risk and very high-risk prostate cancer: Outcomes after 5-year follow-up. J. Radiat. Res. 2014, 55, 509–517. [Google Scholar] [CrossRef]
- Wang, C.; Kishan, A.U.; Kamrava, M.; Steinberg, M.L.; King, C.R. External beam radiation therapy with a brachytherapy boost versus radical prostatectomy in Gleason pattern 5 prostate cancer: A population-based cohort study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1045–1052. [Google Scholar] [CrossRef]
- Kasahara, T.; Ishizaki, F.; Kazama, A.; Yuki, E.; Yamana, K.; Maruyama, R.; Oshikane, T.; Kaidu, M.; Aoyama, H.; Bilim, V.; et al. High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term androgen deprivation therapy for very high-risk prostate cancer. Int. J. Urol. 2020, 27, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Suzuki, G.; Masui, K.; Aibe, N.; Shimizu, D.; Kimoto, T.; Yoshida, K.; Nakamura, S.; Okabe, H. Radiotherapy for Clinically Localized T3b or T4 Very-High-Risk Prostate Cancer-Role of Dose Escalation Using High-Dose-Rate Brachytherapy Boost or High Dose Intensity Modulated Radiotherapy. Cancers 2021, 13, 1856. [Google Scholar] [CrossRef]
- Kishan, A.U.; Cook, R.R.; Ciezki, J.P.; Ross, A.E.; Pomerantz, M.M.; Nguyen, P.L.; Shaikh, T.; Tran, P.T.; Sandler, K.A.; Stock, R.G.; et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9-10 Prostate Cancer. JAMA 2018, 319, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Shu, M.; Yang, L.; Di, X.; Liu, P.; Liu, Z.; Zhou, J.; Dong, Q. The Prognosis of Radical Prostatectomy, External Beam Radiotherapy plus Brachytherapy, and External Beam Radiotherapy Alone for Patients above 70 Years with Very High-Risk Prostate Cancer: A Population-Matched Study. Urol. Int. 2022, 106, 11–19. [Google Scholar] [CrossRef]
- Muralidhar, V.; Mahal, B.A.; Butler, S.; Lamba, N.; Yang, D.D.; Leeman, J.; D’Amico, A.V.; Nguyen, P.L.; Trinh, Q.D.; Orio, P.F.; et al. Combined External Beam Radiation Therapy and Brachytherapy versus Radical Prostatectomy with Adjuvant Radiation Therapy for Gleason 9-10 Prostate Cancer. J. Urol. 2019, 202, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Sandler, K.A.; Cook, R.R.; Ciezki, J.P.; Ross, A.E.; Pomerantz, M.M.; Nguyen, P.L.; Shaikh, T.; Tran, P.T.; Stock, R.G.; Merrick, G.S.; et al. Clinical Outcomes for Patients With Gleason Score 10 Prostate Adenocarcinoma: Results From a Multi-institutional Consortium Study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Brenner, D.J.; Martinez, A.A.; Edmundson, G.K.; Mitchell, C.; Thames, H.D.; Armour, E.P. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 6–13. [Google Scholar] [CrossRef]
- Moll, M.; Magrowski, Ł.; Mittlböck, M.; Heinzl, H.; Kirisits, C.; Ciepał, J.; Masri, O.; Heilemann, G.; Stando, R.; Krzysztofiak, T.; et al. Biochemical control in intermediate- and high-risk prostate cancer after EBRT with and without brachytherapy boost. Strahlenther. Onkol. 2025, 201, 11–19. [Google Scholar] [CrossRef]
- Abramowitz, M.C.; Li, T.; Buyyounouski, M.K.; Ross, E.; Uzzo, R.G.; Pollack, A.; Horwitz, E.M. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008, 112, 55–60. [Google Scholar] [CrossRef]
- Pompe, R.S.; Karakiewicz, P.I.; Tian, Z.; Mandel, P.; Steuber, T.; Schlomm, T.; Salomon, G.; Graefen, M.; Huland, H.; Tilki, D. Oncologic and Functional Outcomes after Radical Prostatectomy for High or Very High Risk Prostate Cancer: European Validation of the Current NCCN® Guideline. J. Urol. 2017, 198, 354–361. [Google Scholar] [CrossRef]
- Mano, R.; Eastham, J.; Yossepowitch, O. The very-high-risk prostate cancer: A contemporary update. Prostate Cancer Prostatic Dis. 2016, 19, 340–348. [Google Scholar] [CrossRef]
- Demanes, D.J.; Rodriguez, R.R.; Schour, L.; Brandt, D.; Altieri, G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Oshikane, T.; Kaidu, M.; Abe, E.; Ohta, A.; Saito, H.; Nakano, T.; Honda, M.; Tanabe, S.; Utsunomiya, S.; Sasamoto, R.; et al. A comparative study of high-dose-rate brachytherapy boost combined with external beam radiation therapy versus external beam radiation therapy alone for high-risk prostate cancer. J. Radiat. Res. 2021, 62, 525–532. [Google Scholar] [CrossRef]
- Vargas, C.E.; Martinez, A.A.; Boike, T.P.; Spencer, W.; Goldstein, N.; Gustafson, G.S.; Krauss, D.J.; Gonzalez, J. High-dose irradiation for prostate cancer via a high-dose-rate brachytherapy boost: Results of a phase I to II study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef]
- Dayes, I.S.; Parpia, S.; Gilbert, J.; Julian, J.A.; Davis, I.R.; Levine, M.N.; Sathya, J. Long-Term Results of a Randomized Trial Comparing Iridium Implant Plus External Beam Radiation Therapy With External Beam Radiation Therapy Alone in Node-Negative Locally Advanced Cancer of the Prostate. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, H.; Tsang, Y.; Choudhury, A.; Alonzi, R.; Wylie, J.; Ahmed, I.; Henry, A.; Heath, C.; Hoskin, P.J. External Beam Radiation Therapy (EBRT) and High-Dose-Rate (HDR) Brachytherapy for Intermediate and High-Risk Prostate Cancer: The Impact of EBRT Volume. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 525–533. [Google Scholar] [CrossRef]
- Åström, L.; Grusell, E.; Sandin, F.; Turesson, I.; Holmberg, L. Two decades of high dose rate brachytherapy with external beam radiotherapy for prostate cancer. Radiother. Oncol. 2018, 127, 81–87. [Google Scholar] [CrossRef]
- Chin, J.; Rumble, R.B.; Kollmeier, M.; Heath, E.; Efstathiou, J.; Dorff, T.; Berman, B.; Feifer, A.; Jacques, A.; Loblaw, D.A. Brachytherapy for Patients With Prostate Cancer: American Society of Clinical Oncology/Cancer Care Ontario Joint Guideline Update. J. Clin. Oncol. 2017, 35, 1737–1743. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar]
- Oh, J.; Tyldesley, S.; Pai, H.; McKenzie, M.; Halperin, R.; Duncan, G.; Morton, G.; Keyes, M.; Hamm, J.; Morris, W.J. An Updated Analysis of the Survival Endpoints of ASCENDE-RT. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 1061–1070. [Google Scholar] [CrossRef]
- GC, M.; SM, A. High Dose Rate Brachytherapy in High-Risk Localised Disease—Why Do Anything Else? Clin. Oncol. 2020, 32, 163–169. [Google Scholar]
- Vigneault, E.; Mbodji, K.; Carignan, D.; Martin, A.G.; Miksys, N.; Thomson, R.M.; Aubin, S.; Varfalvy, N.; Beaulieu, L. The association of intraprostatic calcifications and dosimetry parameters with biochemical control after permanent prostate implant. Brachytherapy 2019, 18, 787–792. [Google Scholar] [CrossRef]
- Henry, A.; Pieters, B.R.; André Siebert, F.; Hoskin, P. GEC-ESTRO ACROP prostate brachytherapy guidelines. Radiother. Oncol. 2022, 167, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.A.; Yan, Y.; Lee, W.R.; Gillin, M.; Firat, S.; Baikadi, M.; Crook, J.; Kuettel, M.; Morton, G.; Sandler, H. Long-term results of an RTOG Phase II trial (00-19) of external-beam radiation therapy combined with permanent source brachytherapy for intermediate-risk clinically localized adenocarcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e795–e801. [Google Scholar] [CrossRef]
- Hsu, I.C.; Rodgers, J.P.; Shinohara, K.; Purdy, J.; Michalski, J.; Roach, M.; Vigneault, E.; Ivker, R.A.; Pryzant, R.M.; Kuettel, M.; et al. Long-Term Results of NRG Oncology/RTOG 0321: A Phase II Trial of Combined High Dose Rate Brachytherapy and External Beam Radiation Therapy for Adenocarcinoma of the Prostate. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 700–707. [Google Scholar] [CrossRef]
- Shahid, N.; Loblaw, A.; Chung, H.T.; Cheung, P.; Szumacher, E.; Danjoux, C.; Sankreacha, R.; Zhang, L.; Deabreu, A.; Mamedov, A.; et al. Long-term Toxicity and Health-related Quality of Life after Single-fraction High Dose Rate Brachytherapy Boost and Hypofractionated External Beam Radiotherapy for Intermediate-risk Prostate Cancer. Clin. Oncol. 2017, 29, 412–420. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Davis, B.J.; Nguyen, P.L.; Showalter, T.N.; Hoskin, P.J.; Yoshioka, Y.; Morton, G.C.; Horwitz, E.M. The evolution of brachytherapy for prostate cancer. Nat. Rev. Urol. 2017, 14, 415–439. [Google Scholar] [CrossRef]
- Wallis, C.J.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: Systematic review and meta-analysis. BMJ 2016, 352, i851. [Google Scholar] [CrossRef]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.P.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012, 109 (Suppl. 1), 22–29. [Google Scholar] [CrossRef]
- Moris, L.; Cumberbatch, M.G.; Van den Broeck, T.; Gandaglia, G.; Fossati, N.; Kelly, B.; Pal, R.; Briers, E.; Cornford, P.; De Santis, M.; et al. Benefits and Risks of Primary Treatments for High-risk Localized and Locally Advanced Prostate Cancer: An International Multidisciplinary Systematic Review. Eur. Urol. 2020, 77, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Cole, A.P.; Krimphove, M.J.; Nabi, J.; Marchese, M.; Lipsitz, S.R.; Noldus, J.; Choueiri, T.K.; Kibel, A.S.; Trinh, Q.D. Comparative Effectiveness of Radical Prostatectomy Versus External Beam Radiation Therapy Plus Brachytherapy in Patients with High-risk Localized Prostate Cancer. Eur. Urol. 2019, 75, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Hoeh, B.; Würnschimmel, C.; Flammia, R.S.; Horlemann, B.; Sorce, G.; Chierigo, F.; Tian, Z.; Saad, F.; Graefen, M.; Gallucci, M.; et al. Cancer-specific survival after radical prostatectomy versus external beam radiotherapy in high-risk and very high-risk African American prostate cancer patients. Prostate 2022, 82, 120–131. [Google Scholar] [CrossRef]
- Chierigo, F.; Wenzel, M.; Würnschimmel, C.; Flammia, R.S.; Horlemann, B.; Tian, Z.; Saad, F.; Chun, F.K.H.; Graefen, M.; Gallucci, M.; et al. Survival after Radical Prostatectomy versus Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer. J. Urol. 2022, 207, 375–384. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Lardas, M.; Liew, M.; van den Bergh, R.C.; De Santis, M.; Bellmunt, J.; Van den Broeck, T.; Cornford, P.; Cumberbatch, M.G.; Fossati, N.; Gross, T.; et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.E.; Metcalfe, C.; Donovan, J.L.; Lane, J.A.; Davis, M.; Young, G.J.; Dutton, S.J.; Walsh, E.I.; Martin, R.M.; Peters, T.J.; et al. Ten-year Mortality, Disease Progression, and Treatment-related Side Effects in Men with Localised Prostate Cancer from the ProtecT Randomised Controlled Trial According to Treatment Received. Eur. Urol. 2020, 77, 320–330. [Google Scholar] [CrossRef]
- Hoffman, K.E.; Penson, D.F.; Zhao, Z.; Huang, L.C.; Conwill, R.; Laviana, A.A.; Joyce, D.D.; Luckenbaugh, A.N.; Goodman, M.; Hamilton, A.S.; et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA 2020, 323, 149–163. [Google Scholar] [CrossRef]
- Dolezel, M.; Odrazka, K.; Zouhar, M.; Vaculikova, M.; Sefrova, J.; Jansa, J.; Paluska, P.; Kohlova, T.; Vanasek, J.; Kovarik, J. Comparing morbidity and cancer control after 3D-conformal (70/74 Gy) and intensity modulated radiotherapy (78/82 Gy) for prostate cancer. Strahlenther. Onkol. 2015, 191, 338–346. [Google Scholar] [CrossRef]
- Guo, W.; Sun, Y.C.; Zhang, L.Y.; Yin, X.M. Gastrointestinal/genitourinary adverse event after intensity modulated versus three-dimensional primary radiation therapy in the treatment of prostate cancer: Systematic review and meta-analysis. J. Cancer. 2023, 14, 2878–2888. [Google Scholar] [CrossRef]
| Characteristics | Nº Patients (%) |
|---|---|
| Stage | |
| ≤T2c | 13 (14.9%) |
| T3a | 59 (67.8%) |
| T3b-T4 | 15 (17.2%) |
| Gleason score: | |
| ≤6 | 25 (28.7%) |
| 7 | 17 (19.5%) |
| ≥8 | 45 (52%) |
| Pretreatment PSA level (ng/mL) | |
| <10 | 10 (11.5%) |
| 10–20 | 16 (18.4%) |
| >20 | 61 (70.1%) |
| Mean pretreatment PSA level (ng/mL): 24.7/Median: 24.4 (3.4–59.6) | |
| Adjuvant hormonal ablation | |
| Yes | 72 (82.8%) |
| No | 15 (17.2%) |
| Age at diagnosis (years) | |
| ≤60 | 11 (12.6%) |
| 61–70 | 45 (51.7%) |
| >70 | 31 (35.6%) |
| Prognostic factors | |
| One very high-risk criteria | 25 (28.7%) |
| >One very high-risk criteria | 4 (4.6%) |
| ≥Two high-risk criteria | 64 (73.6%) |
| Gland Volume (cc): Mean: 31/Median: 28 (9–69) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prada Gómez, P.J.; Rivero Pérez, A.L.; Carballido Rodríguez, J.; Anchuelo Latorre, J.; Fabregat Borrás, R.; Gutiérrez Ruiz, M.; Rodríguez-Acosta Caballero, C.; Carrascal Gordillo, C.F.; Galdós Barroso, M.P.; Navarrete Solano, P.A. Long-Term Outcomes After High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy in Very High-Risk Prostate Cancer: A 24-Year Follow-Up. Biomedicines 2025, 13, 1310. https://doi.org/10.3390/biomedicines13061310
Prada Gómez PJ, Rivero Pérez AL, Carballido Rodríguez J, Anchuelo Latorre J, Fabregat Borrás R, Gutiérrez Ruiz M, Rodríguez-Acosta Caballero C, Carrascal Gordillo CF, Galdós Barroso MP, Navarrete Solano PA. Long-Term Outcomes After High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy in Very High-Risk Prostate Cancer: A 24-Year Follow-Up. Biomedicines. 2025; 13(6):1310. https://doi.org/10.3390/biomedicines13061310
Chicago/Turabian StylePrada Gómez, Pedro J., Ana L. Rivero Pérez, Joaquín Carballido Rodríguez, Javier Anchuelo Latorre, Rosa Fabregat Borrás, Marina Gutiérrez Ruiz, Cristina Rodríguez-Acosta Caballero, Carlos F. Carrascal Gordillo, Maria P. Galdós Barroso, and Paola A. Navarrete Solano. 2025. "Long-Term Outcomes After High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy in Very High-Risk Prostate Cancer: A 24-Year Follow-Up" Biomedicines 13, no. 6: 1310. https://doi.org/10.3390/biomedicines13061310
APA StylePrada Gómez, P. J., Rivero Pérez, A. L., Carballido Rodríguez, J., Anchuelo Latorre, J., Fabregat Borrás, R., Gutiérrez Ruiz, M., Rodríguez-Acosta Caballero, C., Carrascal Gordillo, C. F., Galdós Barroso, M. P., & Navarrete Solano, P. A. (2025). Long-Term Outcomes After High-Dose-Rate Brachytherapy and Hypofractionated External Beam Radiotherapy in Very High-Risk Prostate Cancer: A 24-Year Follow-Up. Biomedicines, 13(6), 1310. https://doi.org/10.3390/biomedicines13061310







