Mechanism and Predictive Role of NUB1 Protein in Oestrogen Receptor Pathway of FEC-Treated Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Tissue Specimen Collection
2.2. Cell Lines and FEC Treatment
2.3. Cell Cycle Analysis Using Flow Cytometry
2.4. Western Blot
2.5. Micro-Sectioning of Paraffin-Embedded Tissue Sections
2.6. Immunostaining of Paraffin-Embedded Tissue Sections
2.7. Statistical Analysis
3. Results
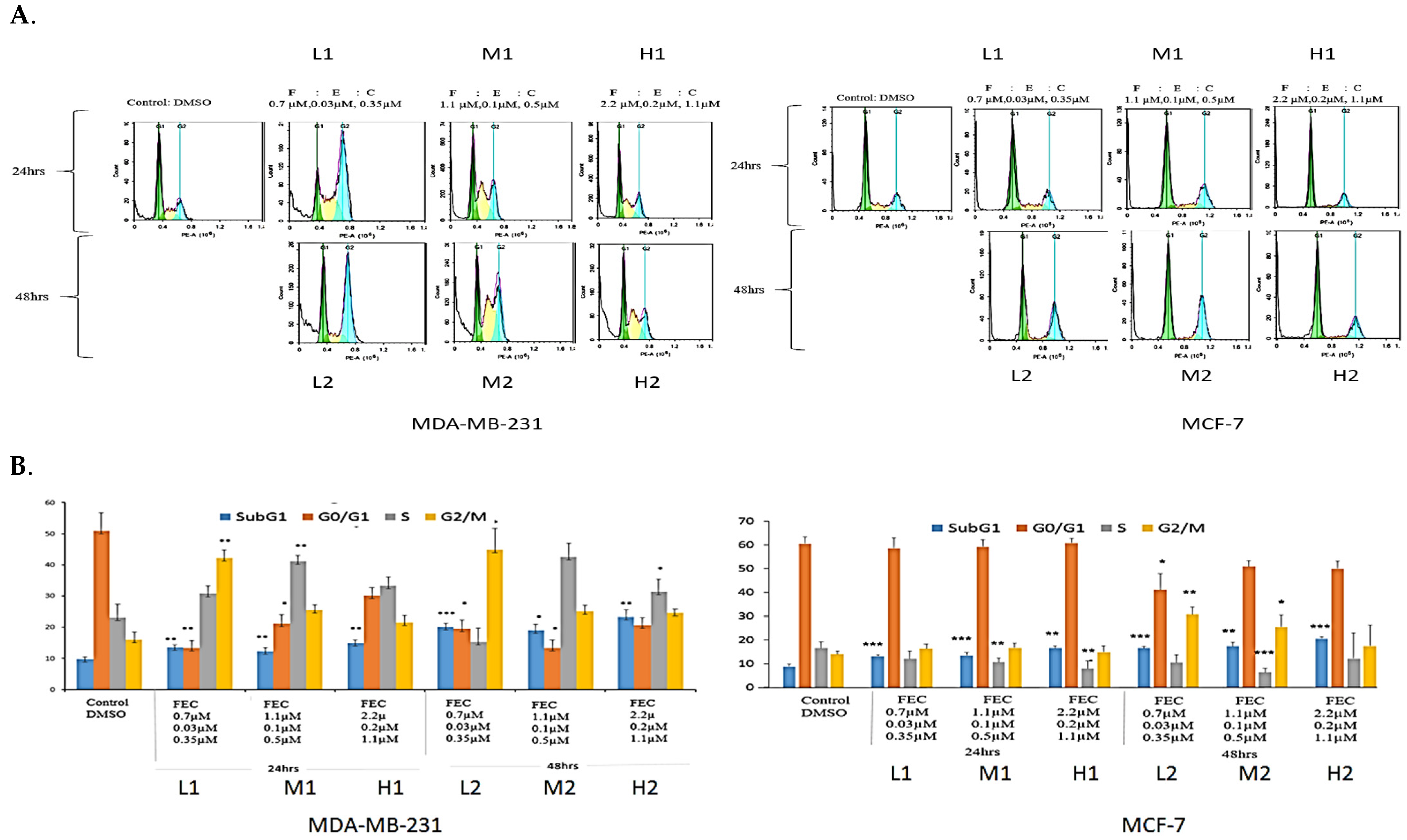
3.1. Anti-Proliferative Effects of FEC Treatment on BC Cells Through the Expression of Cell Cycle Regulatory Proteins
3.2. FEC Upregulated NUB1 and Downregulated ERα Expression
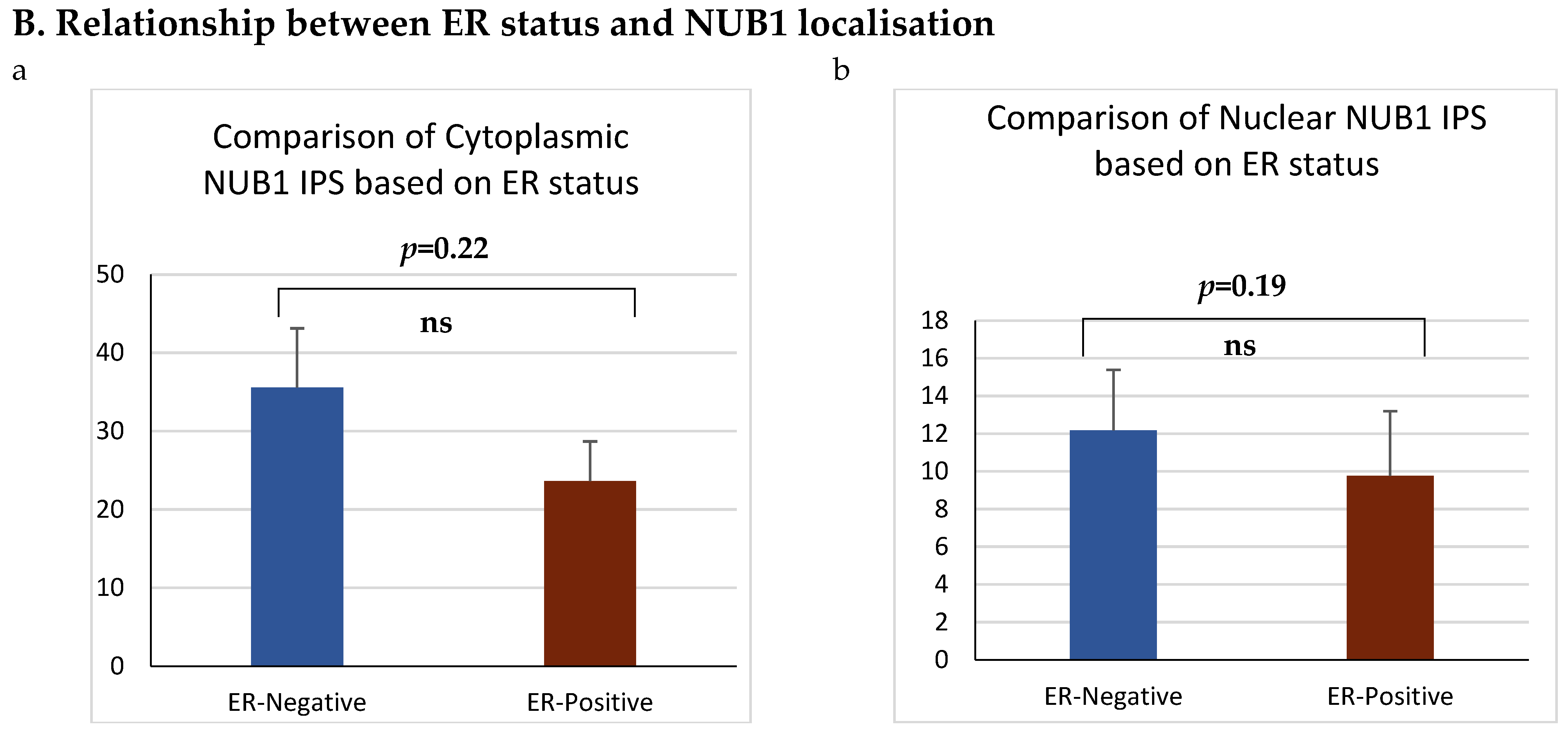
3.3. NUB1 Expression Patterns and Subcellular Localisation in BC
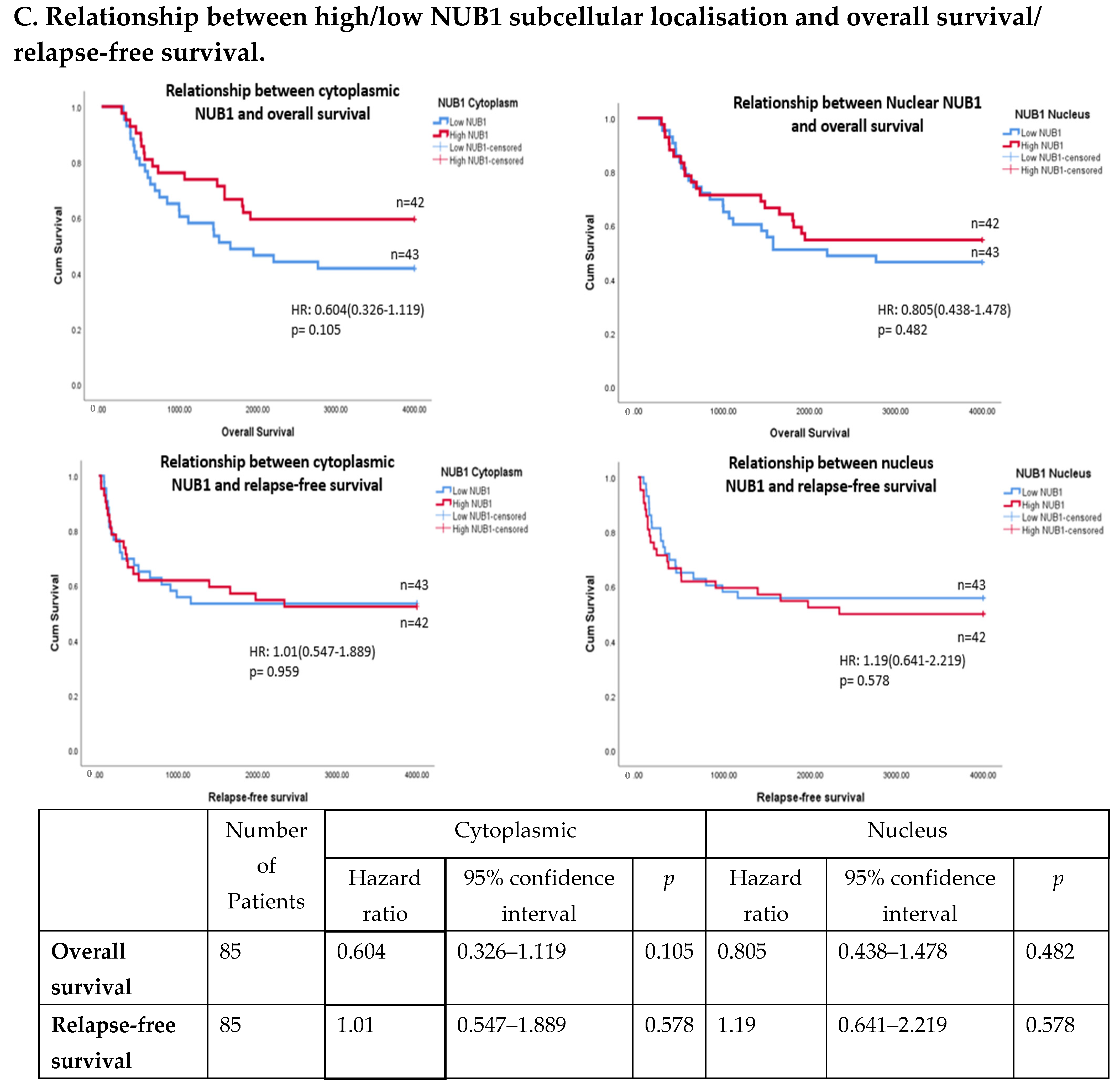
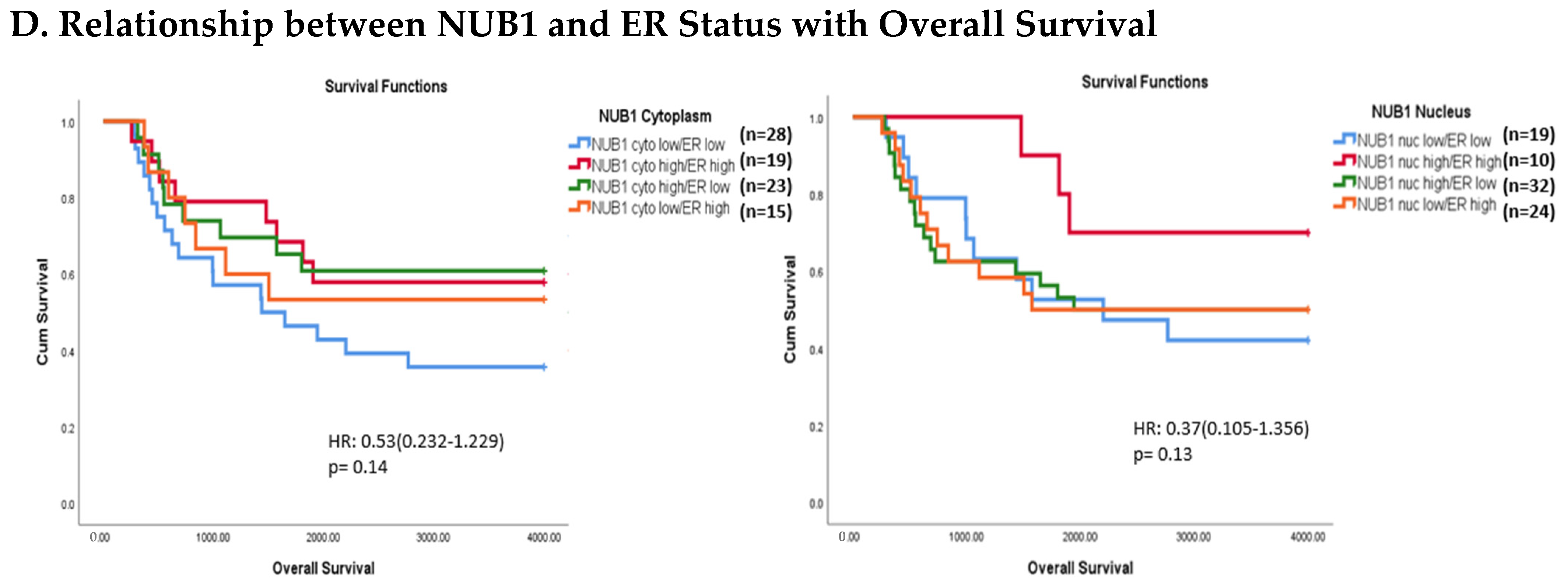
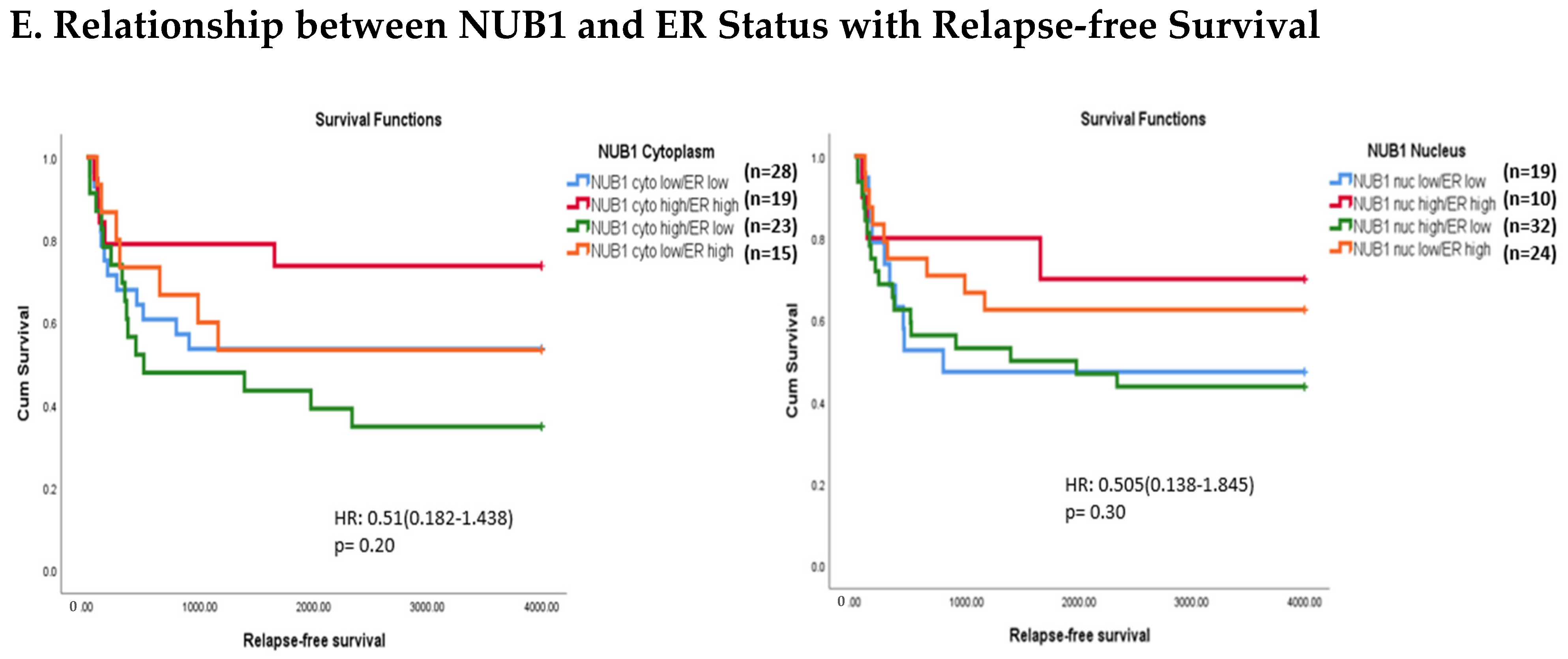
3.4. Multivariate Analysis: NUB1 Expression Association with Survival in BC
4. Discussion
5. Conclusions
6. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BC | Breast Cancer |
| CDK | Cyclin-Dependent Kinase |
| CI | Confidence Interval |
| DAB | Diaminobenzidine |
| DMSO | Dimethyl Sulfoxide |
| ECL | Enhanced Chemiluminescence |
| Erα | Oestrogen Receptor alpha |
| FACS | Fluorescence-Activated Cell Sorting |
| FBS | Fetal Bovine Serum |
| FEC | 5-Fluorouracil, Epirubicin, Cyclophosphamide (chemotherapy regimen) |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| FRGS | Fundamental Research Grant Scheme |
| GLM | General Linear Model |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HR | Hazard Ratio |
| HRP | Horseradish Peroxidase |
| IHC | Immunohistochemistry |
| IPS | Intensity Percentage Score |
| NEDD8 | Neural precursor cell-expressed developmentally downregulated protein |
| NUB1 | NEDD8 Ultimate Buster 1 |
| OS | Overall Survival |
| PBS | Phosphate-Buffered Saline |
| PCNA | Proliferating Cell Nuclear Antigen |
| PI | Propidium Iodide |
| PR | Progesterone Receptor |
| PVDF | Polyvinylidene Difluoride |
| RFS | Relapse-Free Survival |
| RIPA | Radioimmunoprecipitation Assay Buffer |
| RPMI | Roswell Park Memorial Institute medium |
| SCF | Skp, Cullin, F-box containing complex (ubiquitin ligase) |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| TME | Tumour Microenvironment |
References
- Afifi, A.M.; Saad, A.M.; Al-Husseini, M.J.; Elmehrath, A.O.; Northfelt, D.W.; Sonbol, M.B. Causes of death after breast cancer diagnosis: A US population-based analysis. Cancer 2020, 126, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.; Pathy, N.B.; Teo, S. A review of breast cancer research in Malaysia. Med. J. Malays. 2014, 69, 8–22. [Google Scholar]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Tan, K.F.; Adam, F.; Hami, R.; Shariff, N.M.; Mujar, N.M.M. Review of breast cancer in young women. Malays. J. Med. Health Sci. 2020, 16, 370–378. [Google Scholar]
- Freelander, A.; Brown, L.J.; Parker, A.; Segara, D.; Portman, N.; Lau, B.; Lim, E. Molecular biomarkers for contemporary therapies in hormone receptor-positive breast cancer. Genes 2021, 12, 285. [Google Scholar] [CrossRef]
- Gamble, P.; Jaroensri, R.; Wang, H.; Tan, F.; Moran, M.; Brown, T.; Flament-Auvigne, I.; Rakha, E.A.; Toss, M.; Dabbs, D.J. Determining breast cancer biomarker status and associated morphological features using deep learning. Commun. Med. 2021, 1, 14. [Google Scholar] [CrossRef]
- Duffy, M.J.; Walsh, S.; McDermott, E.W.; Crown, J. Biomarkers in breast cancer: Where are we and where are we going? Adv. Clin. Chem. 2015, 71, 1–23. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, W.; Deng, S.; Dou, Q.; Wang, X.; An, Q.; Wang, X.; Ji, H.; Hei, Y.; Chen, Y. Redefining the incidence and profile of fluoropyrimidine-associated cardiotoxicity in cancer patients: A systematic review and meta-analysis. Pharmaceuticals 2023, 16, 510. [Google Scholar] [CrossRef]
- Florescu, M.; Cinteza, M.; Vinereanu, D. Chemotherapy-induced cardiotoxicity. Maedica 2013, 8, 59. [Google Scholar]
- Yang, F.; Kemp, C.J.; Henikoff, S. Anthracyclines induce double-strand DNA breaks at active gene promoters. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2015, 773, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.; Wiklund, T.; Erikstein, B.; Lidbrink, E.; Lindman, H.; Malmström, P.; Kellokumpu-Lehtinen, P.; Bengtsson, N.-O.; Söderlund, G.; Anker, G. Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: A randomised trial. Lancet 2000, 356, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Roché, H.; Fumoleau, P.; Spielmann, M.; Canon, J.-L.; Delozier, T.; Serin, D.; Symann, M.; Kerbrat, P.; Soulié, P.; Eichler, F. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J. Clin. Oncol. 2006, 24, 5664–5671. [Google Scholar] [CrossRef]
- Hesketh, P.J. Chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 2008, 358, 2482–2494. [Google Scholar] [CrossRef]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-term cognitive dysfunction in cancer survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Zimmermann, M.; Arachchige-Don, A.S.; Donaldson, M.S.; Dallapiazza, R.F.; Cowan, C.E.; Horne, M.C. Elevated cyclin G2 expression intersects with DNA damage checkpoint signaling and is required for a potent G2/M checkpoint arrest response to doxorubicin. J. Biol. Chem. 2012, 287, 22838–22853. [Google Scholar] [CrossRef]
- Hengstler, J.; Hengst, A.; Fuchs, J.; Tanner, B.; Pohl, J.; Oesch, F. Induction of DNA crosslinks and DNA strand lesions by cyclophosphamide after activation by cytochrome P450 2B1. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1997, 373, 215–223. [Google Scholar] [CrossRef]
- Bonacci, T.; Audebert, S.; Camoin, L.; Baudelet, E.; Bidaut, G.; Garcia, M.; Witzel, I.-I.; Perkins, N.D.; Borg, J.-P.; Iovanna, J.-L. Identification of new mechanisms of cellular response to chemotherapy by tracking changes in post-translational modifications by ubiquitin and ubiquitin-like proteins. J. Proteome Res. 2014, 13, 2478–2494. [Google Scholar] [CrossRef]
- Kamitani, T.; Kito, K.; Fukuda-Kamitani, T.; Yeh, E.T. Targeting of NEDD8 and its conjugates for proteasomal degradation by NUB1. J. Biol. Chem. 2001, 276, 46655–46660. [Google Scholar] [CrossRef]
- Soucy, T.A.; Dick, L.R.; Smith, P.G.; Milhollen, M.A.; Brownell, J.E. The NEDD8 conjugation pathway and its relevance in cancer biology and therapy. Genes Cancer 2010, 1, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Yeh, E.T.; Kamitani, T. NUB1, a NEDD8-interacting protein, is induced by interferon and down-regulates the NEDD8 expression. J. Biol. Chem. 2001, 276, 20603–20609. [Google Scholar] [CrossRef] [PubMed]
- Hosono, T.; Tanaka, T.; Tanji, K.; Nakatani, T.; Kamitani, T. NUB1, an interferon-inducible protein, mediates anti-proliferative actions and apoptosis in renal cell carcinoma cells through cell-cycle regulation. Br. J. Cancer 2010, 102, 873–882. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, P.; Zhang, Z.; An, W.; Zhang, C.; Pan, S.; Tan, Y.; Xu, H. Overexpression of negative regulator of ubiquitin-like proteins 1 (NUB1) inhibits proliferation and invasion of gastric cancer cells through upregulation of p27Kip1 and inhibition of epithelial-mesenchymal transition. Pathol.-Res. Pract. 2020, 216, 153002. [Google Scholar] [CrossRef]
- Tan, K.-L.; Haider, S.; Zois, C.E.; Hu, J.; Turley, H.; Leek, R.; Buffa, F.; Acuto, O.; Harris, A.L.; Pezzella, F. Low cytoplasmic NUB1 protein exerts hypoxic cell death with poorer prognosis in oestrogen receptor negative breast cancer patients. Transl. Oncol. 2024, 49, 102106. [Google Scholar] [CrossRef]
- Ruengwanichayakun, P. Histochemical scoring assessment (H-score). Asian Arch. Pathol. 2021, 3, 13–14. [Google Scholar]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 125–131. [Google Scholar] [CrossRef]
- Al-Bader, M.; Ford, C.; Al-Ayadhy, B.; Francis, I. Analysis of estrogen receptor isoforms and variants in breast cancer cell lines. Exp. Ther. Med. 2011, 2, 537–544. [Google Scholar] [CrossRef]
- Sinclair, W.D.; Cui, X. The effects of HER2 on CDK4/6 activity in breast cancer. Clin. Breast Cancer 2022, 22, e278–e285. [Google Scholar] [CrossRef]
- Peurala, E.; Koivunen, P.; Haapasaari, K.-M.; Bloigu, R.; Jukkola-Vuorinen, A. The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res. 2013, 15, R5. [Google Scholar] [CrossRef]
- Khanna, O.; Kazerooni, A.F.; Arif, S.; Mahtabfar, A.; Momin, A.A.; Andrews, C.E.; Hafazalla, K.; Baldassari, M.P.; Velagapudi, L.; Garcia, J.A. Radiomic signatures of meningiomas using the Ki-67 proliferation index as a prognostic marker of clinical outcomes. Neurosurg. Focus 2023, 54, E17. [Google Scholar] [CrossRef] [PubMed]
- Al-Qasem, A.J.; Alves, C.L.; Ehmsen, S.; Tuttolomondo, M.; Terp, M.G.; Johansen, L.E.; Vever, H.; Hoeg, L.V.; Elias, D.; Bak, M. Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer. Npj Precis. Oncol. 2022, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Petrossian, K.; Kanaya, N.; Lo, C.; Hsu, P.-Y.; Nguyen, D.; Yang, L.; Yang, L.; Warden, C.; Wu, X.; Pillai, R. ERα-mediated cell cycle progression is an important requisite for CDK4/6 inhibitor response in HR+ breast cancer. Oncotarget 2018, 9, 27736–27751. [Google Scholar] [CrossRef]
- Lamb, R.; Lehn, S.; Rogerson, L.; Clarke, R.B.; Landberg, G. Cell cycle regulators cyclin D1 and CDK4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle 2013, 12, 2384–2394. [Google Scholar] [CrossRef]
- Lee, T.-H.; Chang, H.-C.; Chuang, L.-Y.; Hung, W.-C. Involvement of PKA and Sp1 in the induction of p27Kip1 by tamoxifen. Biochem. Pharmacol. 2003, 66, 371–377. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, J.; Shen, J.; Jin, M.; Xie, S.; Wang, L. The role of estrogen receptor alpha in mediating chemoresistance in breast cancer cells. J. Exp. Clin. Cancer Res. 2012, 31, 42. [Google Scholar] [CrossRef]
- Rogatsky, I.; Trowbridge, J.M.; Garabedian, M.J. Potentiation of human estrogen receptor α transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J. Biol. Chem. 1999, 274, 22296–22302. [Google Scholar] [CrossRef]
- Du, D.; Zhang, W.; Zhang, D.; Liu, L.; Li, J.; Chen, Z.; Yu, X.; Ye, M.; Wang, W.; Li, Z. NUB1 reduction promotes PCNA-mediated tumor growth by disturbing the PCNA polyubiquitination/NEDDylation in hepatocellular carcinoma cells. Cell Death Dis. 2025, 16, 228. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Xin, Q.; Zhang, Y.; Wang, K.; Li, M. Recent progress of CDK4/6 inhibitors’ current practice in breast cancer. Cancer Gene Ther. 2024, 31, 1283–1291. [Google Scholar] [CrossRef]


| Clinicopathological Factors | ER-Negative (n = 51) | ER-Positive (n = 34) | Overall (n = 85) | p-Value |
|---|---|---|---|---|
| HER 2 Receptor Status | ||||
| Negative | 16 (55.17%) | 13 (44.82%) | 29 | 0.354 |
| Positive | 22 (66.66%) | 11 (33.33%) | 33 | |
| Node Status | ||||
| Negative | 6 (75%) | 2 (25%) | 8 | 0.348 |
| Positive | 44 (57.89%) | 32 (42.10%) | 76 | |
| Histologic Grade | ||||
| Grade I | 4 (80%) | 1 (20%) | 5 | 0.814 |
| Grade II | 15 (65.21%) | 8 (34.78%) | 23 | |
| Grade III | 23 (67.64%) | 11(32.34%) | 34 | |
| Age | ||||
| <50 years | 22 (57.89%) | 16 (42.11%) | 38 | 0.824 |
| ≥50 years | 29 (61.7%) | 18 (38.29%) | 47 | |
| Median(Range) | 52 (32–73) | 51(27–70) | 52 (27–73) | |
| Tumour Size | ||||
| ≤2 cm | 12 (70.58%) | 5 (29.14%) | 17 | |
| ˃2 cm | 38 (56.71%) | 29 (43.28%) | 67 | |
| Median(Range) | 4 (0.5–11.3) | 5 (1–16) | 4 (0.5–16) | 0.298 |
| Variable | Events 85 | Cytoplasmic | Nuclear | ||||
|---|---|---|---|---|---|---|---|
| Mean IPS | 95% Confidence Interval | p | Mean IPS | 95% Confidence Interval | p | ||
| Age, year | |||||||
| ≥50 | 47 | 28.9 | 21.470–52.896 | 0.780 | 9.30 | −2.592–15.505 | 0.496 |
| <50 | 38 | 31.8 | 6.815–42.827 | 13.4 | −7.648–15.505 | ||
| Size, cm | |||||||
| ≤2 | 17 | 41.9 | 20.469–72.656 | 0.144 | 17.7 | −3.338–30.213 | 0.096 |
| >2 | 67 | 24.7 | 13.025–39.834 | 7.3 | −5.157–12.078 | ||
| Lymph nodes | |||||||
| Positive | 76 | 29.8 | 13.979–40.351 | 0.652 | 12.1 | −1.994–14.960 | 0.383 |
| Negative | 8 | 37.8 | 21.361–79.055 | 3.1 | −14.379–22.713 | ||
| Grade | |||||||
| 1 | 5 | 88.0 | −43.802–103.802 | 40.0 | −44.948–49.948 | ||
| 2 | 23 | 21.4 | 8.866–46.865 | 0.259 | 0.97 | −10.484–13.945 | 0.214 |
| 3 | 34 | 32.8 | 18.793–50.398 | 8.30 | −0.625–19.694 | ||
| Histology | |||||||
| Ductal | 75 | 31.9 | 18.417–43.395 | 0.280 | 11 | −1.348–14.711 | 0.896 |
| Lobular | 5 | 28.5 | −7.186–97.186 | 0 | −33.551–33.551 | ||
| Other | 5 | 7.0 | −48.802–98.802 | 0 | −47.448–47.448 | ||
| ER | |||||||
| Negative | 51 | 35.0 | 24.520–55.966 | 0.248 | 12.4 | −0.917–14.105 | 0.595 |
| Positive | 34 | 23.0 | 2.647–39.674 | 9.1 | −9.700–14.105 | ||
| HER2 | |||||||
| Negative | 29 | 31.6 | 13.026–47.130 | 0.571 | 8.8 | −5.025–16.900 | 0.927 |
| Positive | 33 | 25.6 | 16.386–50.164 | 9.3 | −4.719–16.997 | ||
| Menopausal status | |||||||
| Pre | 29 | 34.9 | 9.578–50.573 | 0.513 | 14.0 | −8.405–17.951 | 0.487 |
| Post | 56 | 27.8 | 17.663–47.291 | 9.6 | −2.795–16.253 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, M.; Abdullah, A.R.; Ismail, F.; Pezzella, F.; Yahaya, A.; Tan, G.-C.; Chia, S.L.; Md Salleh, M.S.; Abdullah, N.; Tan, K.-L. Mechanism and Predictive Role of NUB1 Protein in Oestrogen Receptor Pathway of FEC-Treated Breast Cancer Patients. Biomedicines 2025, 13, 1307. https://doi.org/10.3390/biomedicines13061307
Arshad M, Abdullah AR, Ismail F, Pezzella F, Yahaya A, Tan G-C, Chia SL, Md Salleh MS, Abdullah N, Tan K-L. Mechanism and Predictive Role of NUB1 Protein in Oestrogen Receptor Pathway of FEC-Treated Breast Cancer Patients. Biomedicines. 2025; 13(6):1307. https://doi.org/10.3390/biomedicines13061307
Chicago/Turabian StyleArshad, Maria, Amira Raudhah Abdullah, Fuad Ismail, Francesco Pezzella, Azyani Yahaya, Geok-Chin Tan, Suet Lin Chia, Md Salzihan Md Salleh, Noraidatulakma Abdullah, and Ka-Liong Tan. 2025. "Mechanism and Predictive Role of NUB1 Protein in Oestrogen Receptor Pathway of FEC-Treated Breast Cancer Patients" Biomedicines 13, no. 6: 1307. https://doi.org/10.3390/biomedicines13061307
APA StyleArshad, M., Abdullah, A. R., Ismail, F., Pezzella, F., Yahaya, A., Tan, G.-C., Chia, S. L., Md Salleh, M. S., Abdullah, N., & Tan, K.-L. (2025). Mechanism and Predictive Role of NUB1 Protein in Oestrogen Receptor Pathway of FEC-Treated Breast Cancer Patients. Biomedicines, 13(6), 1307. https://doi.org/10.3390/biomedicines13061307






