Aberrant Effective Connectivity Within and Between the Default Mode, Executive Control, and Salience Networks in Chronic Insomnia Disorder—Toward Identifying the Hyperarousal State
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic and Clinical Characteristics
3.2. One-Sample Kolmogorov–Smirnov Test Results
3.3. Two-Sample Mann–Whitney Test Results
3.4. Correlation Between Connectivity Strengths, Questionnaires, and PSG Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea-hypopnea index |
| AIR | Right anterior insula |
| BDI | Beck depression inventory |
| CID | Chronic insomnia disorder |
| DCM | Dynamic casual modeling |
| DLPFC | Dorsolateral prefrontal cortex |
| DMN | Default mode network |
| DMPFC | Dorsomedial prefrontal cortex |
| EC | Effective connectivity |
| ECN | Executive control network |
| EPI | Echo Planar Imaging |
| ESS | Epworth sleepiness scale |
| FC | Functional connectivity |
| FDR | False discovery rate |
| HC | Healthy controls |
| Hippocamp R | Right hippocamps |
| ICSD-III TR | International classification of sleep disorders, third edition. Text revision |
| ISI | Insomnia severity index |
| MNI | Montreal Neurological Institute |
| MPFC | Medial prefrontal cortex |
| N1 | Stage 1 Non-REM Sleep |
| N2 | Stage 2 Non-REM Sleep |
| N3 | Stage 3 Non-REM Sleep |
| ODI | Oxygen desaturation index |
| PCC | Posterior cingulate cortex |
| PLMS | Periodic limb movement syndrome |
| PSG | Polysomnography |
| REM | Rapid eye movement sleep |
| ROI | Region of interest |
| Rs-fMRI | Resting-state functional magnetic resonance imaging |
| SE | Sleep efficiency |
| SN | Salience network |
| SOL | Sleep onset latency |
| SPM | Statistical Parametric Mapping |
| TE | Echo time |
| TIB | Time in bed |
| TR | Relaxation time |
| WASO | Wake after sleep onset |
References
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Angst, J.; Gamma, A.; Ajdacic, V.; Eich, D.; Rössler, W. Prevalence, Course, and Comorbidity of Insomnia and Depression in Young Adults. Sleep 2008, 31, 473–480. [Google Scholar] [CrossRef]
- Dressle, R.J.; Riemann, D. Hyperarousal in insomnia disorder: Current evidence and potential mechanisms. J. Sleep Res. 2023, 32, e13928. [Google Scholar] [CrossRef]
- Perlis, M.; Gehrman, P.; Pigeon, W.R.; Findley, J.; Drummond, S. Neurobiologic Mechanisms in Chronic Insomnia. Sleep Med. Clin. 2009, 4, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Gehrman, P.; Sengupta, A.; Harders, E.; Ubeydullah, E.; Pack, A.I.; Weljie, A. Altered diurnal states in insomnia reflect peripheral hyperarousal and metabolic desynchrony: A preliminary study. Sleep 2018, 41, zsy043. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, E.; Nissen, C.; Riemann, D.; Feige, B.; Baglioni, C.; Spiegelhalder, K. The exploratory power of sleep effort, dysfunctional beliefs and arousal for insomnia severity and polysomnography-determined sleep. J. Sleep Res. 2015, 24, 399–406. [Google Scholar] [CrossRef]
- Perlis, M.L.; Merica, H.; Smith, M.T.; Giles, D.E. Beta EEG activity and insomnia. Sleep Med. Rev. 2001, 5, 365–376. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia: State of the science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Cuamatzi-Castelan, A.S.; Tonnu, C.V.; Tran, K.M.; Anderson, J.R.; Roth, T.; Drake, C.L. Hyperarousal and sleep reactivity in insomnia: Current insights. Nat. Sci. Sleep 2018, 10, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, X.; Zhang, J.; Wang, E.; Zhang, H.; Li, Y. Magnetic resonance study on the brain structure and resting-state brain functional connectivity in primary insomnia patients. Medicine 2018, 97, e11944. [Google Scholar] [CrossRef]
- Riemann, D.; Voderholzer, U.; Spiegelhalder, K.; Hornyak, M.; Buysse, D.J.; Nissen, C.; Hennig, J.; Perlis, M.L.; van Elst, L.T.; Feige, B. Chronic Insomnia and MRI-Measured Hippocampal Volumes: A Pilot Study. Sleep 2007, 30, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.R.; Gomes, A.A.; Caetano, G.; Castelo-Branco, M. Insomnia disorder and brain’s default-mode network. Curr. Neurol. Neurosci. Rep. 2018, 18, 45. [Google Scholar] [CrossRef]
- Soehner, A.M.; Chase, H.W.; Bertocci, M.A.; Greenberg, T.; Stiffler, R.; Lockovich, J.C.; Aslam, H.A.; Graur, S.; Bebko, G.; Phillips, M.L. Unstable wakefulness during resting-state fMRI and its associations with network connectivity and affective psychopathology in young adults. J. Affect. Disord. 2019, 258, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sabot, D.; Baumann, O. Neuroimaging Correlates of Cognitive Behavioral Therapy for Insomnia (CBT-I): A Systematic Literature Review. J. Cogn. Psychother. 2022, 37, 82–101. [Google Scholar] [CrossRef]
- Li, Y.; Wang, E.; Zhang, H.; Dou, S.; Liu, L.; Tong, L.; Lei, Y.; Wang, M.; Xu, J.; Shi, D.; et al. Functional connectivity changes between parietal and prefrontal cortices in primary insomnia patients: Evidence from resting-state fMRI. Eur. J. Med. Res. 2014, 19, 32. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.-Q.; Li, Z.-X.; Qu, M.; Liao, D.; Guo, Z.-P.; Li, D.-C.; Liu, C.-H. The impact of insomnia on brain networks topology in depressed patients: A resting-state fMRI study. Brain Res. 2024, 1844, 149169. [Google Scholar] [CrossRef]
- Mars, R.B.; Neubert, F.X.; Noonan, M.A.P.; Sallet, J.; Toni, I.; Rushworth, M.F.S. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef]
- Witt, S.T.; van Ettinger-Veenstra, H.; Salo, T.; Riedel, M.C.; Laird, A.R. What Executive Function Network is that? An Image-Based Meta-Analysis of Network Labels. Brain Topogr. 2021, 34, 598–607. [Google Scholar] [CrossRef]
- Stein, J.; Korb, F.M.; Goschke, T.; Zwosta, K. Salience network resting-state functional connectivity predicts self-controlled decision-making. Sci. Rep. 2025, 15, 16332. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tian, Y.; He, Q.; Qiu, J.; Feng, T.; Chen, H.; Lei, X. Enhanced Anti-correlation Between the Dorsal Attention and Default-mode Networks: A Resting-state fMRI Study of Acute Insomnia. Neuroscience 2021, 467, 47–55. [Google Scholar] [CrossRef]
- Kandilarova, S.; Stoyanov, D.S.; Paunova, R.; Todeva-Radneva, A.; Aryutova, K.; Maes, M. Effective connectivity between major nodes of the limbic system, salience and frontoparietal networks differentiates schizophrenia and mood disorders from healthy controls. J. Pers. Med. 2021, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Zhan, Y.; Zhang, Y.; Guo, R.; Wang, J.; Guo, X.; Liu, Y.; Wang, Z.; Li, K. Aberrant functional connectivity architecture in participants with chronic insomnia disorder accompanying cognitive dysfunction: A whole-brain, data-driven analysis. Front. Neurosci. 2017, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, K.; Liu, J.H.; Wang, Y.P. Altered Default Mode and Sensorimotor Network Connectivity With Striatal Subregions in Primary Insomnia: A Resting-State Multi-Band fMRI Study. Front. Neurosci. 2018, 12, 917. [Google Scholar] [CrossRef]
- Wang, T.; Yan, J.; Li, S.; Zhan, W.; Ma, X.; Xia, L.; Li, M.; Lin, C.; Tian, J.; Li, C.; et al. Increased insular connectivity with emotional regions in primary insomnia patients: A resting-state fMRI study. Eur. Radiol. 2017, 27, 3703–3709. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Zou, Z.; Wu, X.; Gao, H.; Wang, C.; Zhou, J.; Qi, F.; Zhang, M.; He, J.; et al. Real-Time fMRI Neurofeedback Training Changes Brain Degree Centrality and Improves Sleep in Chronic Insomnia Disorder: A Resting-State fMRI Study. Front. Mol. Neurosci. 2022, 15, 825286. [Google Scholar] [CrossRef]
- Yu, S.; Guo, B.; Shen, Z.; Wang, Z.; Kui, Y.; Hu, Y.; Feng, F. The imbalanced anterior and posterior default mode network in the primary insomnia. J. Psychiatr. Res. 2018, 103, 97–103. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, N.; Lee, J.J.; Cho, S.E.; Na, K.S.; Kang, S.G. Brain reactivity using fMRI to insomnia stimuli in insomnia patients with discrepancy between subjective and objective sleep. Sci. Rep. 2021, 11, 1592. [Google Scholar] [CrossRef]
- Elberse, J.D.; Saberi, A.; Ahmadi, R.; Changizi, M.; Bi, H.; Hoffstaedter, F.; Mander, B.A.; Eickhoff, S.B.; Tahmasian, M.; Initiative, A.D.N. The interplay between insomnia symptoms and Alzheimer’s disease across three main brain networks. Sleep 2024, 47, zsae145. [Google Scholar] [CrossRef]
- Wei, Y.; Leerssen, J.; Wassing, R.; Stoffers, D.; Perrier, J.; Van Someren, E.J.W. Reduced dynamic functional connectivity between salience and executive brain networks in insomnia disorder. J. Sleep Res. 2020, 29, e12953. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Spiegelhalder, K.; Regen, W.; Feige, B.; Nissen, C.; Lombardo, C.; Violani, C.; Hennig, J.; Riemann, D. Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 2014, 37, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Qin, H.; Wu, T.; Hu, H.; Liao, K.; Cheng, F.; Gao, D.; Lei, X. Rest but busy: Aberrant resting-state functional connectivity of triple network model in insomnia. Brain Behav. 2018, 8, e00876. [Google Scholar] [CrossRef] [PubMed]
- Stephan, K.E.; Friston, K.J. Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 1, 446–459. [Google Scholar] [CrossRef]
- Li, C.; Dong, M.; Yin, Y.; Hua, K.; Fu, S.; Jiang, G. Aberrant effective connectivity of the right anterior insula in primary insomnia. Front. Neurol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Guo, R.; Wu, X.; Hu, F.; Liu, M.; Zhang, L.; Wang, Z.; Li, K. Altered regional homogeneity in chronic insomnia disorder with or without cognitive impairment. Am. J. Neuroradiol. 2018, 39, 742–747. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Q.; Zhang, C.; Wen, Z.; Zhou, X. The Effect of sequential bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. Brain Behav. 2019, 9, e01206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, X.; Wang, C.; Liang, K. Alteration of gamma-aminobutyric acid in the left dorsolateral prefrontal cortex of individuals with chronic insomnia: A combined transcranial magnetic stimulation-magnetic resonance spectroscopy study. Sleep Med. 2022, 92, 34–40. [Google Scholar] [CrossRef]
- Bechara, A.; Tranel, D.; Damasio, H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 2000, 123, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.S.; Laubach, M. Top-Down Control of Motor Cortex Ensembles by Dorsomedial Prefrontal Cortex. Neuron 2006, 52, 921–931. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Akbudak, E.; Shulman, G.L.; Raichle, M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Jilka, S.R.; Scott, G.; Ham, T.; Pickering, A.; Bonnelle, V.; Braga, R.M.; Leech, R.; Sharp, D.J. Damage to the salience network and interactions with the default mode network. J. Neurosci. 2014, 34, 10798–10807. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chang, C.; Glover, G.H.; Gotlib, I.H. Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 2014, 97, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A. Revisiting the Psychobiological Inhibition Model: A conceptual framework for understanding and treating insomnia using cognitive and behavioural therapeutics (CBTx). J. Sleep Res. 2023, 32, e13841. [Google Scholar] [CrossRef]
- Espie, C.A.; Broomfield, N.M.; MacMahon, K.M.A.; Macphee, L.M.; Taylor, L.M. The attention-intention-effort pathway in the development of psychophysiologic insomnia: A theoretical review. Sleep Med. Rev. 2006, 10, 215–245. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Long, Z.; Cheng, F. Age effect on functional connectivity changes of right anterior insula after partial sleep deprivation. Neuroreport 2019, 30, 1246–1250. [Google Scholar] [CrossRef]
- Xia, G.; Hu, Y.; Chai, F.; Wang, Y.; Liu, X. Abnormalities of the Default Mode Network Functional Connectivity in Patients with Insomnia Disorder. Contrast Media Mol. Imaging 2022, 2022. [Google Scholar] [CrossRef]
- Wang, T.; Ye, Y.; Li, S.; Jiang, G. Altered functional connectivity of anterior cingulate cortex in chronic insomnia: A resting-state fMRI study. Sleep Med. 2023, 102, 46–51. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R.; Guan, M.; Wang, E.; Qian, T.; Zhao, C.; Zou, Z.; Beck, T.; Shi, D.; Wang, M.; et al. Disrupted brain network topology in chronic insomnia disorder: A resting-state fMRI study. Neuroimage Clin. 2018, 18, 178–185. [Google Scholar] [CrossRef]
- Bagherzadeh-Azbari, S.; Khazaie, H.; Zarei, M.; Spiegelhalder, K.; Walter, M.; Leerssen, J.; Van Someren, E.J.; Sepehry, A.A.; Tahmasian, M. Neuroimaging insights into the link between depression and Insomnia: A systematic review. J. Affect. Disord. 2019, 258, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, B.; Wang, S.; Chen, C.; Liu, Z.; Ji, Y.; Liu, K.; Niu, Y. The brain in chronic insomnia and anxiety disorder: A combined structural and functional fMRI study. Front. Psychiatry 2024, 15, 1364713. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.S.; Ganesan, S.; Tay, J.; Elliott, E.S.; Misaki, M.; White, E.J.; Paulus, M.P.; Guinjoan, S.M.; Tsuchiyagaito, A. Increased Insular Functional Connectivity During Repetitive Negative Thinking in Major Depression and Healthy Volunteers. Biol. Psychiatry 2024, 95, S200. [Google Scholar] [CrossRef]
- Barbey, A.K.; Koenigs, M.; Grafman, J. Dorsolateral prefrontal contributions to human working memory. Cortex 2013, 49, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Wu, P.; Wang, C.; Wei, M.; Li, Y.; Xue, Y.; Li, X.; Jiang, J.; Bi, Y.; Dai, J.; Jiang, W. Prefrontal cortex functional connectivity changes during verbal fluency test in adults with short-term insomnia disorder: A functional near-infrared spectroscopy study. Front. Neurosci. 2023, 17, 1277690. [Google Scholar] [CrossRef]
- Boon, M.E.; van Hooff, M.L.M.; Vink, J.M.; Geurts, S.A.E. The effect of fragmented sleep on emotion regulation ability and usage. Cogn. Emot. 2023, 37, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.E.; Harris, A.L.; Falco, A.; Edinger, J.D. The relation between insomnia symptoms, mood and rumination about insomnia symptoms. J. Clin. Sleep Med. 2013, 9, 567–575. [Google Scholar] [CrossRef]
- Jansson-Fröjmark, M.; Norell-Clarke, A.; Linton, S.J. The role of emotion dysregulation in insomnia: Longitudinal findings from a large community sample. Br. J. Health Psychol. 2016, 21, 93–113. [Google Scholar] [CrossRef]
- Patel, M.; Teferi, M.; Gura, H.; Casalvera, A.; Lynch, K.G.; Nitchie, F.; Makhoul, W.; Sheline, Y.I.; Oathes, D.J.; Balderston, N.L. Interleaved TMS/fMRI shows that threat decreases dlPFC-mediated top-down regulation of emotion processing. NPP—Digit. Psychiatry Neurosci. 2024, 2, 6. [Google Scholar] [CrossRef]
- Nofzinger, E.A.; Buysse, D.J.; Germain, A.; Price, J.C.; Miewald, J.M.; David Kupfer, B.J. Functional Neuroimaging Evidence for Hyperarousal in Insomnia. Am. J. Psychiatry 2004, 161, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kong, X.; Zhang, X.; Zhang, Y.; Li, X.; Ge, Y.J. The impact of insomnia on prefrontal activation during a verbal fluency task in patients with major depressive disorder: A preliminary fNIRS study. Sleep Med. 2025, 125, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, L.; Da, H.; Ji, B.; Xiao, Q.; Shi, H. The role of dorsolateral and orbitofrontal cortex in depressed with insomniacs population: A large-scale fNIRS study. Curr. Psychol. 2025. [Google Scholar] [CrossRef]
| Region of Interest | X | Y | Z | Brodmann Area |
|---|---|---|---|---|
| Ventromedial Prefrontal Cortex (VMPFC) | 44 | 52 | −2 | 25 |
| Dorsomedial Prefrontal Cortex (DMPFC) | 4 | 30 | 46 | 32 |
| Medial Prefrontal Cortex (MPFC) | 3 | 54 | −2 | 14;11 |
| Right Anterior Insula (AIR) | 38 | 22 | 3 | 13 |
| Right Hippocampus | 24 | −12 | −20 | 28 |
| Dorsolateral Prefrontal Cortex (DLPFC) | −37 | 27 | 44 | 46 |
| Posterior Cingulate Cortex (PCC) | 0 | −52 | 26 | 23;31 |
| Precuneus | −10 | −64 | 24 | 7 |
| Variable | Insomnia (n = 31) Mean (±SD) | Healthy Control (n = 24) Mean (SD) | Between-Group Differences p-Value ϯ |
|---|---|---|---|
| Age | 34.00 (±9.08) | 30 (±7.01) | 0.092 |
| Sex (F/M) | 22/9 | 15/9 | Χ2 = 0.579 |
| Education (secondary/higher) | 10/21 | 6/19 | Χ2 = 0.496 |
| ISI | 18 (±4.00) | 4 (±2.85) | <0.001 ** |
| BDI | 13 (±7.57) | 5 (±4.72) | <0.001 ** |
| ESS | 4 (±3.72) | 6 (±3.52) | 0.040 * |
| SOL (min) | 18 (±15.42) | 12.9 (±8.29) | 0.325 |
| TIB (min) | 474.8 (±60.01) | 411.6 (±55.81) | <0.001 ** |
| TST (min) | 393.2 (±65.73) | 368.8 (±53.42) | 0.105 |
| SE | 82.3 (±10.65) | 79.9 (±27.34) | 0.062 |
| WASO (min) | 63.6 (±37.08) | 30.9 (±17.19) | <0.001 ** |
| N1 (min) | 5.6 (±4.37) | 4.8 (±4.31) | 0.216 |
| N1 (%) | 1.4 (±1.17) | 1.3 (±1.21) | 0.312 |
| N2 (min) | 222.7 (±50.08) | 183.0 (±51.29) | 0.003 * |
| N2 (%) | 56.9 (±9.68) | 49.3 (±9.68) | 0.010 * |
| N3 (min) | 91.8 (±39.11) | 110.8 (±31.40) | 0.047 * |
| N3 (%) | 23.2 (±9.73) | 30.7 (±9.57) | 0.007 * |
| REM (min) | 72.3 (±28.29) | 70.2 (±26.23) | 0.882 |
| REM (%) | 18.1 (±4.78) | 18.7 (±5.90) | 0.465 |
| REM Latency | 115.7 (±61.86) | 95.0 (±42.35) | 0.243 |
| AHI | 2.6 (±2.29) | 3.1 (±2.51) | 0.191 |
| ODI | 1.8 (±1.67) | 2.5 (±3.0) | 0.347 |
| PLMS Index | 3.4 (±5.46) | 6.3 (±9.65) | 0.176 |
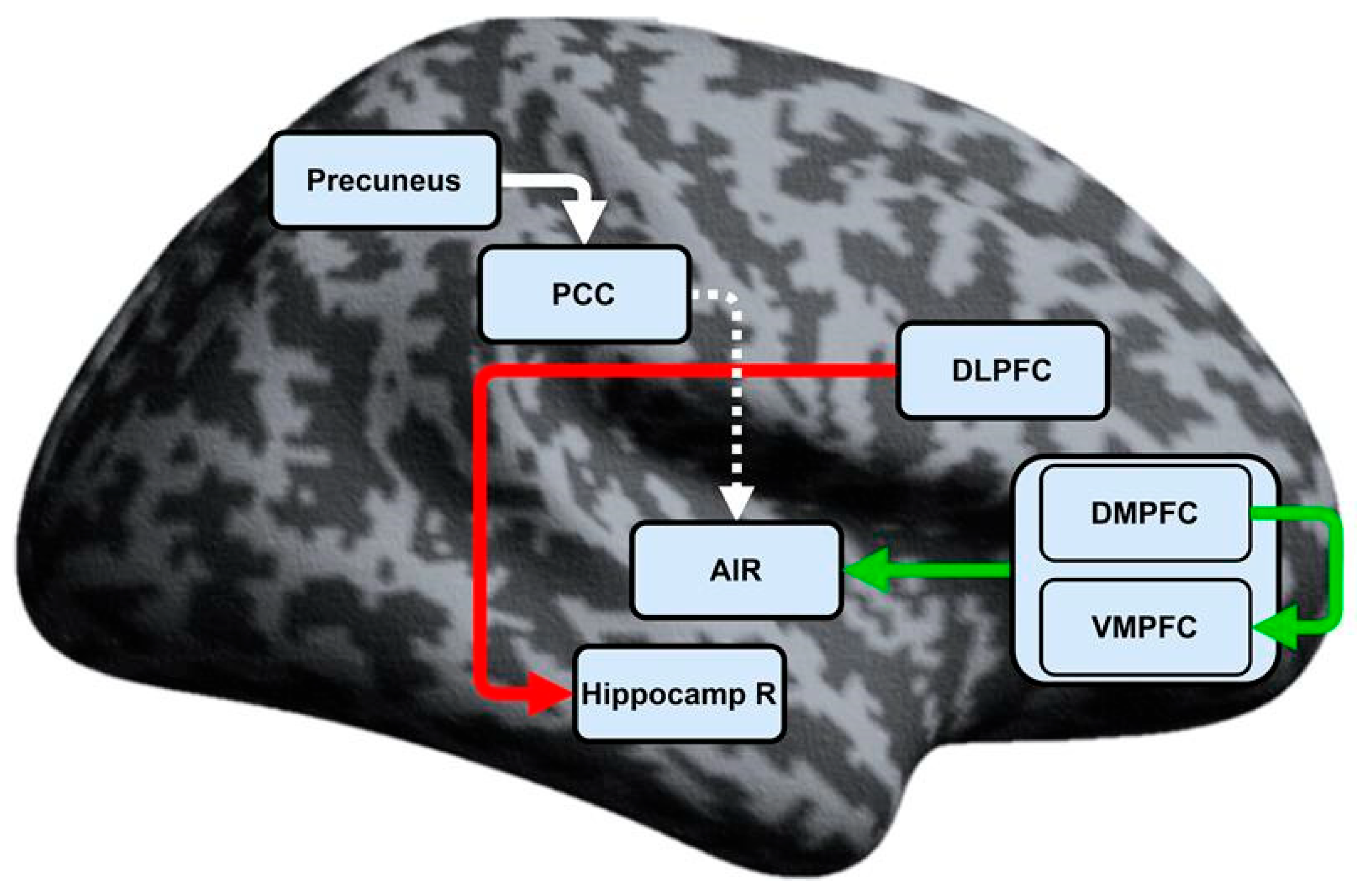
| Connections | CID Mean ± SD | HC Mean ± SD | Significance U |
|---|---|---|---|
| DMPFC → VMPFC | 0.20608 ±0.342 | 0.35446 a ±0.369 | 0.049 |
| MPFC → AIR | 0.06669 ±0.172 | −0.06238 a ±0.221 | 0.014 |
| DLPFC → Hippocamp R | −0.11002 ±0.258 | 0.18370 a ±0.204 | <0.001 |
| PCC → AIR b | −0.01701 a ±0.269 | 0.14839 ±0.242 | 0.039 |
| Precuneus → PCC | 0.30175 a ±0.302 | 0.44117 ±0.388 | 0.040 |
| Correlation | Significance r |
|---|---|
| DLPFC → Hippocamp R–ISI | −0.507 ** |
| DLPFC → Hippocamp R–BDI | −0.272 * |
| DLPFC → Hippocamp R–Total wake time | −0.320 * |
| DLPFC → Hippocamp R–WASO | −0.304 * |
| ISI-BDI | 0.703 ** |
| ISI–Total wake time | 0.494 ** |
| ISI-SOL | 0.297 * |
| ISI-WASO | 0.448 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, T.; Paunova, R.; Todeva-Radneva, A.; Avramov, K.; Draganova, A.; Kandilarova, S.; Terziyski, K. Aberrant Effective Connectivity Within and Between the Default Mode, Executive Control, and Salience Networks in Chronic Insomnia Disorder—Toward Identifying the Hyperarousal State. Biomedicines 2025, 13, 1293. https://doi.org/10.3390/biomedicines13061293
Georgiev T, Paunova R, Todeva-Radneva A, Avramov K, Draganova A, Kandilarova S, Terziyski K. Aberrant Effective Connectivity Within and Between the Default Mode, Executive Control, and Salience Networks in Chronic Insomnia Disorder—Toward Identifying the Hyperarousal State. Biomedicines. 2025; 13(6):1293. https://doi.org/10.3390/biomedicines13061293
Chicago/Turabian StyleGeorgiev, Todor, Rositsa Paunova, Anna Todeva-Radneva, Krasimir Avramov, Aneliya Draganova, Sevdalina Kandilarova, and Kiril Terziyski. 2025. "Aberrant Effective Connectivity Within and Between the Default Mode, Executive Control, and Salience Networks in Chronic Insomnia Disorder—Toward Identifying the Hyperarousal State" Biomedicines 13, no. 6: 1293. https://doi.org/10.3390/biomedicines13061293
APA StyleGeorgiev, T., Paunova, R., Todeva-Radneva, A., Avramov, K., Draganova, A., Kandilarova, S., & Terziyski, K. (2025). Aberrant Effective Connectivity Within and Between the Default Mode, Executive Control, and Salience Networks in Chronic Insomnia Disorder—Toward Identifying the Hyperarousal State. Biomedicines, 13(6), 1293. https://doi.org/10.3390/biomedicines13061293






