THSD1 Is a Multifaceted Regulator in Health and Disease
Abstract
1. Introduction
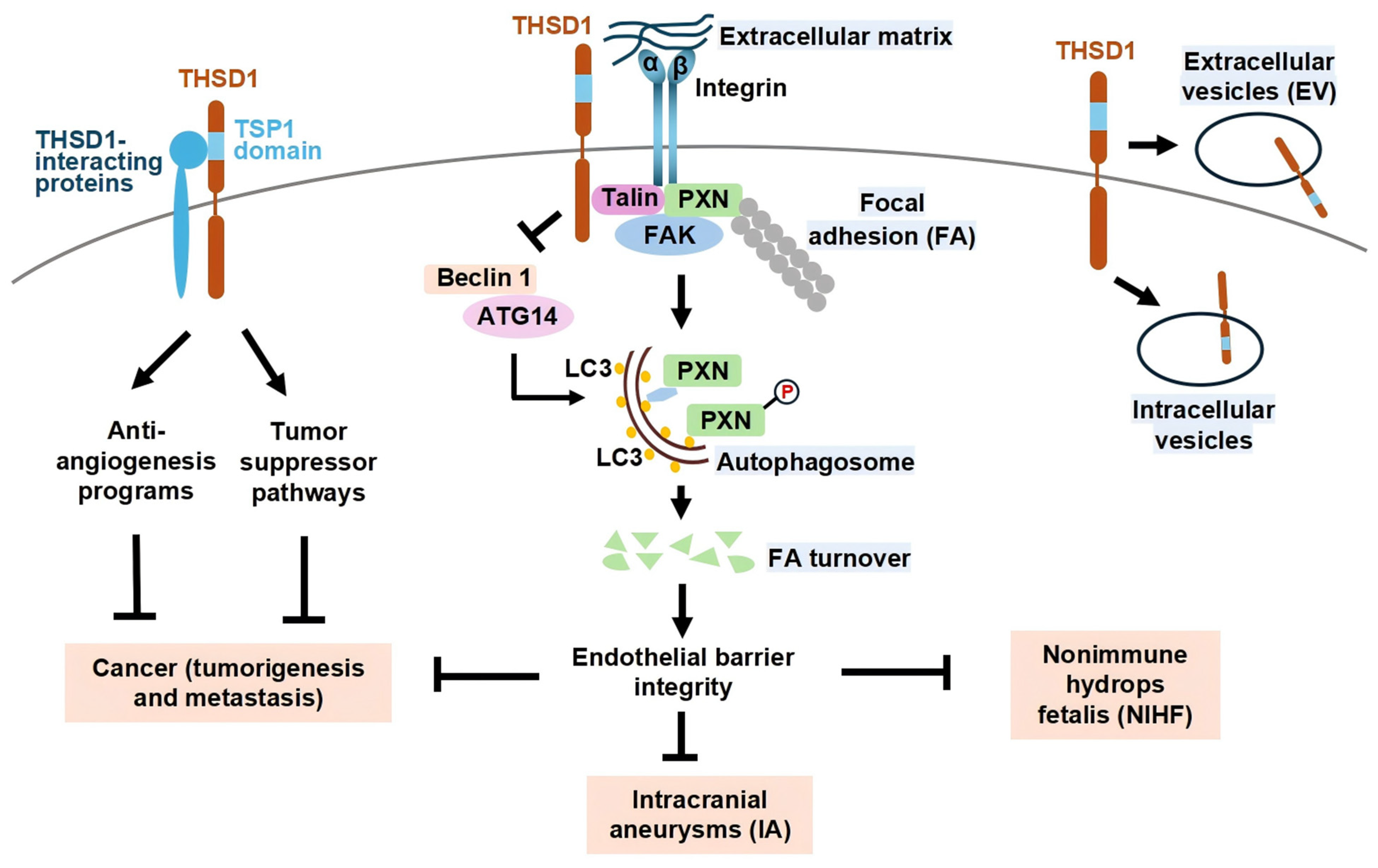
2. THSD1 in Vascular Integrity and Intracranial Aneurysms
3. THSD1 in Developmental and Perinatal Disorders
4. THSD1 in Cancer and Emerging Roles
5. Conclusions/Discussion
Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| EV | Extracellular vesicle |
| FA | Focal adhesion |
| IA | Intracranial aneurysm |
| NIHF | Nonimmune hydrops fetalis |
| THSD1 | Thrombospondin Type 1 Domain-Containing Protein 1 |
References
- Takayanagi, S.; Hiroyama, T.; Yamazaki, S.; Nakajima, T.; Morita, Y.; Usui, J.; Eto, K.; Motohashi, T.; Shiomi, K.; Keino-Masu, K.; et al. Genetic marking of hematopoietic stem and endothelial cells: Identification of the Tmtsp gene encoding a novel cell surface protein with the thrombospondin-1 domain. Blood 2006, 107, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Rui, Y.N.; Hagan, J.P.; Kim, D.H. Intracranial Aneurysms: Pathology, Genetics, and Molecular Mechanisms. NeuroMolecular Med. 2019, 21, 325–343. [Google Scholar] [CrossRef]
- Santiago-Sim, T.; Fang, X.; Hennessy, M.L.; Nalbach, S.V.; DePalma, S.R.; Lee, M.S.; Greenway, S.C.; McDonough, B.; Hergenroeder, G.W.; Patek, K.J.; et al. THSD1 (Thrombospondin Type 1 Domain Containing Protein 1) Mutation in the Pathogenesis of Intracranial Aneurysm and Subarachnoid Hemorrhage. Stroke 2016, 47, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Gu, X.; Liu, F.; Sun, C.; Mu, J.; Jin, D.; Sui, X.; Geng, D.; Li, Q.; Jiang, Y.; et al. SNP rs3803264 polymorphisms in THSD1 and abnormally expressed mRNA are associated with hemorrhagic stroke. Front. Aging Neurosci. 2023, 15, 1144364. [Google Scholar] [CrossRef] [PubMed]
- Sauvigny, T.; Alawi, M.; Krause, L.; Renner, S.; Spohn, M.; Busch, A.; Kolbe, V.; Altmuller, J.; Loscher, B.S.; Franke, A.; et al. Exome sequencing in 38 patients with intracranial aneurysms and subarachnoid hemorrhage. J. Neurol. 2020, 267, 2533–2545. [Google Scholar] [CrossRef]
- Roberts, W.; Magwenzi, S.; Aburima, A.; Naseem, K.M. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood 2010, 116, 4297–4306. [Google Scholar] [CrossRef]
- Rui, Y.N.; Xu, Z.; Fang, X.; Menezes, M.R.; Balzeau, J.; Niu, A.; Hagan, J.P.; Kim, D.H. The Intracranial Aneurysm Gene THSD1 Connects Endosome Dynamics to Nascent Focal Adhesion Assembly. Cell Physiol. Biochem. 2017, 43, 2200–2211. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, J.; Gao, S.; Rui, Y.N. THSD1 Suppresses Autophagy-Mediated Focal Adhesion Turnover by Modulating the FAK-Beclin 1 Pathway. Int. J. Mol. Sci. 2024, 25, 2139. [Google Scholar] [CrossRef]
- Zhang, J. Teaching the basics of autophagy and mitophagy to redox biologists--mechanisms and experimental approaches. Redox Biol. 2015, 4, 242–259. [Google Scholar] [CrossRef]
- Xu, Z.; Rui, Y.N.; Hagan, J.P.; Kim, D.H. Precision Tagging: A Novel Seamless Protein Tagging by Combinational Use of Type II and Type IIS Restriction Endonucleases. Bio-Protoc. 2018, 8, e2721. [Google Scholar] [CrossRef]
- Haasdijk, R.A.; Den Dekker, W.K.; Cheng, C.; Tempel, D.; Szulcek, R.; Bos, F.L.; Hermkens, D.M.; Chrifi, I.; Brandt, M.M.; Van Dijk, C.; et al. THSD1 preserves vascular integrity and protects against intraplaque haemorrhaging in ApoE-/- mice. Cardiovasc. Res. 2016, 110, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, G.; Sovis, R.; Shellvacumar, S.; Dissanayake, V.H.W. Spontaneous coronary artery dissection with leucoencephalopathy associated with thrombospondin Type 1 domain containing 1 gene mutation: A case report. Eur. Heart J. Case Rep. 2023, 7, ytad419. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, H.E.; Tulbah, M.; Kurdi, W.; Nemer, M.; Alsahan, N.; Al Mardawi, E.; Khalifa, O.; Hashem, A.; Kurdi, A.; Babay, Z.; et al. Identification of embryonic lethal genes in humans by autozygosity mapping and exome sequencing in consanguineous families. Genome Biol. 2015, 16, 116. [Google Scholar] [CrossRef]
- Abdelrahman, H.A.; Al-Shamsi, A.; John, A.; Hertecant, J.; Lootah, A.; Ali, B.R.; Al-Gazali, L. A recessive truncating variant in thrombospondin-1 domain containing protein 1 gene THSD1 is the underlying cause of nonimmune hydrops fetalis, congenital cardiac defects, and haemangiomas in four patients from a consanguineous family. Am. J. Med. Genet. A 2018, 176, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Al Rawi, W.N.; Ibrahim, F.H.; El Nakeib, O.A.S.; Al Zidgali, F.M. Manifestations of thrombospondin type-1 domain-containing protein 1 gene mutation in an extremely premature infant with nonimmune hydrops fetalis. Am. J. Med. Genet. A 2021, 185, 1598–1601. [Google Scholar] [CrossRef]
- Saxena, D.; Tiwari, A.K.; Prasad, R.; Srivastav, S. Resolving fetal hydrops—A rare entity. Eur. J. Med. Genet. 2023, 66, 104888. [Google Scholar] [CrossRef]
- Hall, V.; Vadakekut, E.S.; Avulakunta, I.D. Hemolytic Disease of the Fetus and Newborn. In StatPearls; StatPearls Publishing LLC.: Petersburg, FL, USA, 2025. [Google Scholar]
- Liu, H.; Li, F.; Zhu, Y.; Li, T.; Huang, H.; Lin, T.; Hu, Y.; Qi, X.; Yu, J.; Li, G. Whole-exome sequencing to identify somatic mutations in peritoneal metastatic gastric adenocarcinoma: A preliminary study. Oncotarget 2016, 7, 43894–43906. [Google Scholar] [CrossRef]
- Jin, Y.; Lei, Z.; Li, P.; Lyu, G. Proteome-wide Mendelian randomization and single-cell sequencing analysis identify the association between plasma proteins and gastric cancer. J. Gastrointest. Oncol. 2024, 15, 1464–1474. [Google Scholar] [CrossRef]
- Tang, W.; Ma, X. Application of large-scale and multicohort plasma proteomics data to discover novel causal proteins in gastric cancer. Discov. Oncol. 2024, 15, 570. [Google Scholar] [CrossRef]
- Khamas, A.; Ishikawa, T.; Mogushi, K.; Iida, S.; Ishiguro, M.; Tanaka, H.; Uetake, H.; Sugihara, K. Genome-wide screening for methylation-silenced genes in colorectal cancer. Int. J. Oncol. 2012, 41, 490–496. [Google Scholar] [CrossRef]
- Ko, J.M.; Chan, P.L.; Yau, W.L.; Chan, H.K.; Chan, K.C.; Yu, Z.Y.; Kwong, F.M.; Miller, L.D.; Liu, E.T.; Yang, L.C.; et al. Monochromosome transfer and microarray analysis identify a critical tumor-suppressive region mapping to chromosome 13q14 and THSD1 in esophageal carcinoma. Mol. Cancer Res. 2008, 6, 592–603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, W.; Ju, D.; Jie, Z.; Zhang, A.; Xing, X.; Yang, Q. Aberrant CpG-methylation affects genes expression predicting survival in lung adenocarcinoma. Cancer Med. 2018, 7, 5716–5726. [Google Scholar] [CrossRef]
- Kis, E.; Szatmari, T.; Keszei, M.; Farkas, R.; Esik, O.; Lumniczky, K.; Falus, A.; Safrany, G. Microarray analysis of radiation response genes in primary human fibroblasts. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1506–1514. [Google Scholar] [CrossRef]
- Monroe, J.D.; Moolani, S.A.; Irihamye, E.N.; Speed, J.S.; Gibert, Y.; Smith, M.E. RNA-Seq Analysis of Cisplatin and the Monofunctional Platinum(II) Complex, Phenanthriplatin, in A549 Non-Small Cell Lung Cancer and IMR90 Lung Fibroblast Cell Lines. Cells 2020, 9, 2637. [Google Scholar] [CrossRef] [PubMed]
- Danuta, G.; Tobias, M.; Marcus, D.; Miriam, E.; Nergiz, K.; Olesja, S.; Steffen, R.; Tanja, Z.; Christian, M.; Thomas, H.; et al. Molecular karyotyping and gene expression analysis in childhood cancer patients. J. Mol. Med. 2020, 98, 1107–1123. [Google Scholar] [CrossRef]
- Petrik, J.; Lauks, S.; Garlisi, B.; Lawler, J. Thrombospondins in the tumor microenvironment. Semin. Cell Dev. Biol. 2024, 155, 3–11. [Google Scholar] [CrossRef]
- Conkright, W.R.; Beckner, M.E.; Sterczala, A.J.; Mi, Q.; Lovalekar, M.; Sahu, A.; Krajewski, K.T.; Martin, B.J.; Flanagan, S.D.; Greeves, J.P.; et al. Resistance exercise differentially alters extracellular vesicle size and subpopulation characteristics in healthy men and women: An observational cohort study. Physiol. Genom. 2022, 54, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Conkright, W.R.; Beckner, M.E.; Sahu, A.; Mi, Q.; Clemens, Z.J.; Lovalekar, M.; Flanagan, S.D.; Martin, B.J.; Ferrarelli, F.; Ambrosio, F.; et al. Men and women display distinct extracellular vesicle biomarker signatures in response to military operational stress. J. Appl. Physiol. 2022, 132, 1125–1136. [Google Scholar] [CrossRef]
- Beckner, M.E.; Conkright, W.R.; Sahu, A.; Mi, Q.; Clemens, Z.J.; Martin, B.J.; Flanagan, S.D.; Ferrarelli, F.; Ambrosio, F.; Nindl, B.C. Utility of extracellular vesicles as a potential biological indicator of physiological resilience during military operational stress. Physiol. Rep. 2022, 10, e15219. [Google Scholar] [CrossRef]
- Beckner, M.E.; Conkright, W.R.; Mi, Q.; Martin, B.; Sahu, A.; Flanagan, S.D.; Ledford, A.K.; Wright, M.; Susmarski, A.; Ambrosio, F.; et al. Neuroendocrine, inflammatory, and extracellular vesicle responses during the Navy Special Warfare Screener Selection Course. Physiol. Genom. 2022, 54, 283–295. [Google Scholar] [CrossRef]
| Study | Disease/Context | Experimental Model/Method | Key Findings | Quantitative Data |
|---|---|---|---|---|
| Santiago-Sim et al. (2016) [3] | IA and SAH | Genetic sequencing of 507 IA probands | THSD1 mutation is a potential genetic risk factor for IA/SAH | Variant frequency: 1.6% in IA probands vs. 0 in 89,040 controls |
| Rui et al. (2017) [7] | Focal adhesions | siRNA knockdown in HUVECs | THSD1 stabilizes focal adhesions via interaction with FA proteins | FA area decreased; endothelial detachment increased |
| Chen et al. (2023) [4] | Hemorrhagic stroke | Case-control study in Chinese Han population | SNP rs3803264 and abnormal THSD1 mRNA associated with stroke | Allele frequency OR > 1.5; p < 0.05 |
| Xu et al. (2024) [8] | Vascular autophagy | HUVECs and mouse models | THSD1 suppresses Beclin 1-mediated autophagy of focal adhesions | Beclin1 Y233 phosphorylation decreased after THSD1 loss |
| Sauvigny et al. (2020) [5] | IA | Exome sequencing (n = 38) | THSD1 variants detected in IA+SAH patients | Multiple variants with CADD > 20 |
| Haasdijk et al. (2016) [11] | Atherosclerosis | Mouse model with THSD1 overexpression | THSD1 preserves vascular integrity and reduces intraplaque hemorrhage | Intraplaque hemorrhage reduced by 45% in THSD1-OE mice |
| Ranasinghe et al. (2023) [12] | Spontaneous coronary artery dissection (SCAD) with leukoencephalopathy | Case report: single patient with rare THSD1 mutation | Rare THSD1 variant may be associated with SCAD and neurological phenotype | 1 patient with SCAD and white matter changes + THSD1 mutation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, M.; Qu, K.; Liu, S.; Xu, Z.; Rui, Y.-N. THSD1 Is a Multifaceted Regulator in Health and Disease. Biomedicines 2025, 13, 1292. https://doi.org/10.3390/biomedicines13061292
Dai M, Qu K, Liu S, Xu Z, Rui Y-N. THSD1 Is a Multifaceted Regulator in Health and Disease. Biomedicines. 2025; 13(6):1292. https://doi.org/10.3390/biomedicines13061292
Chicago/Turabian StyleDai, Mengjun, Kuizhi Qu, Sophie Liu, Zhen Xu, and Yan-Ning Rui. 2025. "THSD1 Is a Multifaceted Regulator in Health and Disease" Biomedicines 13, no. 6: 1292. https://doi.org/10.3390/biomedicines13061292
APA StyleDai, M., Qu, K., Liu, S., Xu, Z., & Rui, Y.-N. (2025). THSD1 Is a Multifaceted Regulator in Health and Disease. Biomedicines, 13(6), 1292. https://doi.org/10.3390/biomedicines13061292






