Coronary Microvascular Disease Early After Myocardial Infarction: Diagnostic Approach and Prognostic Value—A Narrative Review
Abstract
1. Introduction
2. Search Methods
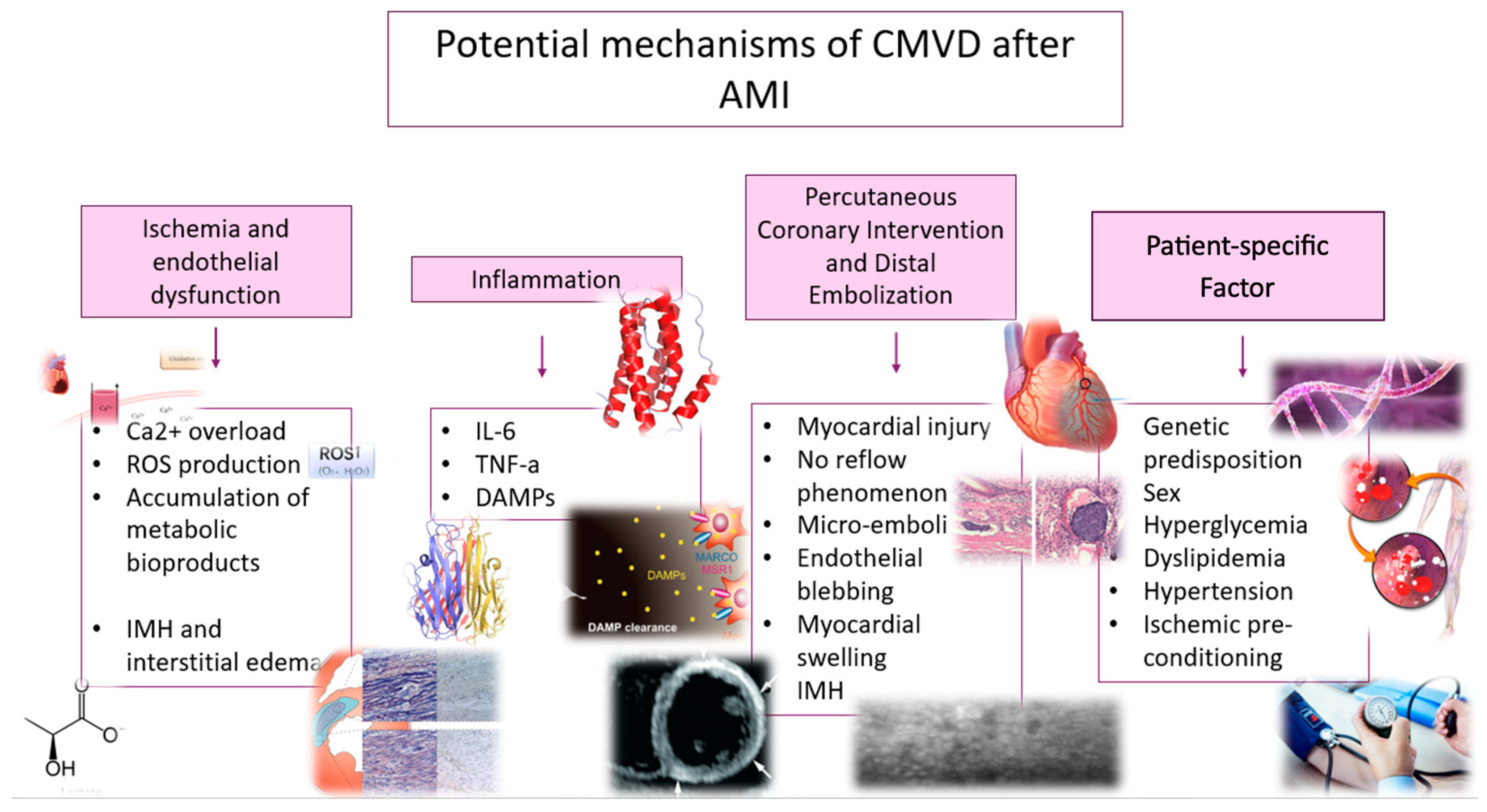
3. Potential Mechanisms of CMVD After AMI
3.1. Ischemia and Endothelial Dysfunction
3.2. Inflammation
3.3. Percutaneous Coronary Intervention and Distal Embolization
3.4. Patient-Specific Factors
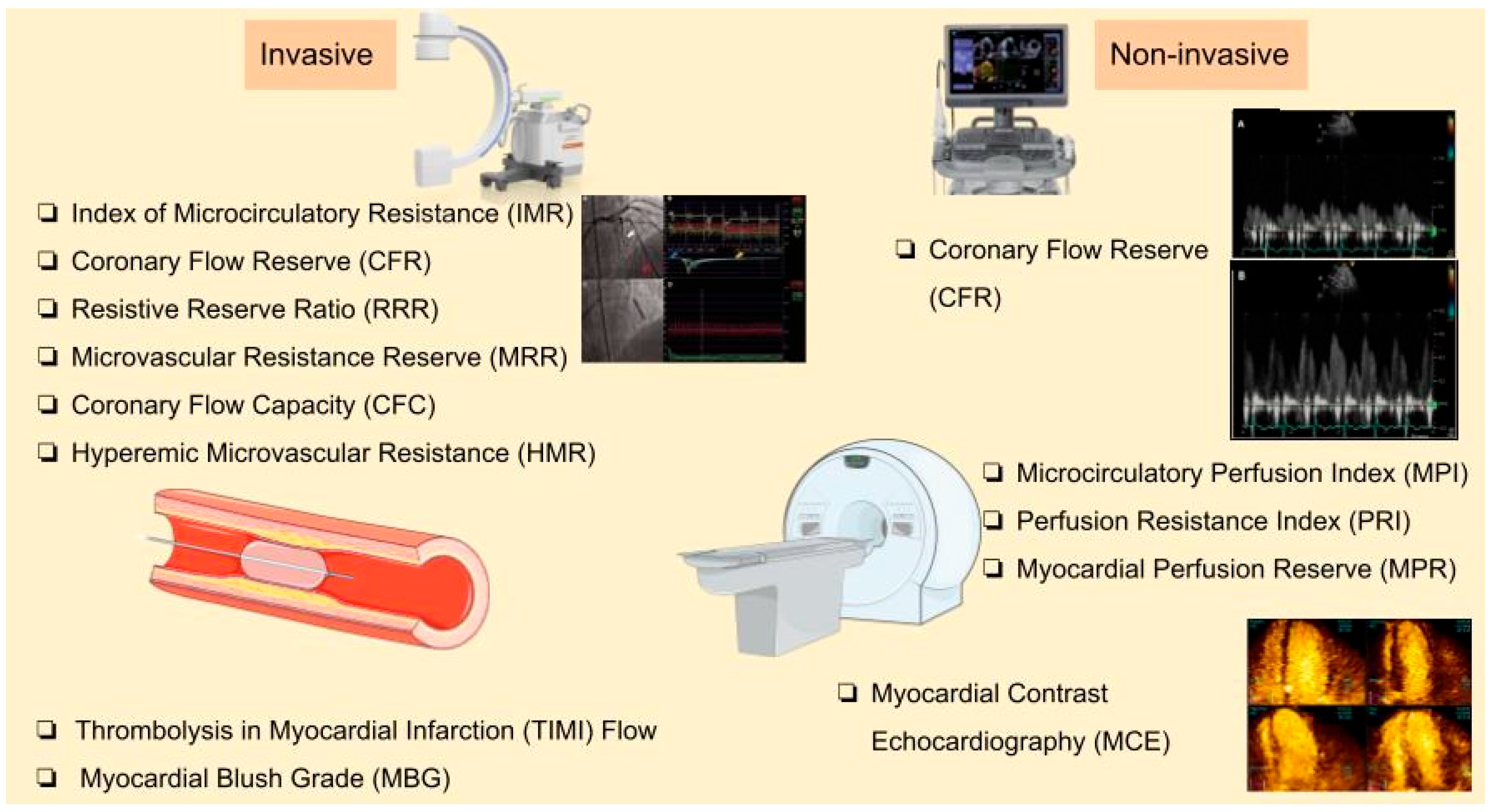
4. Diagnostic Methods
4.1. Angiography-Based Techniques
4.2. Non-Invasive Methods
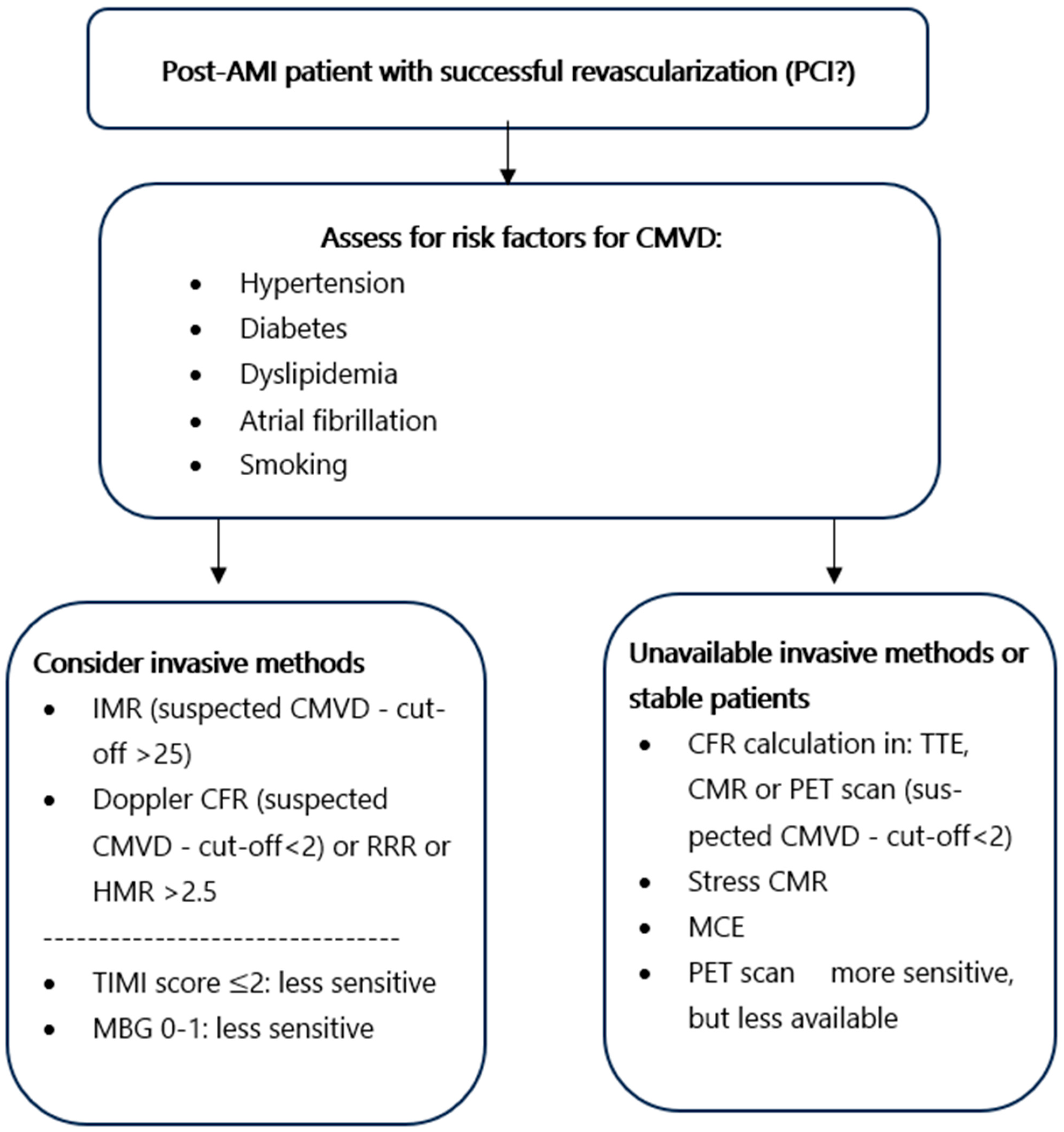
4.3. Diagnostic Approach of Early Post-AMI CMVD
5. Prognostic Value of CMVD
5.1. Prognostic Value Based on Methods
5.2. Other Prognostic Considerations and Pharmaceutical Implications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18F-FDG | Fluorodeoxyglucose F-18 |
| AHA/ACC | American Heart Association/American College of Cardiology |
| ACS | Acute Coronary Syndrome |
| AMI | Acute Myocardial Infarction |
| ATP | Adenosine Triphosphate |
| CAD | Coronary Artery Disease |
| CDKN2B-AS1 | Cyclin-Dependent Kinase Inhibitor 2B Antisense RNA 1 |
| CFC | Coronary Flow Capacity |
| CFR | Coronary Flow Reserve |
| CFVR | Coronary Flow Velocity Reserve |
| CI | Confidence Interval |
| CMVD | Coronary Microvascular Disease |
| CMR | Cardiac Magnetic Resonance |
| CTFC | Corrected TIMI Frame Count |
| DAMPs | Damage-Associated Molecular Patterns |
| DE-CMR | Delayed Enhancement Cardiac Magnetic Resonance |
| ESC | European Society of Cardiology |
| FFR | Fractional Flow Reserve |
| FPP | First-Pass Perfusion |
| HF | Heart Failure |
| HMR | Hyperemic Microvascular Resistance |
| HR | Hazard Ratio |
| IL-6 | Interleukin-6 |
| IMH | Intramyocardial Hemorrhage |
| IMR | Index of Microcirculatory Resistance |
| IMRangio | Angiography-Derived Index of Microcirculatory Resistance |
| INOCA | Ischemia with Non-Obstructive Coronary Arteries |
| IRA | Infarct-Related Artery |
| LAD | Left Anterior Descending Artery |
| LGE | Late Gadolinium Enhancement |
| LV | Left Ventricular |
| LVEF | Left Ventricular Ejection Fraction |
| MACE | Major Adverse Cardiovascular Events |
| MBG | Myocardial Blush Grade |
| MCE | Myocardial Contrast Echocardiography |
| MINOCA | Myocardial Infarction with Non-Obstructed Coronary Arteries |
| MPI | Microcirculatory Perfusion Index |
| MPRI | Myocardial Perfusion Reserve Index |
| MRR | Microvascular Resistance Reserve |
| MVO | Microvascular Obstruction |
| MYH15 | Myosin Heavy Chain 15 |
| NH-IMRangio | Non-Hyperemic Angiography-Derived Index of Microcirculatory Resistance |
| NO | Nitric Oxide |
| NT5E | 5′-Nucleotidase Ecto |
| OR | Odds Ratio |
| PCI | Percutaneous Coronary Intervention |
| PET | Positron Emission Tomography |
| PPCI | Primary Percutaneous Coronary Intervention |
| QFR | Quantitative Flow Ratio |
| ROS | Reactive Oxygen Species |
| RRR | Resistive Reserve Ratio |
| SE | Stress Echocardiography |
| SPECT | Single-Photon Emission Computed Tomography |
| STEMI | ST-segment Elevation Myocardial Infarction |
| STR | ST-segment Resolution |
| TTE | Transthoracic Echocardiography |
| TIMI | Thrombolysis in Myocardial Infarction |
| TNF-α | Tumor Necrosis Factor-alpha |
| VEGFA | Vascular Endothelial Growth Factor A |
References
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Stiermaier, T.; Fuernau, G.; Pöss, J.; De Waha-Thiele, S.; Desch, S.; Thiele, H.; Eitel, I. Impact of Direct Stenting on Myocardial Injury Assessed by Cardiac Magnetic Resonance Imaging and Prognosis in ST-Elevation Myocardial Infarction. Int. J. Cardiol. 2019, 283, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Abdu, F.A.; Mohammed, A.-Q.; Zhang, W.; Liu, L.; Yin, G.; Feng, Y.; Mohammed, A.A.; Mareai, R.M.; Lv, X.; et al. Prognostic Impact of Coronary Microvascular Dysfunction Assessed by caIMR in Overweight with Chronic Coronary Syndrome Patients. Front. Endocrinol. 2022, 13, 922264. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Badimon, L.; Bugiardini, R.; Camici, P.G.; Dorobantu, M.; Duncker, D.J.; Escaned, J.; Koller, A.; Piek, J.J.; De Wit, C. Coronary Vascular Regulation, Remodelling, and Collateralization: Mechanisms and Clinical Implications on Behalf of the Working Group on Coronary Pathophysiology and Microcirculation. Eur. Heart J. 2015, 36, 3134–3146. [Google Scholar] [CrossRef]
- Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review|JACC. Available online: https://www.jacc.org/doi/10.1016/j.jacc.2018.09.042 (accessed on 31 March 2025).
- Canu, M.; Khouri, C.; Marliere, S.; Vautrin, E.; Piliero, N.; Ormezzano, O.; Bertrand, B.; Bouvaist, H.; Riou, L.; Djaileb, L.; et al. Prognostic Significance of Severe Coronary Microvascular Dysfunction Post-PCI in Patients with STEMI: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0268330. [Google Scholar] [CrossRef]
- El Aidi, H.; Adams, A.; Moons, K.G.M.; Den Ruijter, H.M.; Mali, W.P.; Doevendans, P.A.; Nagel, E.; Schalla, S.; Bots, M.L.; Leiner, T. Cardiac Magnetic Resonance Imaging Findings and the Risk of Cardiovascular Events in Patients With Recent Myocardial Infarction or Suspected or Known Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 63, 1031–1045. [Google Scholar] [CrossRef]
- Scarica, V.; Rinaldi, R.; Animati, F.M.; Manzato, M.; Montone, R.A. Coronary Microvascular Dysfunction: Pathophysiology, Diagnosis, and Therapeutic Strategies across Cardiovascular Diseases. EXCLI J. 2025, 24, 454–478. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Camici, P. Advances in Coronary Microvascular Dysfunction. Heart Lung Circ. 2009, 18, 19–27. [Google Scholar] [CrossRef]
- Galli, M.; Niccoli, G.; De Maria, G.; Brugaletta, S.; Montone, R.A.; Vergallo, R.; Benenati, S.; Magnani, G.; D’Amario, D.; Porto, I.; et al. Coronary Microvascular Obstruction and Dysfunction in Patients with Acute Myocardial Infarction. Nat. Rev. Cardiol. 2024, 21, 283–298. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary Microvascular Obstruction in Acute Myocardial Infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial Ischaemia–Reperfusion Injury and Cardioprotection in Perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Dai, N.; Li, Y.; Kim, J.; Shin, D.; Lee, S.H.; Joh, H.S.; Kim, H.K.; Jeon, K.-H.; Ha, S.J.; et al. Functional Coronary Angiography–Derived Index of Microcirculatory Resistance in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 1670–1684. [Google Scholar] [CrossRef]
- Zhao, B.-H.; Ruze, A.; Zhao, L.; Li, Q.-L.; Tang, J.; Xiefukaiti, N.; Gai, M.-T.; Deng, A.-X.; Shan, X.-F.; Gao, X.-M. The Role and Mechanisms of Microvascular Damage in the Ischemic Myocardium. Cell. Mol. Life Sci. 2023, 80, 341. [Google Scholar] [CrossRef]
- Heusch, G. Treatment of Myocardial Ischemia/Reperfusion Injury by Ischemic and Pharmacological Postconditioning. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1123–1145. ISBN 978-0-470-65071-4. [Google Scholar]
- Maslov, L.N.; Popov, S.V.; Naryzhnaya, N.V.; Mukhomedzyanov, A.V.; Kurbatov, B.K.; Derkachev, I.A.; Boshchenko, A.A.; Khaliulin, I.; Prasad, N.R.; Singh, N.; et al. The Regulation of Necroptosis and Perspectives for the Development of New Drugs Preventing Ischemic/Reperfusion of Cardiac Injury. Apoptosis 2022, 27, 697–719. [Google Scholar] [CrossRef]
- Doherty, D.J.; Sykes, R.; Mangion, K.; Berry, C. Predictors of Microvascular Reperfusion After Myocardial Infarction. Curr. Cardiol. Rep. 2021, 23, 21. [Google Scholar] [CrossRef]
- Reffelmann, T.; Kloner, R.A. The No-Reflow Phenomenon: A Basic Mechanism of Myocardial Ischemia and Reperfusion. Basic Res. Cardiol. 2006, 101, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; Van Geuns, R.-J.M.; Berry, C.; Riksen, N.P.; Escaned, J.; Van Royen, N. Pathophysiology and Diagnosis of Coronary Microvascular Dysfunction in ST-Elevation Myocardial Infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Dörge, H.; Schulz, R.; Belosjorow, S.; Post, H.; Van De Sand, A.; Konietzka, I.; Frede, S.; Hartung, T.; Vinten-Johansen, J.; Youker, K.A.; et al. Coronary Microembolization: The Role of TNF- α in Contractile Dysfunction. J. Mol. Cell. Cardiol. 2002, 34, 51–62. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, B.; Peng, W.; Xu, Z. Protective Effect of Glycyrrhizin on Coronary Microembolization-induced Myocardial Dysfunction in Rats. Pharmacol. Res. Perspect. 2021, 9, e00714. [Google Scholar] [CrossRef]
- Loubeyre, C.; Morice, M.-C.; Lefèvre, T.; Piéchaud, J.-F.; Louvard, Y.; Dumas, P. A Randomized Comparison of Direct Stenting with Conventional Stent Implantation in Selected Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2002, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Selvanayagam, J.B.; Cheng, A.S.H.; Jerosch-Herold, M.; Rahimi, K.; Porto, I.; Van Gaal, W.; Channon, K.M.; Neubauer, S.; Banning, A.P. Effect of Distal Embolization on Myocardial Perfusion Reserve After Percutaneous Coronary Intervention: A Quantitative Magnetic Resonance Perfusion Study. Circulation 2007, 116, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Heusch, G. A Fresh Look at Coronary Microembolization. Nat. Rev. Cardiol. 2022, 19, 265–280. [Google Scholar] [CrossRef]
- Wu, K.C. CMR of Microvascular Obstruction and Hemorrhage in Myocardial Infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 72. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The Pathophysiology of Acute Myocardial Infarction and Strategies of Protection beyond Reperfusion: A Continual Challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, S.; Cilluffo, R.; Best, P.J.M.; Atkinson, E.J.; Aoki, T.; Cunningham, J.M.; De Andrade, M.; Choi, B.-J.; Lerman, L.O.; Lerman, A. Single Nucleotide Polymorphisms Associated with Abnormal Coronary Microvascular Function. Coron. Artery Dis. 2014, 25, 281–289. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Ikushima, M.; Kawano, S.; Okamura, A.; Asano, K.; Kuroda, T.; Tanaka, K.; Masuyama, T.; Hori, M.; et al. Association between Hyperglycemia and the No-Reflow Phenomenon Inpatients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2003, 41, 1–7. [Google Scholar] [CrossRef]
- Dąbrowska, E.; Narkiewicz, K. Hypertension and Dyslipidemia: The Two Partners in Endothelium-Related Crime. Curr. Atheroscler. Rep. 2023, 25, 605–612. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Andreadou, I.; Baxter, G.F.; Bøtker, H.E.; Davidson, S.M.; Dobrev, D.; Gersh, B.J.; Heusch, G.; Lecour, S.; Ruiz-Meana, M.; et al. Interaction of Cardiovascular Nonmodifiable Risk Factors, Comorbidities and Comedications With Ischemia/Reperfusion Injury and Cardioprotection by Pharmacological Treatments and Ischemic Conditioning. Pharmacol. Rev. 2023, 75, 159–216. [Google Scholar] [CrossRef]
- Scarsini, R.; Portolan, L.; Della Mora, F.; Marin, F.; Mainardi, A.; Ruzzarin, A.; Levine, M.B.; Banning, A.P.; Ribichini, F.; Garcia Garcia, H.M.; et al. Angiography-Derived and Sensor-Wire Methods to Assess Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2023, 16, 965–981. [Google Scholar] [CrossRef]
- Ohara, Y. Relation between the TIMI Frame Count and the Degree of Microvascular Injury after Primary Coronary Angioplasty in Patients with Acute Anterior Myocardial Infarction. Heart 2005, 91, 64–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marra, M.P.; Corbetti, F.; Cacciavillani, L.; Tarantini, G.; Ramondo, A.B.; Napodano, M.; Basso, C.; Lacognata, C.; Marzari, A.; Maddalena, F.; et al. Relationship between Myocardial Blush Grades, Staining, and Severe Microvascular Damage after Primary Percutaneous Coronary Intervention. Am. Heart J. 2010, 159, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wu, X.; Liu, H.; Zheng, D.; Xia, L. Index of Microcirculatory Resistance: State-of-the-Art and Potential Applications in Computational Simulation of Coronary Artery Disease. J. Zhejiang Univ. Sci. B 2022, 23, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; OxAMI Study Investigators; Ribichini, F.; Ferreira, V.M.; et al. Angiography-Derived Index of Microcirculatory Resistance (IMRangio) as a Novel Pressure-Wire-Free Tool to Assess Coronary Microvascular Dysfunction in Acute Coronary Syndromes and Stable Coronary Artery Disease. Int. J. Cardiovasc. Imaging 2021, 37, 1801–1813. [Google Scholar] [CrossRef]
- Garcia, D.; Harbaoui, B.; Van De Hoef, T.P.; Meuwissen, M.; Nijjer, S.S.; Echavarria-Pinto, M.; Davies, J.E.; Piek, J.J.; Lantelme, P. Relationship between FFR, CFR and Coronary Microvascular Resistance—Practical Implications for FFR-Guided Percutaneous Coronary Intervention. PLoS ONE 2019, 14, e0208612. [Google Scholar] [CrossRef]
- Doucette, J.W.; Corl, P.D.; Payne, H.M.; Flynn, A.E.; Goto, M.; Nassi, M.; Segal, J. Validation of a Doppler Guide Wire for Intravascular Measurement of Coronary Artery Flow Velocity. Circulation 1992, 85, 1899–1911. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.M.; Park, J.; Choi, K.H.; Hwang, D.; Doh, J.; Nam, C.; Shin, E.; Hoshino, M.; Murai, T.; et al. Prognostic Implications of Resistive Reserve Ratio in Patients With Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015846. [Google Scholar] [CrossRef]
- De Waard, G.A.; Fahrni, G.; De Wit, D.; Kitabata, H.; Williams, R.; Patel, N.; Teunissen, P.F.; Van De Ven, P.M.; Umman, S.; Knaapen, P.; et al. Hyperaemic Microvascular Resistance Predicts Clinical Outcome and Microvascular Injury after Myocardial Infarction. Heart 2018, 104, 127–134. [Google Scholar] [CrossRef]
- Van De Hoef, T.P.; Echavarría-Pinto, M.; Van Lavieren, M.A.; Meuwissen, M.; Serruys, P.W.J.C.; Tijssen, J.G.P.; Pocock, S.J.; Escaned, J.; Piek, J.J. Diagnostic and Prognostic Implications of Coronary Flow Capacity. JACC Cardiovasc. Interv. 2015, 8, 1670–1680. [Google Scholar] [CrossRef]
- Simova, I. Coronary Flow Velocity Reserve Assessment with Transthoracic Doppler Echocardiography. Eur. Cardiol. Rev. 2015, 10, 12. [Google Scholar] [CrossRef]
- Dwivedi, G.; Janardhanan, R.; Hayat, S.A.; Lim, T.K.; Greaves, K.; Senior, R. Relationship between Myocardial Perfusion with Myocardial Contrast Echocardiography and Function Early after Acute Myocardial Infarction for the Prediction of Late Recovery of Function. Int. J. Cardiol. 2010, 140, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Dirschinger, R.J.; Nekolla, S.G.; Kossmann, H.; Nicolosi, S.; Hanus, F.; Van Marwick, S.; Kunze, K.P.; Meinicke, A.; Götze, K.; et al. Prospective Evaluation of 18F-Fluorodeoxyglucose Uptake in Postischemic Myocardium by Simultaneous Positron Emission Tomography/Magnetic Resonance Imaging as a Prognostic Marker of Functional Outcome. Circ Cardiovasc. Imaging 2016, 9, e004316. [Google Scholar] [CrossRef]
- Dutta, U.; Sinha, A.; Demir, O.M.; Ellis, H.; Rahman, H.; Perera, D. Coronary Slow Flow Is Not Diagnostic of Microvascular Dysfunction in Patients With Angina and Unobstructed Coronary Arteries. J. Am. Heart Assoc. 2023, 12, e027664. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular Obstruction and the No-Reflow Phenomenon After Percutaneous Coronary Intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef]
- Kest, M.; Ágoston, A.; Szabó, G.T.; Kiss, A.; Üveges, Á.; Czuriga, D.; Komócsi, A.; Hizoh, I.; Kőszegi, Z. Angiography-Based Coronary Microvascular Assessment with and without Intracoronary Pressure Measurements: A Systematic Review. Clin. Res. Cardiol. 2024, 113, 1609–1621. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.O.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel Index for Invasively Assessing the Coronary Microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- Ng, M.K.C.; Yeung, A.C.; Fearon, W.F. Invasive Assessment of the Coronary Microcirculation: Superior Reproducibility and Less Hemodynamic Dependence of Index of Microcirculatory Resistance Compared with Coronary Flow Reserve. Circulation 2006, 113, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, 22. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; Carberry, J.; Yue May, V.T.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Hood, S.; et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients with an Acute ST-Segment–Elevation Myocardial Infarction. Circulation 2016, 134, 1833–1847. [Google Scholar] [CrossRef]
- De Waard, G.A.; Hollander, M.R.; Teunissen, P.F.A.; Jansen, M.F.; Eerenberg, E.S.; Beek, A.M.; Marques, K.M.; Van De Ven, P.M.; Garrelds, I.M.; Danser, A.H.J.; et al. Changes in Coronary Blood Flow After Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2016, 9, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Fuyuki, H.; Kubo, T.; Iwata, K.; Taguchi, H.; Toda, I.; Yoshikawa, J. Noninvasive Diagnosis of Ischemic and Nonischemic Cardiomyopathy Using Coronary Flow Velocity Measurements of the Left Anterior Descending Coronary Artery by Transthoracic Doppler Echocardiography. J. Am. Soc. Echocardiogr. 2006, 19, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Sadauskiene, E.; Zakarkaite, D.; Ryliskyte, L.; Celutkiene, J.; Rudys, A.; Aidietiene, S.; Laucevicius, A. Non-Invasive Evaluation of Myocardial Reperfusion by Transthoracic Doppler Echocardiography and Single-Photon Emission Computed Tomography in Patients with Anterior Acute Myocardial Infarction. Cardiovasc. Ultrasound 2011, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Maznyczka, A.M.; Oldroyd, K.G.; Greenwood, J.P.; McCartney, P.J.; Cotton, J.; Lindsay, M.; McEntegart, M.; Rocchiccioli, J.P.; Good, R.; Robertson, K.; et al. Comparative Significance of Invasive Measures of Microvascular Injury in Acute Myocardial Infarction. Circ. Cardiovasc. Interv. 2020, 13, e008505. [Google Scholar] [CrossRef]
- Toya, T.; Ahmad, A.; Corban, M.T.; Özcan, I.; Sara, J.D.; Sebaali, F.; Escaned, J.; Lerman, L.O.; Lerman, A. Risk Stratification of Patients With NonObstructive Coronary Artery Disease Using Resistive Reserve Ratio. J. Am. Heart Assoc. 2021, 10, e020464. [Google Scholar] [CrossRef]
- Scarsini, R.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Ferreira, V.M.; Ribichini, F.; Channon, K.M.; et al. Incremental Value of Coronary Microcirculation Resistive Reserve Ratio in Predicting the Extent of Myocardial Infarction in Patients with STEMI. Insights from the Oxford Acute Myocardial Infarction (OxAMI) Study. Cardiovasc. Revascularization Med. 2019, 20, 1148–1155. [Google Scholar] [CrossRef]
- Gallinoro, E.; Candreva, A.; Colaiori, I.; Kodeboina, M.; Fournier, S.; Nelis, O.; Di Gioia, G.; Sonck, J.; Van ’T Veer, M.; Pijls, N.H.J.; et al. Thermodilution-Derived Volumetric Resting Coronary Blood Flow Measurement in Humans. EuroIntervention 2021, 17, e672–e679. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Aldujeli, A.; Haq, A.; Knokneris, A.; Briedis, K.; Hughes, D.; Unikas, R.; Renkens, M.; Revaiah, P.C.; Tobe, A.; et al. The Impact of Microvascular Resistance Reserve on the Outcome of Patients with STEMI. JACC Cardiovasc. Interv. 2024, 17, 1214–1227. [Google Scholar] [CrossRef]
- Van De Hoef, T.P.; Bax, M.; Meuwissen, M.; Damman, P.; Delewi, R.; De Winter, R.J.; Koch, K.T.; Schotborgh, C.; Henriques, J.P.S.; Tijssen, J.G.P.; et al. Impact of Coronary Microvascular Function on Long-Term Cardiac Mortality in Patients with Acute ST-Segment–Elevation Myocardial Infarction. Circ Cardiovasc. Interv. 2013, 6, 207–215. [Google Scholar] [CrossRef]
- Van Lavieren, M.A.; Stegehuis, V.E.; Bax, M.; Echavarría-Pinto, M.; Wijntjens, G.W.M.; De Winter, R.J.; Koch, K.T.; Henriques, J.P.; Escaned, J.; Meuwissen, M.; et al. Time Course of Coronary Flow Capacity Impairment in ST-Segment Elevation Myocardial Infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 516–522. [Google Scholar] [CrossRef]
- Wijnbergen, I.; Van ’T Veer, M.; Lammers, J.; Ubachs, J.; Pijls, N.H.J. Absolute Coronary Blood Flow Measurement and Microvascular Resistance in ST-Elevation Myocardial Infarction in the Acute and Subacute Phase. Cardiovasc. Revascularization Med. 2016, 17, 81–87. [Google Scholar] [CrossRef]
- Konstantinou, K.; Karamasis, G.V.; Davies, J.R.; Alsanjari, O.; Tang, K.H.; Gamma, R.A.; Kelly, P.R.; Pijls, N.H.J.; Keeble, T.R.; Clesham, G.J. Absolute Microvascular Resistance by Continuous Thermodilution Predicts Microvascular Dysfunction after ST-Elevation Myocardial Infarction. Int. J. Cardiol. 2020, 319, 7–13. [Google Scholar] [CrossRef]
- Kogame, N.; Ono, M.; Kawashima, H.; Tomaniak, M.; Hara, H.; Leipsic, J.; Andreini, D.; Collet, C.; Patel, M.R.; Tu, S.; et al. The Impact of Coronary Physiology on Contemporary Clinical Decision Making. JACC Cardiovasc. Interv. 2020, 13, 1617–1638. [Google Scholar] [CrossRef]
- Tu, S.; Westra, J.; Yang, J.; Von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef]
- Oxford Acute Myocardial Infarction (OXAMI) Study Investigators; De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Angiography-Derived Index of Microcirculatory Resistance as a Novel, Pressure-Wire-Free Tool to Assess Coronary Microcirculation in ST Elevation Myocardial Infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef]
- Fernández-Peregrina, E.; Garcia-Garcia, H.M.; Sans-Rosello, J.; Sanz-Sanchez, J.; Kotronias, R.; Scarsini, R.; Echavarria-Pinto, M.; Tebaldi, M.; De Maria, G.L. Angiography-derived versus Invasively-determined Index of Microcirculatory Resistance in the Assessment of Coronary Microcirculation: A Systematic Review and Meta-analysis. Cathet. Cardio. Interv. 2022, 99, 2018–2025. [Google Scholar] [CrossRef]
- Parwani, P.; Kang, N.; Safaeipour, M.; Mamas, M.A.; Wei, J.; Gulati, M.; Naidu, S.S.; Merz, N.B. Contemporary Diagnosis and Management of Patients with MINOCA. Curr. Cardiol. Rep. 2023, 25, 561–570. [Google Scholar] [CrossRef]
- Almeida, A.G. MINOCA and INOCA: Role in Heart Failure. Curr. Heart Fail. Rep. 2023, 20, 139–150. [Google Scholar] [CrossRef]
- Ahn, S.; Suh, J.; Hung, O.; Lee, H.S.; Bouchi, Y.; Zeng, W.; Gandhi, R.; Eshtehardi, P.; Gogas, B.; Samady, H. Discordance Between Fractional Flow Reserve and Coronary Flow Reserve. JACC Cardiovasc. Interv. 2017, 10, 999–1007. [Google Scholar] [CrossRef]
- Galiuto, L.; Garramone, B.; Scarà, A.; Rebuzzi, A.G.; Crea, F.; La Torre, G.; Funaro, S.; Madonna, M.; Fedele, F.; Agati, L. The Extent of Microvascular Damage During Myocardial Contrast Echocardiography Is Superior to Other Known Indexes of Post-Infarct Reperfusion in Predicting Left Ventricular Remodeling. J. Am. Coll. Cardiol. 2008, 51, 552–559. [Google Scholar] [CrossRef]
- Abdelmoneim, S.S.; Dhoble, A.; Bernier, M.; Erwin, P.J.; Korosoglou, G.; Senior, R.; Moir, S.; Kowatsch, I.; Xian, S.; Muro, T.; et al. Quantitative Myocardial Contrast Echocardiography during Pharmacological Stress for Diagnosis of Coronary Artery Disease: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. Eur. J. Echocardiogr. 2009, 10, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Bekkers, S.C.A.M.; Yazdani, S.K.; Virmani, R.; Waltenberger, J. Microvascular Obstruction: Underlying Pathophysiology and Clinical Diagnosis. J. Am. Coll. Cardiol. 2010, 55, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Herling De Oliveira, L.L.; Correia, V.M.; Nicz, P.F.G.; Soares, P.R.; Scudeler, T.L. MINOCA: One Size Fits All? Probably Not—A Review of Etiology, Investigation, and Treatment. J. Clin. Med. 2022, 11, 5497. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, D.D.; Janssen, C.H.C.; Kuijpers, D.; Van Dijkman, P.R.M.; Overbosch, J.; Willems, T.P.; Oudkerk, M. The Additional Value of First Pass Myocardial Perfusion Imaging during Peak Dose of Dobutamine Stress Cardiac MRI for the Detection of Myocardial Ischemia. Int. J. Cardiovasc. Imaging 2008, 24, 69–76. [Google Scholar] [CrossRef]
- Tonet, E.; Pompei, G.; Faragasso, E.; Cossu, A.; Pavasini, R.; Passarini, G.; Tebaldi, M.; Campo, G. Coronary Microvascular Dysfunction: PET, CMR and CT Assessment. J. Clin. Med. 2021, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Adjedj, J.; Picard, F.; Durand-Viel, G.; Sigal-Cinqualbre, A.; Daou, D.; Diebold, B.; Varenne, O. Coronary Microcirculation in Acute Myocardial Ischaemia: From Non-Invasive to Invasive Absolute Flow Assessment. Arch. Cardiovasc. Dis. 2018, 111, 306–315. [Google Scholar] [CrossRef]
- Mayala, H.A.; Yan, W.; Jing, H.; Shuang-ye, L.; Gui-wen, Y.; Chun-xia, Q.; Ya, W.; Xiao-li, L.; Zhao-hui, W. Clinical Characteristics and Biomarkers of Coronary Microvascular Dysfunction and Obstructive Coronary Artery Disease. J. Int. Med. Res. 2019, 47, 6149–6159. [Google Scholar] [CrossRef]
- Mayala, H.A.; Bakari, K.H.; Mkangala, A.; Magesa, M.; Mghanga, F.P.; ZhaoHui, W. The Association of 18F-FDG PET/CT and Biomarkers in Confirming Coronary Microvascular Dysfunction. BMC Res. Notes 2018, 11, 796. [Google Scholar] [CrossRef]
- Nayfeh, M.; Ahmed, A.I.; Saad, J.M.; Alahdab, F.; Al-Mallah, M. The Role of Cardiac PET in Diagnosis and Prognosis of Ischemic Heart Disease: Optimal Modality Across Different Patient Populations. Curr. Atheroscler. Rep. 2023, 25, 351–357. [Google Scholar] [CrossRef]
- Rashid, H.; Rashid, A.; Mattoo, A.; Guru, F.R.; Mehvish, S.; Kakroo, S.A.; Lone, A.A.; Aslam, K.; Hafeez, I.; Rather, H. Left Ventricular Diastolic Function and Cardiotoxic Chemotherapy. Egypt. Heart J. 2024, 76, 45. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Morrone, D.; Pizzi, C.; Tritto, I.; Bergamaschi, L.; De Vita, A.; Villano, A.; Crea, F. Diagnostic approach for coronary microvascular dysfunction in patients with chest pain and no obstructive coronary artery disease. Trends Cardiovasc. Med. 2022, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Solomon, S.D.; Shah, A.M.; Desai, A.S.; Groarke, J.D.; Osborne, M.T.; Hainer, J.; Bibbo, C.F.; Dorbala, S.; Blankstein, R.; et al. Coronary Microvascular Dysfunction and Future Risk of Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2018, 39, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.P.S.; Zijlstra, F.; Van ‘T Hof, A.W.J.; De Boer, M.-J.; Dambrink, J.-H.E.; Gosselink, M.; Hoorntje, J.C.A.; Suryapranata, H. Angiographic Assessment of Reperfusion in Acute Myocardial Infarction by Myocardial Blush Grade. Circulation 2003, 107, 2115–2119. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Shaw, L.J.; Cook, N.R.; Murthy, V.L.; Shah, N.R.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Di Carli, M.F. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation 2017, 135, 566–577. [Google Scholar] [CrossRef]
- Ćorović, A.; Nus, M.; Mallat, Z.; Rudd, J.H.F.; Tarkin, J.M. PET Imaging of Post-Infarct Myocardial Inflammation. Curr. Cardiol. Rep. 2021, 23, 99. [Google Scholar] [CrossRef]
- Symons, R.; Pontone, G.; Schwitter, J.; Francone, M.; Iglesias, J.F.; Barison, A.; Zalewski, J.; De Luca, L.; Degrauwe, S.; Claus, P.; et al. Long-Term Incremental Prognostic Value of Cardiovascular Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 813–825. [Google Scholar] [CrossRef]
- Hamirani, Y.S.; Wong, A.; Kramer, C.M.; Salerno, M. Effect of Microvascular Obstruction and Intramyocardial Hemorrhage by CMR on LV Remodeling and Outcomes After Myocardial Infarction. JACC Cardiovasc. Imaging 2014, 7, 940–952. [Google Scholar] [CrossRef]
- Sun, W.; Sun, L.; Yang, F.; Zhao, X.; Cai, R.; Yuan, W. Evaluation of Myocardial Viability in Myocardial Infarction Patients by Magnetic Resonance Perfusion and Delayed Enhancement Imaging. Herz 2019, 44, 735–742. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Dorbala, S.; Di Carli, M.F. Cardiac PET Perfusion: Prognosis, Risk Stratification, and Clinical Management. Semin. Nucl. Med. 2014, 44, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Vera Cruz, P.; Palmes, P.; Bacalangco, N. Prognostic Value of Myocardial Blush Grade in ST-Elevation MI: A Systematic Review and Meta-Analysis. Interv. Cardiol. 2022, 17, e10. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; Wang, Y. Mechanism of Coronary Microcirculation Obstruction after Acute Myocardial Infarction and Cardioprotective Strategies. Rev. Cardiovasc. Med. 2024, 25, 367. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.G.; Arslan, F.; Abaci, A.; Heijden, G.V.; Timurkay-Nak, T.; Cengel, A. Myocardial Blush Grade: A Predictor for Major Adverse Cardiac Events after Primary PTCA with Stent Implantation for Acute Myocardial Infarction. Acta Cardiol. 2007, 445–451. [Google Scholar] [CrossRef]
- Angiography-Derived and Sensor-Wire Methods to Assess Coronary Microvascular Dysfunction in Patients with Acute Myocardial Infarction|JACC: Cardiovascular Imaging. Available online: https://www.jacc.org/doi/10.1016/j.jcmg.2023.01.017 (accessed on 3 April 2025).
- Scarsini, R.; Shanmuganathan, M.; De Maria, G.L.; Borlotti, A.; Kotronias, R.A.; Burrage, M.K.; Terentes-Printzios, D.; Langrish, J.; Lucking, A.; Fahrni, G.; et al. Coronary Microvascular Dysfunction Assessed by Pressure Wire and CMR After STEMI Predicts Long-Term Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1948–1959. [Google Scholar] [CrossRef]
- Mahmoudi Hamidabad, N.; Kanaji, Y.; Ozcan, I.; Sara, J.D.S.; Ahmad, A.; Lerman, L.O.; Lerman, A. Prognostic Implications of Resistive Reserve Ratio in Patients With Nonobstructive Coronary Artery Disease With Myocardial Bridging. J. Am. Heart Assoc. 2024, 13, e035000. [Google Scholar] [CrossRef]
- Eerdekens, R.; El Farissi, M.; De Maria, G.L.; Van Royen, N.; Van ‘T Veer, M.; Van Leeuwen, M.A.H.; Hoole, S.P.; Marin, F.; Carrick, D.; Tonino, P.A.L.; et al. Prognostic Value of Microvascular Resistance Reserve After Percutaneous Coronary Intervention in Patients With Myocardial Infarction. J. Am. Coll. Cardiol. 2024, 83, 2066–2076. [Google Scholar] [CrossRef]
- Milasinovic, D.; Nedeljkovic, O.; Maksimovic, R.; Sobic-Saranovic, D.; Dukic, D.; Zobenica, V.; Jelic, D.; Zivkovic, M.; Dedovic, V.; Stankovic, S.; et al. Coronary Microcirculation: The Next Frontier in the Management of STEMI. J. Clin. Med. 2023, 12, 1602. [Google Scholar] [CrossRef]
- Nolte, F.; van de Hoef, T.P.; Meuwissen, M.; Voskuil, M.; Chamuleau, S.A.; Henriques, J.P.; Verberne, H.J.; van Eck-Smit, B.L.; Koch, K.T.; de Winter, R.J.; et al. Increased Hyperaemic Coronary Microvascular Resistance Adds to the Presence of Myocardial Ischaemia. Available online: https://eurointervention.pcronline.com/article/increased-hyperaemic-coronary-microvascular-resistance-adds-to-the-presence-of-myocardial-ischaemia (accessed on 1 February 2025).
- Zhang, Y.; Pu, J.; Niu, T.; Fang, J.; Chen, D.; Yidilisi, A.; Zheng, Y.; Lu, J.; Hu, Y.; Koo, B.-K.; et al. Prognostic Value of Coronary Angiography–Derived Index of Microcirculatory Resistance in Non–ST-Segment Elevation Myocardial Infarction Patients. JACC Cardiovasc. Interv. 2024, 17, 1874–1886. [Google Scholar] [CrossRef]
- Rinaldi, R.; Salzillo, C.; Caffè, A.; Montone, R.A. Invasive Functional Coronary Assessment in Myocardial Ischemia with Non-Obstructive Coronary Arteries: From Pathophysiological Mechanisms to Clinical Implications. Rev. Cardiovasc. Med. 2022, 23, 371. [Google Scholar] [CrossRef]
- Jensen, S.M.; Prescott, E.I.B.; Abdulla, J. The Prognostic Value of Coronary Flow Reserve in Patients with Non-Obstructive Coronary Artery Disease and Microvascular Dysfunction: A Systematic Review and Meta-Analysis with Focus on Imaging Modality and Sex Difference. Int. J. Cardiovasc. Imaging 2023, 39, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Gdowski, M.A.; Murthy, V.L.; Doering, M.; Monroy-Gonzalez, A.G.; Slart, R.; Brown, D.L. Association of Isolated Coronary Microvascular Dysfunction With Mortality and Major Adverse Cardiac Events: A Systematic Review and Meta-Analysis of Aggregate Data. J. Am. Heart Assoc. 2020, 9, e014954. [Google Scholar] [CrossRef] [PubMed]
- Montisci, R.; Marchetti, M.F.; Ruscazio, M.; Biddau, M.; Secchi, S.; Zedda, N.; Casula, R.; Tuveri, F.; Kerkhof, P.L.; Meloni, L.; et al. Non-Invasive Coronary Flow Velocity Reserve Assessment Predicts Adverse Outcome in Women with Unstable Angina without Obstructive Coronary Artery Stenosis. J. Public Health Res. 2023, 12, 22799036231181716. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R. Prognostic Impact of Early ST-Segment Resolution in Acute ST-Elevation Myocardial Infarction. Circulation 2004, 110, e506–e510. [Google Scholar] [CrossRef]
- Nijveldt, R.; Van Der Vleuten, P.A.; Hirsch, A.; Beek, A.M.; Tio, R.A.; Tijssen, J.G.P.; Piek, J.J.; Van Rossum, A.C.; Zijlstra, F. Early Electrocardiographic Findings and MR Imaging-Verified Microvascular Injury and Myocardial Infarct Size. JACC Cardiovasc. Imaging 2009, 2, 1187–1194. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; Lindsay, M.M.; Davie, A.; et al. Myocardial Hemorrhage After Acute Reperfused ST-Segment–Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ Cardiovasc. Imaging 2016, 9, e004148. [Google Scholar] [CrossRef]
- Kaski, J.-C.; Crea, F.; Gersh, B.J.; Camici, P.G. Reappraisal of Ischemic Heart Disease: Fundamental Role of Coronary Microvascular Dysfunction in the Pathogenesis of Angina Pectoris. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef]
- 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Available online: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Acute-Coronary-Syndromes-ACS-Guidelines (accessed on 3 April 2025).
- Tu, Y.; Li, Q.; Zhou, Y.; Ye, Z.; Wu, C.; Xie, E.; Li, Y.; Li, P.; Wu, Y.; Guo, Z.; et al. Empagliflozin Inhibits Coronary Microvascular Dysfunction and Reduces Cardiac Pericyte Loss in Db/Db Mice. Front. Cardiovasc. Med. 2022, 9, 995216. [Google Scholar] [CrossRef]
- Bourcier, L.; Bellemare, M.; Tremblay-Gravel, M.; Henri, C.; White, M.; Bouabdallaoui, N. Effects of COLchicine on Inflammation, Myocardial Damage and Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction—The COLpEF Trial. Arch. Cardiovasc. Dis. Suppl. 2023, 15, 53. [Google Scholar] [CrossRef]
- A Review of Cardio-Pulmonary Microvascular Dysfunction in Pulmonary Hypertension. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 26, 100255. [CrossRef]
- Galiuto, L.; Garramone, B.; Burzotta, F.; Lombardo, A.; Barchetta, S.; Rebuzzi, A.G.; Crea, F. Thrombus Aspiration Reduces Microvascular Obstruction After Primary Coronary Intervention: A Myocardial Contrast Echocardiography Substudy of the REMEDIA Trial. J. Am. Coll. Cardiol. 2006, 48, 1355–1360. [Google Scholar] [CrossRef]
- Tersalvi, G.; Attinger-Toller, A.; Kalathil, D.; Winterton, D.; Cioffi, G.M.; Madanchi, M.; Seiler, T.; Stadelmann, M.; Goffredo, F.; Fankhauser, P.; et al. Trajectories of Cardiac Function Following Treatment With an Impella Device in Patients with Acute Anterior ST-Elevation Myocardial Infarction. CJC Open 2022, 5, 77–85. [Google Scholar] [CrossRef]
- Merz, C.N.B.; Marbán, E. Stem Cell Therapy Targets Repêchage! Circ. Res. 2022, 130, 339–342. [Google Scholar] [CrossRef]
- P887 Trimetazidine Improves Symptoms and Reduces Microvascular Dysfunction in Patients with Microvascular Angina | European Heart Journal|Oxford Academic. Available online: https://academic.oup.com/eurheartj/article-abstract/38/suppl_1/ehx501.P887/4088195 (accessed on 12 March 2025).
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Paschaliori, C.; Galiatsatos, N.; Tsioufis, K.; Tousoulis, D. Inflammation in Coronary Microvascular Dysfunction. Int. J. Mol. Sci. 2021, 22, 13471. [Google Scholar] [CrossRef]
- Andreoli, F.; Carabetta, N.; De, L.; Gabrielli, D. Coronary Microvascular Obstruction and Dysfunction from Pathophysiology. Interv. Cardiol. 2024, 3, 850–860. [Google Scholar]

| Diagnostic Method | Pathophysiology | Clinical Insights and Evaluation |
|---|---|---|
| Invasive | ||
| TIMI Flow Grade (≤2) [33] | Reduced blood flow in angiography cine loops | Clinical outcomes: Association with in-hospital mortality and adverse events. Pros: Simple calculation, cost-effective, performed during every PCI. Cons: Qualitative, poor reproducibility, does not directly assess microvascular function. |
| Myocardial Blush Grade (≤1) [34] | Reduced myocardial perfusion grading the intensity and washout of contrast within the myocardium during angiography | Clinical outcomes: Association with infarct size and adverse remodeling. Pros: Insights into myocardial perfusion. Cons: Subjective interpretation, limited sensitivity and specificity. |
| Corrected TIMI Frame Count (CTFC) [33] | Delayed contrast transit in angiography measured by frame count indicates impaired perfusion | Clinical outcomes: Linked to worse outcomes compared to those without CMVD. Cons: Time-consuming, technical confounders. |
| Index of Microcirculatory Resistance (IMR > 25) [35] | Elevated microvascular resistance assessed via thermodilution during hyperemia | Clinical outcomes: Association with MACEs. Pros: Quantitative gold standard of invasive methods, reproducible, specific to microvascular function. Cons: Hyperemia induction, specialized equipment, and expertise. |
| Angiography-derived IMR (IMRangio) (>25) [36] | Computational fluid dynamics model based on angiographic images simulates IMR | Clinical outcomes: Strong correlation with invasive IMR and MVO. Independently predicts MACEs post-PCI in STEMI and NSTEMI. Pros: Wire-free, faster, no hyperemia needed (in NH-IMRangio), lower procedural risk. Cons: Depends on image quality and validated software, limited real-world data, less validated than invasive IMR. |
| Coronary Flow Reserve (CFR < 2, invasive) [37] CFR (Doppler < 2.1) [38] | Impaired capacity of coronary circulation to augment flow during hyperemia Reduced coronary flow velocity reserve using Doppler guidewire | Clinical outcomes: Association with MACEs. Pros: Evaluates both epicardial and microvascular function. Cons: Cannot distinguish between epicardial and microvascular dysfunction, influenced by hemodynamic variables. |
| Resistive Reserve Ratio (RRR ≤ 2.62) [39] | Reduced functional vasodilatory reserve of microvasculature during stress-induced hyperemia | Clinical outcomes: Association with adverse events. Pros: Insights into microvascular functional reserve. Cons: Invasive measurements and hyperemia induction. |
| Hyperemic Microvascular Resistance (HMR ≥ 2.5 mmHg/cm/s) [40] | Increased resistance to flow during hyperemia at distal coronary microvasculature | Clinical outcomes: Association with adverse events. Pros: Specific assessment of microvascular resistance. Cons: Technically challenging, hyperemia induction and Doppler expertise required. |
| Coronary Flow Capacity (CFC < 2.8) [41] | Combined impairment in both CFR and absolute flow capacity | Clinical outcomes: Association with adverse events. Pros: Comprehensive assessment of whole coronary flow. Cons: Requires advanced imaging techniques and expertise. |
| Non-invasive | ||
| Transthoracic Doppler Echocardiography (TTE) CFR (<2) [42] | Reduced coronary flow velocity in LAD measured by Doppler echocardiography during hyperemia | Clinical outcomes: Association with MACEs. Pros: Non-invasive, easily accessible. Cons: Operator-dependent, limited in patients with suboptimal acoustic windows. |
| Myocardial Contrast Echocardiography (MCE) [43] | Uses microbubble contrast agents to visualize myocardial perfusion, assessing microvascular integrity and perfusion defects | Clinical outcomes: Association with adverse events. Pros: Bedside applicability, real-time imaging. Cons: Limited availability, expertise required. |
| Cardiac Magnetic Resonance (CMR) with MVO ≥ 2.6% of LV mass [26] | Detects microvascular obstruction and perfusion abnormalities using gadolinium contrast | Limited data available |
| PET CFR (<2.0–2.6) [44] | Decreased hyperemic myocardial blood flow on PET indicating microvascular dysfunction | Clinical outcomes: Strong predictor of MACEs. Pros: Quantitative gold standard of non-invasive methods. Cons: High cost, limited availability. |
| Index | Studied Population | Timing | Methodology | Clinical Outcomes in Patients with CMVD |
|---|---|---|---|---|
| TIMI Flow (≤2) [33] | STEMI patients | Immediate post-reperfusion | Angiographic qualitative assessment of coronary flow | ↑ In-hospital mortality |
| MBG (≤1) [94] | AMI patients | Immediate post-PCI | Qualitative myocardial perfusion assessment via angiography | ↑ Infarct area, adverse remodeling, ↑ hospitalization for heart failure |
| IMR (>25, or >40) [68] | AMI patients post-PCI | Immediate post-reperfusion | Thermodilution-based coronary microvascular resistance measurement | Predictive of MVO, ↑ infarct size, ↑ MACE, ↑ mortality |
| CFR invasive (<2) [37] | AMI patients | Immediate post-PCI | Pressure–temperature guidewire measurement of coronary flow velocity | ↑ In-hospital mortality |
| CFR (<2.1, Doppler) [42] | AMI patients | Post-reperfusion | Doppler-based coronary flow measurement | ↑ Cardiac mortality |
| RRR (≤1.5, alternatives: 1.7, 2.62) [39] | STEMI patients with PPCI | Immediate post-procedure | Functional reserve assessment of coronary microvasculature | ↑ infarct size, ↑ MACE, ↑ mortality |
| CFC (<2.8) [62] | AMI patients | Post-reperfusion | Combines coronary flow measurements with CFR | ↑ risk stratification, ↑ MACE |
| HMR (≥3) [102] | STEMI patients | Post-primary PCI | Measurement combining hyperemic distal pressure and Doppler velocity | ↑ hospitalization for heart failure, ↑ MACE, ↑mortality |
| MCE [43] | AMI patients | Post-reperfusion | Echocardiographic myocardial contrast imaging | ↑ Infarct area |
| CMR: MVO (≥2.6), MPI, MRPI [90] | AMI patients | Post-reperfusion | Magnetic resonance imaging to visualize myocardial perfusion | ↑ Infarct area, ↑ hospitalization for heart failure |
| PET-derived CFR (<2.6, alternative: 2.0) [93] | AMI patients | Post-infarction | Positron emission tomography for myocardial flow quantification | ↑ Long-term cardiovascular events and mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokratous, S.; Mitsis, A.; Khattab, E.; Karelas, D.; Velidakis, N.; Kadoglou, N.P.E. Coronary Microvascular Disease Early After Myocardial Infarction: Diagnostic Approach and Prognostic Value—A Narrative Review. Biomedicines 2025, 13, 1289. https://doi.org/10.3390/biomedicines13061289
Sokratous S, Mitsis A, Khattab E, Karelas D, Velidakis N, Kadoglou NPE. Coronary Microvascular Disease Early After Myocardial Infarction: Diagnostic Approach and Prognostic Value—A Narrative Review. Biomedicines. 2025; 13(6):1289. https://doi.org/10.3390/biomedicines13061289
Chicago/Turabian StyleSokratous, Stefanos, Andreas Mitsis, Elina Khattab, Dimitrios Karelas, Nikolaos Velidakis, and Nikolaos P. E. Kadoglou. 2025. "Coronary Microvascular Disease Early After Myocardial Infarction: Diagnostic Approach and Prognostic Value—A Narrative Review" Biomedicines 13, no. 6: 1289. https://doi.org/10.3390/biomedicines13061289
APA StyleSokratous, S., Mitsis, A., Khattab, E., Karelas, D., Velidakis, N., & Kadoglou, N. P. E. (2025). Coronary Microvascular Disease Early After Myocardial Infarction: Diagnostic Approach and Prognostic Value—A Narrative Review. Biomedicines, 13(6), 1289. https://doi.org/10.3390/biomedicines13061289








