Targeting Phosphodiesterase 4 in Gastrointestinal and Liver Diseases: From Isoform-Specific Mechanisms to Precision Therapeutics
Abstract
1. Introduction
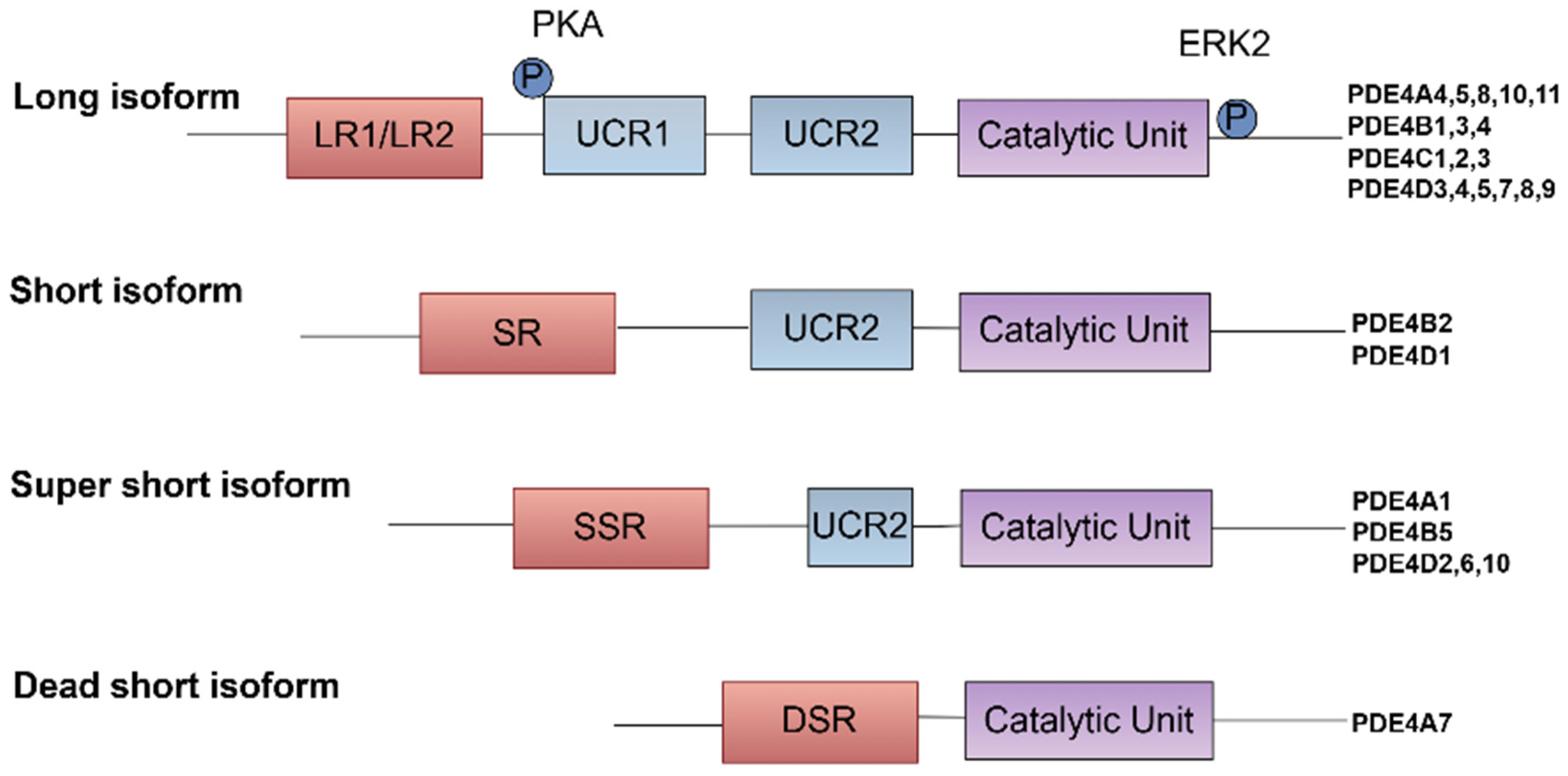
2. Structure, Gene Variants, and Properties of Phosphodiesterase 4
3. Integrated Signaling Network of PDE4-cAMP Axis in GI and Liver Diseases
4. Preclinical Evidence of PDE4 Inhibitors in GI and Liver Disease
4.1. IBD
4.2. NAFLD
4.3. ALD
4.4. Liver Fibrosis and Cirrhosis
4.5. Cancer
4.5.1. HCC
4.5.2. Colorectal Cancer
4.5.3. Gastric Cancer
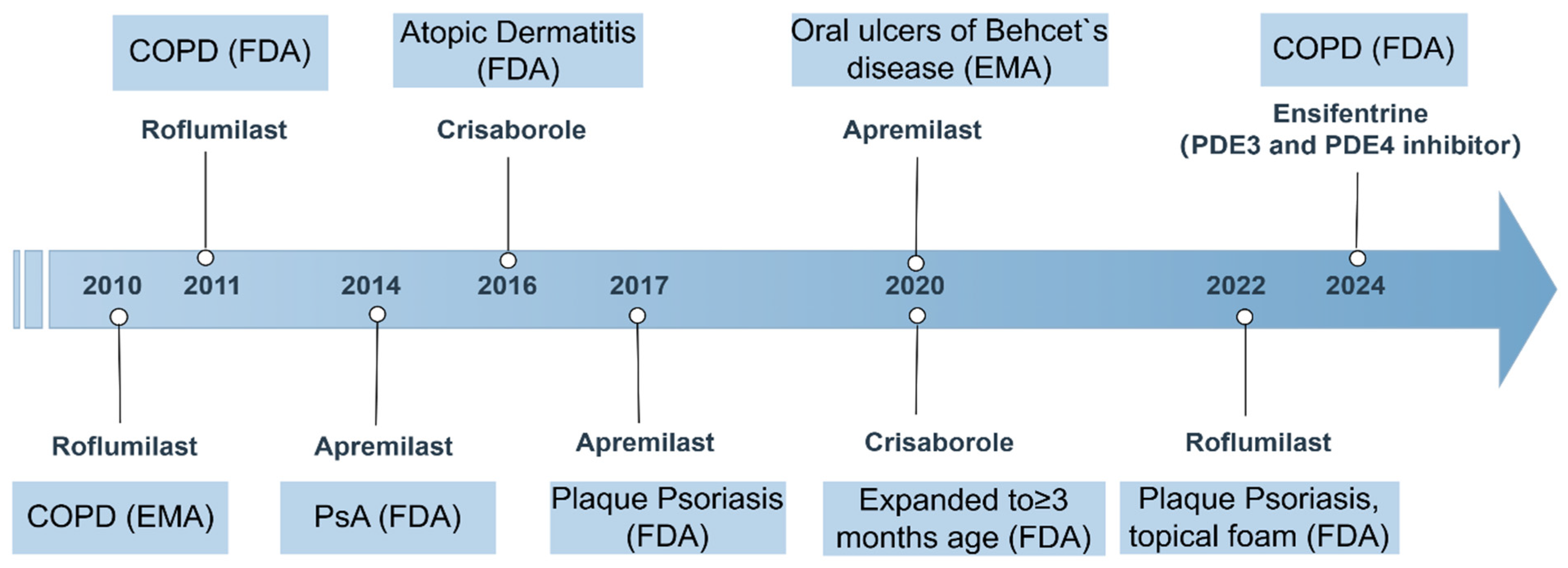
5. PDE4 Inhibitors: Current Status and Challenges
5.1. Approved PDE4 Inhibitors
5.2. Clinical Evidence
5.3. Safety and Tolerability
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wang, Y.; Yan, C.; Xiang, Y.K. Phosphodiesterase in heart and vessels: From physiology to diseases. Physiol. Rev. 2024, 104, 765–834. [Google Scholar] [CrossRef] [PubMed]
- Delhaye, S.; Bardoni, B. Role of phosphodiesterases in the pathophysiology of neurodevelopmental disorders. Mol. Psychiatry 2021, 26, 4570–4582. [Google Scholar] [CrossRef]
- Bhat, A.; Ray, B.; Mahalakshmi, A.M.; Tuladhar, S.; Nandakumar, D.; Srinivasan, M.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J.; Sakharkar, M.K. Phosphodiesterase-4 enzyme as a therapeutic target in neurological disorders. Pharmacol. Res. 2020, 160, 105078. [Google Scholar] [CrossRef]
- Porwal, K.; Pal, S.; Bhagwati, S.; Siddiqi, M.I.; Chattopadhyay, N. Therapeutic potential of phosphodiesterase inhibitors in the treatment of osteoporosis: Scopes for therapeutic repurposing and discovery of new oral osteoanabolic drugs. Eur. J. Pharmacol. 2021, 899, 174015. [Google Scholar] [CrossRef] [PubMed]
- Paes, D.; Schepers, M.; Rombaut, B.; Hove, D.v.D.; Vanmierlo, T.; Prickaerts, J.; Michel, M. The Molecular Biology of Phosphodiesterase 4 Enzymes as Pharmacological Targets: An Interplay of Isoforms, Conformational States, and Inhibitors. Pharmacol. Rev. 2021, 73, 1016–1049. [Google Scholar] [CrossRef]
- Peng, T.; Qi, B.; He, J.; Ke, H.; Shi, J. Advances in the Development of Phosphodiesterase-4 Inhibitors. J. Med. Chem. 2020, 63, 10594–10617. [Google Scholar] [CrossRef]
- Cedervall, P.; Aulabaugh, A.; Geoghegan, K.F.; McLellan, T.J.; Pandit, J. Engineered stabilization and structural analysis of the autoinhibited conformation of PDE4. Proc. Natl. Acad. Sci. USA 2015, 112, E1414–E1422. [Google Scholar] [CrossRef]
- Francis, S.H.; Blount, M.A.; Corbin, J.D. Mammalian Cyclic Nucleotide Phosphodiesterases: Molecular Mechanisms and Physiological Functions. Physiol. Rev. 2011, 91, 651–690. [Google Scholar] [CrossRef]
- Kyurkchieva, E.; Baillie, G.S. Short PDE4 Isoforms as Drug Targets in Disease. Front. Biosci. 2023, 28, 133. [Google Scholar] [CrossRef]
- Xie, M.; Blackman, B.; Scheitrum, C.; Mika, D.; Blanchard, E.; Lei, T.; Conti, M.; Richter, W. The upstream conserved regions (UCRs) mediate homo- and hetero-oligomerization of type 4 cyclic nucleotide phosphodiesterases (PDE4s). Biochem. J. 2014, 459, 539–550. [Google Scholar] [CrossRef]
- Xing, Y.; Hou, Y.; Fan, T.; Gao, R.; Feng, X.; Li, B.; Pang, J.; Guo, W.; Shu, T.; Li, J.; et al. Endothelial phosphodiesterase 4B inactivation ameliorates endothelial-to-mesenchymal transition and pulmonary hypertension. Acta Pharm. Sin. B 2024, 14, 1726–1741. [Google Scholar] [CrossRef]
- Bolger, G.B.; Dunlop, A.J.; Meng, D.; Day, J.P.; Klussmann, E.; Baillie, G.S.; Adams, D.R.; Houslay, M.D. Dimerization of cAMP phosphodiesterase-4 (PDE4) in living cells requires interfaces located in both the UCR1 and catalytic unit domains. Cell. Signal. 2015, 27, 756–769. [Google Scholar] [CrossRef]
- Martinez, A.; Gil, C. cAMP-specific phosphodiesterase inhibitors: Promising drugs for inflammatory and neurological diseases. Expert Opin. Ther. Patents 2014, 24, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.; Elliott, C.; Hoffmann, R.; Baillie, G.S. The activity of cAMP-phosphodiesterase 4D7 (PDE4D7) is regulated by protein kinase A-dependent phosphorylation within its unique N-terminus. FEBS Lett. 2015, 589, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Zaccolo, M.; Kovanich, D. Nanodomain cAMP signaling in cardiac pathophysiology: Potential for developing targeted therapeutic interventions. Physiol. Rev. 2025, 105, 541–591. [Google Scholar] [CrossRef]
- Zaccolo, M.; Zerio, A.; Lobo, M.J.; Garland, C. Subcellular Organization of the cAMP Signaling Pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef]
- Movsesian, M. Novel approaches to targeting PDE3 in cardiovascular disease. Pharmacol. Ther. 2016, 163, 74–81. [Google Scholar] [CrossRef]
- Samidurai, A.; Xi, L.; Das, A.; Iness, A.N.; Vigneshwar, N.G.; Li, P.-L.; Singla, D.K.; Muniyan, S.; Batra, S.K.; Kukreja, R.C. Role of phosphodiesterase 1 in the pathophysiology of diseases and potential therapeutic opportunities. Pharmacol. Ther. 2021, 226, 107858. [Google Scholar] [CrossRef]
- Armani, A.; Marzolla, V.; Rosano, G.M.; Fabbri, A.; Caprio, M. Phosphodiesterase type 5 (PDE5) in the adipocyte: A novel player in fat metabolism? Trends Endocrinol. Metab. 2011, 22, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Sorrentino, S.; Medalia, O.; Korkhov, V.M. The structure of a membrane adenylyl cyclase bound to an activated stimulatory G protein. Science 2019, 364, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Schindler, R. New kids on the block: The Popeye domain containing (POPDC) protein family acting as a novel class of cAMP effector proteins in striated muscle. Cell. Signal. 2017, 40, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; VanSchouwen, B.; Melacini, G. The structure of the apo cAMP-binding domain of HCN4—A stepping stone toward understanding the cAMP-dependent modulation of the hyperpolarization-activated cyclic-nucleotide-gated ion channels. FEBS J. 2018, 285, 2182–2192. [Google Scholar] [CrossRef]
- Li, H.; Fan, C.; Feng, C.; Wu, Y.; Lu, H.; He, P.; Yang, X.; Zhu, F.; Qi, Q.; Gao, Y.; et al. Inhibition of phosphodiesterase-4 attenuates murine ulcerative colitis through interference with mucosal immunity. Br. J. Pharmacol. 2019, 176, 2209–2226. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, M.; Fan, C.; Feng, C.; Lu, Q.; Xiang, C.; Lu, H.; Yang, X.; Wu, B.; et al. Targeting PDE4 as a promising therapeutic strategy in chronic ulcerative colitis through modulating mucosal homeostasis. Acta Pharm. Sin. B 2021, 12, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Woode, R.A.; Strubberg, A.M.; Liu, J.; Walker, N.M.; Clarke, L.L. Increased activity of epithelial Cdc42 Rho GTPase and tight junction permeability in the Cftr knockout intestine. Am. J. Physiol. Liver Physiol. 2024, 327, G545–G557. [Google Scholar] [CrossRef]
- Keely, S.; Kelly, C.J.; Weissmueller, T.; Burgess, A.; Wagner, B.D.; Robertson, C.E.; Harris, J.K.; Colgan, S.P. Activated fluid transport regulates bacterial-epithelial interactions and significantly shifts the murine colonic microbiome. Gut Microbes 2012, 3, 250–260. [Google Scholar] [CrossRef]
- Feng, H.; Chen, J.; Wang, H.; Cheng, Y.; Zou, Z.; Zhong, Q.; Xu, J. Roflumilast reverses polymicrobial sepsis-induced liver damage by inhibiting inflammation in mice. Mod. Pathol. 2017, 97, 1008–1019. [Google Scholar] [CrossRef]
- Lim, J.-H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic Acid Stimulates Complete Oxidation of Fatty Acids through Protein Kinase A-dependent Activation of SIRT1-PGC1α Complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Petrovsky, N. Calcium Signaling As a Therapeutic Target for Liver Steatosis. Trends Endocrinol. Metab. 2019, 30, 270–281. [Google Scholar] [CrossRef]
- Elnagdy, M.; Wang, Y.; Rodriguez, W.; Zhang, J.; Bauer, P.; Wilkey, D.W.; Merchant, M.; Pan, J.; Farooqui, Z.; Cannon, R.; et al. Increased expression of phosphodiesterase 4 in activated hepatic stellate cells promotes cytoskeleton remodeling and cell migration. J. Pathol. 2023, 261, 361–371. [Google Scholar] [CrossRef]
- Milara, J.; Ribera, P.; Marín, S.; Montero, P.; Roger, I.; Tenor, H.; Cortijo, J. Phosphodiesterase 4 is overexpressed in human keloids and its inhibition reduces fibroblast activation and skin fibrosis. Chem. Interactions 2024, 402, 111211. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, V.; Luaces-Regueira, M.; Campos-Toimil, M. The cAMP effectors PKA and Epac activate endothelial NO synthase through PI3K/Akt pathway in human endothelial cells. Biochem. Pharmacol. 2017, 145, 94–101. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Chen, Y.; Ao, Z.; Cheng, Q.; Yang, X.; Tao, H.; Zhao, L.; Shen, A.; Li, P.; Fu, Q. PDE4D binds and interacts with YAP to cooperatively promote HCC progression. Cancer Lett. 2022, 541, 215749. [Google Scholar] [CrossRef]
- Amunjela, J.N.; Tucker, S.J. POPDC proteins as potential novel therapeutic targets in cancer. Drug Discov. Today 2016, 21, 1920–1927. [Google Scholar] [CrossRef]
- Gingold-Belfer, R.; Kessler-Icekson, G.; Morgenstern, S.; Rath-Wolfson, L.; Zemel, R.; Boltin, D.; Levi, Z.; Herman-Edelstein, M. The Transition from Gastric Intestinal Metaplasia to Gastric Cancer Involves POPDC1 and POPDC3 Downregulation. Int. J. Mol. Sci. 2021, 22, 5359. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Sun, B.-Y.; Li, Q.; Dong, L.; Zhang, G.-H.; Grundy, D.; Rong, W.-F. Hyperpolarization-activated cyclic nucleotide-gated cation channel subtypes differentially modulate the excitability of murine small intestinal afferents. World J. Gastroenterol. 2012, 18, 522–531. [Google Scholar] [CrossRef]
- Cosín-Roger, J. Inflammatory Bowel Disease: Immune Function, Tissue Fibrosis and Current Therapies. Int. J. Mol. Sci. 2024, 25, 6416. [Google Scholar] [CrossRef]
- Chen, C.-J.; Hu, H.; Liao, W.-T.; Gaffney, P.; Gaffney, R.; Mravec, B. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2021, 384, 1376–1378. [Google Scholar] [CrossRef]
- Komatsu, K.; Lee, J.-Y.; Miyata, M.; Lim, J.H.; Jono, H.; Koga, T.; Xu, H.; Yan, C.; Kai, H.; Li, J.-D. Inhibition of PDE4B suppresses inflammation by increasing expression of the deubiquitinase CYLD. Nat. Commun. 2013, 4, 1684. [Google Scholar] [CrossRef]
- Scalavino, V.; Piccinno, E.; Labarile, N.; Armentano, R.; Giannelli, G.; Serino, G. Anti-Inflammatory Effects of miR-369-3p via PDE4B in Intestinal Inflammatory Response. Int. J. Mol. Sci. 2024, 25, 8463. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Huang, Y.; Deng, J.; Xie, X.; Zhu, J.; Yuan, Y.; He, Y.-M.; Huang, Y.-Y.; Luo, H.-B.; et al. Discovery of novel PDE4 inhibitors targeting the M-pocket from natural mangostanin with improved safety for the treatment of Inflammatory Bowel Diseases. Eur. J. Med. Chem. 2022, 242, 114631. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, K.; Xie, Y.; Huang, J.; Xia, C.; Bao, Y.-X.; Bi, H.; Wang, J.; Zhou, Z.-Z. Discovery of novel N2-indazole derivatives as phosphodiesterase 4 inhibitors for the treatment of inflammatory bowel disease. Eur. J. Med. Chem. 2024, 277, 116710. [Google Scholar] [CrossRef] [PubMed]
- Bagalagel, A.; Diri, R.; Noor, A.; Almasri, D.; Bakhsh, H.T.; Kutbi, H.I.; Al-Gayyar, M.M.H. Curative effects of fucoidan on acetic acid induced ulcerative colitis in rats via modulating aryl hydrocarbon receptor and phosphodiesterase-4. BMC Complement. Med. Ther. 2022, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-K.; Veeraperumal, S.; Aweya, J.J.; Liu, Y.; Cheong, K.-L. Fucoidan modulates gut microbiota and immunity in Peyer’s patches against inflammatory bowel disease. Carbohydr. Polym. 2024, 342, 122421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Su, J.; Zhong, R.; Yin, S.; Zhao, Z.; Sun, Z. Hypersampsonone H attenuates ulcerative colitis via inhibition of PDE4 and regulation of cAMP/PKA/CREB signaling pathway. Int. Immunopharmacol. 2024, 128, 111490. [Google Scholar] [CrossRef]
- Song, C.; Wu, J.; Wu, J.; Wang, F. MnO2 and roflumilast-loaded probiotic membrane vesicles mitigate experimental colitis by synergistically augmenting cAMP in macrophage. J. Nanobiotechnology 2024, 22, 294. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; A Bolinger, A.; Chen, H.; Liu, Z.; Cong, Y.; Brasier, A.R.; Pinchuk, I.V.; Tian, B.; Zhou, J. Target-Based Small Molecule Drug Discovery Towards Novel Therapeutics for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, S38–S62. [Google Scholar] [CrossRef]
- Spadaccini, M.; D’alessio, S.; Peyrin-Biroulet, L.; Danese, S. PDE4 Inhibition and Inflammatory Bowel Disease: A Novel Therapeutic Avenue. Int. J. Mol. Sci. 2017, 18, 1276. [Google Scholar] [CrossRef] [PubMed]
- Banner, K.H.; Trevethick, M.A. PDE4 inhibition: A novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol. Sci. 2004, 25, 430–436. [Google Scholar] [CrossRef]
- E Powell, E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Y.; Li, Z.; Hua, Q.; Jiang, M.; Fan, X. LncRNA GAS5 Knockdown Mitigates Hepatic Lipid Accumulation via Regulating MiR-26a-5p/PDE4B to Activate cAMP/CREB Pathway. Front. Endocrinol. 2022, 13, 889858. [Google Scholar] [CrossRef]
- Staller, D.W.; Bennett, R.G.; Mahato, R.I. Therapeutic perspectives on PDE4B inhibition in adipose tissue dysfunction and chronic liver injury. Expert Opin. Ther. Targets 2024, 28, 545–573. [Google Scholar] [CrossRef]
- Vollert, S.; Kaessner, N.; Heuser, A.; Hanauer, G.; Dieckmann, A.; Knaack, D.; Kley, H.P.; Beume, R.; Weiss-Haljiti, C. The glucose-lowering effects of the PDE4 inhibitors roflumilast and roflumilast-N-oxide in db/db mice. Diabetologia 2012, 55, 2779–2788. [Google Scholar] [CrossRef]
- Möllmann, J.; Kahles, F.; Lebherz, C.; Kappel, B.; Baeck, C.; Tacke, F.; Werner, C.; Federici, M.; Marx, N.; Lehrke, M. The PDE4 inhibitor roflumilast reduces weight gain by increasing energy expenditure and leads to improved glucose metabolism. Diabetes Obes. Metab. 2017, 19, 496–508. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Yu, S.; Xue, H.; Deng, L.; Zhang, Y.; Zhang, Y.; Liu, Y. Roflumilast ameliorates GAN diet-induced non-alcoholic fatty liver disease by reducing hepatic steatosis and fibrosis in ob/ob mice. Biochem. Biophys. Res. Commun. 2024, 722, 150170. [Google Scholar] [CrossRef]
- Tao, X.; He, H.; Peng, J.; Xu, R.; Fu, J.; Hu, Y.; Li, L.; Yang, X.; Feng, X.; Zhang, C.; et al. Overexpression of PDE4D in mouse liver is sufficient to trigger NAFLD and hypertension in a CD36-TGF-β1 pathway: Therapeutic role of roflumilast. Pharmacol. Res. 2022, 175, 106004. [Google Scholar] [CrossRef] [PubMed]
- Staller, D.W.; Panigrahi, S.S.; Jayasinghe, Y.P.; Dong, Y.; Mahto, S.; Kumar, V.; Ronning, D.R.; Mahato, R.I. A novel phosphodiesterase inhibitor for the treatment of chronic liver injury and metabolic diseases. Hepatology 2024, 81, 1288–1303. [Google Scholar] [CrossRef]
- Tao, X.; Chen, C.; Huang, Z.; Lei, Y.; Wang, M.; Wang, S.; Tian, D. Genetic deletion of phosphodiesterase 4D in the liver improves kidney damage in high-fat fed mice: Liver-kidney crosstalk. Cell Death Dis. 2023, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Ratziu, V.; Bedossa, P.; Francque, S.M.; Larrey, D.; Aithal, G.P.; Serfaty, L.; Voiculescu, M.; Preotescu, L.; Nevens, F.; De Lédinghen, V.; et al. Lack of Efficacy of an Inhibitor of PDE4 in Phase 1 and 2 Trials of Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1724–1730.e5. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. The tribulations of conducting NASH trials. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 274–276. [Google Scholar] [CrossRef]
- Frederick, E. Bacterial toxin linked to severe alcoholic liver disease. Science 2019, 366, 784. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Eguileor, A.; Mrdjen, M.; Pathak, V.; Travers, J.; Nagy, L.E.; Llorente, C. Gut-liver axis: Recent concepts in pathophysiology in alcohol-associated liver disease. Hepatology 2024, 80, 1342–1371. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef]
- Cohen, J.I.; Chen, X.; Nagy, L.E. Redox Signaling and the Innate Immune System in Alcoholic Liver Disease. Antioxid. Redox Signal. 2011, 15, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.V.; Barker, D.F.; Zhang, J.; McClain, C.J.; Barve, S.; Gobejishvili, L. Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J. Pathol. 2016, 240, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, W.E.; Wahlang, B.; Wang, Y.; Zhang, J.; Vadhanam, M.V.; Joshi-Barve, S.; Bauer, P.; Cannon, R.; Ahmadi, A.R.; Sun, Z.; et al. Phosphodiesterase 4 Inhibition as a Therapeutic Target for Alcoholic Liver Disease: From Bedside to Bench. Hepatology 2019, 70, 1958–1971. [Google Scholar] [CrossRef]
- Elnagdy, M.; Barve, S.; McClain, C.; Gobejishvili, L. cAMP Signaling in Pathobiology of Alcohol Associated Liver Disease. Biomolecules 2020, 10, 1433. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Barve, S.; Joshi-Barve, S.; McClain, C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: Relevance to alcoholic liver disease. Am. J. Physiol. Liver Physiol. 2008, 295, G718–G724. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, K.B.; Mangieri, R.A.; Roberts, A.J.; Lopez, M.F.; Firsick, E.J.; Townsley, K.G.; Beneze, A.; Bess, J.; Eisenstein, T.K.; Meissler, J.J.; et al. Preclinical and clinical evidence for suppression of alcohol intake by apremilast. J. Clin. Investig. 2023, 133, e159103. [Google Scholar] [CrossRef]
- Zheng, L.; Aimaiti, Z.; Long, L.; Xia, C.; Wang, W.; Zhou, Z.-Z. Discovery of 4-Ethoxy-6-chloro-5-azaindazoles as Novel PDE4 Inhibitors for the Treatment of Alcohol Use Disorder and Alcoholic Liver Diseases. J. Med. Chem. 2023, 67, 728–753. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kumar, V.; Mahato, R.I. Nanoparticle Delivery of Novel PDE4B Inhibitor for the Treatment of Alcoholic Liver Disease. Pharmaceutics 2022, 14, 1894. [Google Scholar] [CrossRef]
- Wang, S.; Friedman, S.L. Hepatic fibrosis: A convergent response to liver injury that is reversible. J. Hepatol. 2020, 73, 210–211. [Google Scholar] [CrossRef]
- Lackner, C.; Tiniakos, D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019, 70, 294–304. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Z.; Wang, F.-S. Liver fibrosis: Mechanisms of immune-mediated liver injury. Cell. Mol. Immunol. 2011, 9, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Barve, S.; Breitkopf-Heinlein, K.; Li, Y.; Zhang, J.; Avila, D.V.; Dooley, S.; McClain, C.J. Rolipram Attenuates Bile Duct Ligation–Induced Liver Injury in Rats: A Potential Pathogenic Role of PDE4. J. Pharmacol. Exp. Ther. 2013, 347, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ahloulay, M.; Bankir, L.; Lugnier, C.; Le Bec, A.; Poirel, O.; Moreau, R.; Lebrec, D. Cyclic AMP-phosphodiesterases inhibitor improves sodium excretion in rats with cirrhosis and ascites. Liver Int. 2005, 25, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Essam, R.M.; Ahmed, L.A.; Abdelsalam, R.M.; El-Khatib, A.S. Phosphodiestrase-1 and 4 inhibitors ameliorate liver fibrosis in rats: Modulation of cAMP/CREB/TLR4 inflammatory and fibrogenic pathways. Life Sci. 2019, 222, 245–254. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, G.; Ma, L.; Dong, L.; Chen, S.; Shen, X.; Zhang, S.; Xue, R.; Sun, D.; Zhang, S. Smurf2, an E3 ubiquitin ligase, interacts with PDE4B and attenuates liver fibrosis through miR-132 mediated CTGF inhibition. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 297–308. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Zhang, X.; Wu, Y.; Yang, J.; Li, J. Autophagy induces transforming growth factor-β-dependent epithelial-mesenchymal transition in hepatocarcinoma cells through cAMP response element binding signalling. J. Cell. Mol. Med. 2018, 22, 5518–5532. [Google Scholar] [CrossRef]
- Massimi, M.; Cardarelli, S.; Galli, F.; Giardi, M.F.; Ragusa, F.; Panera, N.; Cinque, B.; Cifone, M.G.; Biagioni, S.; Giorgi, M. Increase of Intracellular Cyclic AMP by PDE4 Inhibitors Affects HepG2 Cell Cycle Progression and Survival. J. Cell. Biochem. 2016, 118, 1401–1411. [Google Scholar] [CrossRef]
- Sun, L.; Quan, H.; Xie, C.; Wang, L.; Hu, Y.; Lou, L. Phosphodiesterase 3/4 Inhibitor Zardaverine Exhibits Potent and Selective Antitumor Activity against Hepatocellular Carcinoma Both In Vitro and In Vivo Independently of Phosphodiesterase Inhibition. PLoS ONE 2014, 9, e90627. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Sepanlou, S.G.; Ikuta, K.S.; Bisignano, C.; Salimzadeh, H.; Delavari, A.; Ansari, R.; Roshandel, G.; Merat, S.; Fitzmaurice, C.; et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Ota, T.; Fujimoto, T.; Doi, K.; Tanaka, Y.; Yoshida, Y.; Ogawa, M.; Matsuzaki, H.; Hamabashiri, M.; Tyson, D.R.; et al. Inhibition of Phosphodiesterase-4 (PDE4) activity triggers luminal apoptosis and AKT dephosphorylation in a 3-D colonic-crypt model. Mol. Cancer 2012, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Nam, J.; Cha, M.D.; Kim, S. Inhibition of phosphodiesterase 4D decreases the malignant properties of DLD-1 colorectal cancer cells by repressing the AKT/mTOR/Myc signaling pathway. Oncol. Lett. 2019, 17, 3589–3598. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Liu, J.; Chen, J.; Wang, J.; Hua, H.; Jiang, Y. cAMP-PKA/EPAC signaling and cancer: The interplay in tumor microenvironment. J. Hematol. Oncol. 2024, 17, 5. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Mavropoulos, A.; Bogdanos, D.P. Phosphodiesterase 4 Inhibitors in Immune-mediated Diseases: Mode of Action, Clinical Applications, Current and Future Perspectives. Curr. Med. Chem. 2017, 24, 3054–3067. [Google Scholar] [CrossRef]
- Cooney, J.D.; Aguiar, R.C.T. Phosphodiesterase 4 inhibitors have wide-ranging activity in B-cell malignancies. Blood 2016, 128, 2886–2890. [Google Scholar] [CrossRef]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Tian, Y.; Ao, G. PDE4a predicts poor prognosis and promotes metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. J. Cancer 2018, 9, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Peng, L.; Chen, Z.; Hu, Y.; Tao, L.; Yao, Y.; Wu, Y.; Yang, D.; Xu, T. Recent advances of Phosphodiesterase 4B in cancer. Expert Opin. Ther. Targets 2023, 27, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, J.; Yang, J.; Yan, Y.; Yang, C.; He, X.; Huang, R.; Tan, M.; Wu, D.; Yan, J.; et al. PDE4B Induces Epithelial-to-Mesenchymal Transition in Bladder Cancer Cells and Is Transcriptionally Suppressed by CBX7. Front. Cell Dev. Biol. 2021, 9, 783050. [Google Scholar] [CrossRef]
- Su, R. PDE4B promotes the progression of gastric cancer via the PI3K/AKT/MYC pathway and immune infiltration. Am. J. Cancer Res. 2024, 14, 3451–3467. [Google Scholar] [CrossRef]
- Kashiwagi, E.; Shiota, M.; Yokomizo, A.; Itsumi, M.; Inokuchi, J.; Uchiumi, T.; Naito, S. Downregulation of phosphodiesterase 4B (PDE4B) activates protein kinase A and contributes to the progression of prostate cancer. Prostate 2011, 72, 741–751. [Google Scholar] [CrossRef]
- Wright, T.A.; Gemmell, A.O.; Tejeda, G.S.; Blair, C.M.; Baillie, G.S. Cancer: Phosphodiesterase type 4C (PDE4C), the forgotten subfamily as a therapeutic target. Int. J. Biochem. Cell Biol. 2023, 162, 106453. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Goldstein, J.; Tran, B.; Ensor, J.; Gibbs, P.; Wong, H.L.; Wong, S.F.; Vilar, E.; Tie, J.; Broaddus, R.; Kopetz, S.; et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann. Oncol. 2014, 25, 1032–1038. [Google Scholar] [CrossRef]
- Kim, D.U.; Kwak, B.; Kim, S.-W. Phosphodiesterase 4B is an effective therapeutic target in colorectal cancer. Biochem. Biophys. Res. Commun. 2019, 508, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ai, G.; Wang, D.; Chen, R.; Guo, D.; Yao, Y.; Wang, K.; Liang, G.; Qi, F.; Liu, W.; et al. PDE4 and Epac1 Synergistically Promote Rectal Carcinoma via the cAMP Pathway. Anal. Cell. Pathol. 2019, 2019, 7145198. [Google Scholar] [CrossRef] [PubMed]
- Bevanda, M.; Kelam, N.; Racetin, A.; Filipović, N.; Glibo, D.B.; Bevanda, I.; Vukojević, K. Expression Pattern of PDE4B, PDE4D, and SFRP5 Markers in Colorectal Cancer. Medicina 2024, 60, 1202. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, K.; Liao, J.M.; Zhou, X.; Liao, P.; Zeng, S.X.; He, M.; Chen, L.; He, Y.; Li, W.; et al. Inactivation of oncogenic cAMP-specific phosphodiesterase 4D by miR-139-5p in response to p53 activation. eLife 2016, 5, e15978. [Google Scholar] [CrossRef]
- Nummela, P.; Zafar, S.; Veikkolainen, E.; Ukkola, I.; Cinella, V.; Ayo, A.; Asghar, M.Y.; Välimäki, N.; Törnquist, K.; Karhu, A.; et al. GNAS mutation inhibits growth and induces phosphodiesterase 4D expression in colorectal cancer cell lines. Int. J. Cancer 2024, 154, 1987–1998. [Google Scholar] [CrossRef]
- Xu, N.; Gao, Z.; Wu, D.; Chen, H.; Zhang, Z.; Zhang, L.; Wang, Y.; Lu, X.; Yao, X.; Liu, X.; et al. 5-hydroxymethylcytosine features of portal venous blood predict metachronous liver metastases of colorectal cancer and reveal phosphodiesterase 4 as a therapeutic target. Clin. Transl. Med. 2025, 15, e70189. [Google Scholar] [CrossRef]
- Xu, T.; Xie, M.; Jing, X.; Jiang, H.; Wu, X.; Wang, X.; Shu, Y. Loss of miR-26b-5p promotes gastric cancer progression via miR-26b-5p-PDE4B/CDK8-STAT3 feedback loop. J. Transl. Med. 2023, 21, 77. [Google Scholar] [CrossRef]
- Lin, D.-C.; Xu, L.; Ding, L.-W.; Sharma, A.; Liu, L.-Z.; Yang, H.; Tan, P.; Vadgama, J.; Karlan, B.Y.; Lester, J.; et al. Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc. Natl. Acad. Sci. USA 2013, 110, 6109–6114. [Google Scholar] [CrossRef]
- Li, G.; He, D.; Cai, X.; Guan, W.; Zhang, Y.; Wu, J.-Q.; Yao, H. Advances in the development of phosphodiesterase-4 inhibitors. Eur. J. Med. Chem. 2023, 250, 115195. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Kircik, L.H.; Moore, A.Y.; Gold, L.S.; Draelos, Z.D.; Gooderham, M.J.; Papp, K.A.; Bagel, J.; Bhatia, N.; Del Rosso, J.Q.; et al. Effect of Roflumilast Cream vs Vehicle Cream on Chronic Plaque Psoriasis. JAMA 2022, 328, 1073–1084. [Google Scholar] [CrossRef]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.; Papp, K. Selective Phosphodiesterase Inhibitors for Psoriasis: Focus on Apremilast. BioDrugs 2015, 29, 327–339. [Google Scholar] [CrossRef]
- Deeks, E.D. Apremilast: A Review in Oral Ulcers of Behçet’s Disease. Drugs 2020, 80, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Cather, J.; Gooderham, M.; Poulin, Y.; Mrowietz, U.; Ferrandiz, C.; Crowley, J.; Hu, C.; Stevens, R.; Shah, K.; et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: A phase III, randomized controlled trial (ESTEEM 2). Br. J. Dermatol. 2015, 173, 1387–1399. [Google Scholar] [CrossRef]
- Mease, P.J.; Hatemi, G.; Paris, M.; Cheng, S.; Maes, P.; Zhang, W.; Shi, R.; Flower, A.; Picard, H.; Gold, L.S. Apremilast Long-Term Safety Up to 5 Years from 15 Pooled Randomized, Placebo-Controlled Studies of Psoriasis, Psoriatic Arthritis, and Behçet’s Syndrome. Am. J. Clin. Dermatol. 2023, 24, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Udkoff, J.; Waldman, A.; Borok, J.; Eichenfield, L.F. Phosphodiesterase 4 Inhibitor Therapies for Atopic Dermatitis: Progress and Outlook. Drugs 2017, 77, 1389–1397. [Google Scholar] [CrossRef]
- Keam, S.J. Ensifentrine: First Approval. Drugs 2024, 84, 1157–1163. [Google Scholar] [CrossRef]
- Gobejishvili, L.; E Rodriguez, W.; Bauer, P.; Wang, Y.; Soni, C.; Lydic, T.; Barve, S.; McClain, C.; Maldonado, C. Novel Liposomal Rolipram Formulation for Clinical Application to Reduce Emesis. Drug Des. Dev. Ther. 2022, 16, 1301–1309. [Google Scholar] [CrossRef]

| PDE4 Inhibitor | Disease/Target | Role | References |
|---|---|---|---|
| Apremilast | DSS-induced colitis; PDE4 | Regulates intestinal inflammation, rebuilds the mucosal homeostasis, and remaps gut microbiota | [25,26] |
| 22d | DSS-induced IBD; PDE4, PDE7 | Relives inflammatory injuries, and increases body weight | [45] |
| LZ-14 | IBD; PDE4D7 | Improves the inflammatory response and colon injury | [46] |
| Fucoidan | UC; AHR, PDE4 | Restores the normal weight and length of the colon; enhances antioxidant activity; modulates gut microbiota; anti-inflammation | [47,48] |
| HS-1 | DSS-induced colitis; PDE4D | Protects the integrity of intestinal epithelial barrier and reduces tissue fibrosis; anti-inflammation | [49] |
| MnO2 and roflumilast-loaded probiotic membrane vesicles | DSS-induced colitis; PDE4 | Regulate gut microbe; produce more cAMP and less TNF-α in macrophage | [50] |
| Roflumilast | NAFLD; PDE4, PDE4D | Reduces hepatic steatosis and fibrosis; improves glucose metabolism | [58,59,60,61,63] |
| A33 and MDL3 | Chronic liver injury and metabolic diseases; PDE4B and PDE5A | Ameliorate pathophysiological signs and symptoms of liver injury and inflammation | [62] |
| Rolipram | ALD; PDE4, PDE4B | Regulates FA oxidation, and regulates ER stress and apoptosis | [72,73,75] |
| Apremilast | Alcohol-use disorders; PDE4 | Suppresses alcohol intake | [76] |
| ZL40 | ALD; PDE4 | Attenuates inflammation and decreases alcohol intake | [77] |
| KVA-D88-loaded NPs | ALD; PDE4B | Ameliorate alcohol-induced hepatic injury, steatosis, and inflammation | [78] |
| Rolipram | Bile duct ligation-induced hepatic injury and fibrogenesis; PDE4 | Hepatic inflammatory and profibrotic cytokine expression, injury, and fibrosis | [85] |
| Rolipram | cirrhotic rats with ascites | Increases sodium and phosphate excretion | [86] |
| Roflumilast | DEN-induced liver fibrosis; PDE4 | Modulates cAMP/CREB/TLR4 inflammatory and fibrogenic pathways | [87] |
| Roflumilast | HCC; PDE4 | Inhibits growth, EMT, and invasion of HCC cancer cells | [37,89] |
| Rolipram and DC-TA-46 | HCC; PDE4 | Affect HepG2 cell cycle and survival | [90] |
| Zardaverine | HCC; PDE3/4 | Regulates of Rb or Rb-associated signaling in cell cycles | [91] |
| Resveratrol | CRC; PDE4 | Suppresses tumor | [92] |
| Rolipram | CRC | Disrupts luminal cavity formation and CRC development | [93,94] |
| Drug Name | Sponsor | ID/Status | Indications | Phase |
|---|---|---|---|---|
| ASP9831 | Astellas Pharma Inc. (Tokyo, Japan) | NCT00668070; completed | NASH | Phase 2 |
| Roflumilast | AstraZeneca (Cambridge, UK) | NCT01703260; terminated | NASH | Phase 2 |
| Roflumilast | Tanta University | NCT06677788; completed | NASH | Phase 2 |
| Roflumilast | Tanta University | NCT05684484; not recruiting | UC | Phase 4 |
| PALI-2108 | Palisade Bio (Carlsbad, CA, USA) | NCT06663605; recruiting | UC | Phase 1 |
| Cilostazol | Sadat City University | NCT04761848; active, not recruiting | Fatty liver disease | Phase1/2 |
| Hemay005 | Ganzhou Hemay Pharmaceutical Co., Ltd. (Ganzhou, China) | NCT05486104; recruiting | Moderate-to-severe UC | Phase 2 |
| Tetomilast (OPC-6535) | Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan) | NCT00989573; completed | CD | Phase 3 |
| Tetomilast (OPC-6535) | Otsuka Pharmaceutical Development & Commercialization, Inc. (Rockville, MD, USA) | NCT00064454; completed | UC | Phase 3 |
| OPC-6535 | Otsuka Pharmaceutical Co., Ltd. | NCT00317369; terminated | CD | Phase 2 |
| OPC-6535 | Otsuka Pharmaceutical Co., Ltd. | NCT00317356; terminated | UC | Phase 2 |
| OPC-6535 | Otsuka Pharmaceutical Development & Commercialization, Inc. | NCT00064441; completed | UC | Phase 3 |
| OPC-6535 With Asacol® | Otsuka Pharmaceutical Development & Commercialization, Inc. | NCT00092508; completed | UC | Phase 3 |
| Apremilast | Amgen (Thousand Oaks, CA, USA) | NCT02289417; completed | UC | Phase 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, M.; Tao, X. Targeting Phosphodiesterase 4 in Gastrointestinal and Liver Diseases: From Isoform-Specific Mechanisms to Precision Therapeutics. Biomedicines 2025, 13, 1285. https://doi.org/10.3390/biomedicines13061285
Chen C, Liu M, Tao X. Targeting Phosphodiesterase 4 in Gastrointestinal and Liver Diseases: From Isoform-Specific Mechanisms to Precision Therapeutics. Biomedicines. 2025; 13(6):1285. https://doi.org/10.3390/biomedicines13061285
Chicago/Turabian StyleChen, Can, Mei Liu, and Xiang Tao. 2025. "Targeting Phosphodiesterase 4 in Gastrointestinal and Liver Diseases: From Isoform-Specific Mechanisms to Precision Therapeutics" Biomedicines 13, no. 6: 1285. https://doi.org/10.3390/biomedicines13061285
APA StyleChen, C., Liu, M., & Tao, X. (2025). Targeting Phosphodiesterase 4 in Gastrointestinal and Liver Diseases: From Isoform-Specific Mechanisms to Precision Therapeutics. Biomedicines, 13(6), 1285. https://doi.org/10.3390/biomedicines13061285




