Interleukin-37 Suppresses the Function of Type 2 Follicular Helper T in Allergic Rhinitis
Abstract
1. Introduction
2. Methods
2.1. Patient Recruitment
2.2. Blood Samples Preparation
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Flow Cytometry for Tfh2 Cells
2.6. Cell Sorting
2.7. Mouse Model
2.8. Histologic Analysis
2.9. Statistical Analysis
3. Results
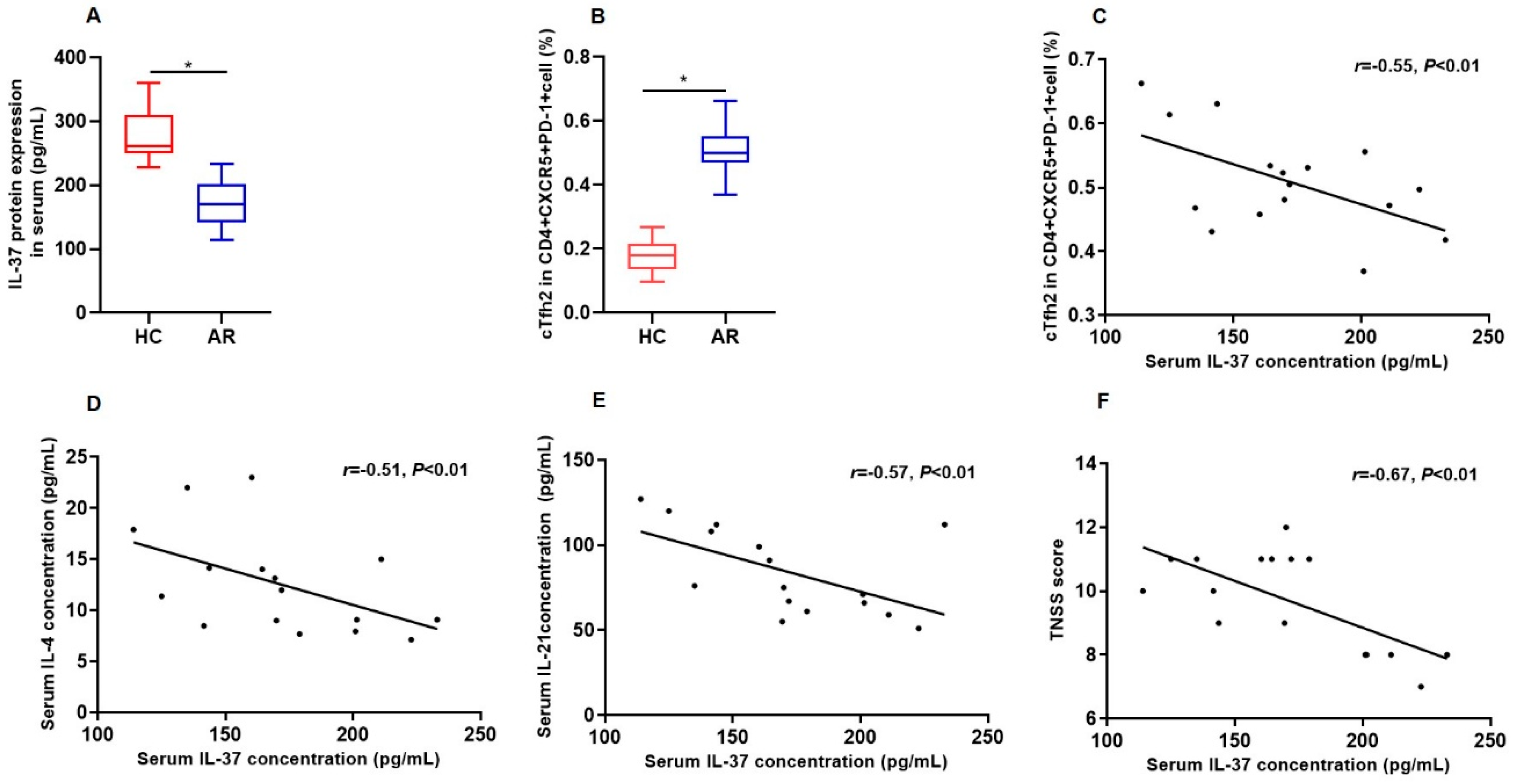
3.1. Serum Protein Concentration of IL-37 and Its Correlation with the Frequencies of Tfh2 in AR
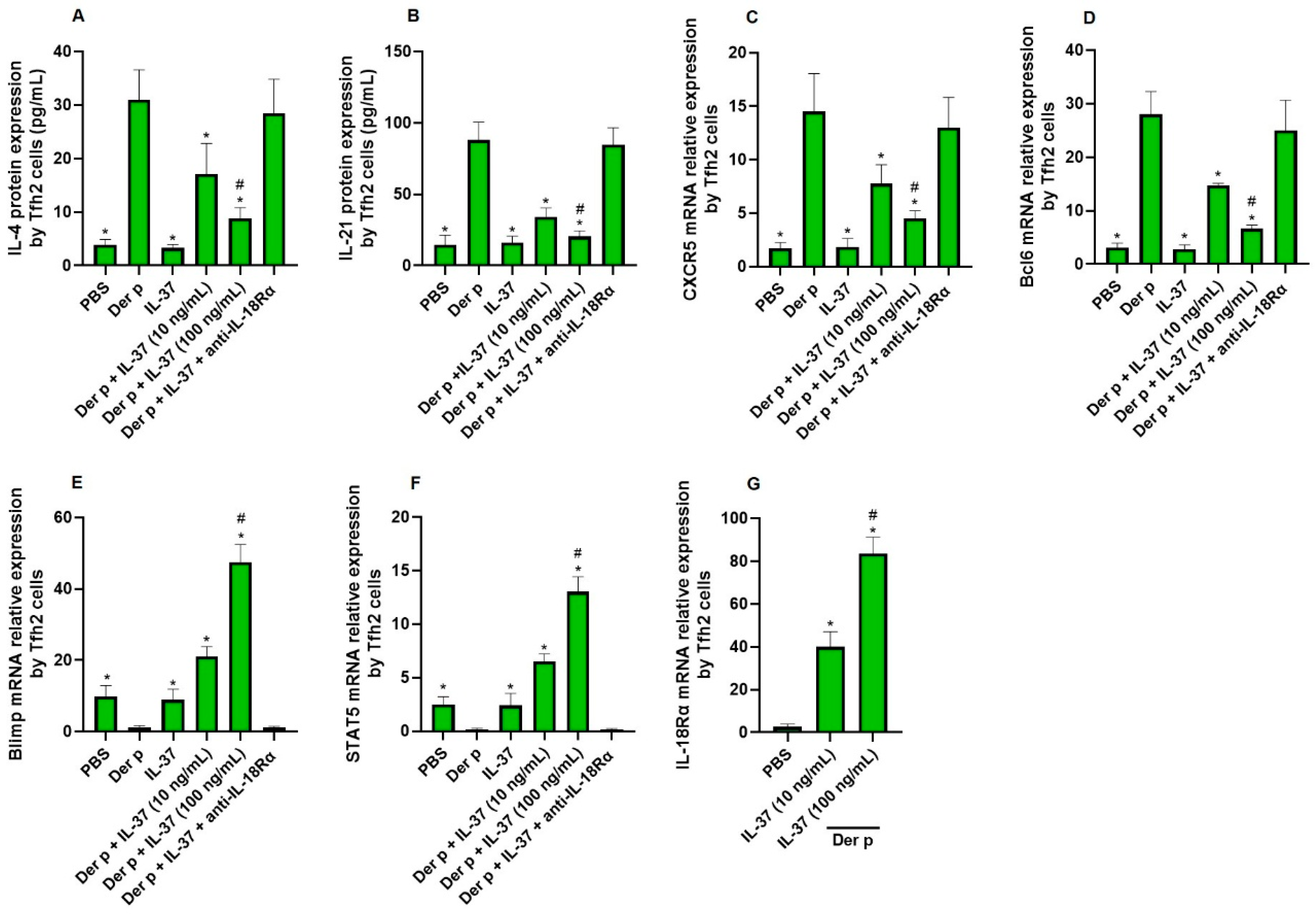
3.2. The Direct Effect of IL-37 on Tfh2 Cells
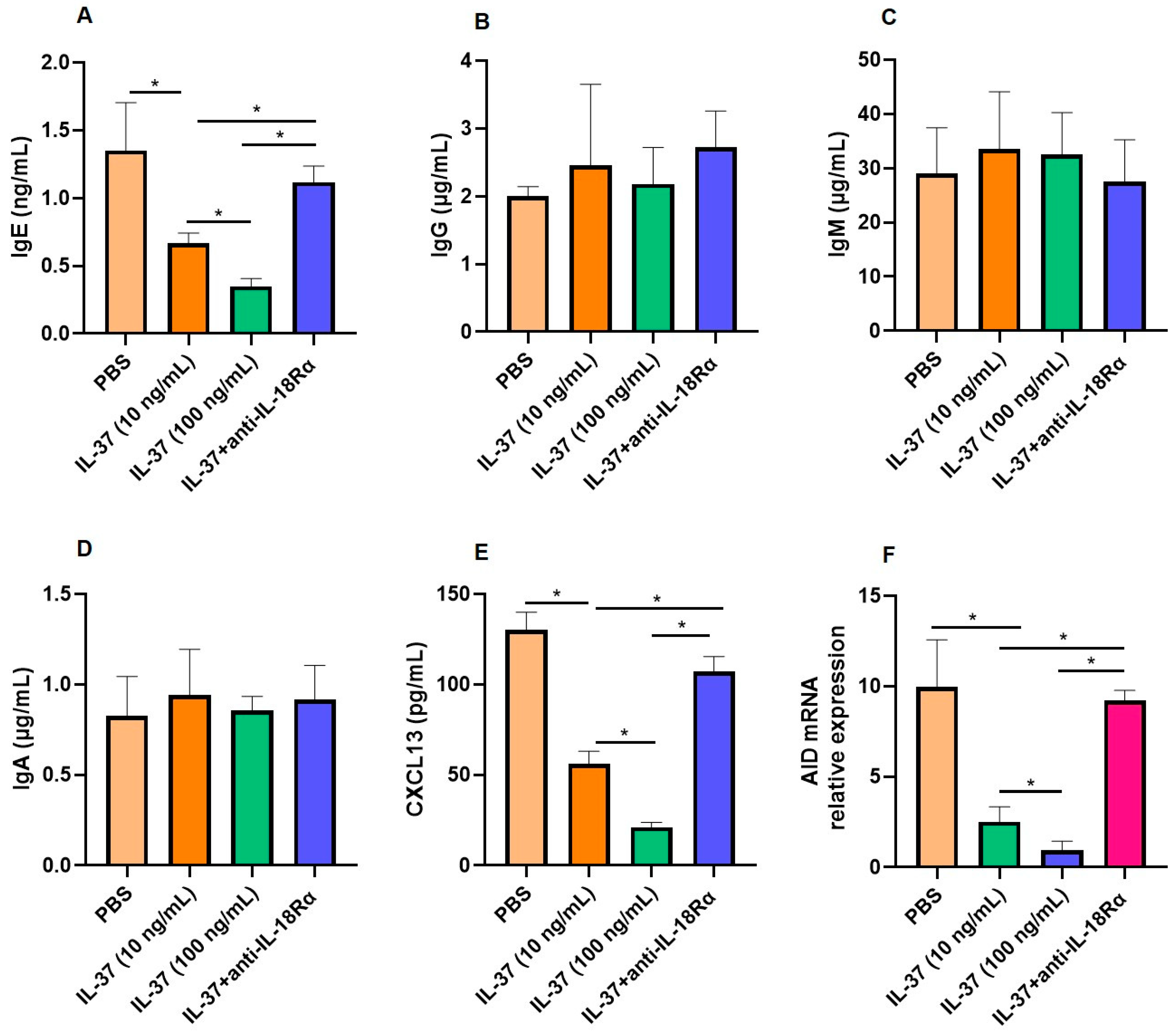
3.3. The Interactions Between Tfh2 and B Cells Regulated by IL-37
3.4. IL-37 Suppresses Tfh2 Response in an Allergic Mouse Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernstein, J.A.; Bernstein, J.S.; Makol, R.; Ward, S. Allergic Rhinitis: A Review. JAMA 2024, 331, 866–877. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Feng, Q.; Zhou, H.; Ma, C.; Lin, C.; Wang, D.; Yin, J. Common Allergens and Immune Responses Associated with Allergic Rhinitis in China. J. Asthma Allergy 2023, 16, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.; Adir, Y.; Al-Ahmad, M.; Celis-Preciado, C.A.; Colodenco, F.D.; Giavina-Bianchi, P.; Lababidi, H.; Ledanois, O.; Mahoub, B.; Perng, D.W.; et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022, 8, 00576–2021. [Google Scholar] [CrossRef] [PubMed]
- Kurata, I.; Matsumoto, I.; Sumida, T. T follicular helper cell subsets: A potential key player in autoimmunity. Immunol. Med. 2021, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Basto, A.P.; Ribeiro, F.; Almeida, S.C.P.; Campos, P.; Peres, C.; Pulvirenti, N.; Al-Khalidi, S.; Kilbey, A.; Tosello, J.; et al. Specialized Tfh cell subsets driving type-1 and type-2 humoral responses in lymphoid tissue. Cell Discov. 2024, 10, 64. [Google Scholar] [CrossRef]
- Varricchi, G.; Harker, J.; Borriello, F.; Marone, G.; Durham, S.R.; Shamji, M.H. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy 2016, 71, 1086–1094. [Google Scholar] [CrossRef]
- Ballesteros-Tato, A.; Randall, T.D.; Lund, F.E.; Spolski, R.; Leonard, W.J.; León, B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity 2016, 44, 259–273. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iijima, K.; Dent, A.L.; Kita, H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol. 2017, 139, 300–313.e7. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, C.L.; Wang, N.; Wang, Z.C.; Ma, J.; Zhu, R.F.; Xu, X.Y.; Zhou, P.C.; Yu, D.; Liu, Z. Correlation of allergen-specific T follicular helper cell counts with specific IgE levels and efficacy of allergen immunotherapy. J. Allergy Clin. Immunol. 2018, 142, 321–324.e10. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Z.C.; Wang, N.; Zhou, P.C.; Chen, C.L.; Song, J.; Pan, L.; Liao, B.; Zhang, X.H.; Yang, Y.S.; et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2019, 144, 118–128. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zhang, W.; Hang, K.; Chen, M.; Hou, W.; Chen, J.; Chen, X.; Chen, E.; Tang, L.; Lu, J.; et al. Extracellular IL-37 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells via activation of the PI3K/AKT signaling pathway. Cell Death Dis. 2019, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, Y.; Li, M.; Tan, S.; Wu, D. IL-37 As a Potential Biotherapeutics of Inflammatory Diseases. Curr. Drug Targets 2020, 21, 855–863. [Google Scholar] [CrossRef]
- Su, Z.; Tao, X. Current Understanding of IL-37 in Human Health and Disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef]
- Zeng, Q.; Zeng, Y.; Xiao, H.; Li, J.; Yang, C.; Luo, R.; Liu, W.; Wen, Y. Interleukin-37b Suppressed ILC2s in Children with Allergic Rhinitis. Mediat. Inflamm. 2023, 2023, 1572891. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shen, Y.; Wang, J.; Ma, Z.X.; Ke, X.; Wang, Z.H.; Hong, S.L.; Hu, G.H. Increased expression of IL-1R8 and a possible immunomodulatory role of its ligand IL-37 in allergic rhinitis patients. Int. Immunopharmacol. 2018, 60, 152–159. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Li, C.; Liu, C.; Wang, Z.H.; Li, Y.S.; Ke, X.; Hu, G.H. IL-37 attenuates allergic process via STAT6/STAT3 pathways in murine allergic rhinitis. Int. Immunopharmacol. 2019, 69, 27–33. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Kim, S.W.; Kang, J.M. Interleukin-37 Relieves Allergic Inflammation in a House Dust Mite Allergic Rhinitis Murine Model. Iran. J. Allergy Asthma Immunol. 2017, 16, 404–417. [Google Scholar]
- Cheng, L.; Chen, J.; Fu, Q.; He, S.; Li, H.; Liu, Z.; Tan, G.; Tao, Z.; Wang, D.; Wen, W.; et al. Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis. Allergy Asthma Immunol. Res. 2018, 10, 300–353. [Google Scholar] [CrossRef]
- Zeng, Y.; Zeng, Q.; Wen, Y.; Li, J.; Xiao, H.; Yang, C.; Luo, R.; Liu, W. Apolipoprotein A-I inhibited group II innate lymphoid cell response mediated by microRNA-155 in allergic rhinitis. J. Allergy Clin. Immunol. Glob. 2024, 3, 100212. [Google Scholar] [CrossRef]
- Ueno, H.; Banchereau, J.; Vinuesa, C.G. Pathophysiology of T follicular helper cells in humans and mice. Nat. Immunol. 2015, 16, 142–152. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, L.; Lu, Z.; Chen, H.; Fan, L.; Xue, Q.; Shi, J.; Li, M.; Li, H.; Gong, J.; et al. IL-37 Represses the Autoimmunity in Myasthenia Gravis via Directly Targeting Follicular Th and B Cells. J. Immunol. 2020, 204, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ke, X.; Yun, L.; Hu, G.H.; Kang, H.Y.; Hong, S.L. Decreased expression of interleukin-37 and its anti-inflammatory effect in allergic rhinitis. Mol. Med. Rep. 2018, 17, 1333–1339. [Google Scholar] [CrossRef]
- Ji, L.S.; Sun, X.H.; Zhang, X.; Zhou, Z.H.; Yu, Z.; Zhu, X.J.; Huang, L.Y.; Fang, M.; Gao, Y.T.; Li, M.; et al. Mechanism of Follicular Helper T Cell Differentiation Regulated by Transcription Factors. J. Immunol. Res. 2020, 2020, 1826587. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Meng, F.L. Taming AID mutator activity in somatic hypermutation. Trends Biochem. Sci. 2024, 49, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Amo-Aparicio, J.; Neff, C.P.; Tengesdal, I.W.; Azam, T.; Palmer, B.E.; López-Vales, R.; Bufler, P.; Dinarello, C.A. Role for nuclear interleukin-37 in the suppression of innate immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 4456–4461. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Nold-Petry, C.; Nold, M.; Fujita, M.; Li, S.; Kim, S.; Bufler, P. Suppression of innate inflammation and immunity by interleukin-37. Eur. J. Immunol. 2016, 46, 1067–1081. [Google Scholar] [CrossRef]
- Li, S.; Neff, C.P.; Barber, K.; Hong, J.; Luo, Y.; Azam, T.; Palmer, B.E.; Fujita, M.; Garlanda, C.; Mantovani, A.; et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. USA 2015, 112, 2497–2502. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]

| Groups | AR | Control |
|---|---|---|
| Number | 16 | 20 |
| Sex (Male:Female) | 8:8 | 11:9 |
| Age (years) | 21.2 ± 2.9 | 22.3 ± 3.6 |
| Duration of symptoms, (years) | 2.1 ± 1.4 | - |
| Serum sIgE level to Der p (IU/mL) Serum sIgE level to Der f (IU/mL) | 21.8 (6.3–599.4) 30.5 (2.4–462.8) | - - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Wen, Y.; Qiu, X.; Zhou, L.; Zeng, Q.; Liu, W. Interleukin-37 Suppresses the Function of Type 2 Follicular Helper T in Allergic Rhinitis. Biomedicines 2025, 13, 1263. https://doi.org/10.3390/biomedicines13051263
Luo X, Wen Y, Qiu X, Zhou L, Zeng Q, Liu W. Interleukin-37 Suppresses the Function of Type 2 Follicular Helper T in Allergic Rhinitis. Biomedicines. 2025; 13(5):1263. https://doi.org/10.3390/biomedicines13051263
Chicago/Turabian StyleLuo, Xi, Yanhui Wen, Xiangqian Qiu, Lifeng Zhou, Qingxiang Zeng, and Wenlong Liu. 2025. "Interleukin-37 Suppresses the Function of Type 2 Follicular Helper T in Allergic Rhinitis" Biomedicines 13, no. 5: 1263. https://doi.org/10.3390/biomedicines13051263
APA StyleLuo, X., Wen, Y., Qiu, X., Zhou, L., Zeng, Q., & Liu, W. (2025). Interleukin-37 Suppresses the Function of Type 2 Follicular Helper T in Allergic Rhinitis. Biomedicines, 13(5), 1263. https://doi.org/10.3390/biomedicines13051263






