Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go
Abstract
1. Introduction
2. Pathophysiology of SCI
3. Industrial Production of EVs
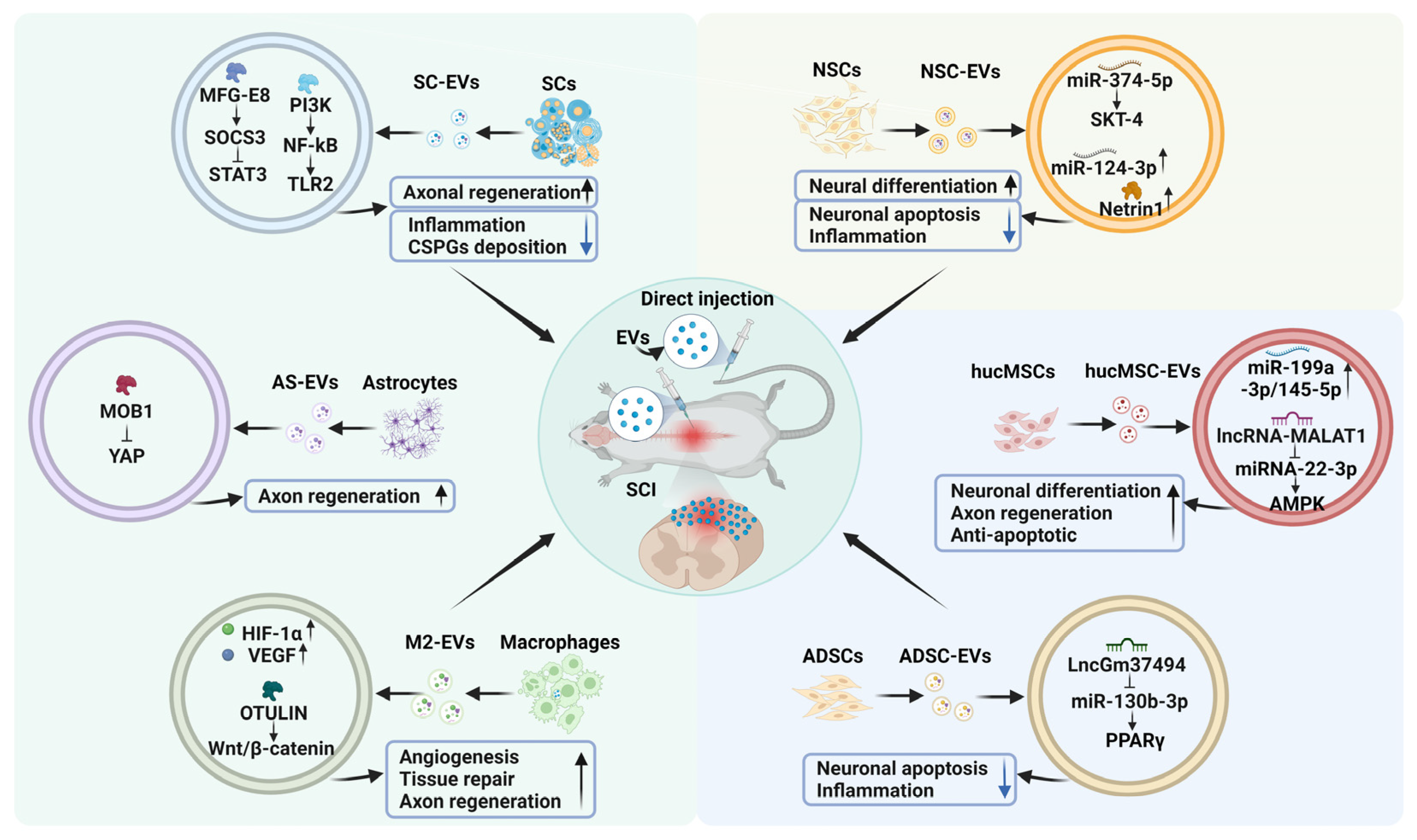
4. The Emerging Roles of EVs from Various Cell Sources in SCI
4.1. NSC-Derived EVs
4.2. MSC-Derived EVs
4.3. EVs Derived from Other Cells
5. Application of Engineered EVs in SCI Treatment
6. EVs Combined with Biomaterials for SCI
6.1. EVs Combined with Fibrin Gels
6.2. EVs Combined with Collagen Scaffolds
6.3. EVs Combined with Bioactive Hydrogels
6.3.1. EVs Combined with Hyaluronic Acid Hydrogels
6.3.2. EVs Combined with FE Hydrogels
6.3.3. EVs Combined with Gelatin Methacrylate Hydrogels
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCI | spinal cord injury |
| EVs | extracellular vesicles |
| MVBs | multivesicular bodies |
| BSCB | blood–spinal cord barrier |
| DAMP | damage-associated molecular patterns |
| ROS | reactive oxygen species |
| BBB | blood‒brain barrier |
| NSCs | neural stem cells |
| MSCs | mesenchymal stem cells |
| hucMSCs | human umbilical cord MSCs |
| ADSCs | adipose-derived stem cells |
| SCs | Schwann cells |
| CNS | central nervous system |
| lncRNA | long non-coding RNA |
| Hyp-sEVs | sEVs under hypoxic conditions |
| AS-sEVs | astrocyte-derived small extracellular vesicles |
| PKC | protein kinase C |
| M2-sEVs | M2 macrophage-derived small extracellular vesicles |
| NGF | nerve growth factor |
| MMP9 | metalloproteinase 9 |
| FE | F127-polycitrate-polyethyleneimine hydrogel |
| PTX | paclitaxel |
| ECM | extracellular matrix |
| HA | hyaluronic acid |
| 3D | three-dimensional |
| MP | methylprednisolone |
| PEG | polyethylene glycol |
| PLA | polylactic acid |
| PVA | polyvinyl alcohol |
References
- Chen, M.; Lin, Y.; Guo, W.; Chen, L. BMSC-derived exosomes carrying miR-26a-5p ameliorate spinal cord injury via negatively regulating EZH2 and activating the BDNF-TrkB-CREB signaling. Mol. Neurobiol. 2024, 61, 8156–8174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, Z.; Zhang, W.; Pang, M.; Hu, X.; Dai, Y.; Shen, R.; Wu, Y.; Liu, C.; Luo, T.; et al. Global incidence and characteristics of spinal cord injury since 2000-2021: A systematic review and meta-analysis. BMC Med. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Oatman, E.; Weinberger, J.; Dixon, A.; Osei-Owusu, P.; Hou, S. The susceptibility of cardiac arrhythmias after spinal cord crush injury in rats. Exp. Neurol. 2022, 357, 114200. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Babajani, A.; Vousooghi, N.; Moghimi, A.; Tarasi, R.; Safaeinejad, F.; Norouzi, S.; Bahrami, S.; Niknejad, H. Therapeutic potential of placenta-derived stem cells cultivated on noggin-loaded nanochitosan/polypyrrole-alginate conductive scaffold to restore spinal cord injury. Stem Cell Res. Ther. 2024, 15, 497. [Google Scholar] [CrossRef]
- Khiar-Fernández, N.; Zian, D.; Vázquez-Villa, H.; Martínez, R.F.; Escobar-Peña, A.; Foronda-Sainz, R.; Ray, M.; Puigdomenech-Poch, M.; Cincilla, G.; Sánchez-Martínez, M.; et al. Novel antagonist of the type 2 lysophosphatidic acid receptor (LPA2), UCM-14216, ameliorates spinal cord injury in mice. J. Med. Chem. 2022, 65, 10956–10974. [Google Scholar] [CrossRef]
- Arasu, U.T.; Kärnä, R.; Härkönen, K.; Oikari, S.; Koistinen, A.; Kröger, H.; Qu, C.; Lammi, M.J.; Rilla, K. Human mesenchymal stem cells secrete hyaluronan-coated extracellular vesicles. Matrix Biol. 2017, 64, 54–68. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Chen, Y.; Yuan, J.; Zhang, H.; Jin, X.; Jiang, Y.; Cao, J.; Wang, Z.; Yang, S.; et al. Distinct molecular properties and functions of small EV subpopulations isolated from human umbilical cord MSCs using tangential flow filtration combined with size exclusion chromatography. J. Extracell. Vesicles 2025, 14, e70029. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Jiang, D.; Wang, L.; Zhao, J.; Yu, L.; Huang, Y.; Wu, X.; Zhu, Y.; Zhao, Y.; Zhao, Q.; et al. VPS28 regulates brain vasculature by controlling neuronal VEGF trafficking through extracellular vesicle secretion. iScience 2022, 25, 104042. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Sheneman, K.R.; Cummins, T.D.; Merchant, M.L.; Hood, J.L.; Uriarte, S.M.; Lawrenz, M.B. Yersinia pestis actively inhibits the production of extracellular vesicles by human neutrophils. J. Extracell. Vesicles 2025, 14, e70074. [Google Scholar] [CrossRef]
- Yanagawa, K.; Yoshimori, T. Rubicon regulates exosome secretion via the non-autophagic pathway. Autophagy 2025, 21, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.C.; Jeon, J.; Rodríguez-delaRosa, A.; Endo, Y.; Kim, D.S.; Madrigal-Salazar, A.D.; Seo, J.W.; Lee, H.; Kim, K.T.; et al. Combinational regenerative inductive effect of bio-adhesive hybrid hydrogels conjugated with hiPSC-derived myofibers and its derived EVs for volumetric muscle regeneration. Bioact. Mater. 2025, 43, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef]
- Karoichan, A.; Boucenna, S.; Tabrizian, M. Therapeutics of the future: Navigating the pitfalls of extracellular vesicles research from an osteoarthritis perspective. J. Extracell. Vesicles 2024, 13, e12435. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death. Dis. 2019, 10, 340. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, C.; Wang, H.; Du, R.; Ji, J.; Xu, T.; Yang, C.; Chen, X. Astrocyte-derived sEVs alleviate fibrosis and promote functional recovery after spinal cord injury in rats. Int. Immunopharmacol. 2022, 113, 109322. [Google Scholar] [CrossRef]
- Zhong, D.; Cao, Y.; Li, C.; Li, M.; Rong, Z.; Jiang, L.; Guo, Z.; Lu, H.; Hu, J. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med 2020, 245, 54–65. [Google Scholar] [CrossRef]
- Huang, J.; Shi, L.; Yang, Y.; Zhao, F.; Chen, R.; Liao, W.; Zhu, J.; Yang, D.; Wu, X.; Han, S. Mesenchymal cell-derived exosomes and miR-29a-3p mitigate renal fibrosis and vascular rarefaction after renal ischemia reperfusion injury. Stem Cell Res. Ther. 2025, 16, 135. [Google Scholar] [CrossRef]
- Kao, Y.; Song, W.; Zhang, R.; Gu, G.; Qiu, H.; Shen, W.; Zhu, H.; Liu, Y.; Yang, Y.; Liu, H.; et al. Synergistic restoration of spinal cord injury through hyaluronic acid conjugated hydrogel-polydopamine nanoparticles combined with human mesenchymal stem cell transplantation. Bioact. Mater. 2025, 46, 569–581. [Google Scholar] [CrossRef]
- Wen, R.; Long, G.; He, X.; Zhang, K.; Ma, W.; Shen, Y.; Xiao, Z.; Zhao, Y.; Liu, D.; Dai, J.; et al. Revisiting the unobtrusive role of exogenous stem cells beyond neural circuits replacement in spinal cord injury repair. Theranostics 2025, 15, 1552–1569. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jang, S.; Kim, C.; Lee, S.; Jeong, H.S. Role of stem cell-derived exosomes and microRNAs in spinal cord injury. Int. J. Mol. Sci. 2023, 24, 13849. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Z.; Zhou, X.; Jin, Q.; Xu, W.; Zhai, X.; Fu, Q.; Qian, H. viaSmall extracellular vesicles derived from mesenchymal stem cell facilitate functional recovery in spinal cord injury by activating neural stem cells the ERK1/2 pathway. Front. Cell. Neurosci. 2022, 16, 954597. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhu, S.; Zhang, W.; Wei, Z.; Yang, F.; Guo, Z.; Ning, G.; Feng, S. Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol. Lett. 2022, 44, 129–142. [Google Scholar] [CrossRef]
- Lin, Y.; Guo, Q.; Liu, Q.; Wang, W.; Lv, A.; Zhang, L.; Li, L.; Gao, J.; Huang, F. An injectable responsive exosome-releasing hydrogel based on sodium alginate restores motor and bladder function by alleviating the injury microenvironment and facilitating distal nerve repair. Int. J. Biol. Macromol. 2025, 304, 140819. [Google Scholar] [CrossRef]
- Li, Q.; Fu, X.; Kou, Y.; Han, N. Engineering strategies and optimized delivery of exosomes for theranostic application in nerve tissue. Theranostics 2023, 13, 4266–4286. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, Y.; Liu, Y.; Zhao, J.; Liu, Q.; Xu, J.; Qin, Y.; He, R.; Yuan, F.; Wu, T.; et al. Targeted delivery of RGD-CD146+CD271+ human umbilical cord mesenchymal stem cell-derived exosomes promotes blood-spinal cord barrier repair after spinal cord injury. ACS Nano 2023, 17, 18008–18024. [Google Scholar] [CrossRef]
- Shen, K.; Li, X.; Huang, G.; Yuan, Z.; Xie, B.; Chen, T.; He, L. High rapamycin-loaded hollow mesoporous Prussian blue nanozyme targets lesion area of spinal cord injury to recover locomotor function. Biomaterials 2023, 303, 122358. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Zheng, S.; Huang, R.; Wen, G.; Zhou, P.; Wang, W.; Zhou, S.; Jiang, X.; Liu, S.; et al. Mesoporous polydopamine delivering 8-gingerol for the target and synergistic treatment to the spinal cord injury. J. Nanobiotechnol. 2023, 21, 192. [Google Scholar] [CrossRef]
- Liu, J.A.; Tam, K.W.; Chen, Y.L.; Feng, X.; Chan, C.W.L.; Lo, A.L.H.; Wu, K.L.; Hui, M.N.; Wu, M.H.; Chan, K.K.; et al. Transplanting human neural stem cells with ≈50% reduction of SOX9 gene dosage promotes tissue repair and functional recovery from severe spinal cord injury. Adv. Sci. 2023, 10, e2205804. [Google Scholar] [CrossRef]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R. Pathophysiology, classification and comorbidities after traumatic spinal cord injury. J. Pers Med. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, H.; Andrabi, S.S.; Petro, M.; Kuang, Y.; Steinmetz, M.P.; Labhasetwar, V. Catalytic antioxidant nanoparticles mitigate secondary injury progression and promote functional recovery in spinal cord injury model. J. Control. Release 2023, 364, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Geng, X.; Wu, Y.; Dai, Y.; Zeng, J.; Wang, Z.; Fang, H.; Sun, Y.; Chen, X. Engineered macrophage membrane-coated nanoparticles with enhanced CCR2 expression promote spinal cord injury repair by suppressing neuroinflammation and neuronal death. Small 2023, 20, e2305659. [Google Scholar] [CrossRef]
- Rong, Y.; Ji, C.; Wang, Z.; Ge, X.; Wang, J.; Ye, W.; Tang, P.; Jiang, D.; Fan, J.; Yin, G.; et al. Small extracellular vesicles encapsulating CCL2 from activated astrocytes induce microglial activation and neuronal apoptosis after traumatic spinal cord injury. J. Neuroinflamm. 2021, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gu, G.; Ren, J.; Song, X.; Li, J.; Wang, C.; Zhang, W.; Huo, Y.; Wang, H.; Jin, L.; et al. Schwann cell-derived exosomes and methylprednisolone composite patch for spinal cord injury repair. ACS Nano 2023, 17, 22928–22943. [Google Scholar] [CrossRef]
- Wang, H.; Lin, F.; Wu, Y.; Guo, W.; Chen, X.; Xiao, C.; Chen, M. Carrier-free nanodrug based on co-assembly of methylprednisolone dimer and rutin for combined treatment of spinal cord injury. ACS Nano 2023, 17, 12176–12187. [Google Scholar] [CrossRef]
- Taylor, E.C.; Fitzpatrick, C.E.; Thompson, S.E.; Justice, S.B. Acute traumatic spinal cord injury. Adv. Emerg. Nurs. J. 2022, 44, 272–280. [Google Scholar] [CrossRef]
- Makrygianni, E.; Chrousos, G. Extracellular vesicles and the stress system. Neuroendocrinology 2022, 113, 120–167. [Google Scholar] [CrossRef]
- Ollen-Bittle, N.; Roseborough, A.; Wang, W.; Wu, J.; Whitehead, S. Mechanisms and biomarker potential of extracellular vesicles in stroke. Biology 2022, 11, 1231. [Google Scholar] [CrossRef]
- Fan, B.; Gu, J.; Wu, J.; Sun, Y.; Huang, R.; Shen, H.; Zhang, X.; Li, Z. Circulating abnormal extracellular vesicles: Their mechanism for crossing blood-brain barrier, effects on central nervous system and detection methods. J. Biomed. Nanotechnol. 2022, 18, 640–659. [Google Scholar] [CrossRef]
- Mirab, S.M.; Omidi, A.; Soleimani, M.; Soufi-Zomorrod, M.; Fekrirad, Z. Human umbilical cord mesenchymal stem cell-derived exosomes boost functional performance in an animal model of multiple sclerosis through recruiting oligodendrocytes and attenuating gliosis. Stem Cell Rev. Rep. 2025. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Qian, J.; Tu, B.Z.; Xia, X.; Jia, C.Y.; Shen, C.L. MiR-124 delivered by extracellular vesicles from mesenchymal stem cell exerts neuroprotective effects by stabilizing the p62-Keap1-Nrf2 pathway after spinal cord injury in rats. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K.; Passaro, A.P.; Latchoumane, C.F.; Spellicy, S.E.; Bowler, M.; Goeden, M.; Martin, W.J.; Holmes, P.V.; Stice, S.L.; Karumbaiah, L. Extracellular vesicles mediate neuroprotection and functional recovery after traumatic brain injury. J. Neurotrauma 2020, 37, 1358–1369. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, L.; Zheng, Z.; Fu, M.; Peng, H.; Ma, S. Microbead encapsulation strategy for efficient production of extracellular vesicles derived from human mesenchymal stem cells. J. Extracell. Vesicles 2025, 14, e70053. [Google Scholar] [CrossRef]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, Y.; Guo, M.; Li, C. Large-scale expansion of human umbilical cord-derived mesenchymal stem cells in a stirred suspension bioreactor enabled by computational fluid dynamics modeling. Bioengineering 2022, 9, 274. [Google Scholar] [CrossRef]
- Khan, N.L.A.; Muhandiram, S.; Dissanayake, K.; Godakumara, K.; Midekessa, G.; Andronowska, A.; Heath, P.R.; Kodithuwakku, S.; Hart, A.R.; Fazeli, A. Effect of 3D and 2D cell culture systems on trophoblast extracellular vesicle physico-chemical characteristics and potency. Front. Cell Dev. Biol. 2024, 12, 1382552. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Neubauer, M.; Gulová, S.; Lacza, Z.; Nehrer, S.; Luna, A. Culture of hoffa fat pad mesenchymal stem/stromal cells on microcarrier suspension in vertical wheel bioreactor for extracellular vesicle production. Stem Cell Res. Ther. 2024, 15, 61. [Google Scholar] [CrossRef]
- Ene, J.; Liu, C.; Syed, F.; Sun, L.; Berry, D.; Durairaj, P.; Liu, Z.L.; Zeng, C.; Jung, S.; Li, Y. Biomanufacturing and lipidomics analysis of extracellular vesicles secreted by human blood vessel organoids in a vertical wheel bioreactor. Stem Cell Res. Ther. 2025, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Son, J.P.; Kim, E.H.; Shin, E.K.; Kim, D.H.; Sung, J.H.; Oh, M.J.; Cha, J.M.; Chopp, M.; Bang, O.Y. Mesenchymal stem cell-extracellular vesicle therapy for stroke: Scalable production and imaging biomarker studies. Stem Cells Transl. Med. 2023, 12, 459–473. [Google Scholar] [CrossRef]
- Wang, J.; Bonacquisti, E.E.; Brown, A.D.; Nguyen, J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bu, T.; Cao, R.; Li, Z.; Wang, C.; Huang, B.; Wei, M.; Yuan, L.; Yang, G. An optimized exosome production strategy for enhanced yield while without sacrificing cargo loading efficiency. J. Nanobiotechnol. 2022, 20, 463. [Google Scholar] [CrossRef] [PubMed]
- Askeland, A.; Borup, A.; Østergaard, O.; Olsen, J.V.; Lund, S.M.; Christiansen, G.; Kristensen, S.R.; Heegaard, N.H.H.; Pedersen, S. Mass-spectrometry based proteome comparison of extracellular vesicle isolation methods: Comparison of ME-kit, size-exclusion chromatography, and high-speed centrifugation. Biomedicines 2020, 8, 246. [Google Scholar] [CrossRef]
- Andriolo, G.; Provasi, E.; Brambilla, A.; Panella, S.; Soncin, S.; Cicero, V.L.; Radrizzani, M.; Turchetto, L.; Barile, L. Methodologies for scalable production of high-quality purified small extracellular vesicles from conditioned medium. Methods Mol. Biol. 2023, 2668, 69–98. [Google Scholar] [CrossRef]
- Lozano, N.; Prescilla-Ledezma, A.; Calabuig, E.; Trelis, M.; Arce, J.M.S.; López Hontangas, J.L.; de Pablos, L.M.; Gomez-Samblas, M.; Osuna, A. Circulating extracellular vesicles in sera of chronic patients as a method for determining active parasitism in Chagas disease. PLoS Negl. Trop. Dis. 2024, 18, e0012356. [Google Scholar] [CrossRef]
- Filipović, L.; Spasojević Savković, M.; Prodanović, R.; Matijašević Joković, S.; Stevanović, S.; Marco, A.; Kosanović, M.; Brajušković, G.; Popović, M. Urinary extracellular vesicles as a readily available biomarker source: A simplified stratification method. Int. J. Mol. Sci. 2024, 25, 8004. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.K.; Powell, R.R.; Marcus, R.K.; Bruce, T.F. Comparison of the capillary-channeled polymer (C-CP) fiber spin-down tip approach to traditional methods for the isolation of extracellular vesicles from human urine. Anal. Bioanal. Chem. 2022, 414, 3813–3825. [Google Scholar] [CrossRef]
- Bu, Y.; Wang, J.; Ni, S.; Lu, Z.; Guo, Y.; Yobas, L. High-performance gel-free and label-free size fractionation of extracellular vesicles with two-dimensional electrophoresis in a microfluidic artificial sieve. Anal. Chem. 2024, 96, 3508–3516. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, B.; Liu, W.; Zhang, J.; Zhu, L.; Yang, C.; Song, Y. Isolation of PD-L1 extracellular vesicle subpopulations using DNA computation mediated microfluidic tandem separation. Small Methods 2023, 7, e2300516. [Google Scholar] [CrossRef]
- Multia, E.; Liangsupree, T.; Jussila, M.; Ruiz-Jimenez, J.; Kemell, M.; Riekkola, M.L. Automated on-line isolation and fractionation system for nanosized biomacromolecules from human plasma. Anal. Chem. 2020, 92, 13058–13065. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Forssén, P.; Fornstedt, T.; Riekkola, M.L. Kinetics and interaction studies of anti-tetraspanin antibodies and ICAM-1 with extracellular vesicle subpopulations using continuous flow quartz crystal microbalance biosensor. Biosens. Bioelectron. 2022, 206, 114151. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ferrer, M.; Villanueva-Badenas, E.; Sánchez-Sánchez, R.; Sánchez-López, C.M.; Baquero, M.C.; Sepúlveda, P.; Dorronsoro, A. HIF-1α and pro-inflammatory signaling improves the immunomodulatory activity of MSC-derived extracellular vesicles. Int. J. Mol. Sci. 2021, 22, 3416. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Wu, D.; Liu, B.; Wang, N.; Rong, L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res. Ther. 2021, 12, 117. [Google Scholar] [CrossRef]
- Shao, M.; Jin, M.; Xu, S.; Zheng, C.; Zhu, W.; Ma, X.; Lv, F. Exosomes from long noncoding RNA-Gm37494-ADSCs repair spinal cord injury via shifting microglial M1/M2 polarization. Inflammation 2020, 43, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhu, B.; Gu, G.; Zhang, W.; Li, J.; Wang, H.; Wang, M.; Song, X.; Wei, Z.; Feng, S. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023, 14, 70. [Google Scholar] [CrossRef]
- Huang, J.H.; He, H.; Chen, Y.N.; Liu, Z.; Romani, M.D.; Xu, Z.Y.; Xu, Y.; Lin, F.Y. Exosomes derived from M2 macrophages improve angiogenesis and functional recovery after spinal cord injury through HIF-1α/VEGF axis. Brain Sci. 2022, 12, 1322. [Google Scholar] [CrossRef]
- Wu, Z.; Han, T.; Dong, Y.; Ying, W.; Fang, H.; Liu, Y.; Song, P.; Shen, C. Acid-sensing ion channel-1 contributes to the failure of myelin sheath regeneration following spinal cord injury by transcellular delivery of PGE2. Cell. Mol. Biol. Lett. 2024, 29, 149. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Lv, C.; Wang, J.; Luo, Y.; Jiang, D.; Li, L.; Zhou, Z.; Zhou, W.; Li, Q.; et al. Neural stem cell small extracellular vesicle-based delivery of 14-3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1. Aging 2019, 11, 7723–7745. [Google Scholar] [CrossRef]
- Ma, L.; Wei, X.; Ma, W.; Liu, Y.; Wang, Y.; He, Y.; Jia, S.; Wang, Y.; Luo, W.; Liu, D.; et al. Neural stem cell-derived exosomal Netrin1 contributes to neuron differentiation of mesenchymal stem cells in therapy of spinal bifida aperta. Stem Cells Transl. Med. 2022, 11, 539–551. [Google Scholar] [CrossRef]
- Zhang, L.; Han, P. Neural stem cell-derived exosomes suppress neuronal cell apoptosis by activating autophagy via miR-374-5p/STK-4 axis in spinal cord injury. J. Musculoskelet. Neuronal Interact. 2022, 22, 411–421. [Google Scholar] [PubMed]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Vismara, I.; Mariani, A.; Barilani, M.; Rimondo, S.; De Paola, M.; Panini, N.; Erba, E.; Mauri, E.; Rossi, F.; et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J. Control. Release 2018, 278, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem cell therapy for spinal cord injury. Cell Transplant. 2021, 30, 963689721989266. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.Y.; Chen, Z.H.; Wang, N.X.; Zhang, L.M.; Chen, Y.Y.; Mo, J.; Dong, J.W.; et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: A phase 1/2 pilot study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Liu, W.; Li, Y.; Ma, X.; Abulimiti, M.; Maimaitiaili, N.; Qin, H. Intrathecal transplantation of human umbilical cord mesenchymal stem cells enhances spinal cord injury recovery: Role of miR-124-3p as a biomarker. Exp. Ther. Med. 2025, 29, 57. [Google Scholar] [CrossRef]
- Akhlaghpasand, M.; Tavanaei, R.; Hosseinpoor, M.; Yazdani, K.O.; Soleimani, A.; Zoshk, M.Y.; Soleimani, M.; Chamanara, M.; Ghorbani, M.; Deylami, M.; et al. Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: A first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res. Ther. 2024, 15, 264. [Google Scholar] [CrossRef]
- Li, K.; Liu, Z.; Wu, P.; Chen, S.; Wang, M.; Liu, W.; Zhang, L.; Guo, S.; Liu, Y.; Liu, P.; et al. Micro electrical fields induced MSC-sEVs attenuate neuronal cell apoptosis by activating autophagy via lncRNA MALAT1/miR-22-3p/SIRT1/AMPK axis in spinal cord injury. J. Nanobiotechnol. 2023, 21, 451. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.L.; Liu, R.Q.; Xu, M.M.; Xie, J.L.; Zhang, X.L.; Xie, G.M.; Han, Y.T.; Zhang, X.M.; Zhang, W.T.; et al. Exosome lncRNA IFNG-AS1 derived from mesenchymal stem cells of human adipose ameliorates neurogenesis and ASD-like behavior in BTBR mice. J. Nanobiotechnol. 2024, 22, 66. [Google Scholar] [CrossRef]
- Rau, C.S.; Wu, S.C.; Kuo, P.J.; Lin, C.W.; Lu, T.H.; Wu, Y.C.; Tsai, C.W.; Hsieh, C.H. Tracking adipose-derived stem cell exosomes applied in a mouse crush injury model: Insights from fluorescent labeling and spatial transcriptomics—An experimental study. Int. J. Surg. 2024, 111, 1860–1873. [Google Scholar] [CrossRef]
- Lu, X.; Lv, C.; Zhao, Y.; Wang, Y.; Li, Y.; Ji, C.; Wang, Z.; Ye, W.; Yu, S.; Bai, J.; et al. TSG-6 released from adipose stem cells-derived small extracellular vesicle protects against spinal cord ischemia reperfusion injury by inhibiting endoplasmic reticulum stress. Stem Cell Res. Ther. 2022, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Seo, M.; Kim, Y.; Kang, K.; Choi, J.; Lee, S.; Sung, M.; Yim, S.; Lim, J.; Seok, H.; et al. Human epidural AD-MSC exosomes improve function recovery after spinal cord injury in rats. Biomedicines 2022, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Fan, B.; Ding, H.; Liu, Y.; Tang, H.; Pan, D.; Shi, J.; Zheng, P.; Shi, H.; Wu, H.; et al. Proteomics analysis of Schwann cell-derived exosomes: A novel therapeutic strategy for central nervous system injury. Mol. Cell. Biochem. 2019, 457, 51–59. [Google Scholar] [CrossRef]
- Pan, D.; Li, Y.; Yang, F.; Lv, Z.; Zhu, S.; Shao, Y.; Huang, Y.; Ning, G.; Feng, S. Increasing toll-like receptor 2 on astrocytes induced by Schwann cell-derived exosomes promotes recovery by inhibiting CSPGs deposition after spinal cord injury. J. Neuroinflamm. 2021, 18, 172. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, Z.; Fang, J.; Wang, J.; Tao, K.; Liu, J.; Liu, S. Exosomes derived from schwann cells alleviate mitochondrial dysfunction and necroptosis after spinal cord injury via AMPK signaling pathway-mediated mitophagy. Free Radic. Biol. Med. 2023, 208, 319–333. [Google Scholar] [CrossRef]
- Sun, R.; Liao, W.; Lang, T.; Qin, K.; Jiao, K.; Shao, L.; Deng, C.; She, Y. Astrocyte-derived exosomal miR-378a-5p mitigates cerebral ischemic neuroinflammation by modulating NLRP3-mediated pyroptosis. Front. Immunol. 2024, 15, 1454116. [Google Scholar] [CrossRef]
- Sun, H.; Cao, X.; Gong, A.; Huang, Y.; Xu, Y.; Zhang, J.; Sun, J.; Lv, B.; Li, Z.; Guan, S.; et al. Extracellular vesicles derived from astrocytes facilitated neurite elongation by activating the Hippo pathway. Exp. Cell Res. 2022, 411, 112937. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Zhou, W.; Ye, P.; Zhang, L.; Sun, W.; Tian, L.; Peng, B.; Hu, M.; Xu, B. LncRNA 4933431K23Rik modulate microglial phenotype via inhibiting miR-10a-5p in spinal cord injury induced neuropathic pain. Sci. Rep. 2025, 15, 11620. [Google Scholar] [CrossRef]
- Luo, Z.; Peng, W.; Xu, Y.; Xie, Y.; Liu, Y.; Lu, H.; Cao, Y.; Hu, J. Exosomal OTULIN from M2 macrophages promotes the recovery of spinal cord injuries via stimulating Wnt/β-catenin pathway-mediated vascular regeneration. Acta Biomater. 2021, 136, 519–532. [Google Scholar] [CrossRef]
- Zeng, J.; Gu, C.; Sun, Y.; Chen, X. Engineering of M2 macrophages-derived exosomes via click chemistry for spinal cord injury repair. Adv. Healthc. Mater. 2023, 12, e2203391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Haney, M.J.; Fallon, J.K.; Rodriguez, M.; Swain, C.J.; Arzt, C.J.; Smith, P.C.; Loop, M.S.; Harrison, E.B.; El-Hage, N.; et al. Using extracellular vesicles released by GDNF-transfected macrophages for therapy of parkinson disease. Cells 2022, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tang, Q.; Müller, S.A.; Gao, P.; Mahlstedt, D.; Zampagni, S.; Tan, Y.; Klingl, A.; Bötzel, K.; Lichtenthaler, S.F.; et al. Fibroblast growth factor 2-mediated regulation of neuronal exosome release depends on VAMP3/cellubrevin in hippocampal neurons. Adv. Sci. 2020, 7, 1902372. [Google Scholar] [CrossRef]
- Guan, P.; Fan, L.; Zhu, Z.; Yang, Q.; Kang, X.; Li, J.; Zhang, Z.; Liu, S.; Liu, C.; Wang, X.; et al. M2 microglia-derived exosome-loaded electroconductive hydrogel for enhancing neurological recovery after spinal cord injury. J. Nanobiotechnol. 2024, 22, 8. [Google Scholar] [CrossRef]
- Kong, G.; Xiong, W.; Li, C.; Xiao, C.; Wang, S.; Li, W.; Chen, X.; Wang, J.; Chen, S.; Zhang, Y.; et al. Treg cells-derived exosomes promote blood-spinal cord barrier repair and motor function recovery after spinal cord injury by delivering miR-2861. J. Nanobiotechnol. 2023, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnol. 2023, 21, 10. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Liu, Q.; Xu, Y.; Qin, Y.; He, R.; Zheng, L.; Xie, Y.; Li, C.; Wu, T.; et al. Intranasal delivery of small extracellular vesicles from specific subpopulation of mesenchymal stem cells mitigates traumatic spinal cord injury. J. Control Release 2024, 369, 335–350. [Google Scholar] [CrossRef]
- Deng, M.; Xie, P.; Xue, H.; Chen, Q.; Zhou, Y.; Ming, J.; Ma, Y.; Liu, J.; Huang, H. Decellularized tissue matrices hydrogels functionalized with extracellular vesicles promote macrophage reprogramming and neural stem cell differentiation for spinal cord injury repair. J. Nanobiotechnol. 2025, 23, 139. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.; Tong, K.; Huang, Z.; Liu, S.; Zhu, H.; Zhong, Y.; Zhou, Z.; Jiao, G.; Wei, F.; et al. Extracellular vesicles released by transforming growth factor-beta 1-preconditional mesenchymal stem cells promote recovery in mice with spinal cord injury. Bioact. Mater. 2024, 35, 135–149. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Zhou, X.; Zeng, W.; Yuan, J.; Ye, J. Multiple strategies enhance the efficacy of MSC-Exos transplantation for spinal cord injury. Exp. Neurol. 2024, 383, 115038. [Google Scholar] [CrossRef]
- Wang, D.; Lyu, Y.; Yang, Y.; Zhang, S.; Chen, G.; Pan, J.; Tian, W. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta. Biomater. 2022, 140, 610–624. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, R.; Mukherjee, N.; Ghosh, S.; Jash, M.; Jana, A.; Ghosh, S. EphA4 targeting peptide-conjugated extracellular vesicles rejuvenates adult neural stem cells and exerts therapeutic benefits in aging rats. ACS Chem. Neurosci. 2024, 15, 3482–3495. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, L.; Chen, R.; Han, J.; Lei, Q.; Chen, Z.; Tang, X.; Wu, W.; Liu, S.; Yao, X. Engineered extracellular vesicles efficiently deliver CRISPR-Cas9 ribonucleoprotein (RNP) to inhibit herpes simplex virus1 infection in vitro and in vivo. Acta Pharm. Sin. B 2024, 14, 1362–1379. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Wang, Z.; Tang, P.; Wang, J.; Ji, C.; Chang, J.; Zhu, Y.; Ye, W.; Bai, J.; Liu, W.; et al. Engineered extracellular vesicles for delivery of siRNA promoting targeted repair of traumatic spinal cord injury. Bioact. Mater. 2023, 23, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, D.; Hu, H.; Wang, Z.; An, J.; Gao, Z.; Zhang, K.; Mei, X.; Wu, C.; Tian, H. Engineered extracellular vesicles derived from primary M2 macrophages with anti-inflammatory and neuroprotective properties for the treatment of spinal cord injury. J. Nanobiotechnol. 2021, 19, 373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, S.; Dong, L.; Wu, P.; Li, K.; Li, X.; Li, X.; Qian, H.; Fu, Q. A tannic acid doped hydrogel with small extracellular vesicles derived from mesenchymal stem cells promotes spinal cord repair by regulating reactive oxygen species microenvironment. Mater. Today Bio 2022, 16, 100425. [Google Scholar] [CrossRef]
- Roh, E.J.; Kim, D.S.; Kim, J.H.; Lim, C.S.; Choi, H.; Kwon, S.Y.; Park, S.Y.; Kim, J.Y.; Kim, H.M.; Hwang, D.Y.; et al. Multimodal therapy strategy based on a bioactive hydrogel for repair of spinal cord injury. Biomaterials 2023, 299, 122160. [Google Scholar] [CrossRef]
- King, V.; Alovskaya, A.; Wei, D.; Brown, R.; Priestley, J. The use of injectable forms of fibrin and fibronectin to support axonal ingrowth after spinal cord injury. Biomaterials 2010, 31, 4447–4456. [Google Scholar] [CrossRef]
- Wang, S.; Wang, R.; Chen, J.; Yang, B.; Shu, J.; Cheng, F.; Tao, Y.; Shi, K.; Wang, C.; Wang, J.; et al. Controlled extracellular vesicles release from aminoguanidine nanoparticle-loaded polylysine hydrogel for synergistic treatment of spinal cord injury. J. Control. Release 2023, 363, 27–42. [Google Scholar] [CrossRef]
- Mu, J.; Wu, J.; Cao, J.; Ma, T.; Li, L.; Feng, S.; Gao, J. Rapid and effective treatment of traumatic spinal cord injury using stem cell derived exosomes. Asian. J. Pharm. Sci. 2021, 16, 806–815. [Google Scholar] [CrossRef]
- He, X.; Yang, L.; Dong, K.; Zhang, F.; Liu, Y.; Ma, B.; Chen, Y.; Hai, J.; Zhu, R.; Cheng, L. Biocompatible exosome-modified fibrin gel accelerates the recovery of spinal cord injury by VGF-mediated oligodendrogenesis. J. Nanobiotechnol. 2022, 20, 360. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Xu, Z.; Xiong, X.; Yu, Y.; Wu, H.; Qiao, H.; Zhong, J.; Zhao, Z.; Dai, J.; et al. A functionalized collagen-I scaffold delivers microRNA 21-loaded exosomes for spinal cord injury repair. Acta Biomater. 2022, 154, 385–400. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, C.; Hao, W.; Zhuang, Y.; Liu, X.; Zhao, Y.; Chen, B.; Xiao, Z.; Chen, Y.; Dai, J. NSCs migration promoted and drug delivered exosomes-collagen scaffold via a bio-specific peptide for one-step spinal cord injury repair. Adv. Healthc. Mater. 2021, 10, e2001896. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Lu, X.; Li, Y.; Liu, Z.; Li, F.; Shang, Z.; Wang, X.; Li, X.; Li, J.; et al. Three-dimensional-cultured MSC-derived exosome-hydrogel hybrid microneedle array patch for spinal cord repair. Nano Lett. 2022, 22, 6391–6401. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xia, K.; Wang, J.; Cheng, F.; Shi, K.; Ying, L.; Yu, C.; Xu, H.; Xiao, S.; et al. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact. Mater. 2021, 6, 2523–2534. [Google Scholar] [CrossRef]
- Mu, J.; Li, L.; Wu, J.; Huang, T.; Zhang, Y.; Cao, J.; Ma, T.; Chen, J.; Zhang, C.; Zhang, X.; et al. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater. Sci. 2022, 10, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Mu, J.; Chen, J.; Zhang, C.; Cao, H.; Gao, J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020, 20, 4298–4305. [Google Scholar] [CrossRef]
- Song, P.; Han, T.; Wu, Z.; Fang, H.; Liu, Y.; Ying, W.; Wang, X.; Shen, C. Transplantation of neural stem cells loaded in an IGF-1 bioactive supramolecular nanofiber hydrogel for the effective treatment of spinal cord injury. Adv. Sci. 2024, 11, e2306577. [Google Scholar] [CrossRef]
- Imamura, H.; Tomimaru, Y.; Kobayashi, S.; Harada, A.; Kita, S.; Sasaki, K.; Iwagami, Y.; Yamada, D.; Noda, T.; Takahashi, H.; et al. Adipose-derived stem cells using fibrin gel as a scaffold enhances post-hepatectomy liver regeneration. Sci. Rep. 2025, 15, 6334. [Google Scholar] [CrossRef]
- Ahmann, K.A.; Weinbaum, J.S.; Johnson, S.L.; Tranquillo, R.T. Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue Eng. Part A 2010, 16, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Bayat, N.; Ebrahimi-Barough, S.; Ardakan, M.; Ai, A.; Kamyab, A.; Babaloo, H.; Ai, J. Differentiation of human endometrial stem cells into Schwann cells in fibrin hydrogel as 3D culture. Mol. Neurobiol. 2016, 53, 7170–7176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Fan, C.; Zhuang, Y.; Yang, W.; Chen, Y.; Shen, H.; Xiao, Z.; Zhao, Y.; Li, X.; et al. Adhesive, stretchable, and spatiotemporal delivery fibrous hydrogels harness endogenous neural stem/progenitor cells for spinal cord injury repair. ACS Nano 2022, 16, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Lou, G.; Mo, Y.; Pan, Y.; Zhang, Z.; Guo, R.; Li, Z. A combination of GDNF and hUCMSC transplantation loaded on SF/AGs composite scaffolds for spinal cord injury repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 230–237. [Google Scholar] [CrossRef]
- Yeh, J.; Wang, D.; Cherng, J.; Wang, Y.; Fan, G.; Liou, N.; Liu, J.; Chou, C. A collagen-based scaffold for promoting neural plasticity in a rat model of spinal cord injury. Polymers 2020, 12, 2245. [Google Scholar] [CrossRef]
- Reinehr, P.; Diel, L.F.; Diz, F.M.; Balbinot, G.S.; Monteiro, W.F.; Ligabue, R.A.; Lamers, M.L.; Kunrath, M.F. Three-dimensional bioactive collagen scaffolds incorporated with titanate nanotubes for tissue regeneration. Colloids Surf. B Biointerfaces 2025, 252, 114638. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Liu, X.; Xu, H.; Jiang, W.; Wang, J.; Chen, F.; Zhang, S.; Li, R.; Chen, X.; et al. The corticospinal tract structure of collagen/silk fibroin scaffold implants using 3D printing promotes functional recovery after complete spinal cord transection in rats. J. Mater. Sci. Mater. Med. 2021, 32, 31. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Y.; Wang, L.; Tai, C.; Chen, D.; Mu, D.; Cui, Y.; Wang, B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural. Regen. Res. 2021, 16, 2284–2292. [Google Scholar] [CrossRef]
- Fan, C.; Yang, W.; Zhang, L.; Cai, H.; Zhuang, Y.; Chen, Y.; Zhao, Y.; Dai, J. Restoration of spinal cord biophysical microenvironment for enhancing tissue repair by injury-responsive smart hydrogel. Biomaterials 2022, 288, 121689. [Google Scholar] [CrossRef] [PubMed]
- Chocarro-Wrona, C.; Pleguezuelos-Beltrán, P.; López de Andrés, J.; Antich, C.; de Vicente, J.; Jiménez, G.; Arias-Santiago, S.; Gálvez-Martín, P.; López-Ruiz, E.; Marchal, J.A. A bioactive three-layered skin substitute based on ECM components effectively promotes skin wound healing and regeneration. Mater. Today Bio 2025, 31, 101592. [Google Scholar] [CrossRef]
- Tran, K.; Jin, Y.; Bouyer, J.; DeOre, B.; Suprewicz, Ł.; Figel, A.; Walens, H.; Fischer, I.; Galie, P. Magnetic alignment of injectable hydrogel scaffolds for spinal cord injury repair. Biomater. Sci. 2022, 10, 2237–2247. [Google Scholar] [CrossRef]
- Zheng, Q.; Yao, J.; Sun, Z.; Li, R.; Zhang, Y.; Jiang, P.; Xie, Y.; Song, X.; Sun, H.; Zhu, D.; et al. Carboxymethyl chitosan/oxidized hyaluronic acid hydrogel-encapsulated hucMSC-derived exosomes for enhanced hepatic regeneration and post-hepatectomy applications. Carbohydr. Polym. 2025, 353, 123248. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Song, H.; Wang, R.; Wang, W.; Xing, J. A hyaluronic acid nanogels based exosome production factory for tumor photothermal therapy and angiogenesis inhibition. Int. J. Biol. Macromol. 2024, 293, 139363. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Wang, Y.; Guan, N.; Zuo, Y.; Lin, L.; Guo, B.; Mo, A.; Wu, Y.; Lin, X.; Cai, W.; et al. Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury. Nat. Commun. 2023, 14, 4011. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Dudko, V.; Khoruzhenko, O.; Hong, X.; Lv, Z.P.; Tunn, I.; Umer, M.; Timonen, J.V.I.; Linder, M.B.; Breu, J.; et al. Stiff and self-healing hydrogels by polymer entanglements in co-planar nanoconfinement. Nat. Mater. 2025, 24, 599–606. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, R.; Xia, Y.; Liu, J.; Tu, R.; Shi, J.; Dai, H. Effect of magnesium and calcium ions on the strength and biofunctionality of GelMA/SAMA composite hydrogels. J. Mater. Chem. B 2024, 12, 10692–10704. [Google Scholar] [CrossRef]
- Galbiati, M.; Maiullari, F.; Ceraolo, M.G.; Bousselmi, S.; Fratini, N.; Gega, K.; Recchia, S.; Ferretti, A.M.; Scala, G.; Costantini, M.; et al. Bioactive hydrogel supplemented with stromal cell-derived extracellular vesicles enhance wound healing. Pharmaceutics 2025, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Milan, M.; Chirivì, M.; Ceraolo, M.G.; Bousselmi, S.; Fratini, N.; Galbiati, M.; Fortunato, O.; Costantini, M.; Brambilla, F.; et al. Enhancing neovascularization post-myocardial infarction through injectable hydrogel functionalized with endothelial-derived EVs. Biofabrication 2024, 16, 045009. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, Z.; Fan, Y.; Yin, M.; Jin, C.; Guo, B.; Yin, Y.; Quan, R.; Zhao, S.; Han, S.; et al. Mimicked spinal cord fibers trigger axonal regeneration and remyelination after injury. ACS Nano 2023, 17, 25591–25613. [Google Scholar] [CrossRef]
- Azizi, M.; Farahmandghavi, F.; Joghataei, M.T.; Zandi, M.; Imani, M.; Bakhtiari, M.; Omidian, H. ChABC-loaded PLGA nanoparticles: A comprehensive study on biocompatibility, functional recovery, and axonal regeneration in animal model of spinal cord injury. Int. J. Pharm. 2020, 577, 119037. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Lin, Z.; Lu, Y.; Chen, H.; Li, B.; Li, Z.; Xia, H.; Li, L.; Zhang, T. Conducting molybdenum sulfide/graphene oxide/polyvinyl alcohol nanocomposite hydrogel for repairing spinal cord injury. J. Nanobiotechnol. 2022, 20, 210. [Google Scholar] [CrossRef]
- Nukolova, N.V.; Aleksashkin, A.D.; Abakumova, T.O.; Morozova, A.Y.; Gubskiy, I.L.; Kirzhanova, E.; Abakumov, M.A.; Chekhonin, V.P.; Klyachko, N.L.; Kabanov, A.V. Multilayer polyion complex nanoformulations of superoxide dismutase 1 for acute spinal cord injury. J. Control. Release 2018, 270, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Nutt, K.; Dombros-Ryan, Z.; Birea, R.; Franks, E.V.; Eastham, S.; Godwin, M.; Adams, C.F.; Chari, D.M.; Jenkins, S.I. Electrospun polycaprolactone (PCL) nanofibers induce elongation and alignment of co-cultured primary cortical astrocytes and neurons. Micromachines 2025, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Geddes, L.; Themistou, E.; Burrows, J.F.; Buchanan, F.J.; Carson, L. Evaluation of the in vitro cytotoxicity and modulation of the inflammatory response by the bioresorbable polymers poly(D,L-lactide-co-glycolide) and poly(L-lactide-co-glycolide). Acta Biomater. 2021, 134, 261–275. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Z.; Yu, M.; Liu, K.; Duan, H.; Zhang, Y.; Huang, X.; Li, M.; Li, C.; Hu, Y.; et al. Injectable ECM-mimetic dynamic hydrogels abolish ferroptosis-induced post-discectomy herniation through delivering nucleus pulposus progenitor cell-derived exosomes. Nat. Commun. 2025, 16, 3131. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Xia, N.; Tian, H.; Li, D.; Lin, J.; Mei, X.; Wu, C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021, 126, 211–223. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Guo, J.; Wu, J.; Lin, L.; Lin, X.; Mu, J.; Huang, T.; Zhu, M.; Ma, L.; et al. An engineering-reinforced extracellular vesicle-integrated hydrogel with an ROS-responsive release pattern mitigates spinal cord injury. Sci. Adv. 2025, 11, eads3398. [Google Scholar] [CrossRef]
- Xia, B.; Gao, X.; Qian, J.; Li, S.; Yu, B.; Hao, Y.; Wei, B.; Ma, T.; Wu, H.; Yang, S.; et al. A novel superparamagnetic multifunctional nerve scaffold: A remote actuation strategy to boost in situ extracellular vesicles production for enhanced peripheral nerve repair. Adv. Mater. 2024, 36, e2305374. [Google Scholar] [CrossRef]
- Nie, X.; Liu, Y.; Yuan, T.; Yu, T.; Yun, Z.; Xue, W.; Yu, T.; An, J.; Dai, A.; Wu, K.; et al. Platelet-rich plasma-derived exosomes promote blood-spinal cord barrier repair and attenuate neuroinflammation after spinal cord injury. J. Nanobiotechnol. 2024, 22, 456. [Google Scholar] [CrossRef]
- Kim, G.; Chen, X.; Yang, Y. Pathogenic extracellular vesicle (EV) signaling in amyotrophic lateral sclerosis (ALS). Neurotherapeutics 2022, 19, 1119–1132. [Google Scholar] [CrossRef]
- Pauwels, J.; Van de Steene, T.; Van de Velde, J.; De Muyer, F.; De Pauw, D.; Baeke, F.; Eyckerman, S.; Gevaert, K. Filter-aided extracellular vesicle enrichment (FAEVEr) for proteomics. Mol. Cell. Proteom. 2025, 24, 100907. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Dong, Y.; Li, G.; Li, J.; Cai, Q.; Yuan, S.; Wang, Y.; Song, H. Surface engineering of HEK293 cell-derived extracellular vesicles for improved pharmacokinetic profile and targeted delivery of IL-12 for the treatment of hepatocellular carcinoma. Int. J. Nanomed. 2023, 18, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Sabani, B.; Brand, M.; Albert, I.; Inderbitzin, J.; Eichenseher, F.; Schmelcher, M.; Rohrer, J.; Riedl, R.; Lehmann, S. A novel surface functionalization platform to prime extracellular vesicles for targeted therapy and diagnostic imaging. Nanomed 2023, 47, 102607. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.H.; Jensen, I.S.; Svenningsen, P. Benchmarking transcriptome deconvolution methods for estimating tissue- and cell-type-specific extracellular vesicle abundances. J. Extracell. Vesicles 2024, 13, e12511. [Google Scholar] [CrossRef] [PubMed]

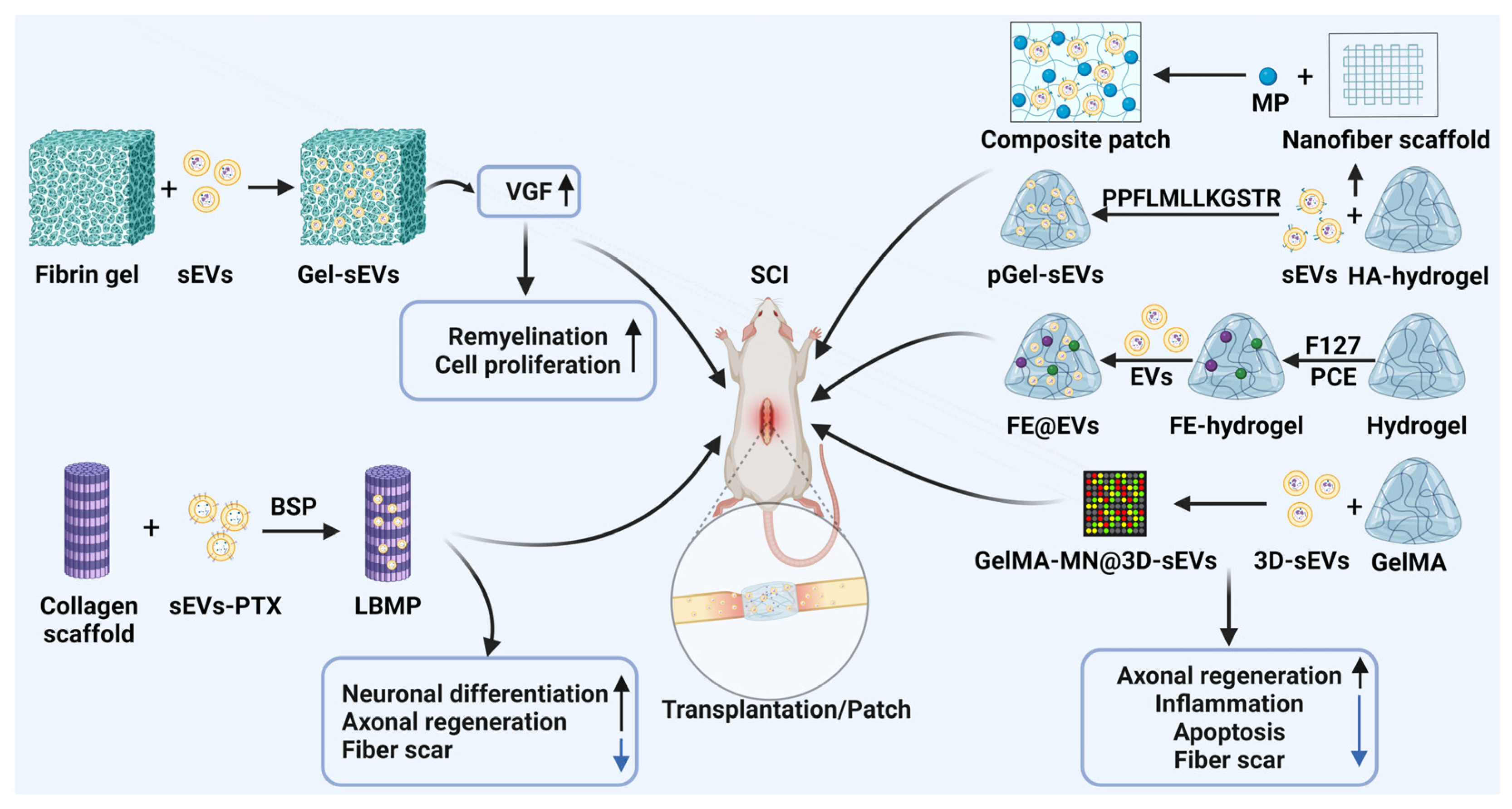
| Material | Method of Modification | SCI Model | Outcome | Reference |
|---|---|---|---|---|
| Fibrin gel | Human umbilical cord MSC-sEV and in situ gelate-encapsulated fibrin glue | Spinal cord injury (T9–T11) | Alleviate inflammatory and oxidative microenvironment Induce effective nerve tissue repair and functional recovery | [110] |
| Fibrin gel | Encapsulation of sEV | Spinal cord injury (T8–T9) | Enhance neurogenesis Remyelination | [111] |
| Collagen-I (Col-I) scaffold | Immobilization of CBD peptide fused in Lamp2b on the sEV surface | Spinal cord injury (T10) | Inhibit pro-apoptotic genes Provide enough space for cell growth or migration | [112] |
| Collagen | BSP specific binding to MSC-sEVs | Spinal cord injury (T8) | Enhance neural regeneration Reduce scar deposition | [113] |
| Gelatin methacryloyl hydrogel | 3D-sEV hybrid culture | Spinal cord injury (T9–T11) | Induce inflammation and glial scar | [114] |
| F127-polycitrate-polyethyleneimine hydrogel (FE) | Encapsulation of EV | Spinal cord injury (T10) | Suppress fibrotic scar formation Promote remyelination and axonal regeneration | [115] |
| Hyaluronic acid-hydrogel | Encapsulation of MSC-derived sEV, DBM, PDRN, et al. | Spinal cord injury (T10) | Increase regenerative capacity Reduce pro-inflammatory responses and restore BSCB disrupted by SCI Enhance remyelination | [107] |
| Hyaluronic acid-hydrogel | Encapsulation of hypo-sEV | Spinal cord injury (T9–T10) | Reduce pro-inflammatory responses Increase angiogenesis capacity and nerve regeneration | [116] |
| Nanofiber scaffold and hyaluronic acid hydrogel composite patch | Loaded with MP and SCs-sEVs | Spinal cord injury (T10) | Reduce neuronal apoptosis Inhibit inflammatory reaction | [35] |
| Peptide-modified adhesive hydrogel (pGel) | Encapsulation of hMSC-derived sEV | Spinal cord injury (T9–T10) | Mitigate inflammation and oxidation Improve nerve recovery and urinary tissue preservation | [117] |
| NCT Number | Study Type | Study Phase | Recruiting Status | Biomaterials | SCI Model | Ages | Enrollment |
|---|---|---|---|---|---|---|---|
| ChiCTR2100043838 | Interventional | 0 | Recruiting | Nerve regeneration collagen scaffold | Spinal cord injury (C6–T12) | 18 years to 60 years | 30 |
| ChiCTR-INR-17012152 | Interventional | New treatment measure clinical study | Active, not recruiting | Functional scaffold | Complete spinal cord injury (T1–T11) | 18 years to 60 years | 6 |
| NCT03762655 | Interventional | Not applicable | Active, not recruiting | Neuro-spinal scaffold | Complete spinal cord injury (T2–T12) | 16 years to 70 years | 20 |
| NCT02352077 | Interventional | Phase 1 | Unknown status | Neural regeneration collagen scaffold | Complete spinal cord injury (C5–T12) | 18 years to 65 years | 30 |
| NCT03966794 | Interventional | Phase 2 | Unknown status | Functional neural regeneration scaffold | Complete spinal cord injury (C4–T12/L1) | 18 years to 60 years | 9 |
| NCT03933072 | Interventional | Phase 2 | Unknown status | Collagen scaffold | Complete spinal cord injury (C5–T10) | 16 years to 65 years | 2 |
| NCT05967325 | Interventional | Not applicable | Recruiting | Functional self-assembling peptide nanofiber hydrogels | Complete spinal cord injury (T1–T12) | 18 years to 60 years | 15 |
| NCT04132596 | Interventional | Not applicable | Completed | Transcutaneous hydrogel electrodes | Spinal cord injury (C4–T12) | 18 years and older | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Wang, Z.; Bao, Q.; Chen, S.; Xu, W.; Jiang, J. Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go. Biomedicines 2025, 13, 1262. https://doi.org/10.3390/biomedicines13051262
Jiang J, Wang Z, Bao Q, Chen S, Xu W, Jiang J. Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go. Biomedicines. 2025; 13(5):1262. https://doi.org/10.3390/biomedicines13051262
Chicago/Turabian StyleJiang, Jiali, Ziyi Wang, Qinghua Bao, Shenyuan Chen, Wenrong Xu, and Jiajia Jiang. 2025. "Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go" Biomedicines 13, no. 5: 1262. https://doi.org/10.3390/biomedicines13051262
APA StyleJiang, J., Wang, Z., Bao, Q., Chen, S., Xu, W., & Jiang, J. (2025). Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go. Biomedicines, 13(5), 1262. https://doi.org/10.3390/biomedicines13051262






