New Insights into the Diagnosis and Treatment of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Pathophysiology of HCC
3. Early Diagnosis of HCC
3.1. Imaging
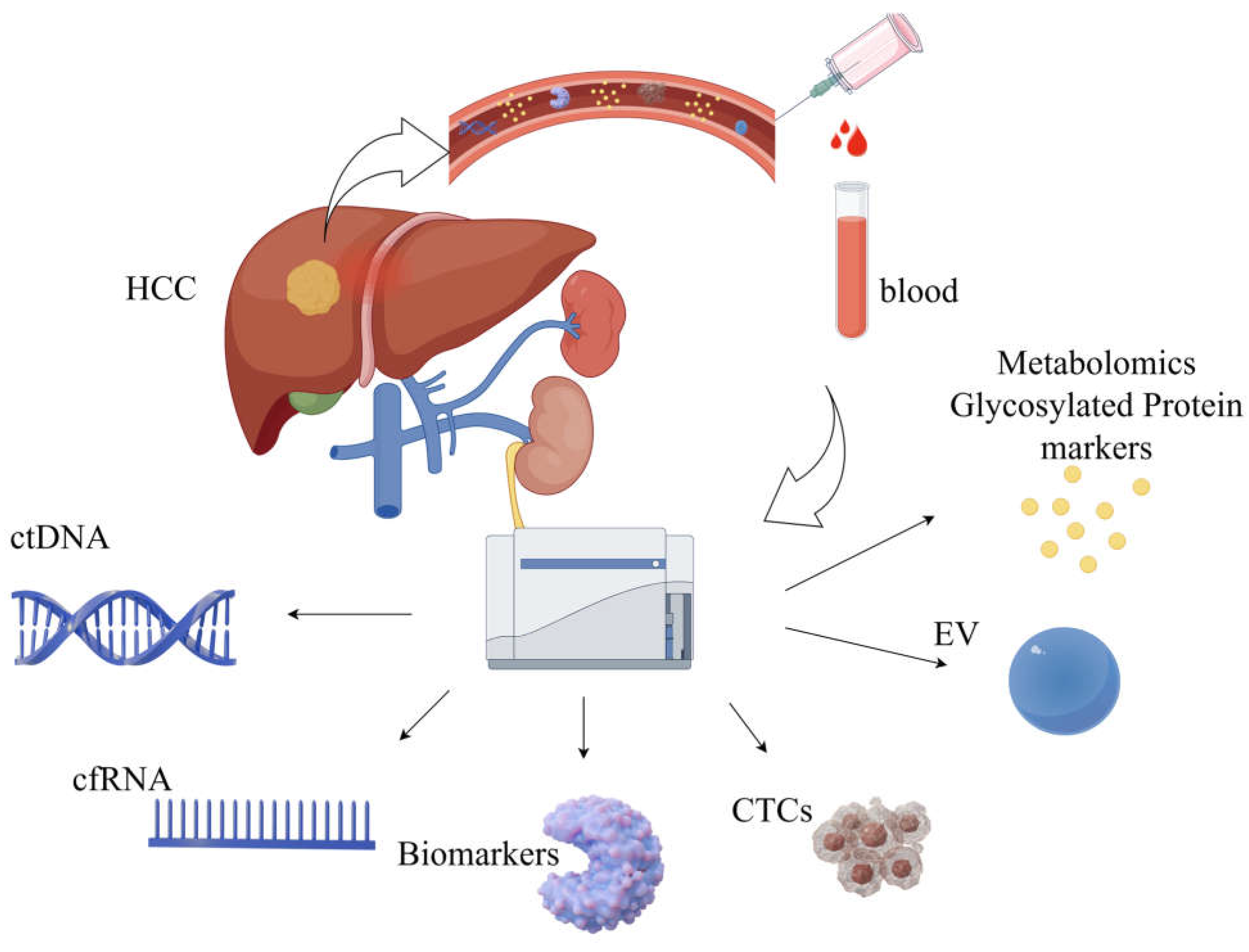
3.2. Liquid Biopsy and New Biomarkers
3.2.1. New Biomarkers
3.2.2. Liquid Biopsy
4. Treatment of HCC
4.1. Immunotherapy for HCC
4.2. Interventional Therapy for HCC
4.3. Other Therapy for HCC
5. The Application of AI to HCC
5.1. AI in HCC Research
5.2. AI in HCC Early Diagnosis
5.2.1. AI in Imaging Diagnosis
5.2.2. AI in Histopathology
5.3. AI in Drug Research and Development
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| AFP | Alpha-fetoprotein |
| DCP | Des-γ-carboxy prothrombin |
| NAFLD | Non-alcoholic fatty liver disease |
| AI | Artificial intelligence |
| DC | Dendritic cells |
| TMB | Tumor mutational burden |
| TGFβ | Transforming growth factor beta |
| VEGF | Vascular endothelial growth factor |
| IFNγ | Interferon gamma |
| Gd-EOB-DTPA | Gadoxetic acid disodium |
| PIVKA-II | Vitamin K absence or antagonist-II |
| GPC3 | Glypican-3 |
| OPN | Osteopontin |
| GP73 | Golgi protein 73 |
| TME | Tumor microenvironment |
| HSP70 | Heat shock protein 70 |
| CTCs | Circulating tumor cells |
| ctDNA | Circulating tumor DNA |
| cfDNA | Cell-free DNA |
| NGS | Next-generation sequencing |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| lncRNA | Long non-coding RNA |
| cfRNA | Cell-free RNA |
| cf-mRNA | Cell-free mRNA |
| EV | Extracellular Vesicles |
| TKIs | Tyrosine kinase inhibitors |
| ICIs | Immune checkpoint inhibitors |
| CIs | Monoclonal antibodies |
| CTLA4 | Cytotoxic T lymphocyte-associated antigen 4 |
| PDL1 | Programmed death-ligand 1 |
| LAG3 | Lymphocyte activation gene 3 |
| TIM3 | T-cell immunoglobulin and mucin-domain containing-3 |
| CSCs | Cancer stem cells |
| TAE | Transarterial therapies, including bland embolization |
| TACE | Transarterial chemoembolization |
| DEB–TACE | Drug-eluting beads—transarterial chemoembolization |
| SIRT | Selective internal radioembolization therapy |
| HAIC | Hepatic artery infusion chemotherapy |
| DEBs | Drug-eluting beads |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| CDK20 | Cyclin-dependent kinase 20 |
| DCNN | Deep convolutional neural network |
| US | Ultrasound |
| DL | Deep learning (DL) |
| CEUS | Contrast-enhanced ultrasound |
| CAD | Computer-aided diagnosis |
| DCCA-MKL | Deep canonical correlation analysis with multiple kernel learning |
| 3DIRCAD | 3D-image reconstruction for comparison of algorithm database |
| CNN | Convolutional neural network |
| DLS | Deep-learning system (DLS) |
| CCA | Cholangiocarcinoma |
| AUC | Area under the curve |
References
- Tsukuma, H.; Hiyama, T.; Tanaka, S.; Nakao, M.; Yabuuchi, T.; Kitamura, T.; Nakanishi, K.; Fujimoto, I.; Inoue, A.; Yamazaki, H.; et al. Risk Factors for Hepatocellular Carcinoma among Patients with Chronic Liver Disease. N. Engl. J. Med. 1993, 328, 1797–1801. [Google Scholar] [CrossRef]
- Ha, N.B.; Ha, N.B.; Ahmed, A.; Ayoub, W.; Daugherty, T.J.; Chang, E.T.; Lutchman, G.A.; Garcia, G.; Cooper, A.D.; Keeffe, E.B.; et al. Risk Factors for Hepatocellular Carcinoma in Patients with Chronic Liver Disease: A Case-Control Study. Cancer Causes Control 2012, 23, 455–462. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.J.; Ferlay, O.; Lesi, C.J.; Cabasag, J.; Vignat, M.; Laversanne, K.; McGlynn, A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Calderaro, J.; Seraphin, T.P.; Luedde, T.; Simon, T.G. Artificial Intelligence for the Prevention and Clinical Management of Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 1348–1361. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A., 3rd; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting Neoantigens to Augment Antitumour Immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and Functional Alterations of Tumor-Associated Antigen-Specific Cd8+ T-Cell Responses in Hepatocellular Carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, C.; Xue, R.; Liu, M.; Bai, J.; Bao, J.; Wang, Y.; Jiang, N.; Li, Z.; Wang, W.; et al. Deep Whole-Genome Analysis of 494 Hepatocellular Carcinomas. Nature 2024, 627, 586–593. [Google Scholar] [CrossRef]
- Qian, Z.; Liang, J.; Huang, R.; Song, W.; Ying, J.; Bi, X.; Zhao, J.; Shi, Z.; Liu, W.; Liu, J.; et al. Hbv Integrations Reshaping Genomic Structures Promote Hepatocellular Carcinoma. Gut 2024, 73, 1169–1182. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. Vegf in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Lee, I.C.; Huang, Y.H.; Chau, G.Y.; Huo, T.I.; Su, C.W.; Wu, J.C.; Lin, H.C. Serum Interferon Gamma Level Predicts Recurrence in Hepatocellular Carcinoma Patients after Curative Treatments. Int. J. Cancer 2013, 133, 2895–2902. [Google Scholar] [CrossRef]
- Cha, C.; DeMatteo, R.P. Molecular Mechanisms in Hepatocellular Carcinoma Development. Best. Pract. Res. Clin. Gastroenterol. 2005, 19, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, B. Hepatocellular Carcinoma: Molecular Mechanism, Targeted Therapy, and Biomarkers. Cancer Metastasis Rev. 2023, 42, 629–652. [Google Scholar] [CrossRef]
- Li, Q.; Ni, Y.; Zhang, L.; Jiang, R.; Xu, J.; Yang, H.; Hu, Y.; Qiu, J.; Pu, L.; Tang, J.; et al. Hif-1α-Induced Expression of M6a Reader Ythdf1 Drives Hypoxia-Induced Autophagy and Malignancy of Hepatocellular Carcinoma by Promoting Atg2a and Atg14 Translation. Signal Transduct. Target. Ther. 2021, 6, 76. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, Q. Autophagy, an Accomplice or Antagonist of Drug Resistance in Hcc? Cell Death Dis. 2021, 12, 266. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The Integrated Stress Response: From Mechanism to Disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in Cancer Therapy: A Novel Approach to Reversing Drug Resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Chen, J.; Xia, C.C.; Cao, L.K.; Duan, T.; Song, B. Non-invasive Imaging of Hepatocellular Carcinoma: From Diagnosis to Prognosis. World J. Gastroenterol. 2018, 24, 2348–2362. [Google Scholar] [CrossRef]
- Yang, D.; Chen, X.; Huang, L.; Wang, X.; Mao, L.; Lin, L.; Han, H.; Lu, Q. Correlation between Ceus Li-Rads Categorization of Hcc < 20 Mm and Clinic-Pathological Features. Insights Imaging 2024, 15, 110. [Google Scholar]
- Sugimoto, K.; Kamiyama, N.; Kakegawa, T.; Takahashi, H.; Wada, T.; Abe, M.; Yoshimasu, Y.; Takeuchi, H.; Itoi, T. Modified Ceus Li-Rads Using Sonazoid for the Diagnosis of Hepatocellular Carcinoma. Ultrasonography 2023, 42, 388–399. [Google Scholar] [CrossRef]
- Paisant, A.; Boursier, J.; Dabli, D.; Lebigot, J.; Oberti, F.; Michalak, S.; Vilgrain, V.; Aubé, C. Two-Phase Mdct Protocol for the Screening of Small Hepatocellular Carcinoma. J. Clin. Med. 2022, 11, 4282. [Google Scholar] [CrossRef]
- Bhandari, A.; Koppen, J.; Wastney, T.; Hacking, C. A Systematic Review and Meta-Analysis of Spectral Ct to Differentiate Focal Liver Lesions. Clin. Radiol. 2023, 78, 430–436. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Li, M.; Liu, X.; Xu, Y. Prediction of Histologic Grade of Hepatocellular Carcinoma Using Dual-Layer Spectral-Detector Computed Tomography (Ct): Comparison of Two Region of Interest Plotting Methods. Quant. Imaging Med. Surg. 2024, 14, 3887–3900. [Google Scholar] [CrossRef]
- Lewin, M.; Laurent-Bellue, A.; Desterke, C.; Radu, A.; Feghali, J.A.; Farah, J.; Agostini, H.; Nault, J.C.; Vibert, E.; Guettier, C. Evaluation of Perfusion Ct and Dual-Energy Ct for Predicting Microvascular Invasion of Hepatocellular Carcinoma. Abdom Radiol. 2022, 47, 2115–2127. [Google Scholar] [CrossRef]
- Fujita, K.; Kinukawa, H.; Ohno, K.; Ito, Y.; Saegusa, H.; Yoshimura, T. Development and Evaluation of Analytical Performance of a Fully Automated Chemiluminescent Immunoassay for Protein Induced by Vitamin K Absence or Antagonist II. Clin. Biochem. 2015, 48, 1330–1336. [Google Scholar] [CrossRef]
- Mohammed, A.F.; Chen, X.; Li, C. Clinical Utility of Biomarkers of Hepatocellular Carcinoma. Bratisl. Lek. Listy 2024, 125, 102–106. [Google Scholar] [CrossRef]
- Zhu, M.; Zheng, J.; Wu, F.; Kang, B.; Liang, J.; Heskia, F.; Zhang, X.; Shan, Y. Opn Is a Promising Serological Biomarker for Hepatocellular Carcinoma Diagnosis. J. Med. Virol. 2020, 92, 3596–3603. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Zhou, S.; Sun, T.; Shen, F.; Zeng, L. Golgi Protein 73 Promotes Angiogenesis in Hepatocellular Carcinoma. Research 2024, 7, 0425. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Tealeb, A.-S.M. The Role of Heat Shock Protein 70 and Glypican 3 Expression in Early Diagnosis of Hepatocellular Carcinoma. Egypt. J. Pathol. 2022, 42, 112–116. [Google Scholar] [CrossRef]
- Qi, L.N.; Xiang, B.D.; Wu, F.X.; Ye, J.Z.; Zhong, J.H.; Wang, Y.Y.; Chen, Y.Y.; Chen, Z.S.; Ma, L.; Chen, J.; et al. Circulating Tumor Cells Undergoing Emt Provide a Metric for Diagnosis and Prognosis of Patients with Hepatocellular Carcinoma. Cancer Res. 2018, 78, 4731–4744. [Google Scholar] [CrossRef]
- Campani, C.; Imbeaud, S.; Couchy, G.; Ziol, M.; Hirsch, T.Z.; Rebouissou, S.; Noblet, B.; Nahon, P.; Hormigos, K.; Sidali, S.; et al. Circulating Tumour DNA in Patients with Hepatocellular Carcinoma across Tumour Stages and Treatments. Gut 2024, 73, 1870–1882. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Cho, E.J.; Lee, J.Y.; Kim, J.; Kwon, C.; Kim-Ha, J.; Hong, S.K.; Choi, Y.; Yi, N.J.; et al. A Circulating Cell-Free DNA Methylation Signature for the Detection of Hepatocellular Carcinoma. Mol. Cancer 2023, 22, 164. [Google Scholar] [CrossRef]
- Roskams-Hieter, B.; Kim, H.J.; Anur, P.; Wagner, J.T.; Callahan, R.; Spiliotopoulos, E.; Kirschbaum, C.W.; Civitci, F.; Spellman, P.T.; Thompson, R.F.; et al. Plasma Cell-Free Rna Profiling Distinguishes Cancers from Pre-Malignant Conditions in Solid and Hematologic Malignancies. NPJ Precis. Oncol. 2022, 6, 28. [Google Scholar] [CrossRef]
- Toh, T.B.; Lim, J.J.; Chow, E.K. Epigenetics of Hepatocellular Carcinoma. Clin. Transl. Med. 2019, 8, 13. [Google Scholar] [CrossRef]
- Hua, D.; Hu, Y.; Wu, Y.-Y.; Cheng, Z.-H.; Yu, J.; Du, X.; Huang, Z.-H. Quantitative Methylation Analysis of Multiple Genes Using Methylation-Sensitive Restriction Enzyme-Based Quantitative Pcr for the Detection of Hepatocellular Carcinoma. Exp. Mol. Pathol. 2011, 91, 455–460. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The Biology, Function, and Applications of Exosomes in Cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Kimhofer, T.; Fye, H.; Taylor-Robinson, S.; Thursz, M.; Holmes, E. Proteomic and Metabonomic Biomarkers for Hepatocellular Carcinoma: A Comprehensive Review. Br. J. Cancer 2015, 112, 1141–1156. [Google Scholar] [CrossRef]
- Zhu, J.; Warner, E.; Parikh, N.D.; Lubman, D.M. Glycoproteomic Markers of Hepatocellular Carcinoma-Mass Spectrometry Based Approaches. Mass. Spectrom. Rev. 2019, 38, 265–290. [Google Scholar] [CrossRef]
- Fujiki, M.; Aucejo, F.; Kim, R. General Overview of Neo-Adjuvant Therapy for Hepatocellular Carcinoma before Liver Transplantation: Necessity or Option? Liver Int. 2011, 31, 1081–1089. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in Immunotherapy for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Du, W.; Yang, M.; Turner, A.; Xu, C.; Ferris, R.L.; Huang, J.; Kane, L.P.; Lu, B. Tim-3 as a Target for Cancer Immunotherapy and Mechanisms of Action. Int. J. Mol. Sci. 2017, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-Like Protein 1 Is a Major Immune Inhibitory Ligand of Lag-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef]
- Becht, R.; Kiełbowski, K.; Wasilewicz, M.P. New Opportunities in the Systemic Treatment of Hepatocellular Carcinoma-Today and Tomorrow. Int. J. Mol. Sci. 2024, 25, 1456. [Google Scholar] [CrossRef]
- Yamamoto, A.; Chervoneva, I.; Sullivan, K.L.; Eschelman, D.J.; Gonsalves, C.F.; Mastrangelo, M.J.; Berd, D.; Shields, J.A.; Shields, C.L.; Terai, M.; et al. High-Dose Immunoembolization: Survival Benefit in Patients with Hepatic Metastases from Uveal Melanoma. Radiology 2009, 252, 290–298. [Google Scholar] [CrossRef]
- Bucalau, A.M.; Tancredi, I.; Verset, G. In the Era of Systemic Therapy for Hepatocellular Carcinoma Is Transarterial Chemoembolization Still a Card to Play? Cancers 2021, 13, 5129. [Google Scholar] [CrossRef]
- Dawoud, M.A.; Mohamed, R.E.; El Waraki, M.S.; Gabr, A.M. Single-Session Combined Radiofrequency Ablation and Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. Egypt. J. Radiol. Nucl. Med. 2017, 48, 935–946. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Mu, H.; Yu, G.; Xing, W.; Wang, L.; Zhang, T. Surgical Conversion for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Using a Triple Combination of Angiogenesis Inhibitors, Anti-Pd-1 Antibodies, and Hepatic Arterial Infusion Chemotherapy: A Retrospective Study. Front. Oncol. 2021, 11, 729764. [Google Scholar] [CrossRef]
- Verma, Y.; Perera Molligoda Arachchige, A.S. Advances in Tumor Management: Harnessing the Potential of Histotripsy. Radiol. Imaging Cancer 2024, 6, e230159. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-Thermal Histotripsy Tumor Ablation Promotes Abscopal Immune Responses That Enhance Cancer Immunotherapy. J. Immunother. Cancer 2020, 8, 1. [Google Scholar] [CrossRef]
- Pun, F.W.; Ozerov, I.V.; Zhavoronkov, A. Ai-Powered Therapeutic Target Discovery. Trends Pharmacol. Sci. 2023, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, K.; Cheng, R.; Nonaka, H.; Tamura, T.; Hamachi, I. Chemical Tools for Endogenous Protein Labeling and Profiling. Cell Chem. Biol. 2020, 27, 970–985. [Google Scholar] [CrossRef]
- Viñas, R.; Andrés-Terré, H.; Liò, P.; Bryson, K. Adversarial Generation of Gene Expression Data. Bioinformatics 2022, 38, 730–737. [Google Scholar] [CrossRef]
- Song, J.; Xu, Z.; Cao, L.; Wang, M.; Hou, Y.; Li, K. The Discovery of New Drug-Target Interactions for Breast Cancer Treatment. Molecules 2021, 26, 7474. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Sun, L.; Xia, Y.; Qin, T.; Zhang, S.; Poon, H.; Liu, T.Y. Biogpt: Generative Pre-Trained Transformer for Biomedical Text Generation and Mining. Brief. Bioinform. 2022, 23, bbac409. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.C.; Yang, J.J.; Mathias, S.L.; Ursu, O.; Mani, S.; Waller, A.; Schürer, S.C.; Jensen, L.J.; Sklar, L.A.; Bologa, C.G.; et al. Tin-X: Target Importance and Novelty Explorer. Bioinformatics 2017, 33, 2601–2603. [Google Scholar] [CrossRef]
- Ren, F.; Ding, X.; Zheng, M.; Korzinkin, M.; Cai, X.; Zhu, W.; Mantsyzov, A.; Aliper, A.; Aladinskiy, V.; Cao, Z.; et al. Alphafold Accelerates Artificial Intelligence Powered Drug Discovery: Efficient Discovery of a Novel Cdk20 Small Molecule Inhibitor. Chem. Sci. 2023, 14, 1443–1452. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, J.; Hao, X.; Kong, D.; Yu, X.; Jiang, T.; Xi, J.; Cai, W.; Luo, Y.; Jing, X.; et al. Improving B-Mode Ultrasound Diagnostic Performance for Focal Liver Lesions Using Deep Learning: A Multicentre Study. EBioMedicine 2020, 56, 102777. [Google Scholar] [CrossRef]
- Guo, L.H.; Wang, D.; Qian, Y.Y.; Zheng, X.; Zhao, C.K.; Li, X.L.; Bo, X.W.; Yue, W.W.; Zhang, Q.; Shi, J.; et al. A Two-Stage Multi-View Learning Framework Based Computer-Aided Diagnosis of Liver Tumors with Contrast Enhanced Ultrasound Images. Clin. Hemorheol. Microcirc. 2018, 69, 343–354. [Google Scholar] [CrossRef]
- Shukla, P.K.; Zakariah, M.; Hatamleh, W.A.; Tarazi, H.; Tiwari, B. Ai-Driven Novel Approach for Liver Cancer Screening and Prediction Using Cascaded Fully Convolutional Neural Network. J. Healthc. Eng. 2022, 2022, 4277436. [Google Scholar] [CrossRef]
- Mokrane, F.; Lu, L.; Vavasseur, A.; Otal, P.; Peron, J.M.; Luk, L.; Yang, H.; Ammari, S.; Saenger, Y.; Rousseau, H.; et al. Radiomics Machine-Learning Signature for Diagnosis of Hepatocellular Carcinoma in Cirrhotic Patients with Indeterminate Liver Nodules. Eur. Radiol. 2020, 30, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.A.; Wang, C.J.; Savic, L.J.; Ferrante, M.; Schobert, I.; Schlachter, T.; Lin, M.; Duncan, J.S.; Weinreb, J.C.; Chapiro, J.; et al. Deep Learning for Liver Tumor Diagnosis Part I: Development of a Convolutional Neural Network Classifier for Multi-Phasic MRI. Eur. Radiol. 2019, 29, 3338–3347. [Google Scholar] [CrossRef]
- Patel, S.K.; George, B.; Rai, V. Artificial Intelligence to Decode Cancer Mechanism: Beyond Patient Stratification for Precision Oncology. Front. Pharmacol. 2020, 11, 1177. [Google Scholar] [CrossRef]
- Liao, H.; Long, Y.; Han, R.; Wang, W.; Xu, L.; Liao, M.; Zhang, Z.; Wu, Z.; Shang, X.; Li, X.; et al. Deep Learning-Based Classification and Mutation Prediction from Histopathological Images of Hepatocellular Carcinoma. Clin. Transl. Med. 2020, 10, e102. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Uyumazturk, B.; Rajpurkar, P.; Wang, A.; Gao, R.; Jones, E.; Yu, Y.; Langlotz, C.P.; Ball, R.L.; Montine, T.J.; et al. Impact of a Deep Learning Assistant on the Histopathologic Classification of Liver Cancer. NPJ Digit. Med. 2020, 3, 23. [Google Scholar] [CrossRef]
- Verghese, G.; Lennerz, J.K.; Ruta, D.; Ng, W.; Thavaraj, S.; Siziopikou, K.P.; Naidoo, T.; Rane, S.; Salgado, R.; Pinder, S.E.; et al. Computational Pathology in Cancer Diagnosis, Prognosis, and Prediction—Present Day and Prospects. J. Pathol. 2023, 260, 551–563. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Bejnordi, B.E.; Trinh, V.Q.; Chan, L.; Hasan, D.; Li, X.; Yang, S.; Kim, T.; Zhang, H.; Wu, T.; et al. Computational Pathology: A Survey Review and the Way Forward. J. Pathol. Inform. 2024, 15, 100357. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-Omics Approach: A New Frontier in Cancer Research. Biomed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef]
- Gulfidan, G.; Soylu, M.; Demirel, D.; Erdonmez, H.B.C.; Beklen, H.; Sarica, P.O.; Arga, K.Y.; Turanli, B. Systems Biomarkers for Papillary Thyroid Cancer Prognosis and Treatment through Multi-Omics Networks. Arch. Biochem. Biophys. 2022, 715, 109085. [Google Scholar] [CrossRef]
- Weberpals, J.I.; Pugh, T.J.; Marco-Casanova, P.; Goss, G.D.; Wright, N.A.; Rath, P.; Torchia, J.; Jones, F.G.; Roudier, M.P.; Bernard, L.; et al. Tumor Genomic, Transcriptomic, and Immune Profiling Characterizes Differential Response to First-Line Platinum Chemotherapy in High Grade Serous Ovarian Cancer. Cancer Med. 2021, 10, 3045–3058. [Google Scholar] [CrossRef]
- Thompson, B.; Howe, N.P. Alphafold 3.0: The Ai Protein Predictor Gets an Upgrade. Nature 2024. [Google Scholar] [CrossRef]
- Kuhlman, B.; Bradley, P. Advances in Protein Structure Prediction and Design. Nat. Rev. Mol. Cell Biol. 2019, 20, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Kantliwala, S.V.; Vybhavi, J.; Ravi, R.; Patel, H.; Patel, J. Review of Alphafold 3: Transformative Advances in Drug Design and Therapeutics. Cureus 2024, 16, e63646. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Zhang, M.; Liu, Y.; Jang, H. Alphafold, Allosteric, and Orthosteric Drug Discovery: Ways Forward. Drug Discov. Today 2023, 28, 103551. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, H.; Steeg, G.V.; Godzik, A. Advances in Ai for Protein Structure Prediction: Implications for Cancer Drug Discovery and Development. Biomolecules 2024, 14, 339. [Google Scholar] [CrossRef]
- Calderaro, J.; Žigutytė, L.; Truhn, D.; Jaffe, A.; Kather, J.N. Artificial Intelligence in Liver Cancer—New Tools for Research and Patient Management. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 585–599. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Lu, B.; Deng, B. New Insights into the Diagnosis and Treatment of Hepatocellular Carcinoma. Biomedicines 2025, 13, 1244. https://doi.org/10.3390/biomedicines13051244
Li C, Lu B, Deng B. New Insights into the Diagnosis and Treatment of Hepatocellular Carcinoma. Biomedicines. 2025; 13(5):1244. https://doi.org/10.3390/biomedicines13051244
Chicago/Turabian StyleLi, Chengbo, Bingjiu Lu, and Baocheng Deng. 2025. "New Insights into the Diagnosis and Treatment of Hepatocellular Carcinoma" Biomedicines 13, no. 5: 1244. https://doi.org/10.3390/biomedicines13051244
APA StyleLi, C., Lu, B., & Deng, B. (2025). New Insights into the Diagnosis and Treatment of Hepatocellular Carcinoma. Biomedicines, 13(5), 1244. https://doi.org/10.3390/biomedicines13051244




