Renalase Overexpression-Mediated Excessive Metabolism of Peripheral Dopamine, DOPAL Accumulation, and α-Synuclein Aggregation in Baroreflex Afferents Contribute to Neuronal Degeneration and Autonomic Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Animals
2.3. 6-OHDA-Induced PD Model
2.4. Behavioral Examination
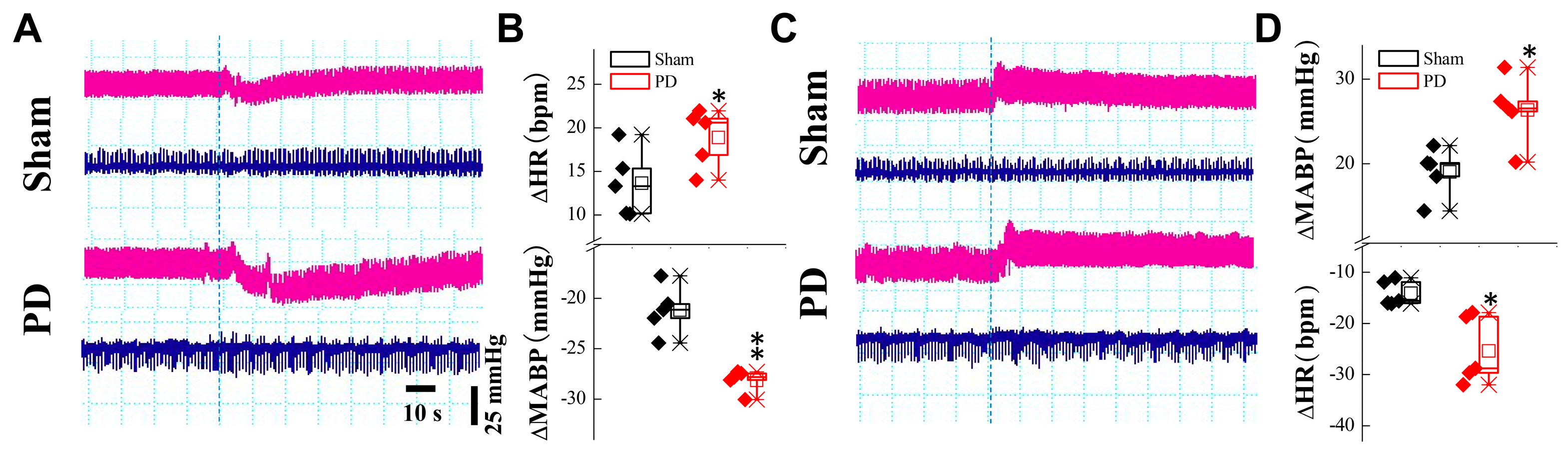
2.5. BP Measurements While Changing Position
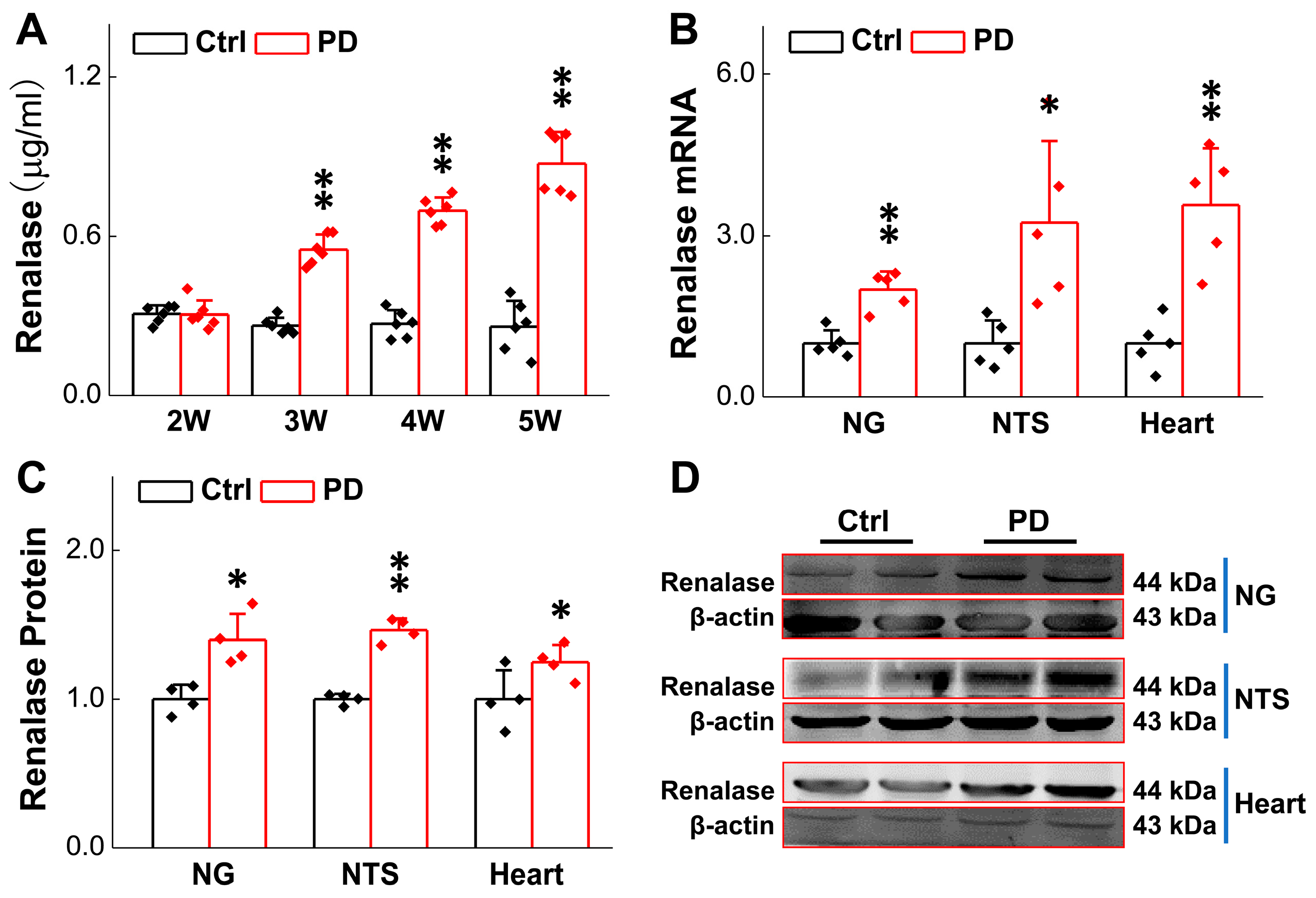
2.6. Elisa Kit Detection of Serum RNLS
2.7. Tissue Collection
2.8. Cell Culture and Transfection
2.9. Western Blot
2.10. Immunohistochemical Analysis
2.11. High Performance Liquid Chromatography (HPLC)
2.12. Transmission Electron Microscopy
2.13. JC-1 and Calcein-AM/PI Double Staining
2.14. CCK-8 Assay
2.15. Statistical Analyses
3. Results
3.1. Up-Regulated RNLS Expression in 6-OHDA-Treated Rats
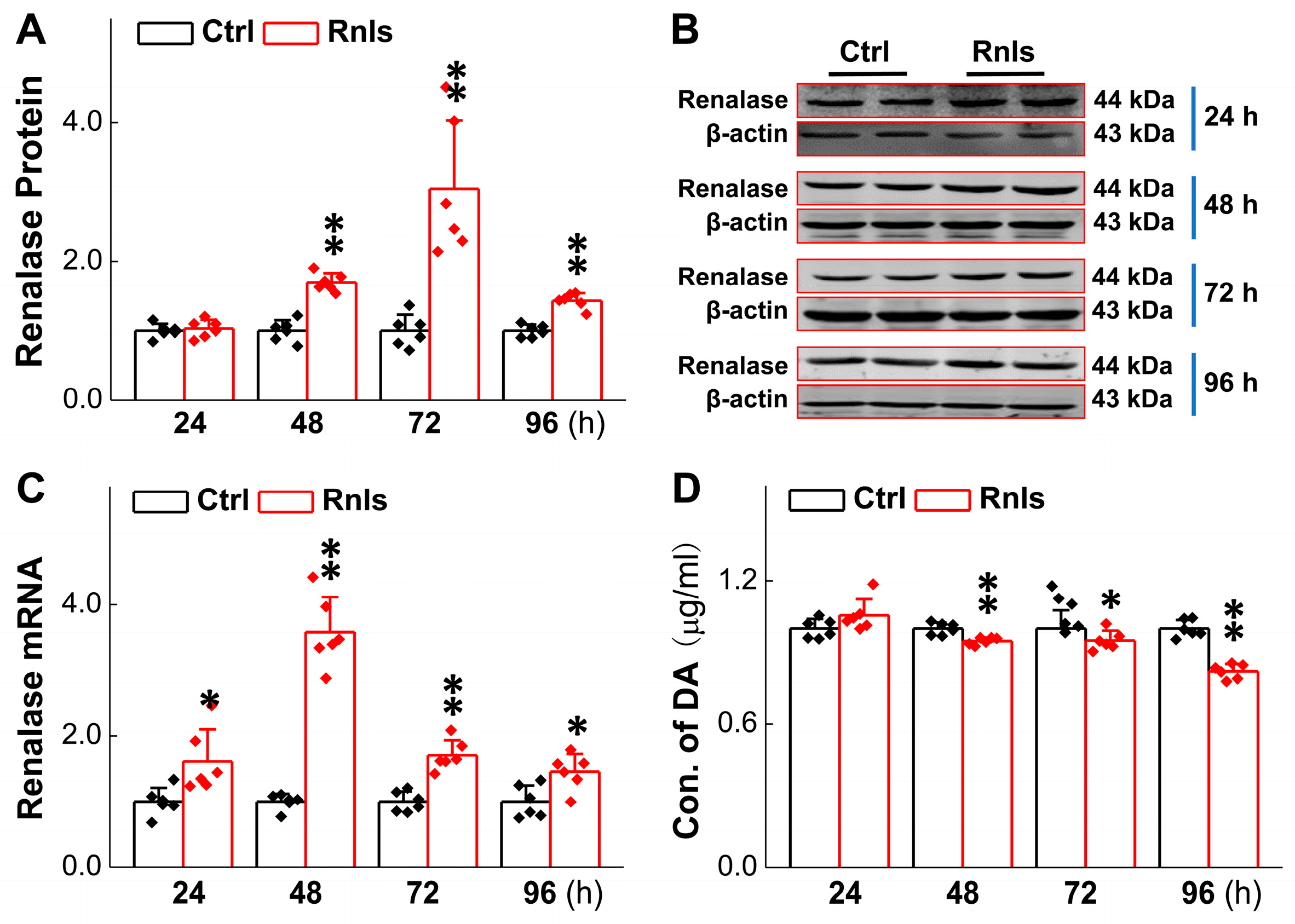
3.2. Decreased DA Concentration in PC12 Cells in Parallel with RNLS Overexpression
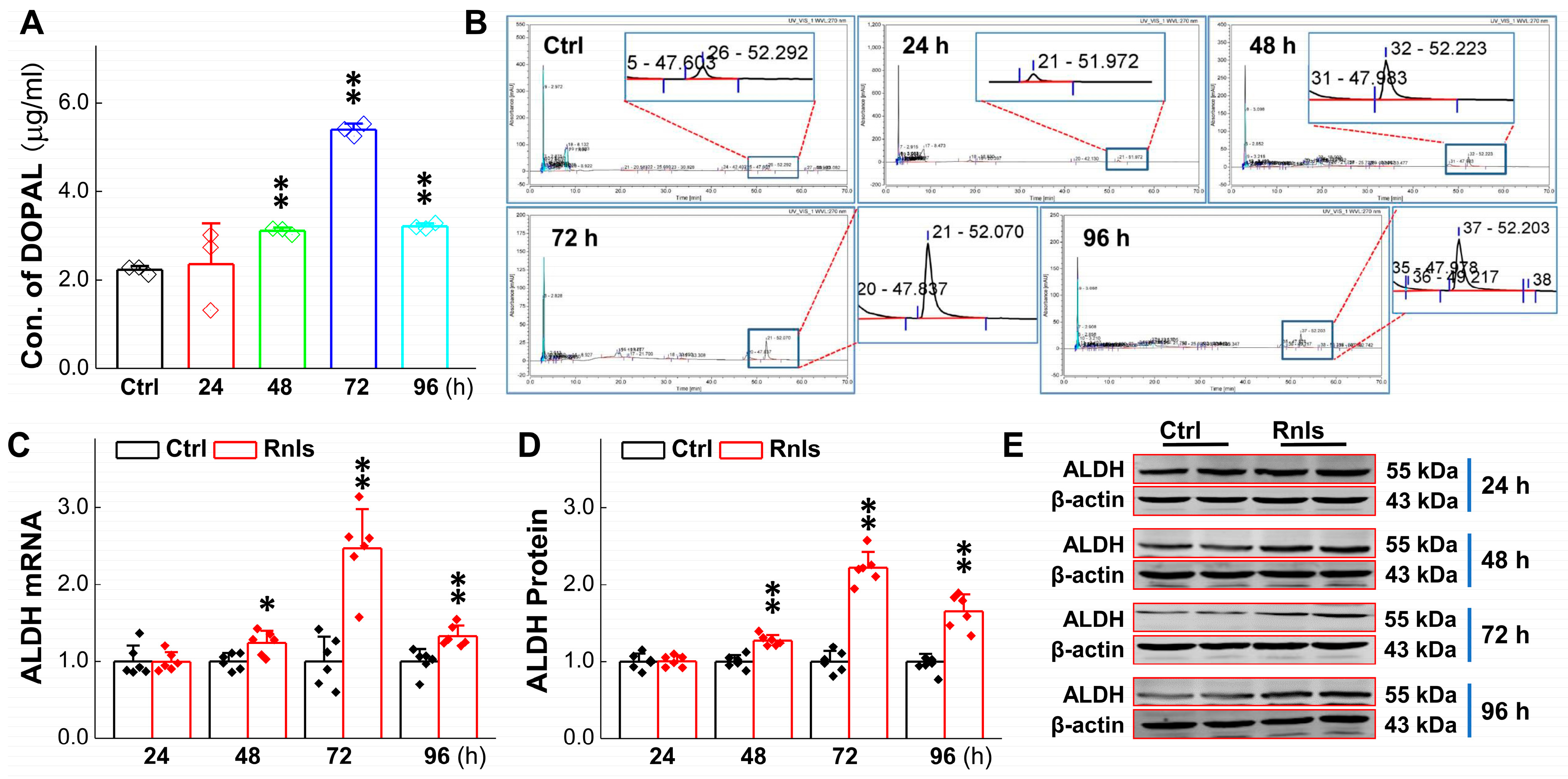
3.3. RNLS-Mediated DA Catalyzation with Increase in Its Metabolite Content
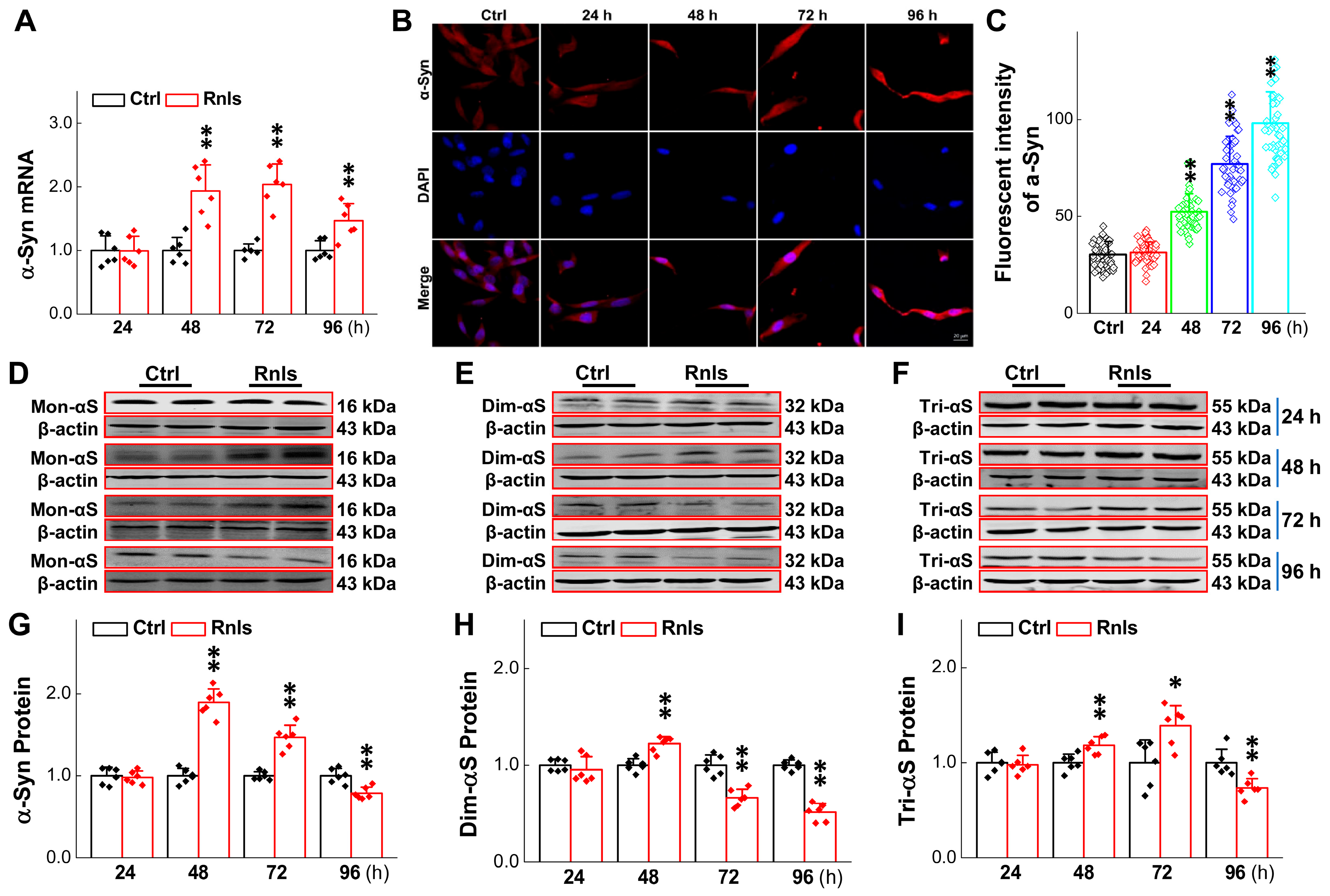
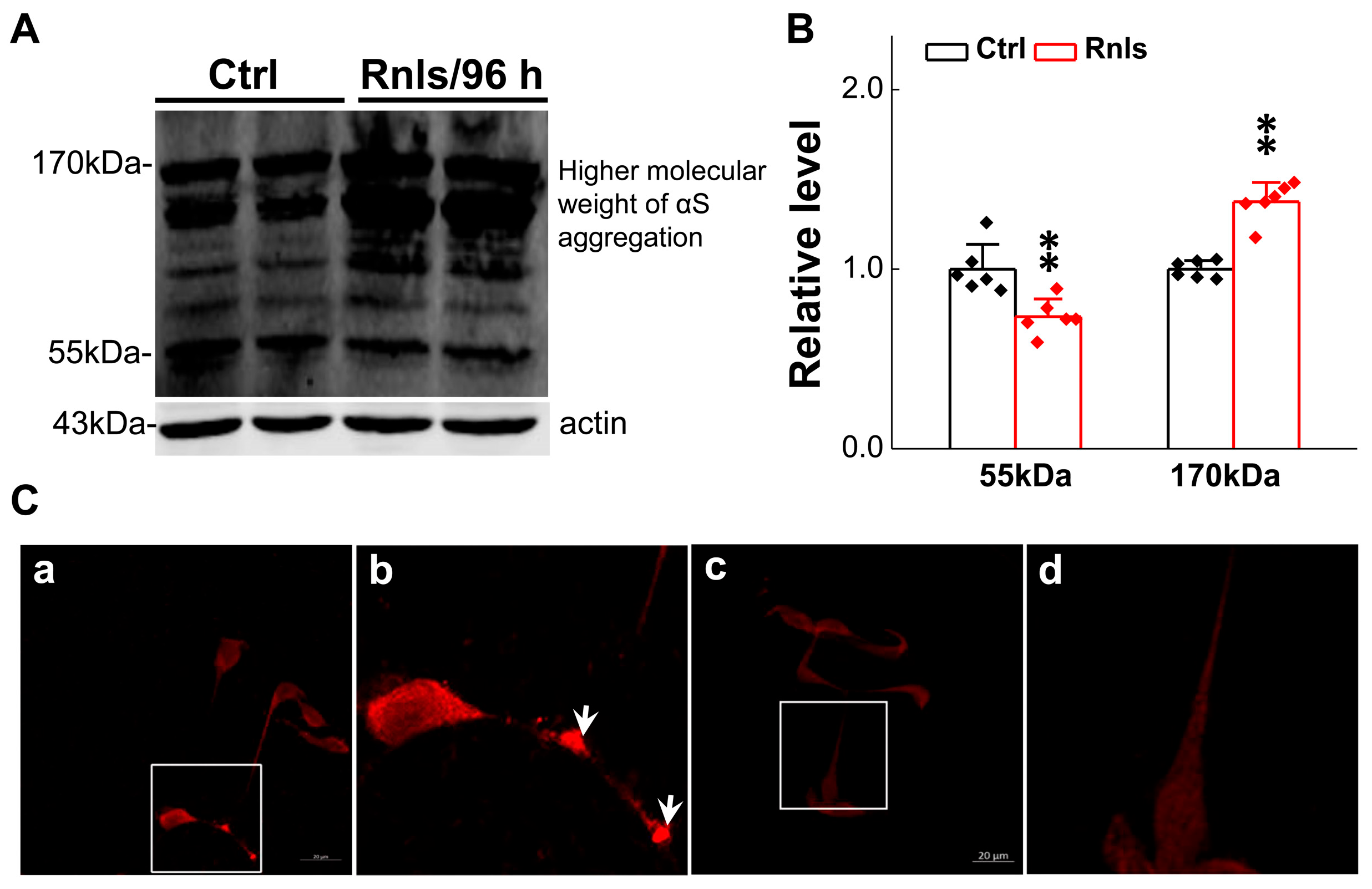
3.4. Increased α-Syn Aggregation over the Time of RNLS Overexpression
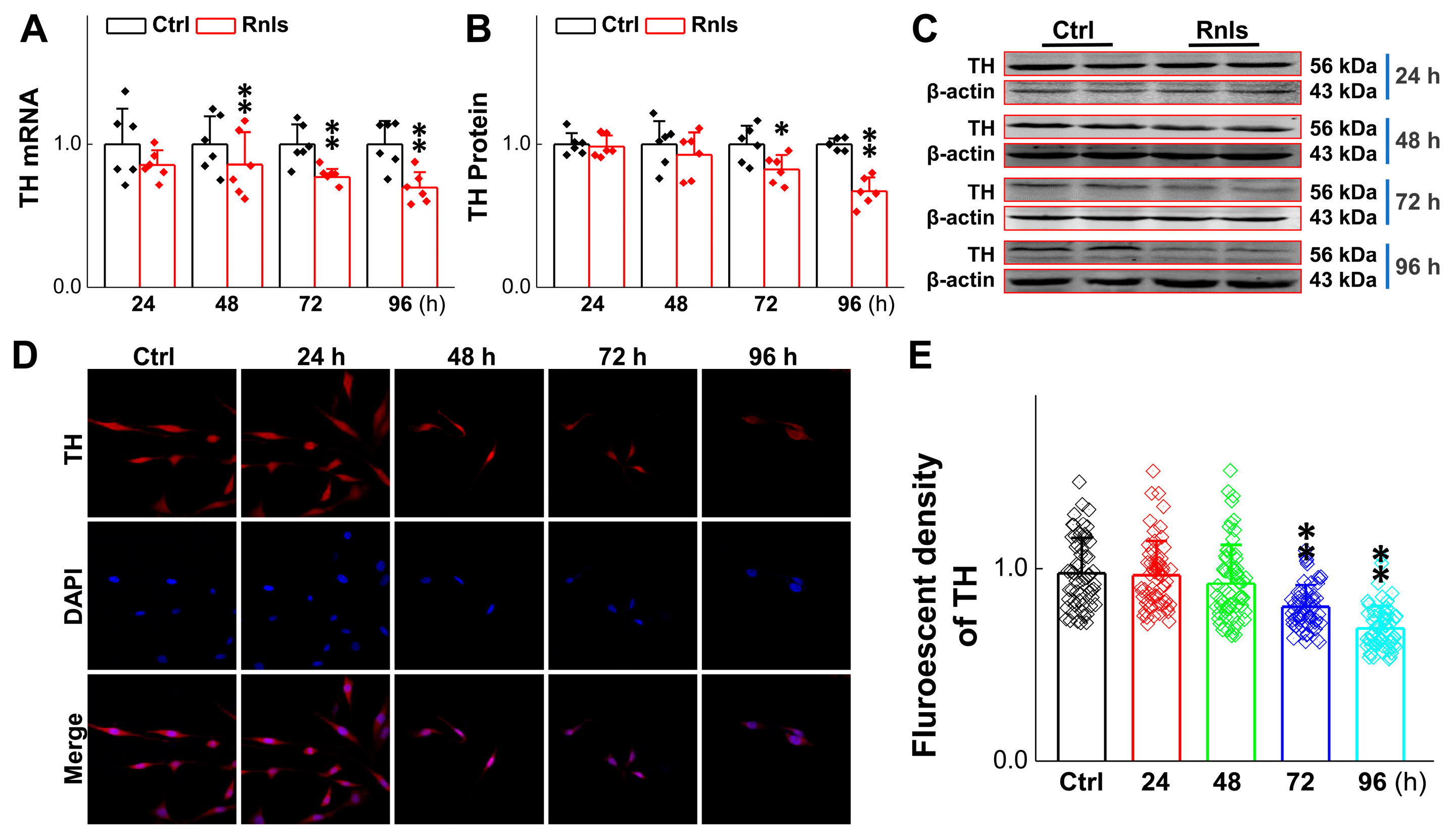
3.5. Reduced Activity of TH-Labeled Dopaminergic Neurons and PC12 Cells After RNLS overexpression
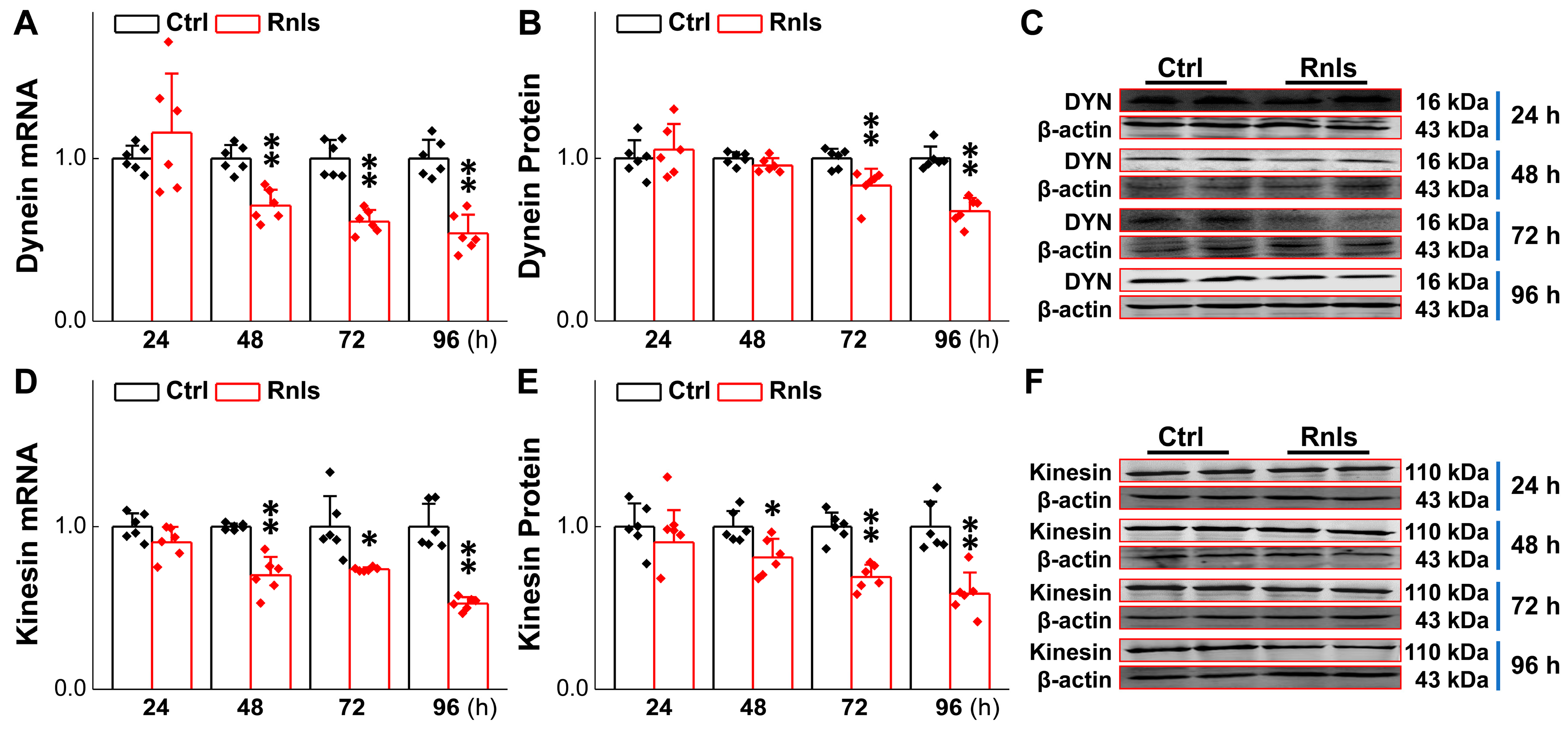
3.6. Impaired Axonal Transport over Time in PC12 Cells Followed by Transfection
3.7. Ultrastructure Changes in PC12 Cell After Transfection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-Hydroxydopamine |
| AD | Alzheimer’s Disease |
| ALDH | Aldehyde Dehydrogenase |
| ANOVA | Analysis of Variance |
| α-Syn | Alpha-Synuclein |
| BP | Blood Pressure |
| BRS | Baroreflex Sensitivity |
| cDNA | Complementary Deoxyribonucleic Acid |
| CNS | Central Nervous System |
| Ctrl | Control |
| DA | Dopamine |
| DAPI | 4′6-diamidino-2-phenylindole, |
| DBP | Diastolic Blood Pressure |
| Dim-αS | Dimer of Alpha-Synuclein |
| DLB | Dementia with Lewy Bodies |
| DOPAC | 3,4-Dihydroxyphenylacetic Acid |
| DOPAL | 3,4-Dihydroxyphenylacetaldehyde |
| DYN | Dynein |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FAD | Flavin Adenine Dinucleotide |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| HPLC | High-Performance Liquid Chromatography |
| HR | Heart Rate |
| IF | Immunofluorescence |
| KHC | Kinesin Heavy Chain |
| LB | Lewy Bodies |
| MAO | Monoamine Oxidase |
| MAP | Mean Arterial Pressure |
| Mon-αS | Monomer of Alpha-Synuclein |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mRNA | Messenger Ribonucleic Acid |
| MSA | Multiple System Atrophy |
| NC | Negative Control |
| NG | Nodose Ganglion |
| NTS | Nucleus Tractus Solitarius |
| O.D. | Optical Density |
| OH | Orthostatic Hypotension |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PD | Parkinson’s Disease |
| PNS | Peripheral Nervous System |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| RNLS | Renalase |
| SBP | Systolic Blood Pressure |
| SD | Sprague Dawley |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| SH | Supine Hypertension |
| SNpc | Substantia Nigra Pars Compacta |
| TH | Tyrosine Hydroxylase |
| Tri-αS | Trimer of Alpha-Synuclein |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling |
| VTA | Ventral Tegmental Area |
| ∆Ψm | Mitochondrial Membrane Potential |
References
- Goedert, M. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Osmanovic Barilar, J.; Papic, V.; Farkas, V.; Rubic, I.; Meglic, P.; Bagaric, R.; Babic Perhoc, A.; Virag, D.; Homolak, J.; Salkovic-Petrisic, M.; et al. Modeling of Parkinson’s disease by intrastriatal administration of streptozotocin. Neuropharmacology 2025, 265, 110246. [Google Scholar] [CrossRef] [PubMed]
- Roytman, S.; Paalanen, R.; Carli, G.; Marusic, U.; Kanel, P.; van Laar, T.; Bohnen, N.I. Multisensory mechanisms of gait and balance in Parkinson’s disease: An integrative review. Neural Regen. Res. 2025, 20, 82–92. [Google Scholar] [CrossRef]
- Jiang, Y.; Qi, Z.; Zhu, H.; Shen, K.; Liu, R.; Fang, C.; Lou, W.; Jiang, Y.; Yuan, W.; Cao, X.; et al. Role of the globus pallidus in motor and non-motor symptoms of Parkinson’s disease. Neural Regen. Res. 2025, 20, 1628–1643. [Google Scholar] [CrossRef]
- Campagnolo, M.; Weis, L.; Sandre, M.; Tushevski, A.; Russo, F.P.; Savarino, E.; Carecchio, M.; Stocco, E.; Macchi, V.; De Caro, R.; et al. Immune landscape of the enteric nervous system differentiates Parkinson’s disease patients from controls: The PADUA-CESNE cohort. Neurobiol. Dis. 2024, 200, 106609. [Google Scholar] [CrossRef]
- Tanaka, H.; Martinez-Valbuena, I.; Forrest, S.L.; Couto, B.; Reyes, N.G.; Morales-Rivero, A.; Lee, S.; Li, J.; Karakani, A.M.; Tang-Wai, D.F.; et al. Distinct involvement of the cranial and spinal nerves in progressive supranuclear palsy. Brain 2024, 147, 1399–1411. [Google Scholar] [CrossRef]
- Garcia-Yague, A.J.; Lastres-Becker, I.; Stefanis, L.; Vassilatis, D.K.; Cuadrado, A. alpha-Synuclein Induces the GSK-3-Mediated Phosphorylation and Degradation of NURR1 and Loss of Dopaminergic Hallmarks. Mol. Neurobiol. 2021, 58, 6697–6711. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Won, S.J.; Wang, J.; Fong, R.; Butler, N.J.M.; Moss, A.; Wong, C.; Pan, J.; Sanchez, J.; Huynh, A.; et al. alpha-synuclein aggregates induce c-Abl activation and dopaminergic neuronal loss by a feed-forward redox stress mechanism. Prog. Neurobiol. 2021, 202, 102070. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.Y.; Jo, A.; Kim, J.H.; Park, S.; Choi, J.Y.; Kang, H.C.; Dawson, V.L.; Dawson, T.M.; Shin, J.H.; et al. Parkin interacting substrate phosphorylation by c-Abl drives dopaminergic neurodegeneration. Brain 2021, 144, 3674–3691. [Google Scholar] [CrossRef]
- Chi, X.; Yin, S.; Sun, Y.; Kou, L.; Zou, W.; Wang, Y.; Jin, Z.; Wang, T.; Xia, Y. Astrocyte-neuron communication through the complement C3-C3aR pathway in Parkinson’s disease. Brain Behav. Immun. 2025, 123, 229–243. [Google Scholar] [CrossRef]
- Serra, M.; Faustini, G.; Brembati, V.; Casu, M.A.; Pizzi, M.; Morelli, M.; Pinna, A.; Bellucci, A. Early alpha-synuclein/synapsin III co-accumulation, nigrostriatal dopaminergic synaptopathy and denervation in the MPTPp mouse model of Parkinson’s Disease. Exp. Neurol. 2025, 383, 115040. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.e627. [Google Scholar] [CrossRef] [PubMed]
- Emmi, A.; Sandre, M.; Russo, F.P.; Tombesi, G.; Garri, F.; Campagnolo, M.; Carecchio, M.; Biundo, R.; Spolverato, G.; Macchi, V.; et al. Duodenal alpha-Synuclein Pathology and Enteric Gliosis in Advanced Parkinson’s Disease. Mov. Disord. 2023, 38, 885–894. [Google Scholar] [CrossRef]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded alpha-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Kang, S.S.; Liu, X.; Chen, G.; Zhang, Z.; Chandrasekharan, B.; Alam, A.M.; Neish, A.S.; Cao, X.; Ye, K. Initiation of Parkinson’s disease from gut to brain by delta-secretase. Cell Res. 2020, 30, 70–87. [Google Scholar] [CrossRef]
- Konings, B.; Villatoro, L.; Van den Eynde, J.; Barahona, G.; Burns, R.; McKnight, M.; Hui, K.; Yenokyan, G.; Tack, J.; Pasricha, P.J. Gastrointestinal syndromes preceding a diagnosis of Parkinson’s disease: Testing Braak’s hypothesis using a nationwide database for comparison with Alzheimer’s disease and cerebrovascular diseases. Gut 2023, 72, 2103–2111. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Wei, M.; Li, S.; Jia, C.; Cheng, C.; Al-Nusaif, M.; Zhang, J.; Liu, C.; Le, W. Intrastriatal injection of Parkinson’s disease intestine and vagus lysates initiates alpha-synucleinopathy in rat brain. Cell Death Dis. 2023, 14, 4. [Google Scholar] [CrossRef]
- Espinosa-Oliva, A.M.; Ruiz, R.; Soto, M.S.; Boza-Serrano, A.; Rodriguez-Perez, A.I.; Roca-Ceballos, M.A.; Garcia-Revilla, J.; Santiago, M.; Serres, S.; Economopoulus, V.; et al. Inflammatory bowel disease induces pathological alpha-synuclein aggregation in the human gut and brain. Neuropathol. Appl. Neurobiol. 2024, 50, e12962. [Google Scholar] [CrossRef]
- Boura, I.; Poplawska-Domaszewicz, K.; Limbachiya, N.; Trivedi, D.; Batzu, L.; Chaudhuri, K.R. Prodromal Parkinson’s Disease: A Snapshot of the Landscape. Neurol. Clin. 2025, 43, 209–228. [Google Scholar] [CrossRef]
- Masato, A.; Andolfo, A.; Favetta, G.; Bellini, E.N.; Cogo, S.; Dalla Valle, L.; Boassa, D.; Greggio, E.; Plotegher, N.; Bubacco, L. Sequestosome-1 (SQSTM1/p62) as a target in dopamine catabolite-mediated cellular dyshomeostasis. Cell Death Dis. 2024, 15, 424. [Google Scholar] [CrossRef]
- Kang, S.S.; Zhang, Z.; Liu, X.; Manfredsson, F.P.; Benskey, M.J.; Cao, X.; Xu, J.; Sun, Y.E.; Ye, K. TrkB neurotrophic activities are blocked by alpha-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 10773–10778. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; He, C.; Yan, Q.; Wang, H.; Li, K.; Sun, X.; Feng, Y.; Zha, R.; Cui, C.; Xiong, X.; et al. Parkinson-like early autonomic dysfunction induced by vagal application of DOPAL in rats. CNS Neurosci. Ther. 2021, 27, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Plotegher, N.; Berti, G.; Ferrari, E.; Tessari, I.; Zanetti, M.; Lunelli, L.; Greggio, E.; Bisaglia, M.; Veronesi, M.; Girotto, S.; et al. DOPAL derived alpha-synuclein oligomers impair synaptic vesicles physiological function. Sci. Rep. 2017, 7, 40699. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Isonaka, R.; Sharabi, Y.; Goldstein, D.S. 3,4-Dihydroxyphenylacetaldehyde Is More Efficient than Dopamine in Oligomerizing and Quinonizing alpha-Synuclein. J. Pharmacol. Exp. Ther. 2020, 372, 157–165. [Google Scholar] [CrossRef]
- Burke, W.J.; Kumar, V.B.; Pandey, N.; Panneton, W.M.; Gan, Q.; Franko, M.W.; O’Dell, M.; Li, S.W.; Pan, Y.; Chung, H.D.; et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008, 115, 193–203. [Google Scholar] [CrossRef]
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.R.; Hoag, M.R. The enzyme: Renalase. Arch. Biochem. Biophys. 2017, 632, 66–76. [Google Scholar] [CrossRef]
- Desir, G.V. Role of renalase in the regulation of blood pressure and the renal dopamine system. Curr. Opin. Nephrol. Hypertens. 2011, 20, 31–36. [Google Scholar] [CrossRef]
- Quelhas-Santos, J.; Serrao, M.P.; Soares-Silva, I.; Fernandes-Cerqueira, C.; Simoes-Silva, L.; Pinho, M.J.; Remiao, F.; Sampaio-Maia, B.; Desir, G.V.; Pestana, M. Renalase regulates peripheral and central dopaminergic activities. Am. J. Physiol. Renal Physiol. 2015, 308, F84–F91. [Google Scholar] [CrossRef]
- Cui, C.P.; Xiong, X.; Zhao, J.X.; Fu, D.H.; Zhang, Y.; Ma, P.B.; Wu, D.; Li, B.Y. Piezo1 channel activation facilitates baroreflex afferent neurotransmission with subsequent blood pressure reduction in control and hypertension rats. Acta Pharmacol. Sin. 2024, 45, 76–86. [Google Scholar] [CrossRef]
- Goldstein, D.S. The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know. Int. J. Mol. Sci. 2021, 22, 5999. [Google Scholar] [CrossRef] [PubMed]
- Zohoorian-Abootorabi, T.; Meratan, A.A.; Jafarkhani, S.; Muronetz, V.; Haertle, T.; Saboury, A.A. Modulation of cytotoxic amyloid fibrillation and mitochondrial damage of alpha-synuclein by catechols mediated conformational changes. Sci. Rep. 2023, 13, 5275. [Google Scholar] [CrossRef]
- Isonaka, R.; Sullivan, P.; Goldstein, D.S. Pathophysiological Significance of alpha-Synuclein in Sympathetic Nerves: In Vivo Observations. Neurology 2025, 104, e210215. [Google Scholar] [CrossRef] [PubMed]
- Prots, I.; Grosch, J.; Brazdis, R.M.; Simmnacher, K.; Veber, V.; Havlicek, S.; Hannappel, C.; Krach, F.; Krumbiegel, M.; Schutz, O.; et al. alpha-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc. Natl. Acad. Sci. USA 2018, 115, 7813–7818. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Scanavachi, G.; Somerville, E.; Saminathan, A.; Nair, A.; Bango Da Cunha Correia, R.F.; Aylan, B.; Sitarska, E.; Oikonomou, A.; Hatzakis, N.S.; et al. Neuronal constitutive endolysosomal perforations enable alpha-synuclein aggregation by internalized PFFs. J. Cell Biol. 2025, 224, e202401136. [Google Scholar] [CrossRef]
- Mao, Q.; Qin, W.Z.; Zhang, A.; Ye, N. Recent advances in dopaminergic strategies for the treatment of Parkinson’s disease. Acta Pharmacol. Sin. 2020, 41, 471–482. [Google Scholar] [CrossRef]
- Guo, Y.J.; Xiong, H.; Chen, K.; Zou, J.J.; Lei, P. Brain regions susceptible to alpha-synuclein spreading. Mol. Psychiatry 2021, 27, 758–770. [Google Scholar] [CrossRef]
- Bassil, F.; Meymand, E.S.; Brown, H.J.; Xu, H.; Cox, T.O.; Pattabhiraman, S.; Maghames, C.M.; Wu, Q.; Zhang, B.; Trojanowski, J.Q.; et al. alpha-Synuclein modulates tau spreading in mouse brains. J. Exp. Med. 2021, 218, e20192193. [Google Scholar] [CrossRef]
- Marrinan, S.; Emmanuel, A.V.; Burn, D.J. Delayed gastric emptying in Parkinson’s disease. Mov. Disord. 2014, 29, 23–32. [Google Scholar] [CrossRef]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Catak, Z.; Kocdemir, E.; Ugur, K.; Yardim, M.; Sahin, I.; Kaya, H.; Aydin, S. A Novel Biomarker Renalase and Its Relationship with its Substrates in Schizophrenia. J. Med. Biochem. 2019, 38, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, M.T.; Mizia-Stec, K. Renalase and Biomarkers of Contrast-Induced Acute Kidney Injury. Cardiorenal Med. 2015, 6, 25–36. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, N.; Dong, S.; Hu, Y. Involvement of GPR12 in the induction of neurite outgrowth in PC12 cells. Brain Res. Bull. 2012, 87, 30–36. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Transm. 2019, 126, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Krainc, D. alpha-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Mu, F.; Jiao, Q.; Du, X.; Jiang, H. Association of orthostatic hypotension with Parkinson’s disease: A meta-analysis. Neurol. Sci. 2020, 41, 1419–1426. [Google Scholar] [CrossRef]
- Watano, C.; Shiota, Y.; Onoda, K.; Sheikh, A.M.; Mishima, S.; Nitta, E.; Yano, S.; Yamaguchi, S.; Nagai, A. Evaluation of autonomic functions of patients with multiple system atrophy and Parkinson’s disease by head-up tilt test. J. Neural Transm. 2018, 125, 153–162. [Google Scholar] [CrossRef]
- Kotagal, V.; Lineback, C.; Bohnen, N.I.; Albin, R.L.; Investigators, C.-P.P.S.G. Orthostatic hypotension predicts motor decline in early Parkinson disease. Park. Relat. Disord. 2016, 32, 127–129. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Xu, Y.-Z.; Zhang, Y.; Zhang, H.-F.; Dou, T.-M.; Li, X.-Y.; Xu, Z.-Y.; Cui, C.-P.; Li, X.-L.; Li, B.-Y. Renalase Overexpression-Mediated Excessive Metabolism of Peripheral Dopamine, DOPAL Accumulation, and α-Synuclein Aggregation in Baroreflex Afferents Contribute to Neuronal Degeneration and Autonomic Dysfunction. Biomedicines 2025, 13, 1243. https://doi.org/10.3390/biomedicines13051243
Xiong X, Xu Y-Z, Zhang Y, Zhang H-F, Dou T-M, Li X-Y, Xu Z-Y, Cui C-P, Li X-L, Li B-Y. Renalase Overexpression-Mediated Excessive Metabolism of Peripheral Dopamine, DOPAL Accumulation, and α-Synuclein Aggregation in Baroreflex Afferents Contribute to Neuronal Degeneration and Autonomic Dysfunction. Biomedicines. 2025; 13(5):1243. https://doi.org/10.3390/biomedicines13051243
Chicago/Turabian StyleXiong, Xue, Yin-Zhi Xu, Yan Zhang, Hong-Fei Zhang, Tian-Min Dou, Xing-Yu Li, Zhao-Yuan Xu, Chang-Peng Cui, Xue-Lian Li, and Bai-Yan Li. 2025. "Renalase Overexpression-Mediated Excessive Metabolism of Peripheral Dopamine, DOPAL Accumulation, and α-Synuclein Aggregation in Baroreflex Afferents Contribute to Neuronal Degeneration and Autonomic Dysfunction" Biomedicines 13, no. 5: 1243. https://doi.org/10.3390/biomedicines13051243
APA StyleXiong, X., Xu, Y.-Z., Zhang, Y., Zhang, H.-F., Dou, T.-M., Li, X.-Y., Xu, Z.-Y., Cui, C.-P., Li, X.-L., & Li, B.-Y. (2025). Renalase Overexpression-Mediated Excessive Metabolism of Peripheral Dopamine, DOPAL Accumulation, and α-Synuclein Aggregation in Baroreflex Afferents Contribute to Neuronal Degeneration and Autonomic Dysfunction. Biomedicines, 13(5), 1243. https://doi.org/10.3390/biomedicines13051243





