The Potential Role of SP-G and PLUNC in Tumor Pathogenesis and Wound Healing in the Human Larynx †
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Cell Culture
2.3. mRNA Extraction and cDNA Synthesis
2.4. Polymerase Chain Reaction (PCR)
2.5. Quantitative Real-Time PCR
2.6. In Vitro Wound Healing Assay Using Electric Cell–Substrate Impedance Sensing (ECIS)
2.7. Immunohistochemistry (IHC) and Immunocytochemistry (ICC)
2.8. Western Blot
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
3.1. Localization of SP-G, PLUNC Within the Tissue of the Human Larynx and in Cultured FaDu Cells
3.2. Detection of SP-G, PLUNC in the Human Vocal Fold Mucosa and in Cultured FaDu Cells
3.3. Effects of Bombesin, Cortisol, Serotonin, and Mechanical Scratch on SP-G and PLUNC Expression in FaDu Cells
3.4. In Vitro Wound Healing Assay (ECIS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| BPI | Bactericidal/permeability-increasing |
| BSA | Bovine serum albumin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| cDNA | Complementary deoxyribonucleic acid |
| CSF | Cerebrospinal fluid |
| ECIS | Electric cell–substrate impedance sensing |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMEM | Eagle’s Minimum Essential Medium |
| ER | Endoplasmic reticulum |
| FCS | Fetal calf serum |
| HNC | Head and neck cancer |
| ICC | Immunocytochemistry |
| IHC | Immunohistochemistry |
| LPS | Lipopolysaccharide |
| mRNA | Messenger ribonucleic acid |
| NCBI | National Center for Biotechnology Information |
| NSCLC | Non-small-cell lung cancer |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PD-L1 | Programmed Death-Ligand 1 |
| PLUNC | Palate, lung, and nasal epithelium clone |
| qPCR | Quantitative Real-Time reverse transcription polymerase chain reaction |
| SCC | Squamous cell carcinoma |
| SEM | Standard error of the mean |
| SP | Surfactant protein |
| UPL | Universal Probe Library |
References
- Noordzij, J.P.; Ossoff, R.H. Anatomy and physiology of the larynx. Otolaryngol. Clin. N. Am. 2006, 39, 1–10. [Google Scholar] [CrossRef]
- Sasaki, C.T.; Weaver, E.M. Physiology of the larynx. Am. J. Med. 1997, 103, 9S–18S. [Google Scholar] [CrossRef] [PubMed]
- Benner, A.; Sharma, P.; Sharma, S. Anatomy, Head and Neck: Cervical, Respiratory, Larynx, and Cricoarytenoid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wienecke, A.; Kraywinkel, K. Epidemiologie von Kopf-Hals-Tumoren in Deutschland. Der Onkol. 2019, 25, 190–200. [Google Scholar] [CrossRef]
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on larynx cancer epidemiology. Chin. J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef]
- Cohen, N.; Fedewa, S.; Chen, A.Y. Epidemiology and Demographics of the Head and Neck Cancer Population. Oral. Maxillofac. Surg. Clin. N. Am. 2018, 30, 381–395. [Google Scholar] [CrossRef]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An update on larynx cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca-Gonzalez, I.; Bernal-Sprekelsen, M.; Blanch-Alejandro, J.L.; Moragas-Lluis, M. Complications in transoral CO2 laser surgery for carcinoma of the larynx and hypopharynx. Head Neck 2003, 25, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Sheats, M.; Schroder, H.; Rausch, F.; Bohr, C.; Kisslinger, F.; de Tristan, J.; Iro, H.; Garreis, F.; Paulsen, F.; Schicht, M.; et al. Surfactant proteins of the human larynx. Ann. Anat. 2016, 208, 135–141. [Google Scholar] [CrossRef]
- Rausch, F.; Schicht, M.; Paulsen, F.; Ngueya, I.; Brauer, L.; Brandt, W. “SP-G”, a putative new surfactant protein--tissue localization and 3D structure. PLoS ONE 2012, 7, e47789. [Google Scholar] [CrossRef]
- Schicht, M.; Riedlova, K.; Kukulka, M.; Li, W.; Scheer, A.; Garreis, F.; Jacobi, C.; Paulsen, F.; Cwiklik, L.; Brauer, L. The Potential Role of SP-G as Surface Tension Regulator in Tear Film: From Molecular Simulations to Experimental Observations. Int. J. Mol. Sci. 2022, 23, 5783. [Google Scholar] [CrossRef]
- Bingle, L.; Cross, S.S.; High, A.S.; Wallace, W.A.; Devine, D.A.; Havard, S.; Campos, M.A.; Bingle, C.D. SPLUNC1 (PLUNC) is expressed in glandular tissues of the respiratory tract and in lung tumours with a glandular phenotype. J. Pathol. 2005, 205, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Schicht, M.; Rausch, F.; Beron, M.; Jacobi, C.; Garreis, F.; Hartjen, N.; Beileke, S.; Kruse, F.; Brauer, L.; Paulsen, F. Palate Lung Nasal Clone (PLUNC), a Novel Protein of the Tear Film: Three-Dimensional Structure, Immune Activation, and Involvement in Dry Eye Disease (DED). Invest. Ophthalmol. Vis. Sci. 2015, 56, 7312–7323. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.A.; Gakhar, L.; Penterman, J.; Singh, P.K.; Mallampalli, R.K.; Porter, E.; McCray, P.B., Jr. PLUNC: A multifunctional surfactant of the airways. Biochem. Soc. Trans. 2011, 39, 1012–1016. [Google Scholar] [CrossRef]
- Li, N.; Zhai, Z.; Chen, Y.; Li, X. Transcriptomic and immunologic implications of the epithelial-mesenchymal transition model reveal a novel role of SFTA2 in prognosis of non-small-cell lung carcinoma. Front. Genet. 2022, 13, 911801. [Google Scholar] [CrossRef] [PubMed]
- Luyapan, J.; Bossé, Y.; Li, Z.; Xiao, X.; Rosenberger, A.; Hung, R.J.; Lam, S.; Zienolddiny, S.; Liu, G.; Kiemeney, L.A.; et al. Candidate pathway analysis of surfactant proteins identifies CTSH and SFTA2 that influences lung cancer risk. Hum. Mol. Genet. 2023, 32, 2842–2855. [Google Scholar] [CrossRef]
- Feng, R.; Guo, Y.; Chen, M.; Tian, Z.; Liu, Y.; Jiang, S.; Zhou, J.; Liu, Q.; Li, X.; Xiong, W.; et al. PLUNC downregulates the expression of PD-L1 by inhibiting the interaction of DDX17/β-catenin in nasopharyngeal carcinoma. J. Pathol. Transl. Med. 2025, 59, 68–83. [Google Scholar] [CrossRef]
- Rangan, S.R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972, 29, 117–121. [Google Scholar] [CrossRef]
- De Blasio, B.F.; Laane, M.; Walmann, T.; Giaever, I. Combining optical and electrical impedance techniques for quantitative measurement of confluence in MDCK-I cell cultures. Biotechniques 2004, 36, 650–662. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Yang, J.M.; Chen, S.W.; Yang, J.H.; Hsu, C.C.; Wang, J.S. A quantitative cell modeling and wound-healing analysis based on the Electric Cell-substrate Impedance Sensing (ECIS) method. Comput. Biol. Med. 2016, 69, 134–143. [Google Scholar] [CrossRef]
- Zhang, Z.; Henzel, W.J. Signal peptide prediction based on analysis of experimentally verified cleavage sites. Protein Sci. 2004, 13, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Bingle, C.D.; Bingle, L. Characterisation of the human plunc gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochim. Biophys. Acta 2000, 1493, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Gakhar, L.; Bartlett, J.A.; Penterman, J.; Mizrachi, D.; Singh, P.K.; Mallampalli, R.K.; Ramaswamy, S.; McCray, P.B., Jr. PLUNC is a novel airway surfactant protein with anti-biofilm activity. PLoS ONE 2010, 5, e9098. [Google Scholar] [CrossRef] [PubMed]
- Floros, J. Human surfactant protein A (SP-A) variants: Why so many, why such a complexity? Swiss Med. Wkly. 2001, 131, 87–90. [Google Scholar] [CrossRef]
- Glasser, S.W.; Korfhagen, T.R.; Perme, C.M.; Pilot-Matias, T.J.; Kister, S.E.; Whitsett, J.A. Two SP-C genes encoding human pulmonary surfactant proteolipid. J. Biol. Chem. 1988, 263, 10326–10331. [Google Scholar] [CrossRef]
- Voorhout, W.F.; Veenendaal, T.; Haagsman, H.P.; Weaver, T.E.; Whitsett, J.A.; van Golde, L.M.; Geuze, H.J. Intracellular processing of pulmonary surfactant protein B in an endosomal/lysosomal compartment. Am. J. Physiol. 1992, 263, L479–L486. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Hull, W.M.; Ohning, B.; Ross, G.; Weaver, T.E. Immunologic identification of a pulmonary surfactant-associated protein of molecular weight = 6000 daltons. Pediatr. Res. 1986, 20, 744–749. [Google Scholar] [CrossRef]
- Mittal, R.A.; Hammel, M.; Schwarz, J.; Heschl, K.M.; Bretschneider, N.; Flemmer, A.W.; Herber-Jonat, S.; Konigshoff, M.; Eickelberg, O.; Holzinger, A. SFTA2--a novel secretory peptide highly expressed in the lung--is modulated by lipopolysaccharide but not hyperoxia. PLoS ONE 2012, 7, e40011. [Google Scholar] [CrossRef]
- Beck, D.C.; Ikegami, M.; Na, C.L.; Zaltash, S.; Johansson, J.; Whitsett, J.A.; Weaver, T.E. The role of homodimers in surfactant protein B function in vivo. J. Biol. Chem. 2000, 275, 3365–3370. [Google Scholar] [CrossRef]
- Kairys, V.; Gilson, M.K.; Luy, B. Structural model for an AxxxG-mediated dimer of surfactant-associated protein C. Eur. J. Biochem. 2004, 271, 2086–2092. [Google Scholar] [CrossRef]
- Kutta, H.; Steven, P.; Tillmann, B.N.; Tsokos, M.; Paulsen, F.P. Region-specific immunological response of the different laryngeal compartments: Significance of larynx-associated lymphoid tissue. Cell Tissue Res. 2003, 311, 365–371. [Google Scholar] [CrossRef]
- Jain, N.; Lodha, R.; Kabra, S.K. Upper respiratory tract infections. Indian. J. Pediatr. 2001, 68, 1135–1138. [Google Scholar] [CrossRef]
- Bingle, C.D.; Craven, C.J. PLUNC: A novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum. Mol. Genet. 2002, 11, 937–943. [Google Scholar] [CrossRef]
- Krause, M.; Peukert, N.; Hartig, W.; Emmer, A.; Mahr, C.V.; Richter, C.; Dieckow, J.; Puchta, J.; Pirlich, M.; Hoffmann, K.T.; et al. Localization, Occurrence, and CSF Changes of SP-G, a New Surface Active Protein with Assumable Immunoregulatory Functions in the CNS. Mol. Neurobiol. 2019, 56, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Döllinger, M.; Gröhn, F.; Berry, D.A.; Eysholdt, U.; Luegmair, G. Preliminary Results on the Influence of Engineered Artificial Mucus Layer on Phonation. J. Speech Lang. Hear. Res. 2014, 57, S637–S647. [Google Scholar] [CrossRef]
- Nakagawa, H.; Fukuda, H.; Kawaida, M.; Shiotani, A.; Kanzaki, J. Lubrication mechanism of the larynx during phonation: An experiment in excised canine larynges. Folia Phoniatr. Logop. 1998, 50, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, H.S.; Aikman, A.; Hines, K.; Deliyski, D.D. Vocal fold mucus aggregation in vocally normal speakers. Logoped Phoniatr. Vocol 2008, 33, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, H.S.; White, L.; Kuckhahn, K.; Gerlach, T.T.; Deliyski, D.D. Vocal fold mucus aggregation in persons with voice disorders. J. Commun. Disord. 2012, 45, 304–311. [Google Scholar] [CrossRef]
- Falco, M.; Tammaro, C.; Takeuchi, T.; Cossu, A.M.; Scafuro, G.; Zappavigna, S.; Itro, A.; Addeo, R.; Scrima, M.; Lombardi, A.; et al. Overview on Molecular Biomarkers for Laryngeal Cancer: Looking for New Answers to an Old Problem. Cancers 2022, 14, 1716. [Google Scholar] [CrossRef]
- Bernabe, D.G.; Tamae, A.C.; Miyahara, G.I.; Sundefeld, M.L.; Oliveira, S.P.; Biasoli, E.R. Increased plasma and salivary cortisol levels in patients with oral cancer and their association with clinical stage. J. Clin. Pathol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Lemaire, F.; Millon, R.; Young, J.; Cromer, A.; Wasylyk, C.; Schultz, I.; Muller, D.; Marchal, P.; Zhao, C.; Melle, D.; et al. Differential expression profiling of head and neck squamous cell carcinoma (HNSCC). Br. J. Cancer 2003, 89, 1940–1949. [Google Scholar] [CrossRef]
- Athanasopoulos, M.; Samara, P.; Agrogiannis, G.; Athanasopoulos, I.; Kavantzas, N.; Kyrodimos, E.; Mastronikolis, N.S. Releasing the brakes: The role of immune checkpoint inhibitors in laryngeal cancer. Explor. Target. Antitumor Ther. 2025, 6, 1002292. [Google Scholar] [CrossRef] [PubMed]
- Iwao, K.; Watanabe, T.; Fujiwara, Y.; Takami, K.; Kodama, K.; Higashiyama, M.; Yokouchi, H.; Ozaki, K.; Monden, M.; Tanigami, A. Isolation of a novel human lung-specific gene, LUNX, a potential molecular marker for detection of micrometastasis in non-small-cell lung cancer. Int. J. Cancer 2001, 91, 433–437. [Google Scholar] [CrossRef]
- Yasui, W.; Oue, N.; Sentani, K.; Sakamoto, N.; Motoshita, J. Transcriptome dissection of gastric cancer: Identification of novel diagnostic and therapeutic targets from pathology specimens. Pathol. Int. 2009, 59, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.T.; Gil, Z.; Binenbaum, Y.; Na’ara, S.; Amit, M. An orthotopic mouse model of laryngeal squamous cell carcinoma. Ann. Otol. Rhinol. Laryngol. 2015, 124, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Abreu, A.R.; Nlend, M.C.; Cobas, M.A.; Conner, G.E.; Whitney, P.L. Purification and characterization of PLUNC from human tracheobronchial secretions. Am. J. Respir. Cell Mol. Biol. 2004, 30, 184–192. [Google Scholar] [CrossRef]
- Grier, D.G.; Halliday, H.L. Effects of glucocorticoids on fetal and neonatal lung development. Treat. Respir. Med. 2004, 3, 295–306. [Google Scholar] [CrossRef]
- Nikolić, J.; Vukojević, K.; Šoljić, V.; Mišković, J.; Orlović Vlaho, M.; Saraga-Babić, M.; Filipović, N. Expression Patterns of Serotonin Receptors 5-HT1A, 5-HT2A, and 5-HT3A during Human Fetal Lung Development. Int. J. Mol. Sci. 2023, 24, 2965. [Google Scholar] [CrossRef]
- Gedeon, T.; Bokes, P. Delayed protein synthesis reduces the correlation between mRNA and protein fluctuations. Biophys. J. 2012, 103, 377–385. [Google Scholar] [CrossRef]
- Fournier, M.L.; Paulson, A.; Pavelka, N.; Mosley, A.L.; Gaudenz, K.; Bradford, W.D.; Glynn, E.; Li, H.; Sardiu, M.E.; Fleharty, B.; et al. Delayed correlation of mRNA and protein expression in rapamycin-treated cells and a role for Ggc1 in cellular sensitivity to rapamycin. Mol. Cell Proteom. 2010, 9, 271–284. [Google Scholar] [CrossRef]
- Ashour, K.; Shan, L.; Lee, J.H.; Schlicher, W.; Wada, K.; Wada, E.; Sunday, M.E. Bombesin inhibits alveolarization and promotes pulmonary fibrosis in newborn mice. Am. J. Respir. Crit. Care Med. 2006, 173, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Asokananthan, N.; Cake, M.H. Stimulation of surfactant lipid secretion from fetal type II pneumocytes by gastrin-releasing peptide. Am. J. Physiol. 1996, 270, L331–L337. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, X.; Jiang, D.; Yang, M.; Xue, X.; Li, W.; Ding, Y.; Wang, S. Upregulation of SPLUNC1, a novel prognostic predictor of lung adenocarcinoma, promotes cell proliferation and migration. Int. J. Clin. Exp. Pathol. 2016, 9, 1138–1147. [Google Scholar]
- Sung, Y.K.; Moon, C.; Yoo, J.-Y.; Moon, C.; Pearse, D.; Pevsner, J.; Ronnett, G.V. Plunc, a Member of the Secretory Gland Protein Family, Is Up-regulated in Nasal Respiratory Epithelium after Olfactory Bulbectomy∗. J. Biol. Chem. 2002, 277, 12762–12769. [Google Scholar] [CrossRef]
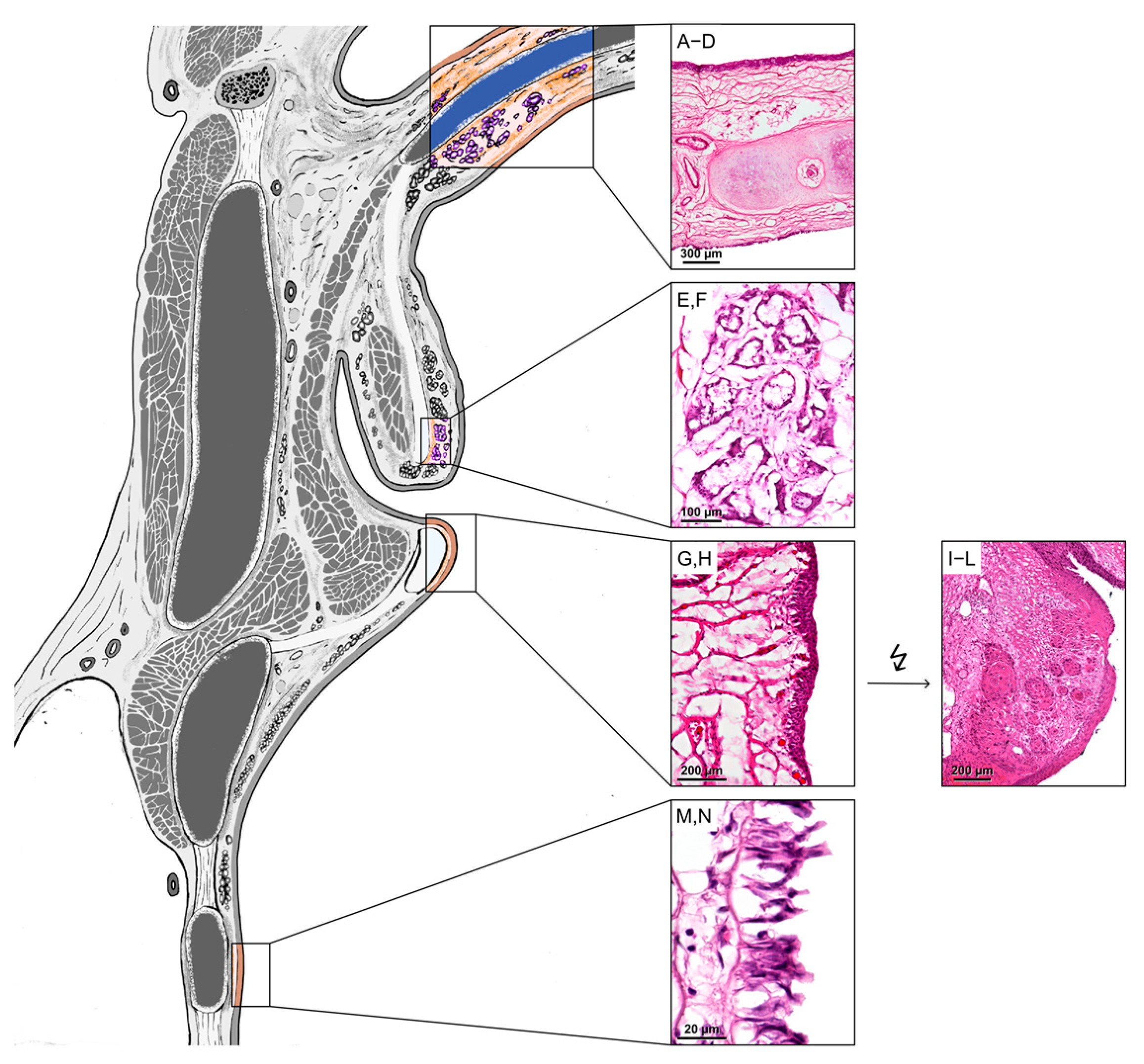
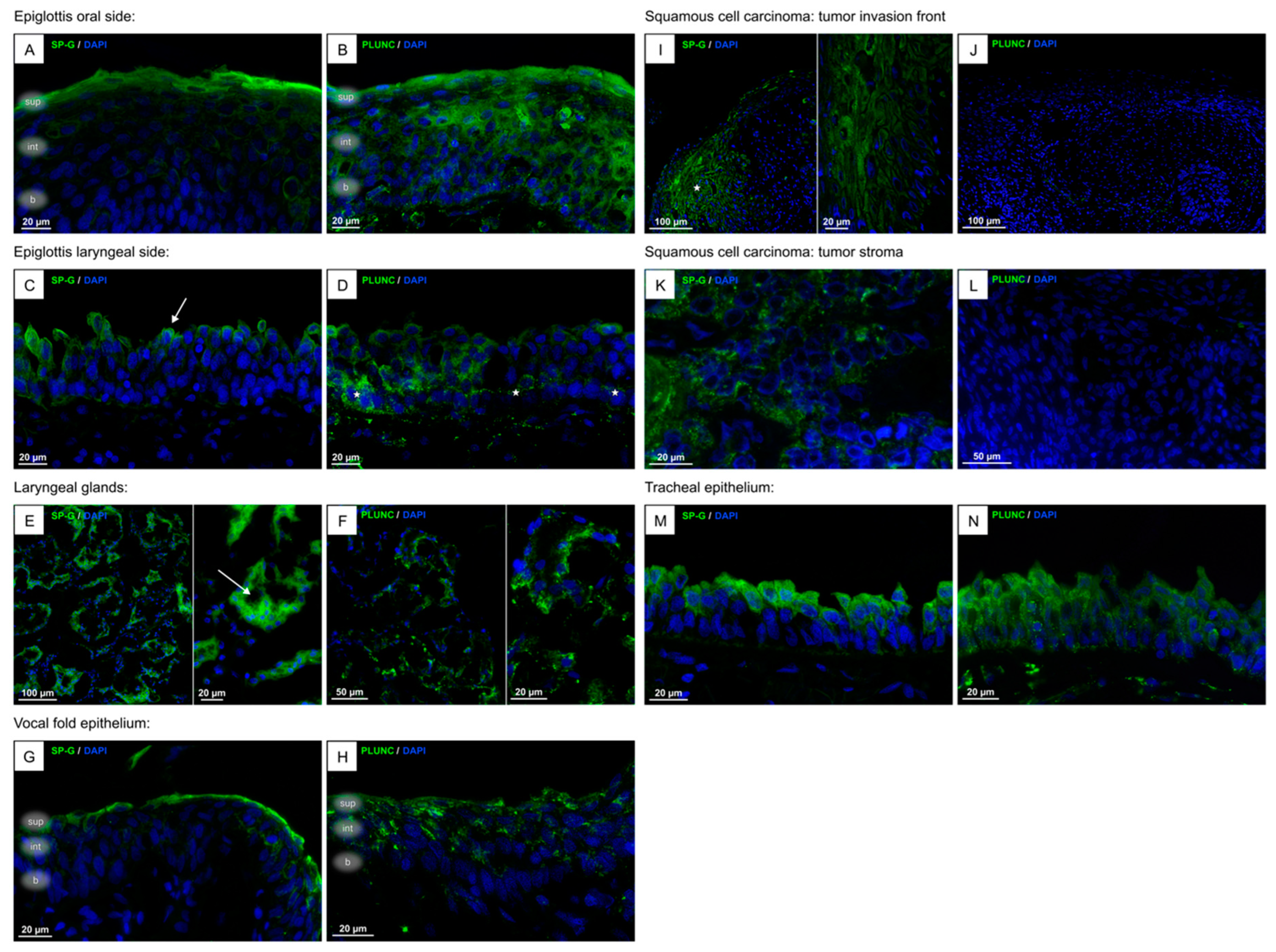

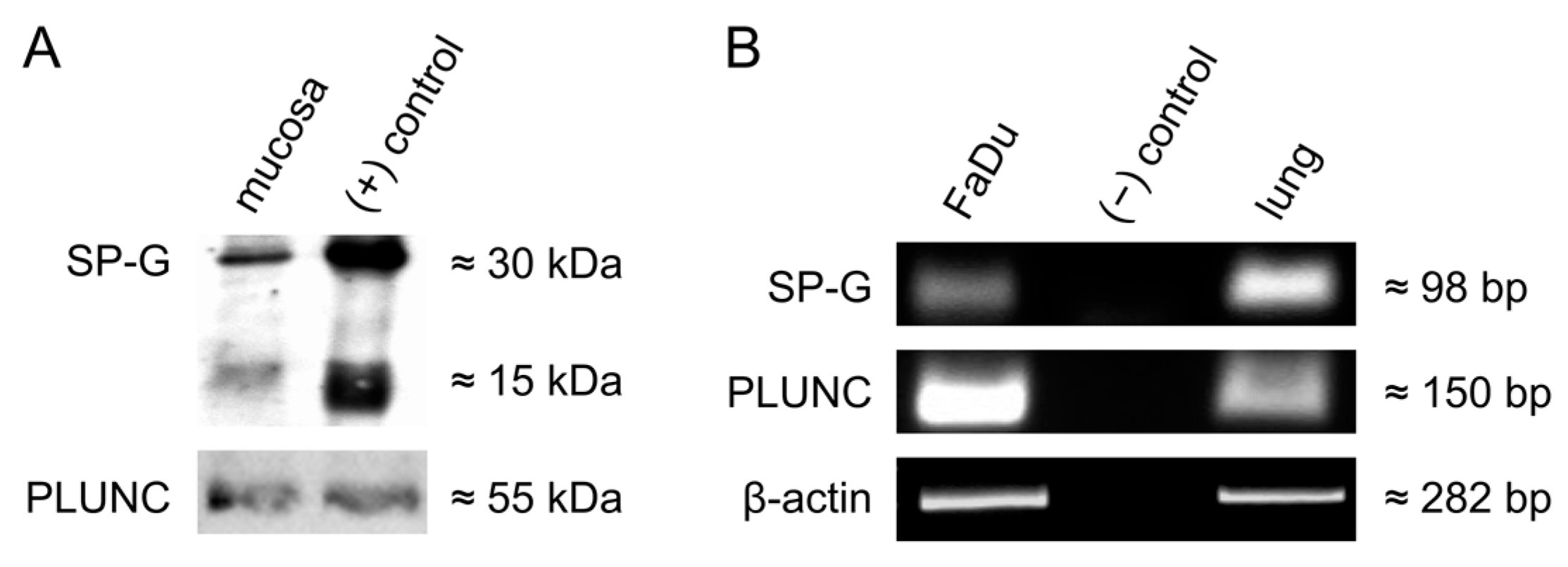
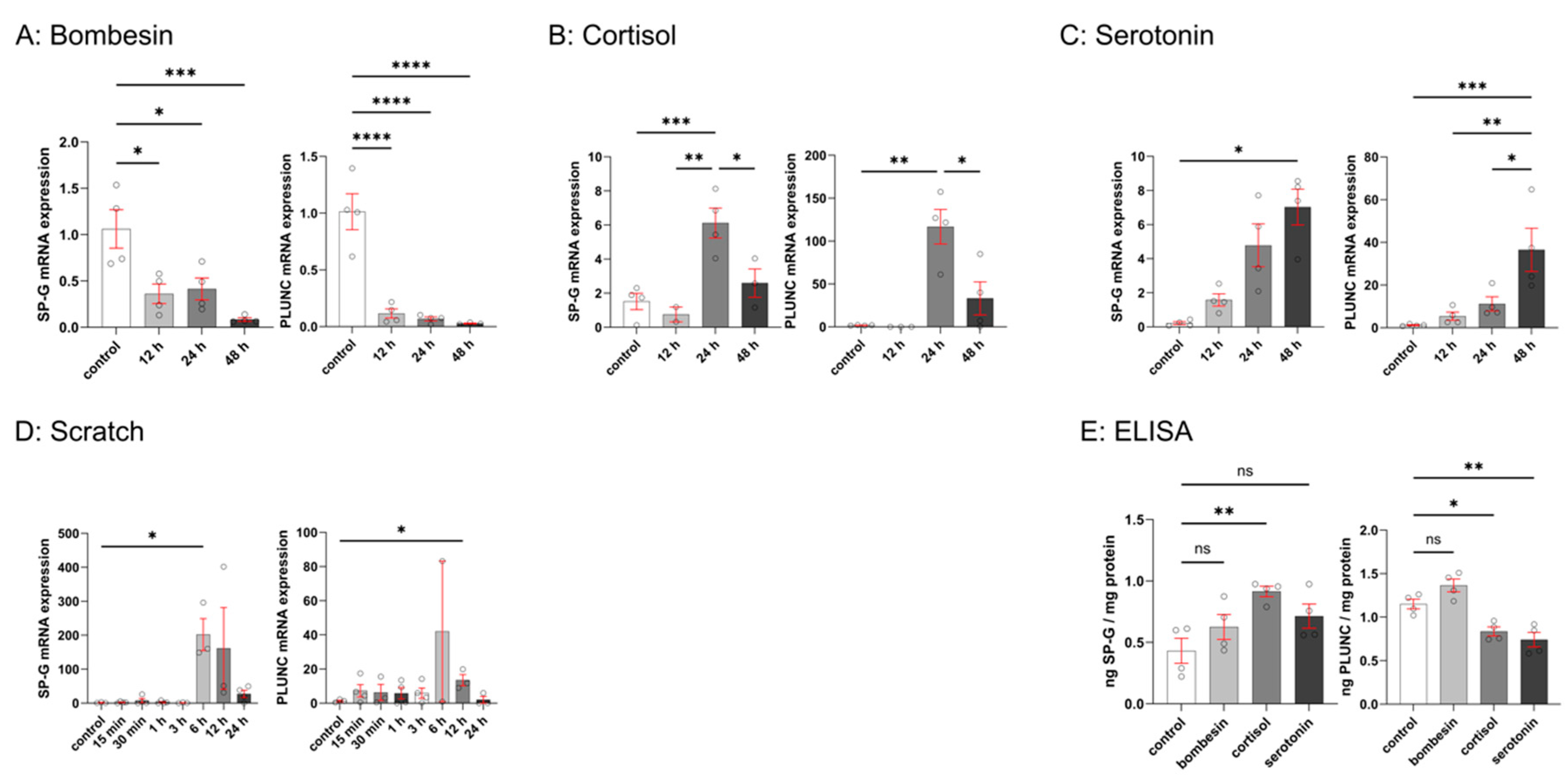

| Characteristics | n (%) |
|---|---|
| Total patients | 15 |
| Male | 13 (86.7) |
| Female | 2 (13.3) |
| Age (in years) | |
| Mean | 62.6 ± 14.1 |
| Median | 68 |
| Range | 28–80 |
| Tumor status | |
| T0 | 2 (13.3) |
| pT1 | 3 (20) |
| pT2 | 1 (6.7) |
| pT3 | 5 (33.3) |
| pT4 | 4 (26.7) |
| Antibody | Method | Dilution | Company, Catalog Number |
|---|---|---|---|
| rabbit anti-SP-G | IF, WB | 1:50, 1:200 | cloud-clone, PAD755Hu01 |
| rabbit anti-PLUNC | IF | 1:50 | santa-cruz, SC-271457 |
| mouse anti-calreticulin | IF | 1:100 | Invitrogen, MA5-15382 |
| goat anti-PLUNC | WB | 1:250 | santa-cruz, SC-49248 |
| goat anti-rabbit, Alexa 488 | IF | 1:1000 | Invitrogen, A11070 |
| goat anti-mouse, Alexa 488 | IF | 1:500 | Invitrogen, A11029 |
| goat anti-rabbit, Alexa 555 | IF | 1:1500 | Invitrogen, A21428 |
| goat anti-mouse, Alexa 555 | IF | 1:500 | Invitrogen, A21424 |
| goat anti-rabbit, HRP-conjugated | WB | 1:5000 | Dako, P0448 |
| donkey anti-goat, HRP-conjugated | WB | 1:5000 | santa-cruz, SC-2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheer, A.; Bräuer, L.; Eckstein, M.; Iro, H.; Paulsen, F.; Garreis, F.; Schicht, M.; Gostian, A.-O. The Potential Role of SP-G and PLUNC in Tumor Pathogenesis and Wound Healing in the Human Larynx. Biomedicines 2025, 13, 1240. https://doi.org/10.3390/biomedicines13051240
Scheer A, Bräuer L, Eckstein M, Iro H, Paulsen F, Garreis F, Schicht M, Gostian A-O. The Potential Role of SP-G and PLUNC in Tumor Pathogenesis and Wound Healing in the Human Larynx. Biomedicines. 2025; 13(5):1240. https://doi.org/10.3390/biomedicines13051240
Chicago/Turabian StyleScheer, Aurelius, Lars Bräuer, Markus Eckstein, Heinrich Iro, Friedrich Paulsen, Fabian Garreis, Martin Schicht, and Antoniu-Oreste Gostian. 2025. "The Potential Role of SP-G and PLUNC in Tumor Pathogenesis and Wound Healing in the Human Larynx" Biomedicines 13, no. 5: 1240. https://doi.org/10.3390/biomedicines13051240
APA StyleScheer, A., Bräuer, L., Eckstein, M., Iro, H., Paulsen, F., Garreis, F., Schicht, M., & Gostian, A.-O. (2025). The Potential Role of SP-G and PLUNC in Tumor Pathogenesis and Wound Healing in the Human Larynx. Biomedicines, 13(5), 1240. https://doi.org/10.3390/biomedicines13051240









