In Vitro Evaluation of the PMN Reaction on a Collagen-Based Purified Reconstituted Bilayer Matrix (PRBM) Using the Autologous Blood Concentrate PRF
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. PRF Preparation
2.3. Histological and Immunohistological Staining of PRF Clots
2.4. Collagen-Based Purified Reconstituted Bilayer Matrix Geistlich Mucograft®
2.5. PRF–PRBM Combination and Cultivation
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Determination of the Recovery Capacity for Cytokines in the Presence of the PRBM
2.8. Statistical Evaluation
3. Results
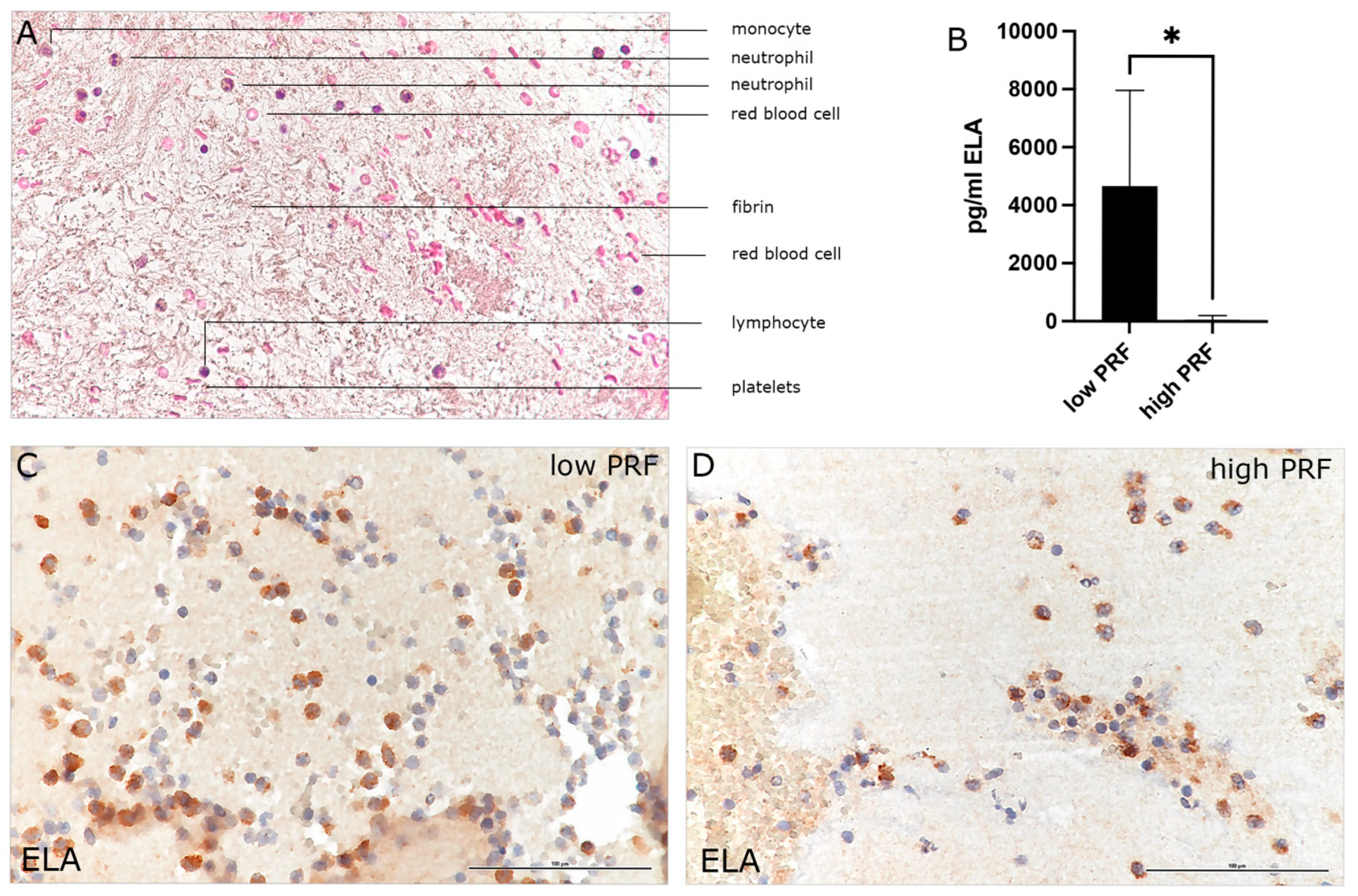
3.1. Characterization of PMNs in High- and Low-RCF PRF
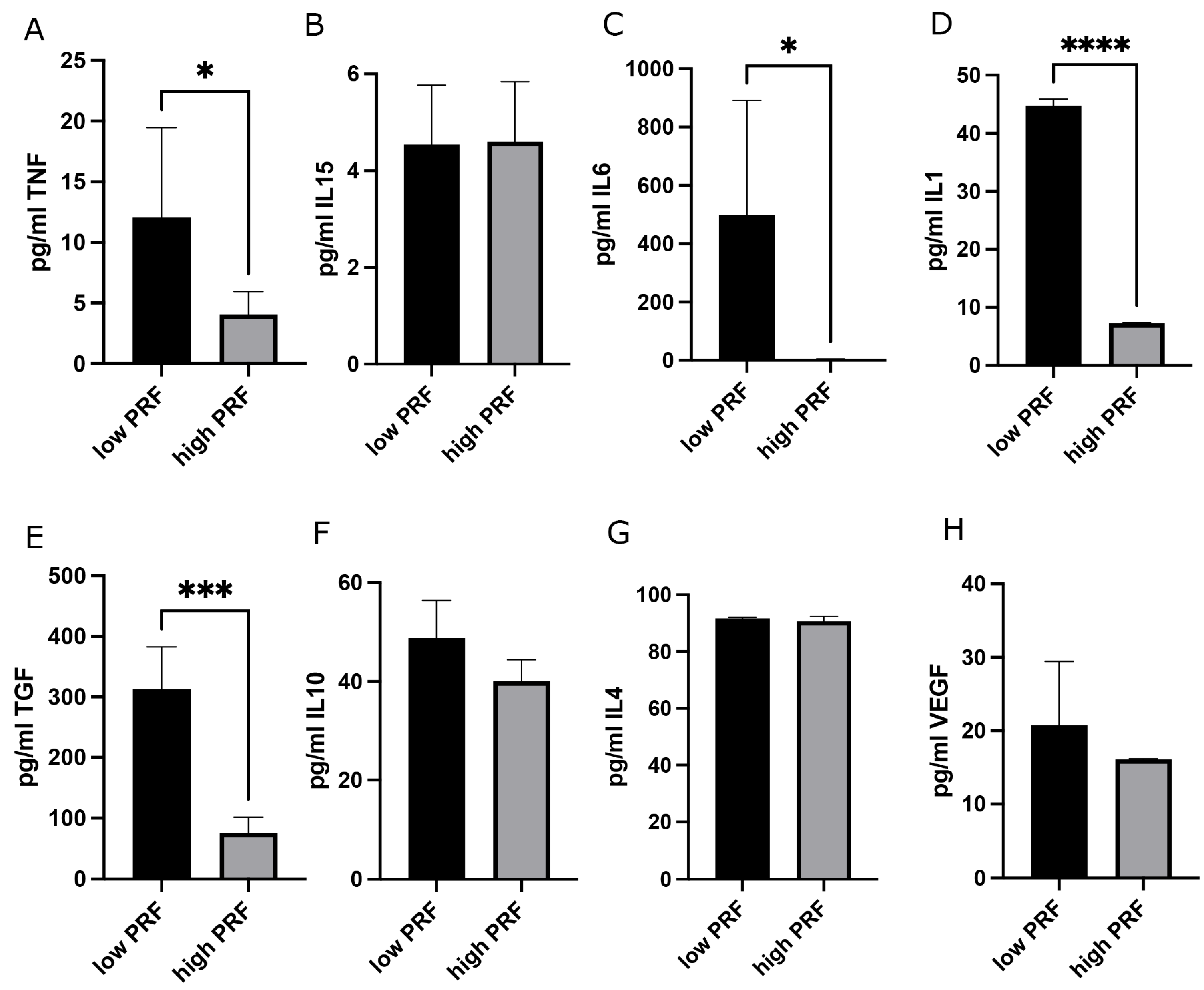
3.2. Combination of PRBM with PRF: Effect on PMNs’ Cytokine Release
3.3. Determination of Cytokine Recovery Capacity in the Presence of PRBM Using the ELISA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMN | Polymorphonuclear cells |
| PRBM | Collagen-based Purified Reconstituted Bilayer Matrix |
| PRF | Platelet-rich fibrin |
| RCF | Relative centrifugal force |
| ELA | Neutrophil Elastase |
| IL | Interleukin |
| TNFα | Tumor Necrosis Factor α |
| TGFß1 | Transforming Growth Factor ß1 |
| VEGF | Vascular endothelial growth factor A |
| SD | Standard Deviation |
References
- Zarember, K.A.; Kuhns, D.B. Editorial: Will the real neutrophil please stand up? J. Leukoc. Biol. 2011, 90, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, D.B.; Priel, D.A.L.; Chu, J.; Zarember, K.A. Isolation and Functional Analysis of Human Neutrophils. Curr. Protoc. Immunol. 2015, 111, 7.23.1–7.23.16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzi, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Dohle, E.; Bischoff, I.; Bose, T.; Marsano, A.; Banfi, A.; Unger, R.E.; Kirkpatrick, C.J. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur. Cell Mater. 2014, 27, 149–164, discussion 164–145. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Perera, P.Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Xie, M.; Hao, Y.; Feng, L.; Wang, T.; Yao, M.; Li, H.; Ma, D.; Feng, J. Neutrophil Heterogeneity and its Roles in the Inflammatory Network after Ischemic Stroke. Curr. Neuropharmacol. 2023, 21, 621–650. [Google Scholar] [CrossRef]
- Cohen, H.C.; Joyce, E.J.; Kao, W.J. Biomaterials selectively modulate interactions between human blood-derived polymorphonuclear leukocytes and monocytes. Am. J. Pathol. 2013, 182, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prosthetic restorations: A randomized prospective clinical trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Takayama, T.; Imamura, K.; Yamano, S. Growth Factor Delivery Using a Collagen Membrane for Bone Tissue Regeneration. Biomolecules 2023, 13, 809. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral Implantol. 2018, 44, 471–492. [Google Scholar] [CrossRef]
- Dohle, E.; El Bagdadi, K.; Sader, R.; Choukroun, J.; James Kirkpatrick, C.; Ghanaati, S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 598–610. [Google Scholar] [CrossRef]
- Herrera-Vizcaino, C.; Dohle, E.; Al-Maawi, S.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Platelet-rich fibrin secretome induces three dimensional angiogenic activation in vitro. Eur. Cell Mater. 2019, 37, 250–264. [Google Scholar] [CrossRef]
- Wend, S.; Kubesch, A.; Orlowska, A.; Al-Maawi, S.; Zender, N.; Dias, A.; Miron, R.J.; Sader, R.; Booms, P.; Kirkpatrick, C.J.; et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J. Mater. Sci. Mater. Med. 2017, 28, 188. [Google Scholar] [CrossRef]
- Venkatachalam, G.; Arumugam, S.; Doble, M. Synthesis, Characterization, and Biological Activity of Aminated Zymosan. ACS Omega 2020, 5, 15973–15982. [Google Scholar] [CrossRef] [PubMed]
- Wesdorp, M.A.; Schwab, A.; Bektas, E.I.; Narcisi, R.; Eglin, D.; Stoddart, M.J.; Van Osch, G.; D’Este, M. A culture model to analyze the acute biomaterial-dependent reaction of human primary neutrophils in vitro. Bioact. Mater. 2023, 20, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a Better Regeneration through Implant-Mediated Immunomodulation: Harnessing the Immune Responses. Adv. Sci. 2021, 8, e2100446. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef] [PubMed]
- Saluja, H.; Dehane, V.; Mahindra, U. Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann. Maxillofac. Surg. 2011, 1, 53–57. [Google Scholar] [CrossRef]
- Egle, K.; Salma, I.; Dubnika, A. From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. Int. J. Mol. Sci. 2021, 22, 11553. [Google Scholar] [CrossRef]
- Dohle, E.; Schmeinck, L.; Parkhoo, K.; Sader, R.; Ghanaati, S. Platelet rich fibrin as a bioactive matrix with proosteogenic and proangiogenic properties on human healthy primary cells in vitro. Platelets 2024, 35, 2316744. [Google Scholar] [CrossRef]
- Ratajczak, J.; Vangansewinkel, T.; Gervois, P.; Merckx, G.; Hilkens, P.; Quirynen, M.; Lambrichts, I.; Bronckaers, A. Angiogenic Properties of ‘Leukocyte- and Platelet-Rich Fibrin’. Sci. Rep. 2018, 8, 14632. [Google Scholar] [CrossRef]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma. Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef]
- Hundhammer, T.; Gruber, M.; Wittmann, S. Paralytic Impact of Centrifugation on Human Neutrophils. Biomedicines 2022, 10, 2896. [Google Scholar] [CrossRef] [PubMed]
- Blanter, M.; Cambier, S.; De Bondt, M.; Vanbrabant, L.; Portner, N.; Abouelasrar Salama, S.; Metzemaekers, M.; Marques, P.E.; Struyf, S.; Proost, P.; et al. Method Matters: Effect of Purification Technology on Neutrophil Phenotype and Function. Front. Immunol. 2022, 13, 820058. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, C.W.; Tambourgi, D.V.; Clark, H.W.; Hoong, S.J.; Spiller, O.B.; McGreal, E.P. Mechanism of Neutrophil Dysfunction: Neutrophil Serine Proteases Cleave and Inactivate the C5a Receptor. J. Immunol. 2014, 192, 1787–1795. [Google Scholar] [CrossRef]
- Chen, C.C.; Manning, A.M. TGF-beta 1, IL-10 and IL-4 differentially modulate the cytokine-induced expression of IL-6 and IL-8 in human endothelial cells. Cytokine 1996, 8, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawi, S.; Herrera-Vizcaino, C.; Orlowska, A.; Willershausen, I.; Sader, R.; Miron, R.J.; Choukroun, J.; Ghanaati, S. Biologization of Collagen-Based Biomaterials Using Liquid-Platelet-Rich Fibrin: New Insights into Clinically Applicable Tissue Engineering. Materials 2019, 12, 3993. [Google Scholar] [CrossRef]
- Nica, C.; Lin, Z.; Sculean, A.; Asparuhova, M.B. Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 2635. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vuckovic, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Luo, D.; McGettrick, H.M.; Stone, P.C.; Rainger, G.E.; Nash, G.B. The roles of integrins in function of human neutrophils after their migration through endothelium into interstitial matrix. PLoS ONE 2015, 10, e0118593. [Google Scholar] [CrossRef]
- Calo, C.J.; Patil, T.; Palizzi, M.; Wheeler, N.; Hind, L.E. Collagen concentration regulates neutrophil extravasation and migration in response to infection in an endothelium dependent manner. Front. Immunol. 2024, 15, 1405364. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Aresta-DaSilva, S.; Tang, K.; Alvarez, D.; Webber, M.J.; Tang, B.C.; Lavin, D.M.; Veiseh, O.; Doloff, J.C.; Bose, S.; et al. Neutrophil Responses to Sterile Implant Materials. PLoS ONE 2015, 10, e0137550. [Google Scholar] [CrossRef] [PubMed]
- Morandini, L.; Avery, D.; Angeles, B.; Winston, P.; Martin, R.K.; Donahue, H.J.; Olivares-Navarrete, R. Reduction of neutrophil extracellular traps accelerates inflammatory resolution and increases bone formation on titanium implants. Acta Biomater. 2023, 166, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273, Table of Contents. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin Immune Landscape: Inside and Outside the Organism. Mediators Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Dohle, E.; Lim, J.; Weigl, P.; Teoh, S.H.; Sader, R.; Ghanaati, S. Biologization of Pcl-Mesh Using Platelet Rich Fibrin (Prf) Enhances Its Regenerative Potential In Vitro. Int. J. Mol. Sci. 2021, 22, 2159. [Google Scholar] [CrossRef]

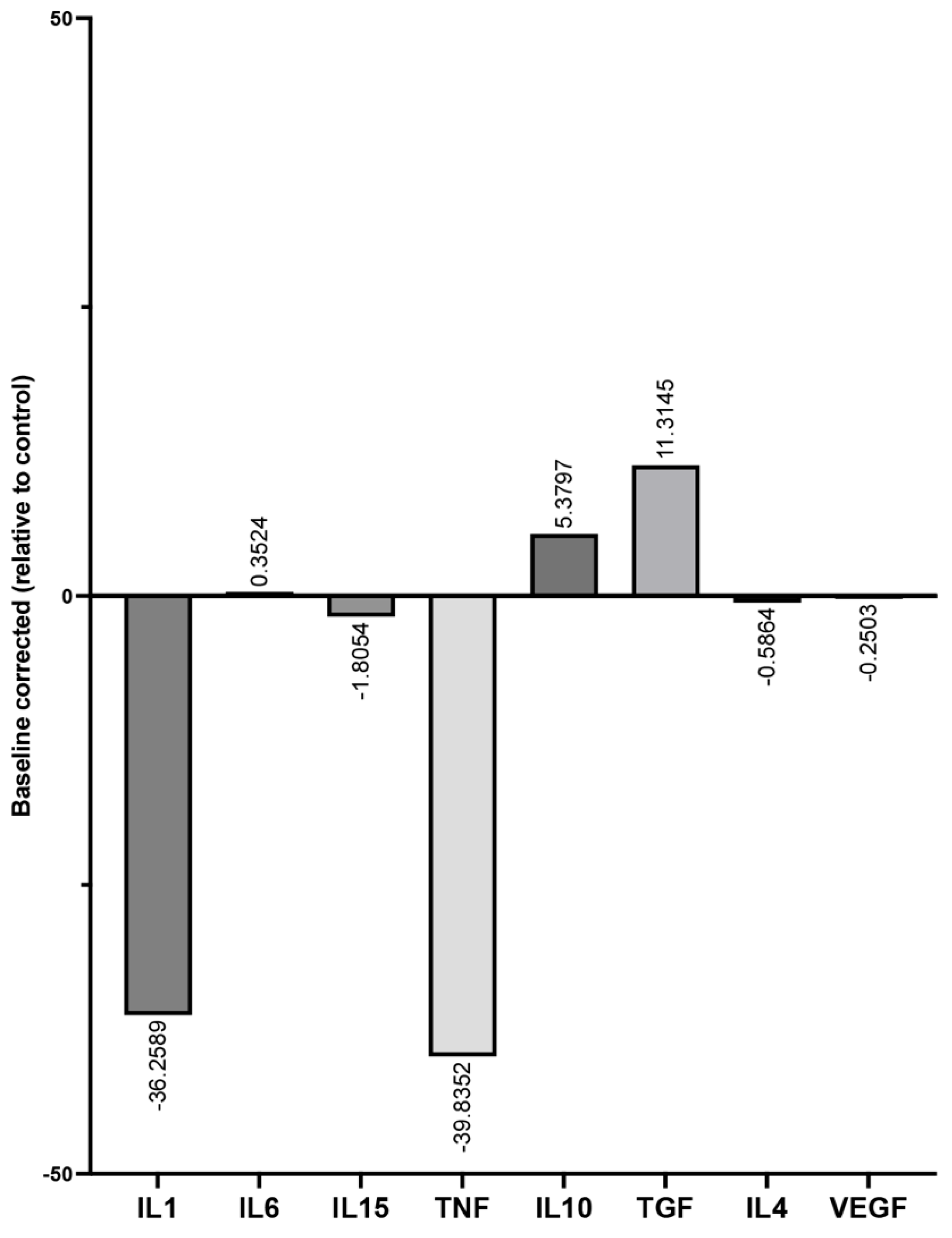
| CYTOKINES/ GROWTH FACTORS (GF) | [c] Input | 4 h | ||
|---|---|---|---|---|
| [c] GF Measured | ∆[c] GF | GF Recovery Rate [%] | ||
| IL15 | 62.5 | 24.38 | −38.12 | 39.00% |
| 250 | 78.35 | −171.65 | 31.34% | |
| 1000 | 156.68 | −843.32 | 15.67% | |
| VEGF | 125 | 20.96 | −104.04 | 16.77% |
| 500 | 71.48 | −428.52 | 14.30% | |
| 2000 | 145.52 | −1854.48 | 7.28% | |
| TGF | 125 | 0.51 | −124.49 | 0.41% |
| 500 | 19.72 | −480.28 | 3.94% | |
| 2000 | 88.99 | −1911.01 | 4.45% | |
| TNF | 62.5 | 27.61 | −34.89 | 44.18% |
| 250 | 93.27 | −156.73 | 37.31% | |
| 1000 | 289.41 | −710.59 | 28.94% | |
| IL1 | 15.6 | 16.42 | 0.82 | 94.76% |
| 62.5 | 62.30 | −0.20 | 99.68% | |
| 250 | 256.36 | 6.36 | 97.45% | |
| IL4 | 125 | 35.15 | −89.85 | 28.12% |
| 500 | 123.37 | −376.63 | 24.67% | |
| 2000 | 861.11 | −1138.89 | 43.06% | |
| IL6 | 37.5 | 40.22 | 2.72 | 92.74% |
| 150 | 134.80 | −15.20 | 89.87% | |
| 600 | 576.51 | −23.49 | 96.08% | |
| IL10 | 125 | 37.30 | −87.70 | 29.84% |
| 500 | 131.05 | −368.95 | 26.21% | |
| 2000 | 691.84 | −1308.16 | 34.59% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dohle, E.; Zuo, H.; Bayrak, B.; Heselich, A.; Schäfer, B.; Sader, R.; Ghanaati, S. In Vitro Evaluation of the PMN Reaction on a Collagen-Based Purified Reconstituted Bilayer Matrix (PRBM) Using the Autologous Blood Concentrate PRF. Biomedicines 2025, 13, 1239. https://doi.org/10.3390/biomedicines13051239
Dohle E, Zuo H, Bayrak B, Heselich A, Schäfer B, Sader R, Ghanaati S. In Vitro Evaluation of the PMN Reaction on a Collagen-Based Purified Reconstituted Bilayer Matrix (PRBM) Using the Autologous Blood Concentrate PRF. Biomedicines. 2025; 13(5):1239. https://doi.org/10.3390/biomedicines13051239
Chicago/Turabian StyleDohle, Eva, Hongyu Zuo, Büşra Bayrak, Anja Heselich, Birgit Schäfer, Robert Sader, and Shahram Ghanaati. 2025. "In Vitro Evaluation of the PMN Reaction on a Collagen-Based Purified Reconstituted Bilayer Matrix (PRBM) Using the Autologous Blood Concentrate PRF" Biomedicines 13, no. 5: 1239. https://doi.org/10.3390/biomedicines13051239
APA StyleDohle, E., Zuo, H., Bayrak, B., Heselich, A., Schäfer, B., Sader, R., & Ghanaati, S. (2025). In Vitro Evaluation of the PMN Reaction on a Collagen-Based Purified Reconstituted Bilayer Matrix (PRBM) Using the Autologous Blood Concentrate PRF. Biomedicines, 13(5), 1239. https://doi.org/10.3390/biomedicines13051239








