Human and Mouse Bone Marrow CD45+ Erythroid Cells Have a Constitutive Expression of Antibacterial Immune Response Signature Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of a Network of Protein–Protein Interactions of the Immune Transcriptome of Human Bone Marrow Erythroid Cells
2.2. Human Bone Marrow Erythroid-Cell Immune Transcriptome Data Re-Analysis
2.3. Bone Marrow Sample Collection and Processing
2.4. Bone Marrow Mononuclear Cell Isolation
2.5. Erythroid-Cell Magnetic Separation
2.6. Erythroid-Cell Culturing
2.7. Erythroid-Cell Culture-Medium Harvesting
2.8. Cytokine Quantification in Culture Medium
2.9. Murine Bone Marrow Hematopoietic Stem-Cell Atlas Re-Analysis
2.10. Mouse Erythroid-Cell Immune Transcriptome Data Re-Analysis
2.11. Flow Cytometry of Human Bone Marrow Erythroid Cells
2.12. Human Bone Marrow Erythroid-Cell Flow Cytometry Data Analysis
2.13. Bacterial Growth Inhibition Assay
3. Results
3.1. Protein-Coding Genes Expressed by the Human Bone Marrow Erythroid Cells Form a Connected Net of Protein–Protein Interactions Involved in the Immune Response to LPS
3.2. Human Bone Marrow Erythroid Cells Secrete Cytokines Involved in the Immune Response to LPS
3.3. Response to LPS Pathway Genes Is Expressed by the CD45+ Murine Bone Marrow Erythroid Cells
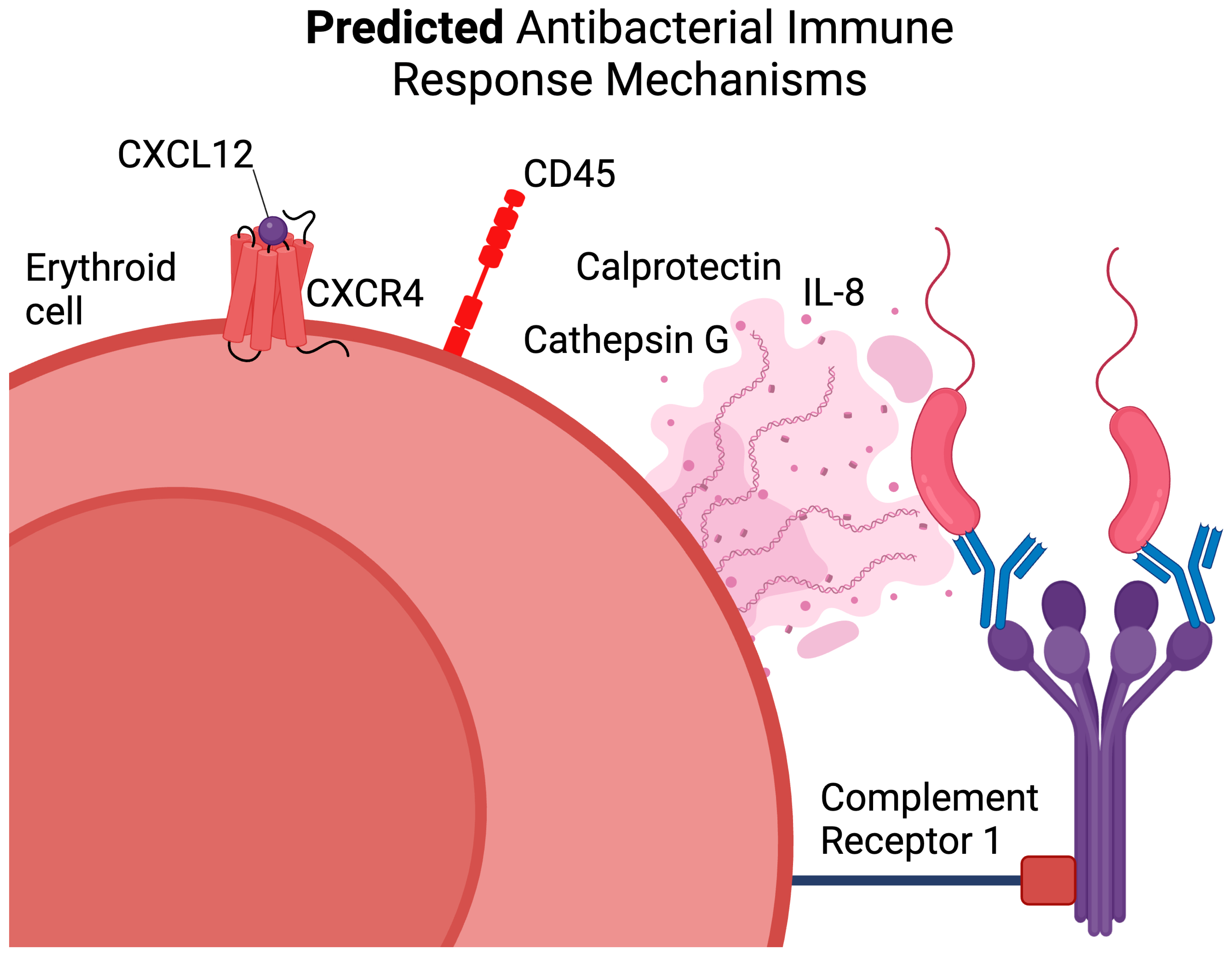
3.4. CD45+ Human Bone Marrow Erythroid-Cell Proteome Maps to a Calprotectin-Positive Cell Population Found in the Human Bone Marrow Erythroid-Cell scRNA-Seq Data
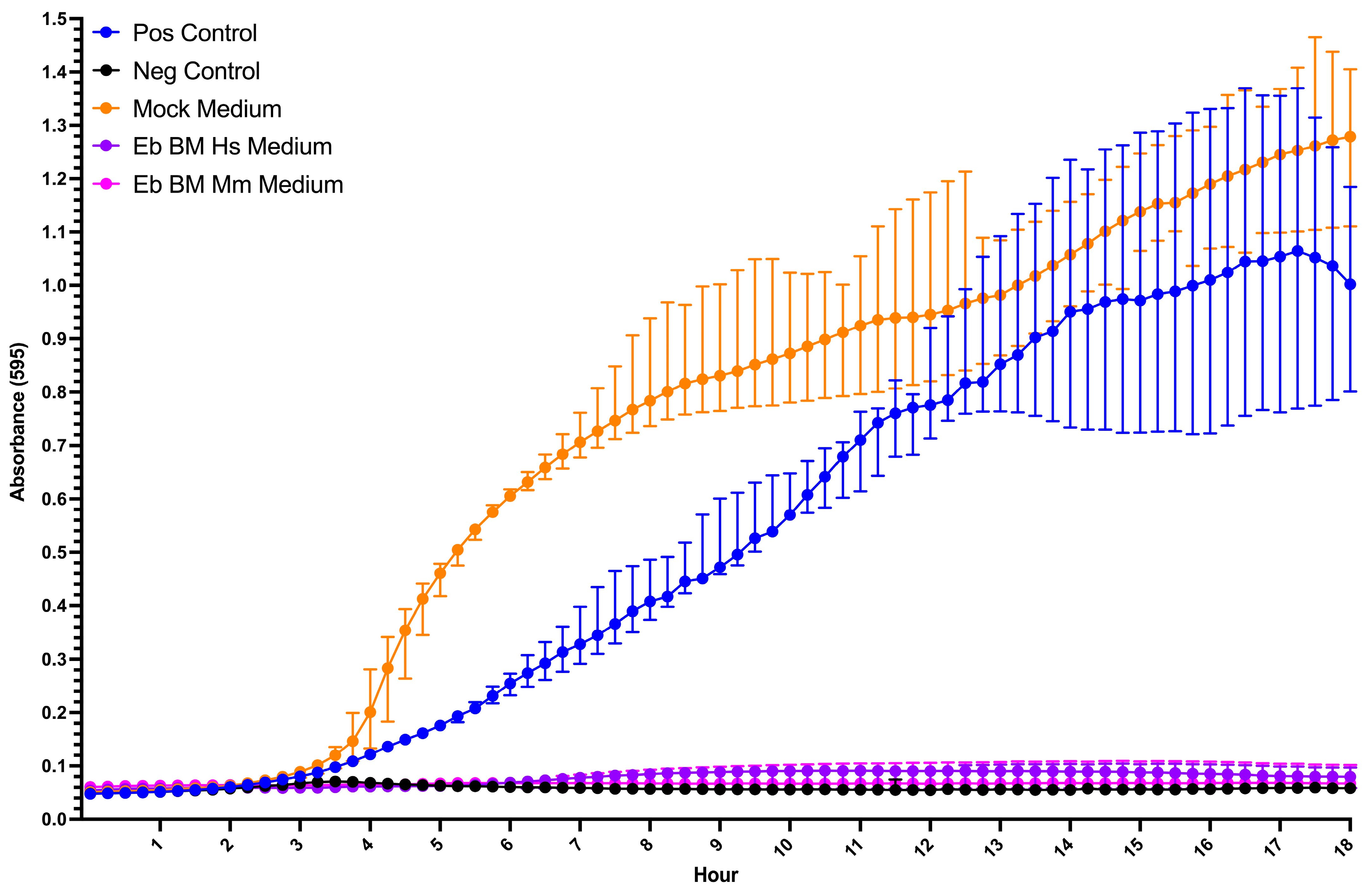
3.5. Human and Mouse Bone Marrow Erythroid-Cell-Conditioned Media Inhibit Bacterial Growth In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zivot, A.; Lipton, J.M.; Narla, A.; Blanc, L. Erythropoiesis: Insights into pathophysiology and treatments in 2017. In Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2018; Volume 24. [Google Scholar] [CrossRef]
- Morera, D.; MacKenzie, S.A. Is there a direct role for erythrocytes in the immune response? In Veterinary Research; Springer: Berlin/Heidelberg, Germany, 2011; Volume 42, p. 89. [Google Scholar] [CrossRef]
- Shahbaz, S.; Bozorgmehr, N.; Koleva, P.; Namdar, A.; Jovel, J.; Fava, R.A.; Elahi, S. CD71+VISTA+ Erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol. 2018, 16, e2006649. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.H.; Lam, Y.W.; Fraser, S.T. Cellular dynamics of mammalian red blood cell production in the erythroblastic island niche. Biophys. Rev. 2019, 11, 873–894. [Google Scholar] [CrossRef]
- Han, H.; Rim, Y.A.; Ju, J.H. Recent updates of stem cell-based erythropoiesis. Hum. Cell 2023, 36, 894–907. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, H.; Zhang, H.; Gao, C.; Guo, X.; Chen, L.; Lobo, C.; Yazdanbakhsh, K.; Zhang, S.; An, X. Analyses of erythropoiesis from embryonic stem cell-CD34+ and cord blood-CD34+ cells reveal mechanisms for defective expansion and enucleation of embryomic stem cell-Erythroid cells. J. Cell. Mol. Med. 2022, 26, 2404–2416. [Google Scholar] [CrossRef]
- Trakarnsanga, K.; Griffiths, R.E.; Wilson, M.C.; Blair, A.; Satchwell, T.J.; Meinders, M.; Cogan, N.; Kupzig, S.; Kurita, R.; Nakamura, Y.; et al. An immortalized adult human Erythroid line facilitates sustainable and scalable generation of functional red cells. Nat. Commun. 2017, 8, 14750. [Google Scholar] [CrossRef]
- Isern, J.; Fraser, S.T.; He, Z.; Baron, M.H. The fetal liver is a niche for maturation of primitive Erythroid cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6662–6667. [Google Scholar] [CrossRef] [PubMed]
- Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Shevchenko, J.; Denisova, V.; Alrhmoun, S.; Volynets, M.; Tereshchenko, V.; Zaitsev, K.; Sennikov, S. Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing. Cells 2022, 11, 3537. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Nowis, D.; Golab, J. The role of CD71+ Erythroid cells in the regulation of the immune response. Pharmacol. Ther. 2021, 228, 107927. [Google Scholar] [CrossRef] [PubMed]
- Allahyani, M.A.; Aljuaid, A.A.; Almehmadi, M.M.; Alghamdi, A.A.; Halawani, I.F.; Aldairi, A.F.; Alharbi, A.M.; Albshri, M.H.; Mutwalli, A.A.; Alhazmi, A.S. Detection of Erythroid progenitors and erythrocytopathies in patients with severe COVID-19 disease. Saudi Med. J. 2022, 43, 899–906. [Google Scholar] [CrossRef]
- Karsten, U.; Butschak, G.; Stahn, R.; Goletz, S. A novel series of anti-human glycophorin A (CD235a) antibodies defining five extra- and intracellular epitopes. Int. Immunopharmacol. 2010, 10, 1354–1360. [Google Scholar] [CrossRef]
- Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Shevchenko, J.; Volynets, M.; Alrhmoun, S.; Nazarov, K.; Denisova, V.; Sennikov, S. A subpopulation of human bone marrow Erythroid cells displays a myeloid gene expression signature similar to that of classic monocytes. PLoS ONE 2024, 19, e0305816. [Google Scholar] [CrossRef]
- Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Nazarov, K.; Denisova, V.; Sennikov, S. Single-cell multi-omics reveal stage of differentiation and trajectory-dependent immunity-related gene expression patterns in human Erythroid cells. Front. Immunol. 2024, 15, 1431303. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; E Peluso, M.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2024, 53, D730–D737. [Google Scholar] [CrossRef]
- Renesh Bedre. Reneshbedre/bioinfokit: Bioinformatics Data Analysis and Visualization Toolkit. Zenodo. 2022. Available online: https://zenodo.org/records/3964972 (accessed on 23 February 2025).
- Fang, Z.; Liu, X.; Peltz, G. GSEApy: A comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics 2022, 39, btac757. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2023, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.-R.; Raychaudhuri, S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Perik-Zavodskaia, O.; Perik-Zavodskii, R.; Nazarov, K.; Volynets, M.; Alrhmoun, S.; Shevchenko, J.; Sennikov, S. Murine Bone Marrow Erythroid Cells Have Two Branches of Differentiation Defined by the Presence of CD45 and a Different Immune Transcriptome Than Fetal Liver Erythroid Cells. Int. J. Mol. Sci. 2023, 24, 15752. [Google Scholar] [CrossRef]
- Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Phenotypic Alterations in Erythroid Nucleated Cells of Spleen and Bone Marrow in Acute Hypoxia. Cells 2023, 12, 2810. [Google Scholar] [CrossRef]
- Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Acute blood loss in mice forces differentiation of both CD45-positive and CD45-negative Erythroid cells and leads to a decreased CCL3 chemokine production by bone marrow Erythroid cells. PLoS ONE 2024, 19, e0309455. [Google Scholar] [CrossRef]
- Alsalloum, A.; Alrhmoun, S.; Perik-Zavosdkaia, O.; Fisher, M.; Volynets, M.; Lopatnikova, J.; Perik-Zavodskii, R.; Shevchenko, J.; Philippova, J.; Solovieva, O.; et al. Decoding NY-ESO-1 TCR T cells: Transcriptomic insights reveal dual mechanisms of tumor targeting in a melanoma murine xenograft model. Front. Immunol. 2024, 15, 1507218. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Nakatani, Y.; Mikami, M. Calprotectin (S100A8/S100A9), an Inflammatory Protein Complex from Neutrophils with a Broad Apoptosis-Inducing Activity. Biol. Pharm. Bull. 2003, 26, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Inciarte-Mundo, J.; Frade-Sosa, B.; Sanmartí, R. From bench to bedside: Calprotectin (S100A8/S100A9) as a biomarker in rheumatoid arthritis. Front. Immunol. 2022, 13, 1001025. [Google Scholar] [CrossRef]
- Sekimoto, R.; Kishida, K.; Nakatsuji, H.; Nakagawa, T.; Funahashi, T.; Shimomura, I. High circulating levels of S100A8/A9 complex (calprotectin) in male Japanese with abdominal adiposity and dysregulated expression of S100A8 and S100A9 in adipose tissues of obese mice. Biochem. Biophys. Res. Commun. 2012, 419, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Machado, L.R.; Ottolini, B. An Evolutionary History of Defensins: A Role for Copy Number Variation in Maximizing Host Innate and Adaptive Immune Responses. Front. Immunol. 2015, 6, 115. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Opi, D.H.; Uyoga, S.; Orori, E.N.; Williams, T.N.; Rowe, J.A. Red blood cell complement receptor one level varies with Knops blood group, α+thalassaemia and age among Kenyan children. Genes Immun. 2016, 17, 171–178. [Google Scholar] [CrossRef][Green Version]
- Pham, B.; Kisserli, A.; Donvito, B.; Duret, V.; Reveil, B.; Tabary, T.; Le Pennec, P.; Peyrard, T.; Rouger, P.; Cohen, J.H. Analysis of complement receptor Type 1 expression on red blood cells in negative phenotypes of the Knops blood group system, according to CR1 gene allotype polymorphisms. Transfusion 2010, 50, 1435–1443. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Stojkov, D.; Wachowska, M.; Surmiak, M. Editorial: Neutrophil extracellular traps: Mechanistic and functional insight. Front. Immunol. 2024, 15, 1407232. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, J.; Kang, W.; Yan, M.; Pan, J.; Zhang, X. Neutrophil extracellular traps in autoimmune diseases: Analysis of the knowledge map. Front. Immunol. 2023, 14, 1095421. [Google Scholar] [CrossRef]
- Schajnovitz, A.; Itkin, T.; D’Uva, G.; Kalinkovich, A.; Golan, K.; Ludin, A.; Cohen, D.; Shulman, Z.; Avigdor, A.; Nagler, A.; et al. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat. Immunol. 2011, 12, 391–398. [Google Scholar] [CrossRef]
- Aoki, K.; Kurashige, M.; Ichii, M.; Higaki, K.; Sugiyama, T.; Kaito, T.; Ando, W.; Sugano, N.; Sakai, T.; Shibayama, H.; et al. Identification of CXCL12-abundant reticular cells in human adult bone marrow. Br. J. Haematol. 2021, 193, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Li, H.; Paterson, A.J.; He, J.; Nagasawa, T.; Bhatia, R. Role of CXCL12-Expressing Bone Marrow Populations in Leukemic Stem Cell Regulation. Blood 2016, 128, 26. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. OncoImmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Bae, J.; Accardi, F.; Hideshima, T.; Tai, Y.-T.; Prabhala, R.; Shambley, A.; Wen, K.; Rowell, S.; Richardson, P.G.; Munshi, N.C.; et al. Targeting LAG3/GAL-3 to overcome immunosuppression and enhance anti-tumor immune responses in multiple myeloma. Leukemia 2021, 36, 138–154. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, R.; Yu, L.; Zheng, X.; Cui, R.; Song, Y.; Wang, D. Roles of galectin-3 in the tumor microenvironment and tumor metabolism (Review). Oncol. Rep. 2020, 44, 1799–1809. [Google Scholar] [CrossRef]
- Zhang, L.; Woltering, I.; Holzner, M.; Brandhofer, M.; Schaefer, C.-C.; Bushati, G.; Ebert, S.; Yang, B.; Muenchhoff, M.; Hellmuth, J.C.; et al. CD74 is a functional MIF receptor on activated CD4+ T cells. Cell. Mol. Life Sci. 2024, 81, 296. [Google Scholar] [CrossRef]
- Alibashe-Ahmed, M.; Roger, T.; Serre-Beinier, V.; Berishvili, E.; Reith, W.; Bosco, D.; Berney, T. Macrophage migration inhibitory factor regulates TLR4 expression and modulates TCR/CD3-mediated activation in CD4+ T lymphocytes. Sci. Rep. 2019, 9, 9380. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitani, A.; Fuss, I.; Pedersen, A.; Harada, N.; Nawata, H.; Strober, W. TGF-β1 Plays an Important Role in the Mechanism of CD4+CD25+ Regulatory T Cell Activity in Both Humans and Mice. J. Immunol. 2004, 172, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, N.; Yopp, A.C.; Chen, D.; Mao, M.; Chen, D.; Zhang, H.; Ding, Y.; Bromberg, J.S. TGF-β Induces Foxp3 + T-Regulatory Cells from CD4+CD25− Precursors. Am. J. Transplant. 2004, 4, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Cottrez, F.; Groux, H. Regulation of TGF-β Response During T Cell Activation Is Modulated by IL-10. J. Immunol. 2001, 167, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, L.; Birnberg, T.; Kim, K.; Jung, S. Dendritic cell-restricted CD80/86 deficiency results in peripheral regulatory T-cell reduction but is not associated with lymphocyte hyperactivation. Eur. J. Immunol. 2011, 41, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Manzotti, C.N.; Liu, M.; Burke, F.; Mead, K.I.; Sansom, D.M. CD86 and CD80 Differentially Modulate the Suppressive Function of Human Regulatory T Cells. J. Immunol. 2004, 172, 2778–2784. [Google Scholar] [CrossRef]
- Williams, J.A.; Tai, X.; Hodes, R.J. CD28-CD80/86 and CD40-CD40L Interactions Promote Thymic Tolerance by Regulating Medullary Epithelial Cell and Thymocyte Development. Crit. Rev. Immunol. 2015, 35, 59–76. [Google Scholar] [CrossRef]
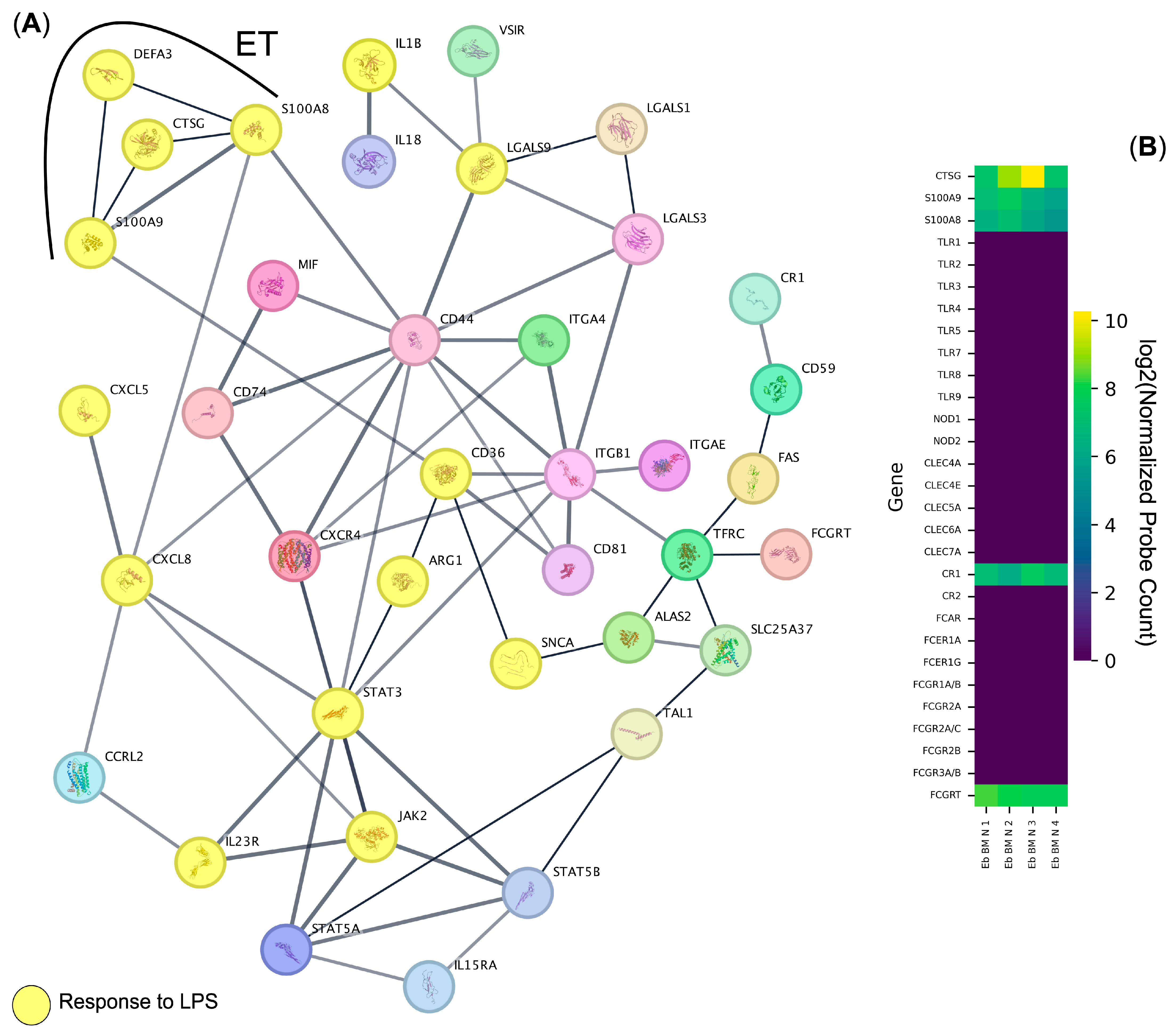
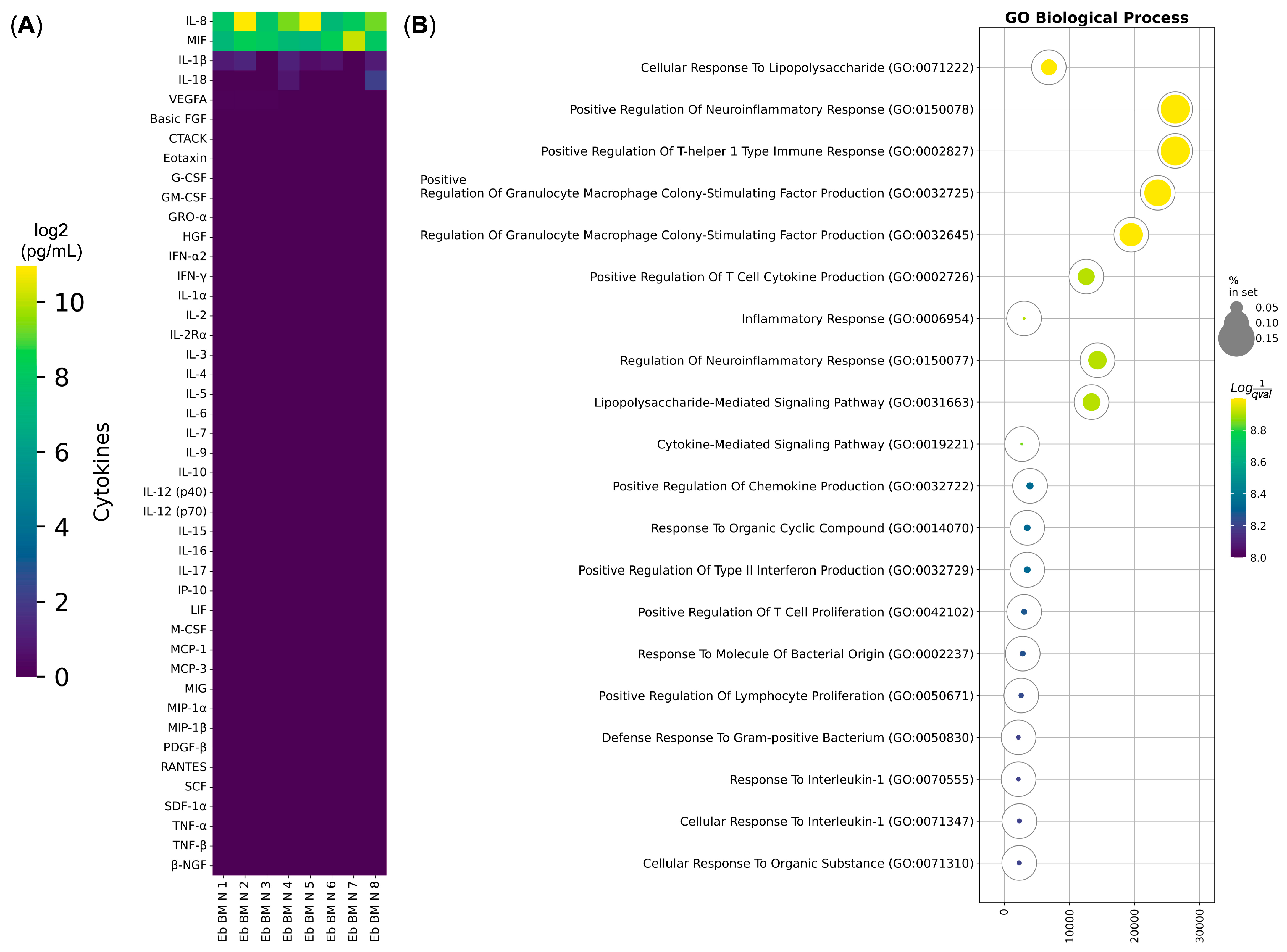
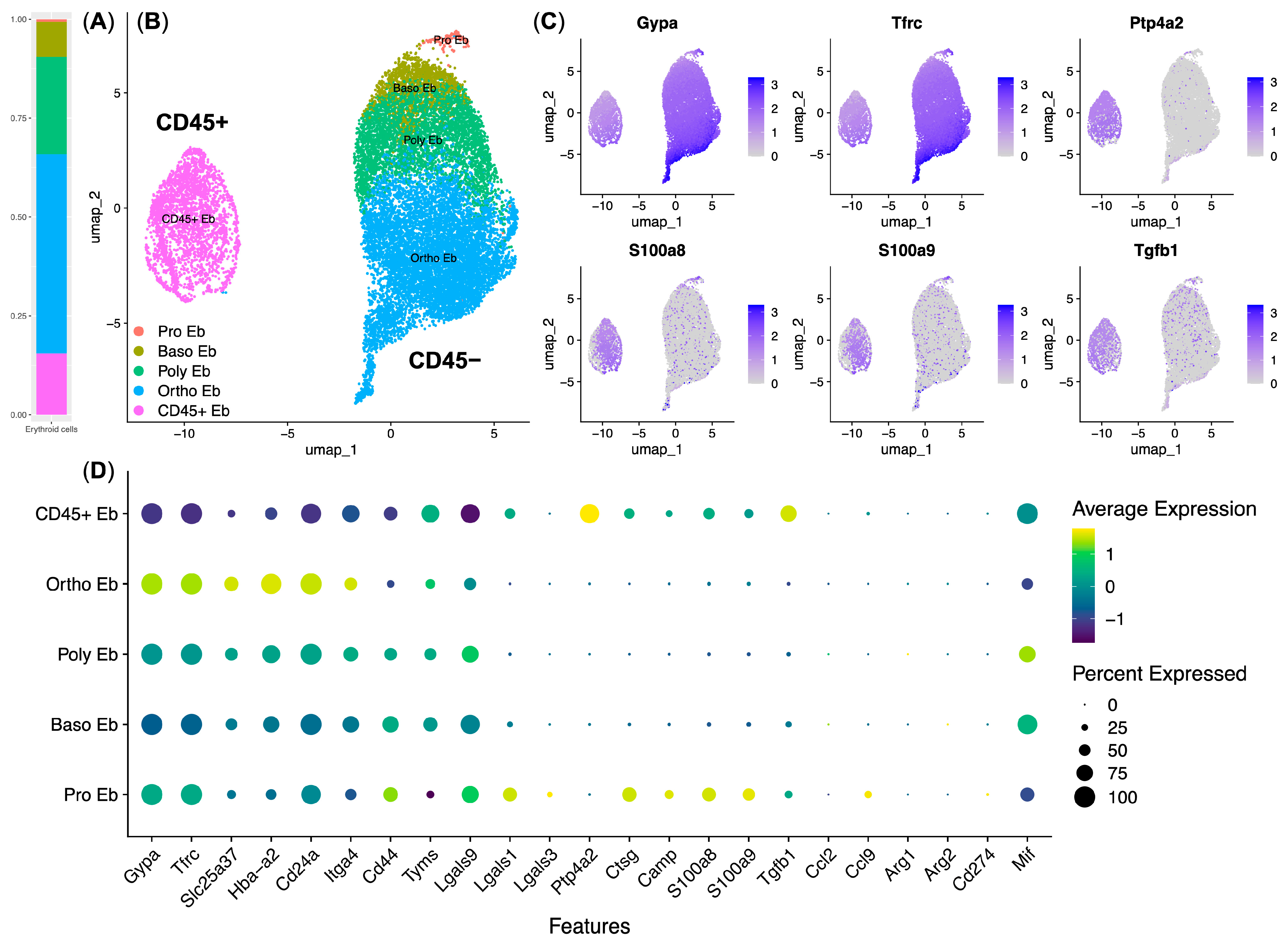
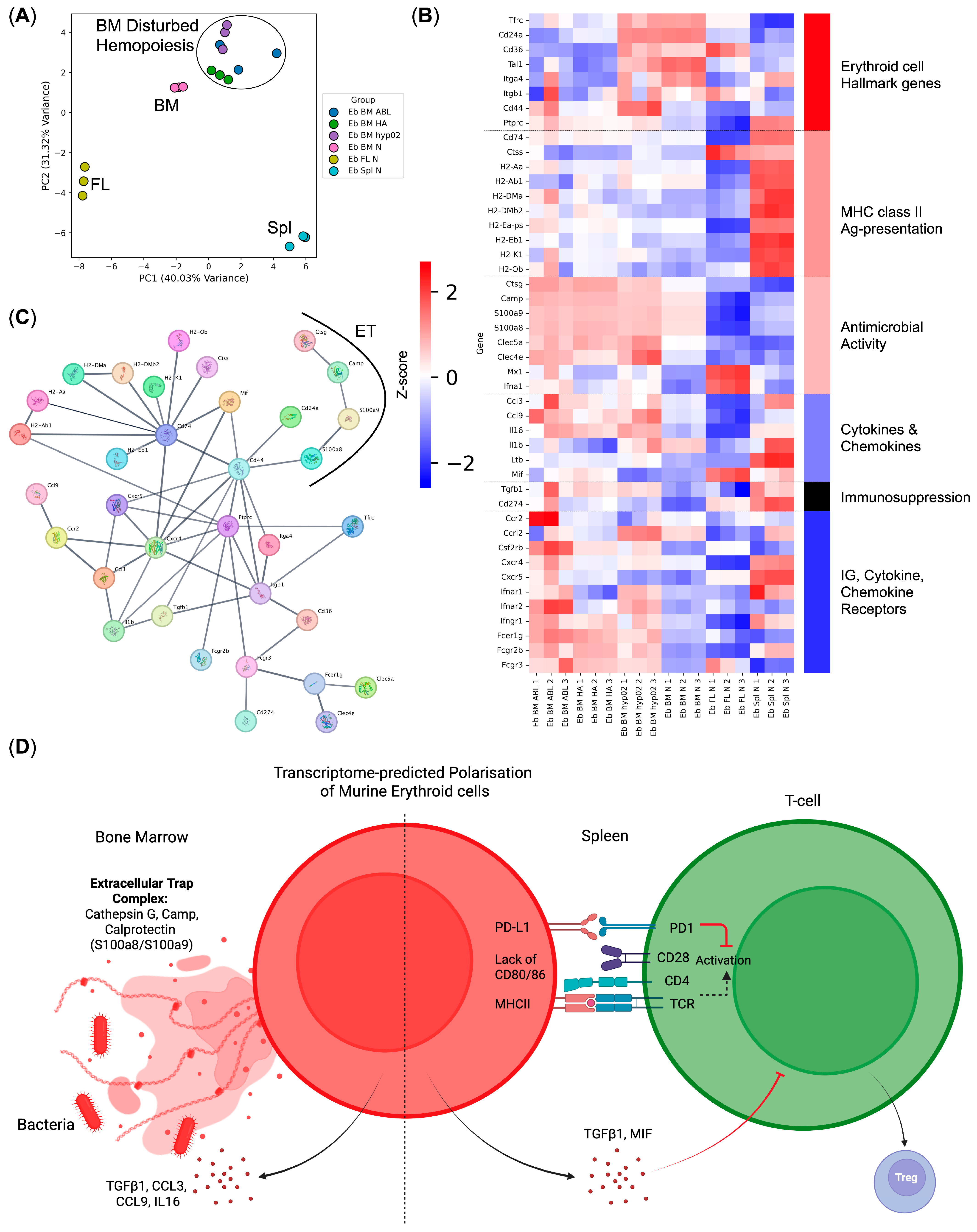

| Gene Ontology Biological Process Term | q-Value | Hits | Out Of | Score | Genes |
|---|---|---|---|---|---|
| Response to lipopolysaccharide | 0.000000001 | 14 | 314 | 446 | CTSG, CXCL5, CXCL8, STAT3, IL1B, IL23R, DEFA3, ARG1, S100A8, S100A9, JAK2, LGALS9, CD36, SNCA |
| Immune cell migration | 0.000000001 | 11 | 249 | 442 | CTSG, LGALS3, STAT5B, CXCL5, CXCL8, S100A8, S100A12, S100A9, ITGB1, ITGA4, CXCR4 |
| Regulation of intercellular adhesion | 0.000000012 | 15 | 368 | 408 | CD74, LGALS1, CTSG, LGALS3, CD81, STAT5B, IL23R, ARG1, CR1, JAK2, TFRC, VSIR, LGALS9, ITGA4, CD44 |
| Positive regulation of the immune effector process | 0.000000038 | 10 | 264 | 379 | CD74, MIF, CD81, STAT5B, IL23R, ARG1, CR1, TFRC, LGALS9, CD36 |
| Humoral immune response | 0.000000697 | 9 | 268 | 336 | CTSG, LGALS3, CD81, CXCL5, CXCL8, DEFA3, CR1, S100A12, S100A9 |
| Positive regulation of the response to an external stimulus | 0.000000732 | 14 | 453 | 309 | CD74, MIF, LGALS1, CD81, STAT5B, CXCL8, ARG1, S100A8, S100A12, S100A9, JAK2, LGALS9, CXCR4, SNCA |
| Activation of immune cells | 0.000001202 | 14 | 574 | 244 | CD74, LGALS1, CTSG, CD81, STAT3, STAT5B, CXCL8, S100A12, JAK2, ITGB1, ITGA4, IL15RA, CD44, SNCA |
| Positive regulation of immune cell migration | 0.000001130 | 11 | 529 | 208 | CD74, LGALS3, STAT3, CXCL8, STAT5A, JAK2, VSIR, LGALS9, ITGB1, ITGA4, CXCR4 |
| Regulation of the apoptotic process | 0.000009600 | 15 | 1462 | 103 | CD74, MIF, LGALS1, LGALS3, STAT5B, S100A8, S100A9, JAK2, TFRC, LGALS9, ITGB1, ITGA4, CD44, FAS, SNCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Shevchenko, J.; Nazarov, K.; Gizbrekht, A.; Alrhmoun, S.; Denisova, V.; Sennikov, S. Human and Mouse Bone Marrow CD45+ Erythroid Cells Have a Constitutive Expression of Antibacterial Immune Response Signature Genes. Biomedicines 2025, 13, 1218. https://doi.org/10.3390/biomedicines13051218
Perik-Zavodskii R, Perik-Zavodskaia O, Shevchenko J, Nazarov K, Gizbrekht A, Alrhmoun S, Denisova V, Sennikov S. Human and Mouse Bone Marrow CD45+ Erythroid Cells Have a Constitutive Expression of Antibacterial Immune Response Signature Genes. Biomedicines. 2025; 13(5):1218. https://doi.org/10.3390/biomedicines13051218
Chicago/Turabian StylePerik-Zavodskii, Roman, Olga Perik-Zavodskaia, Julia Shevchenko, Kirill Nazarov, Anastasia Gizbrekht, Saleh Alrhmoun, Vera Denisova, and Sergey Sennikov. 2025. "Human and Mouse Bone Marrow CD45+ Erythroid Cells Have a Constitutive Expression of Antibacterial Immune Response Signature Genes" Biomedicines 13, no. 5: 1218. https://doi.org/10.3390/biomedicines13051218
APA StylePerik-Zavodskii, R., Perik-Zavodskaia, O., Shevchenko, J., Nazarov, K., Gizbrekht, A., Alrhmoun, S., Denisova, V., & Sennikov, S. (2025). Human and Mouse Bone Marrow CD45+ Erythroid Cells Have a Constitutive Expression of Antibacterial Immune Response Signature Genes. Biomedicines, 13(5), 1218. https://doi.org/10.3390/biomedicines13051218






