The Expression and Molecular Roles of MAMDC2 in MSS Colorectal Cancer with a High Tumor Stromal Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. InterPro Dataset Analysis
2.2. Data Collection and Processing
2.3. Kaplan–Meier Plotter
2.4. Gene-Set Enrichment Analysis
2.5. TIMER Analysis
2.6. Immunohistochemistry
2.7. Western Blotting
2.8. Cell Culture
2.9. Plasmids and siRNAs
2.10. Statistical Analysis
3. Results
3.1. Result 1: Differential Expression and Survival Analysis of MAM Domain-Containing Genes in Colorectal Cancer
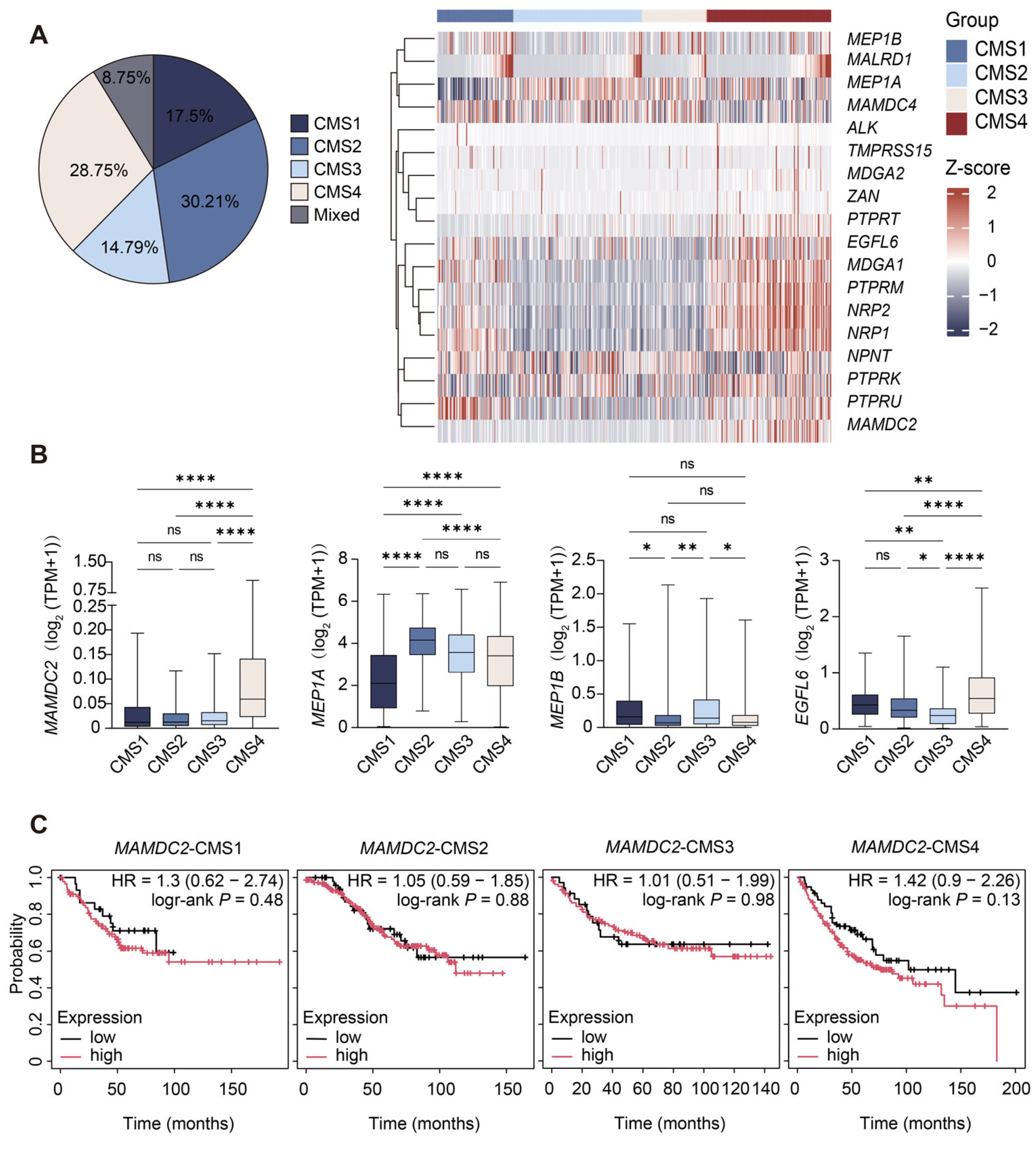
3.2. Result 2: Differential Expression of MAM Domain-Containing Genes and Prognostic Association of MAMDC2 in CMSs
3.3. Result 3: Elevated MAMDC2 Expression in High-TSR Colorectal Cancer
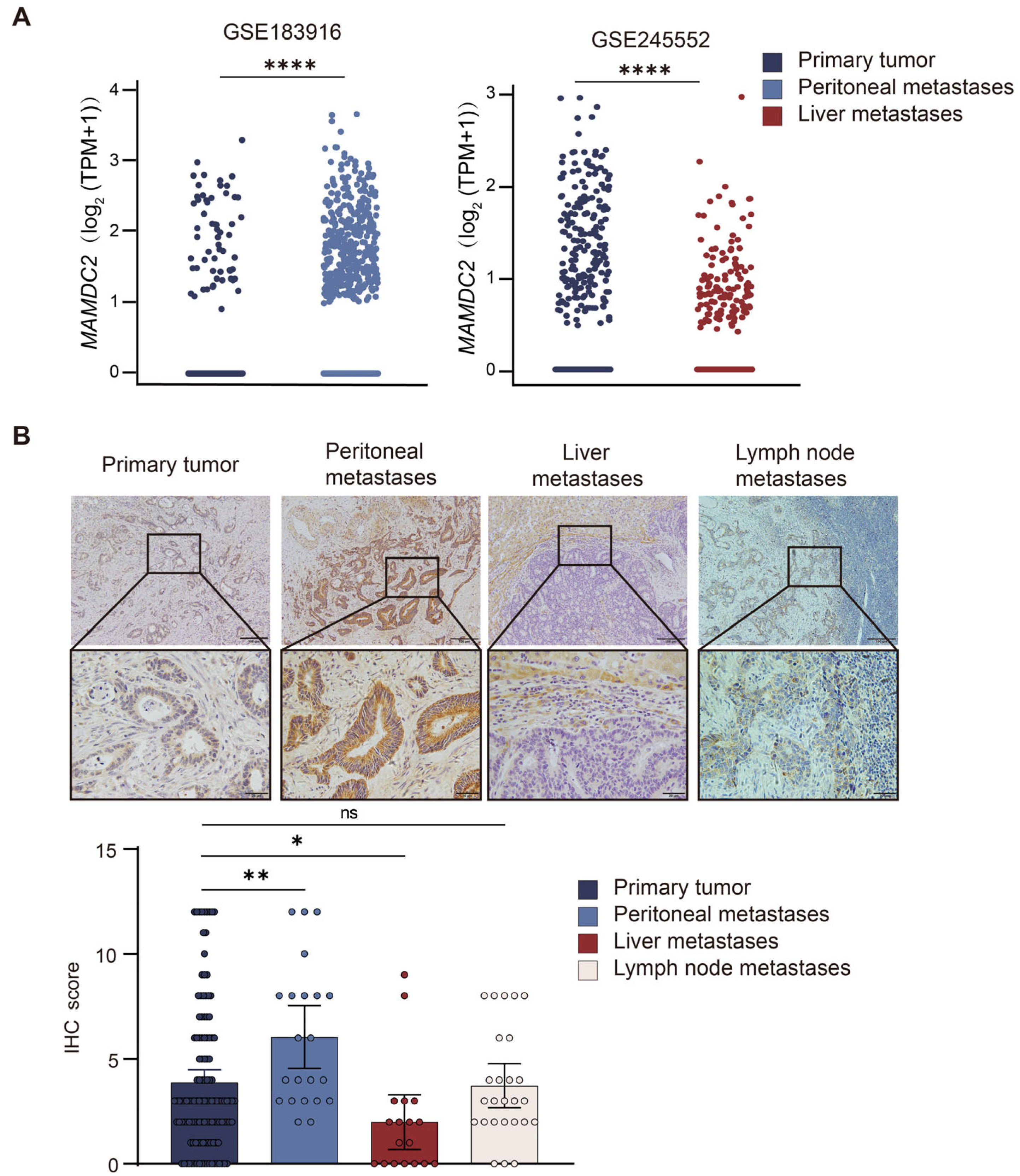
3.4. Result 4: MAMDC2 Expression Patterns in Primary and Metastatic Colorectal Cancer
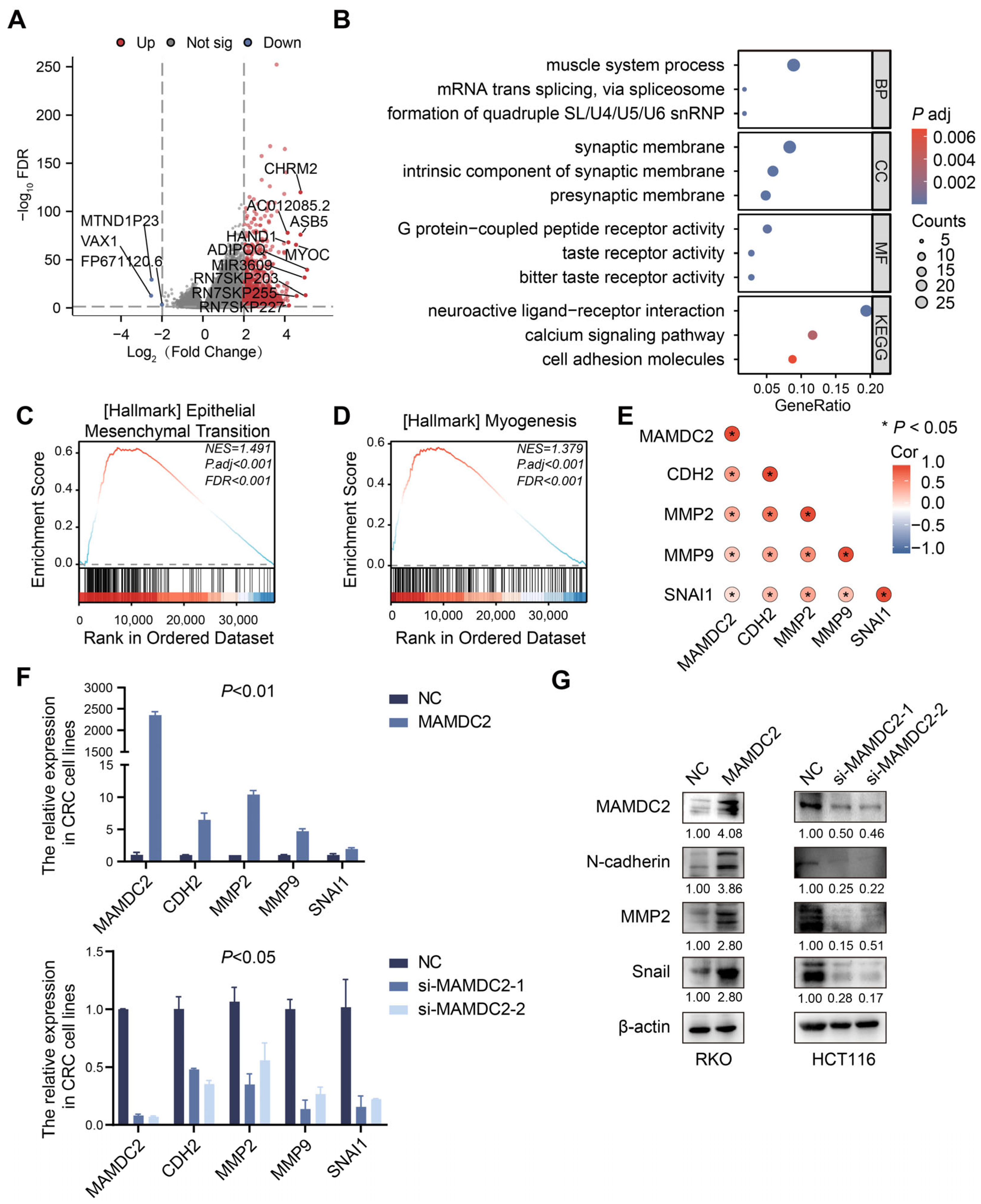
3.5. Result 5: MAMDC2 Regulates Epithelial–Mesenchymal Transition in Colorectal Cancer
3.6. Result 6: Co-Expression Patterns of MAMDC2 and MYLK in Colorectal Cancer
3.7. Result 7: Cancer Cell-Derived MAMDC2 Promotes MYLK Expression in CAFs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| MAM | meprin/A-5 protein/receptor protein-tyrosine phosphatase mu |
| TSR | tumor stroma ratio |
| CMS | consensus molecular subtype |
| CMS4 | The mesenchymal subtype |
| EMT | epithelial–mesenchymal transition |
| CAFs | cancer-associated fibroblasts |
| MAMDC2 | MAM domain-containing 2 |
| MYLK | myosin light-chain kinase |
| LM | liver metastases |
| PM | peritoneal metastases |
| DEG | differentially expressed gene |
| CIN | chromosomal instability |
| MSS | microsatellite stable stability |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Mouillet-Richard, S.; Cazelles, A.; Sroussi, M.; Gallois, C.; Taieb, J.; Laurent-Puig, P. Clinical Challenges of Consensus Molecular Subtype CMS4 Colon Cancer in the Era of Precision Medicine. Clin. Cancer Res. 2024, 30, 2351–2358. [Google Scholar] [CrossRef]
- Melino, G.; Bischof, J.; Chen, W.-L.; Jia, W.; Juhl, H.; Kopeina, G.S.; Mauriello, A.; Novelli, F.; Scimeca, M.; Shi, Y.; et al. New Hope for the World Cancer Day. Biol. Direct 2025, 20, 14. [Google Scholar] [CrossRef]
- Lafarge, M.W.; Domingo, E.; Sirinukunwattana, K.; Wood, R.; Samuel, L.; Murray, G.; Richman, S.D.; Blake, A.; Sebag-Montefiore, D.; Gollins, S.; et al. Image-Based Consensus Molecular Subtyping in Rectal Cancer Biopsies and Response to Neoadjuvant Chemoradiotherapy. NPJ Precis. Oncol. 2024, 8, 89. [Google Scholar] [CrossRef]
- van den Bulk, J.; Verdegaal, E.M.E.; Ruano, D.; Ijsselsteijn, M.E.; Visser, M.; van der Breggen, R.; Duhen, T.; van der Ploeg, M.; de Vries, N.L.; Oosting, J.; et al. Neoantigen-Specific Immunity in Low Mutation Burden Colorectal Cancers of the Consensus Molecular Subtype 4. Genome Med. 2019, 11, 87. [Google Scholar] [CrossRef]
- Peng, S.; Li, Y.; Huang, M.; Tang, G.; Xie, Y.; Chen, D.; Hu, Y.; Yu, T.; Cai, J.; Yuan, Z.; et al. Metabolomics Reveals That CAF-Derived Lipids Promote Colorectal Cancer Peritoneal Metastasis by Enhancing Membrane Fluidity. Int. J. Biol. Sci. 2022, 18, 1912–1932. [Google Scholar] [CrossRef]
- Li, X.; Tang, B.; Yujie, O.; Xu, C.; Yuan, S. Single-Cell RNA Sequencing Analysis Reveals Cancer-Associated Fibroblast Signature for Prediction of Clinical Outcomes and Immunotherapy in Gastric Cancer. J. Immunother. 2025, 48, 63–77. [Google Scholar] [CrossRef]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-Associated Fibroblasts in the Single-Cell Era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Andriani, F.; Merlino, G.; D’Aiuto, F.; Roz, L.; Callari, M. Complexity in the Tumour Microenvironment: Cancer Associated Fibroblast Gene Expression Patterns Identify Both Common and Unique Features of Tumour-Stroma Crosstalk across Cancer Types. Semin. Cancer Biol. 2015, 35, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The Role of Collagen in Cancer: From Bench to Bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Alsaleh, L.; Li, C.; Couetil, J.L.; Ye, Z.; Huang, K.; Zhang, J.; Chen, C.; Johnson, T.S. Spatial Transcriptomic Analysis Reveals Associations between Genes and Cellular Topology in Breast and Prostate Cancers. Cancers 2022, 14, 4856. [Google Scholar] [CrossRef]
- De, P.; Aske, J.; Dey, N. Cancer-Associated Fibroblast Functions as a Road-Block in Cancer Therapy. Cancers 2021, 13, 5246. [Google Scholar] [CrossRef]
- Beckmann, G.; Bork, P. An Adhesive Domain Detected in Functionally Diverse Receptors. Trends Biochem. Sci. 1993, 18, 40–41. [Google Scholar] [CrossRef]
- van der Krogt, J.-A.; Bempt, M.V.; Ferreiro, J.F.; Mentens, N.; Jacobs, K.; Pluys, U.; Doms, K.; Geerdens, E.; Uyttebroeck, A.; Pierre, P.; et al. Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma with the Variant RNF213-, ATIC- and TPM3-ALK Fusions Is Characterized by Copy Number Gain of the Rearranged ALK Gene. Haematologica 2017, 102, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Akbay, E.; Mikse, O.; Tupper, T.; Cheng, K.; Wang, Y.; Tan, X.; Altabef, A.; Woo, S.-A.; Chen, L.; et al. Co-Clinical Trials Demonstrate Superiority of Crizotinib to Chemotherapy in ALK-Rearranged Non-Small Cell Lung Cancer and Predict Strategies to Overcome Resistance. Clin. Cancer Res. 2014, 20, 1204–1211. [Google Scholar] [CrossRef]
- An, J.; Du, Y.; Fan, X.; Wang, Y.; Ivan, C.; Zhang, X.-G.; Sood, A.K.; An, Z.; Zhang, N. EGFL6 Promotes Breast Cancer by Simultaneously Enhancing Cancer Cell Metastasis and Stimulating Tumor Angiogenesis. Oncogene 2019, 38, 2123–2134. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, C.; Lu, T.; Zhang, Y.; Zhang, S.; Chen, Q.; Deng, N. Knockout of EGFL6 by CRISPR/Cas9 Mediated Inhibition of Tumor Angiogenesis in Ovarian Cancer. Front. Oncol. 2020, 10, 1451. [Google Scholar] [CrossRef]
- He, G.; Li, W.; Zhao, W.; Men, H.; Chen, Q.; Hu, J.; Zhang, J.; Zhu, H.; Wang, W.; Deng, M.; et al. Formin-like 2 Promotes Angiogenesis and Metastasis of Colorectal Cancer by Regulating the EGFL6/CKAP4/ERK Axis. Cancer Sci. 2023, 114, 2014–2028. [Google Scholar] [CrossRef] [PubMed]
- Manabe, R.; Tsutsui, K.; Yamada, T.; Kimura, M.; Nakano, I.; Shimono, C.; Sanzen, N.; Furutani, Y.; Fukuda, T.; Oguri, Y.; et al. Transcriptome-Based Systematic Identification of Extracellular Matrix Proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 12849–12854. [Google Scholar] [CrossRef] [PubMed]
- Mavillard, F.; Servian-Morilla, E.; Dofash, L.; Rojas-Marcos, I.; Folland, C.; Monahan, G.; Gutierrez-Gutierrez, G.; Rivas, E.; Hernández-Lain, A.; Valladares, A.; et al. Ablation of the Carboxy-Terminal End of MAMDC2 Causes a Distinct Muscular Dystrophy. Brain 2023, 146, 5235–5248. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wang, X.; Ma, Y.; Huang, L.; Wang, Y. MAMDC2, a Gene Highly Expressed in Microglia in Experimental Models of Alzheimers Disease, Positively Regulates the Innate Antiviral Response during Neurotropic Virus Infection. J. Infect. 2022, 84, 187–204. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.-C.; Soon Kang, J.; Cheon, Y.; Lee, S.; Jae Maeng, P. MAM Domain Containing 2 Is a Potential Breast Cancer Biomarker That Exhibits Tumour-Suppressive Activity. Cell Prolif. 2020, 53, e12883. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2022, 51, D587–D592. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for Analysis of Tumor-Infiltrating Immune Cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Lenos, K.J.; Bach, S.; Ferreira Moreno, L.; Ten Hoorn, S.; Sluiter, N.R.; Bootsma, S.; Vieira Braga, F.A.; Nijman, L.E.; van den Bosch, T.; Miedema, D.M.; et al. Molecular Characterization of Colorectal Cancer Related Peritoneal Metastatic Disease. Nat. Commun. 2022, 13, 4443. [Google Scholar] [CrossRef]
- Chen, K.; Liao, S.; Li, Y.; Jiang, H.; Liu, Y.; Wang, C.; Kuek, V.; Kenny, J.; Li, B.; Huang, Q.; et al. Osteoblast-Derived EGFL6 Couples Angiogenesis to Osteogenesis during Bone Repair. Theranostics 2021, 11, 9738–9751. [Google Scholar] [CrossRef] [PubMed]
- OuYang, H.-Y.; Xu, J.; Luo, J.; Zou, R.-H.; Chen, K.; Le, Y.; Zhang, Y.-F.; Wei, W.; Guo, R.-P.; Shi, M. MEP1A Contributes to Tumor Progression and Predicts Poor Clinical Outcome in Human Hepatocellular Carcinoma. Hepatology 2016, 63, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Huguenin, M.; Müller, E.J.; Trachsel-Rösmann, S.; Oneda, B.; Ambort, D.; Sterchi, E.E.; Lottaz, D. The Metalloprotease Meprinbeta Processes E-Cadherin and Weakens Intercellular Adhesion. PLoS ONE 2008, 3, e2153. [Google Scholar] [CrossRef] [PubMed]
- Arolas, J.L.; Broder, C.; Jefferson, T.; Guevara, T.; Sterchi, E.E.; Bode, W.; Stöcker, W.; Becker-Pauly, C.; Gomis-Rüth, F.X. Structural Basis for the Sheddase Function of Human Meprin β Metalloproteinase at the Plasma Membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 16131–16136. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, B.; Hahn, D.; Pischitzis, A.; Hansen, M.K.; Sterchi, E.E. Human Meprin Beta: O-Linked Glycans in the Intervening Region of the Type I Membrane Protein Protect the C-Terminal Region from Proteolytic Cleavage and Diminish Its Secretion. Biochem. J. 2003, 369, 659–665. [Google Scholar] [CrossRef]
- Dunne, P.D.; McArt, D.G.; Bradley, C.A.; O’Reilly, P.G.; Barrett, H.L.; Cummins, R.; O’Grady, T.; Arthur, K.; Loughrey, M.B.; Allen, W.L.; et al. Challenging the Cancer Molecular Stratification Dogma: Intratumoral Heterogeneity Undermines Consensus Molecular Subtypes and Potential Diagnostic Value in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4095–4104. [Google Scholar] [CrossRef]
- Sirinukunwattana, K.; Domingo, E.; Richman, S.D.; Redmond, K.L.; Blake, A.; Verrill, C.; Leedham, S.J.; Chatzipli, A.; Hardy, C.; Whalley, C.M.; et al. Image-Based Consensus Molecular Subtype (imCMS) Classification of Colorectal Cancer Using Deep Learning. Gut 2021, 70, 544–554. [Google Scholar] [CrossRef]
- Luo, Q.; Quan, Y.; Liu, W.; Wu, Z.; Qiu, W.; Liang, W.; Yang, P.; Huang, Q.; Li, G.; Wei, J.; et al. Seed and Soil: Consensus Molecular Subgroups (CMS) and Tumor Microenvironment Features Between Primary Lesions and Metastases of Different Organ Sites in Colorectal Cancer. Cancer Manag. Res. 2024, 16, 225–243. [Google Scholar] [CrossRef]
- Zhao, J.; Ou, B.; Han, D.; Wang, P.; Zong, Y.; Zhu, C.; Liu, D.; Zheng, M.; Sun, J.; Feng, H.; et al. Tumor-Derived CXCL5 Promotes Human Colorectal Cancer Metastasis through Activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-Catenin Pathways. Mol. Cancer 2017, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Zheng, W.; Bai, B.; Hu, J.; Lv, Y.; Chen, K.; Wang, X.; Pan, Y.; Huang, X.; Zhu, H.; et al. BMAL1 Promotes Colorectal Cancer Cell Migration and Invasion through ERK- and JNK-dependent c-Myc Expression. Cancer Med. 2022, 12, 4472–4485. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.G.; Lemmer, J. Biomechanical Cell Regulatory Networks as Complex Adaptive Systems in Relation to Cancer. Cancer Cell Int. 2017, 17, 16. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in Cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Girigoswami, K.; Saini, D.; Girigoswami, A. Extracellular Matrix Remodeling and Development of Cancer. Stem Cell Rev. Rep. 2021, 17, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, S.; Liu, X.; Guo, F.; Zhai, J.; Li, Z.; Deng, L.; Ge, L.; Qian, H.; Yang, L.; et al. Extracellular Matrix-Derived Mechanical Force Governs Breast Cancer Cell Stemness and Quiescence Transition through Integrin-DDR Signaling. Signal Transduct. Target. Ther. 2023, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lou, C.; Ma, J.; Gong, Q.; Tian, Z.; You, Y.; Ren, G.; Guo, W.; Wang, Y.; He, K.; et al. Single-Cell Transcriptome Analysis Reveals Changes of Tumor Immune Microenvironment in Oral Squamous Cell Carcinoma After Chemotherapy. Front. Cell Dev. Biol. 2022, 10, 914120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Rodansky, E.S.; Sauder, K.L.; Horowitz, J.C.; Mih, J.D.; Tschumperlin, D.J.; Higgins, P.D. Matrix Stiffness Corresponding to Strictured Bowel Induces a Fibrogenic Response in Human Colonic Fibroblasts. Inflamm. Bowel Dis. 2013, 19, 891–903. [Google Scholar] [CrossRef]
- Tito, A.; Niespolo, C.; Monti, M.C.; Colucci, M.G.; Fogliano, V. Oenothera Biennis Cell Culture Produce Lignans Activating Piezo1 Triggering the Myosin Light Chain Kinase Depending Pathways. Biochem. Biophys. Res. Commun. 2023, 681, 36–40. [Google Scholar] [CrossRef]
- Sundararajan, V.; Gengenbacher, N.; Stemmler, M.P.; Kleemann, J.A.; Brabletz, T.; Brabletz, S. The ZEB1/miR-200c Feedback Loop Regulates Invasion via Actin Interacting Proteins MYLK and TKS5. Oncotarget 2015, 6, 27083–27096. [Google Scholar] [CrossRef]
- Shi, X.; Yu, X.; Wang, J.; Bian, S.; Li, Q.; Fu, F.; Zou, X.; Zhang, L.; Bast, R.C.; Lu, Z.; et al. SIK2 Promotes Ovarian Cancer Cell Motility and Metastasis by Phosphorylating MYLK. Mol. Oncol. 2022, 16, 2558–2574. [Google Scholar] [CrossRef]
- Wang, R.; Chen, X.; Huang, C.; Yang, X.; He, H.; OuYang, C.; Li, H.; Guo, J.; Yang, C.; Lin, Z. Identification of Key Genes with Prognostic Value in Gastric Cancer by Bioinformatics Analysis. Front. Genet. 2022, 13, 958213. [Google Scholar] [CrossRef]
- Stadler, S.; Nguyen, C.H.; Schachner, H.; Milovanovic, D.; Holzner, S.; Brenner, S.; Eichsteininger, J.; Stadler, M.; Senfter, D.; Krenn, L.; et al. Colon Cancer Cell-Derived 12(S)-HETE Induces the Retraction of Cancer-Associated Fibroblast via MLC2, RHO/ROCK and Ca2+ Signalling. Cell Mol. Life Sci. 2017, 74, 1907–1921. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Qian, S.; Wei, J.; He, J.; Li, M.; Gao, X.; Cai, H.; Wang, Y.; Han, Y.; Tan, T.; et al. The Expression and Molecular Roles of MAMDC2 in MSS Colorectal Cancer with a High Tumor Stromal Ratio. Biomedicines 2025, 13, 1217. https://doi.org/10.3390/biomedicines13051217
Liu Y, Qian S, Wei J, He J, Li M, Gao X, Cai H, Wang Y, Han Y, Tan T, et al. The Expression and Molecular Roles of MAMDC2 in MSS Colorectal Cancer with a High Tumor Stromal Ratio. Biomedicines. 2025; 13(5):1217. https://doi.org/10.3390/biomedicines13051217
Chicago/Turabian StyleLiu, Yiling, Shengnan Qian, Jia Wei, Jianting He, Minghui Li, Xiaobing Gao, Hong Cai, Yiqing Wang, Yue Han, Tianyuan Tan, and et al. 2025. "The Expression and Molecular Roles of MAMDC2 in MSS Colorectal Cancer with a High Tumor Stromal Ratio" Biomedicines 13, no. 5: 1217. https://doi.org/10.3390/biomedicines13051217
APA StyleLiu, Y., Qian, S., Wei, J., He, J., Li, M., Gao, X., Cai, H., Wang, Y., Han, Y., Tan, T., & Yang, M. (2025). The Expression and Molecular Roles of MAMDC2 in MSS Colorectal Cancer with a High Tumor Stromal Ratio. Biomedicines, 13(5), 1217. https://doi.org/10.3390/biomedicines13051217





