Effects of Collagenase Preconditioning on Partially Incised Rat Tendon Treated with Light-Emitting Diodes and Platelet-Rich Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedure and Interventions
2.3. Observational, Blood Biochemistry and Hematology
2.4. Histological Assessment
2.5. Imaging and Scoring
2.6. Statistical Analysis
3. Results
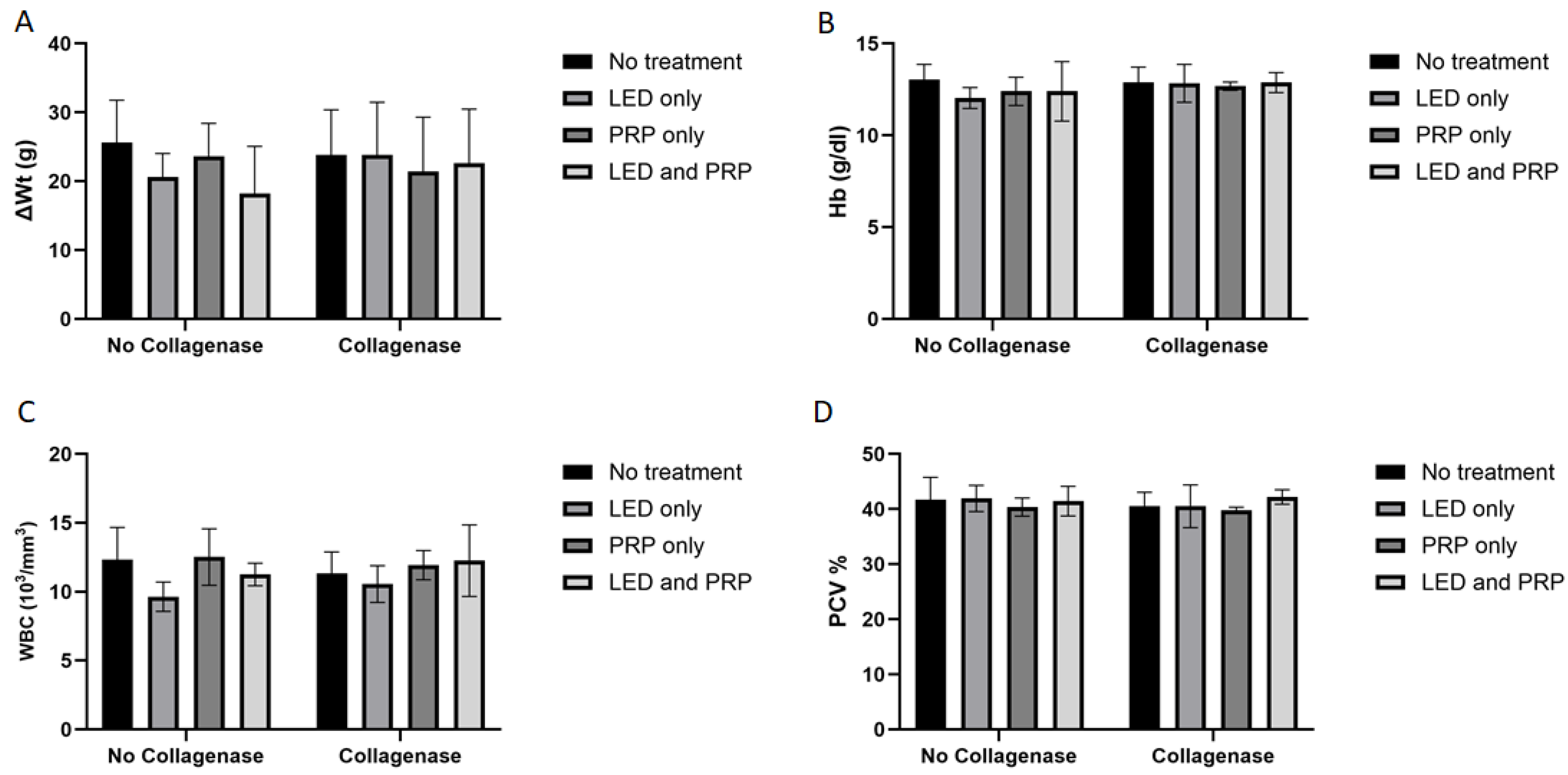
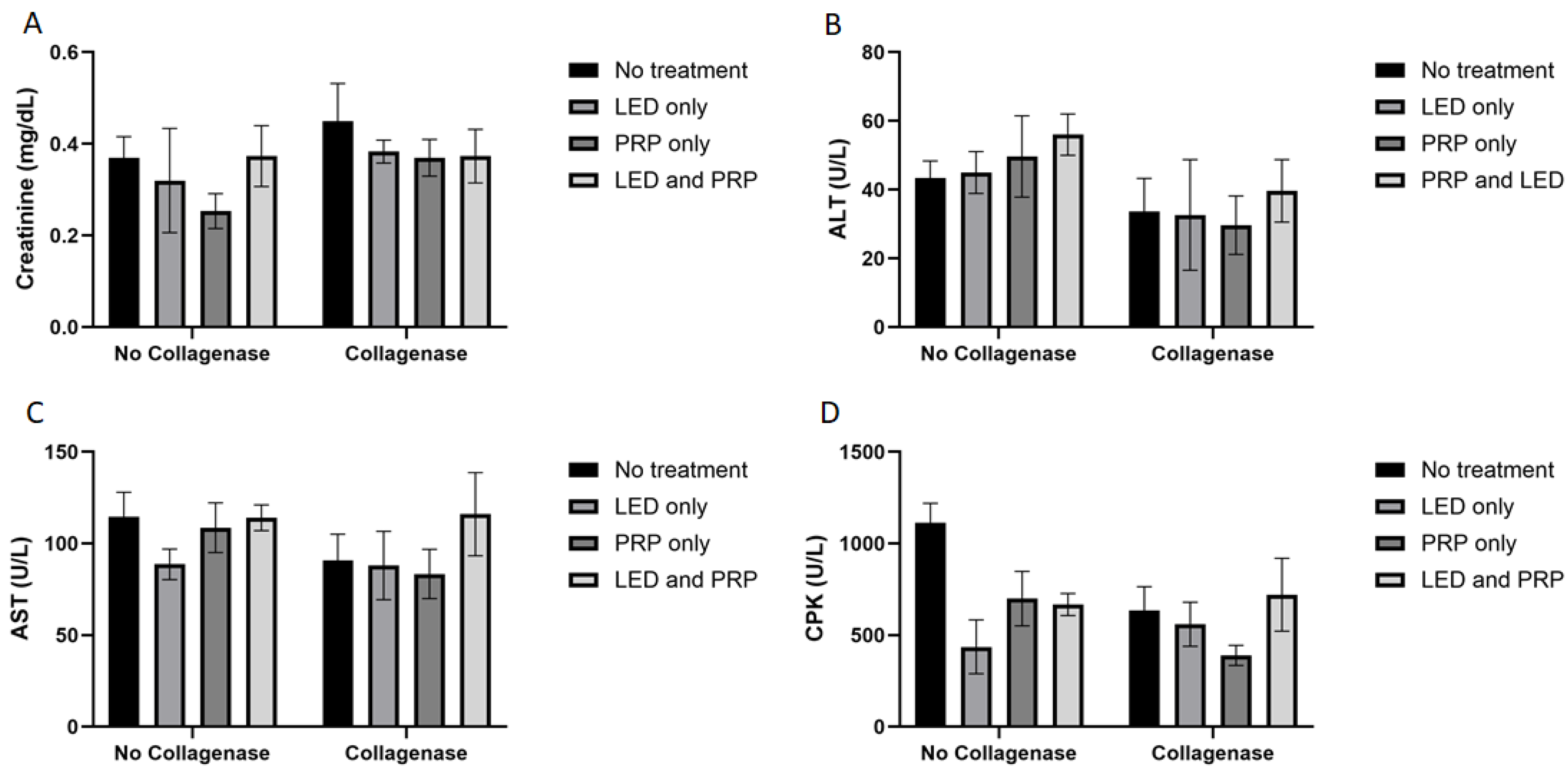
3.1. Observational Changes, Blood Biochemistry, and Hematology Analysis
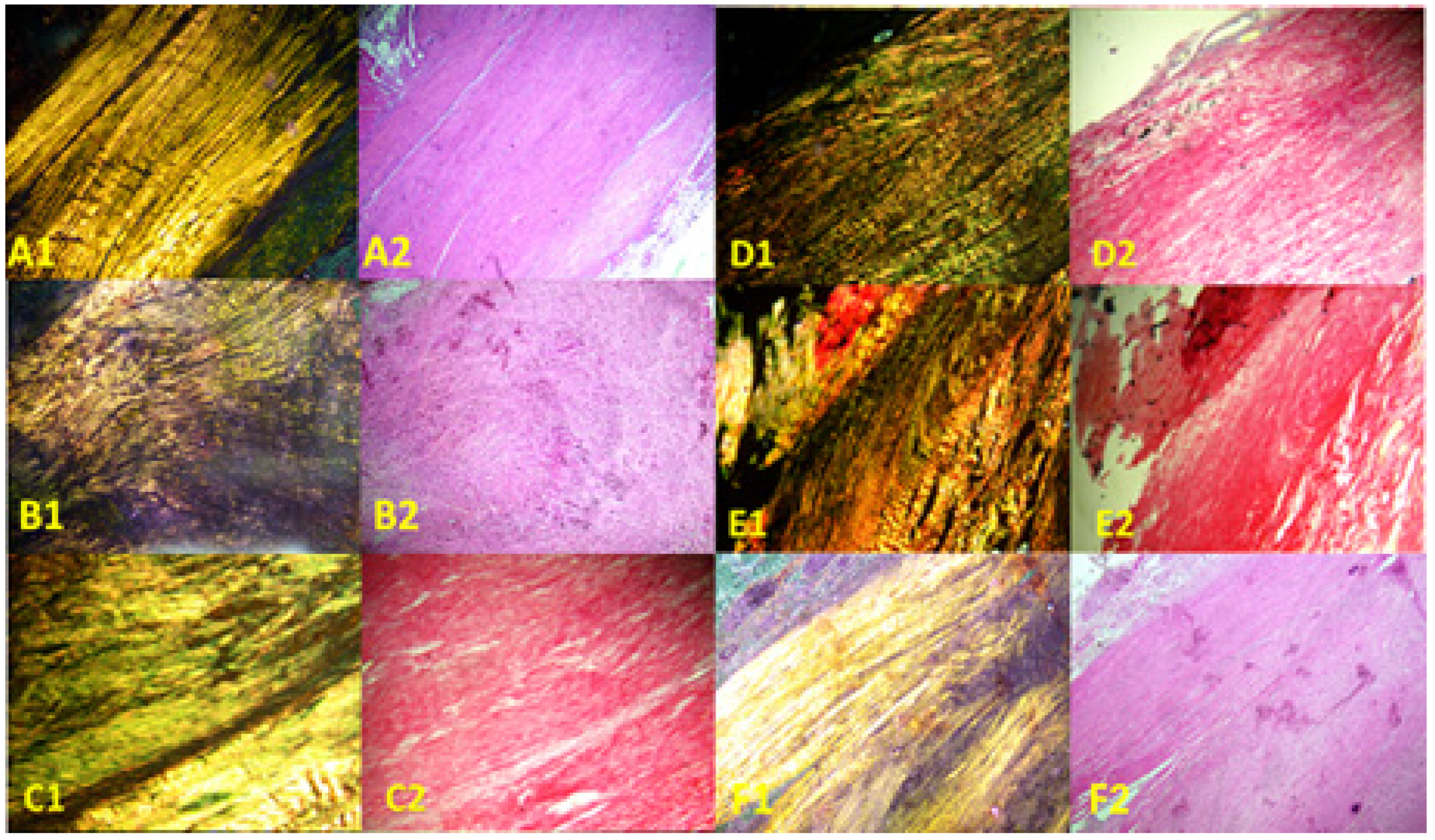
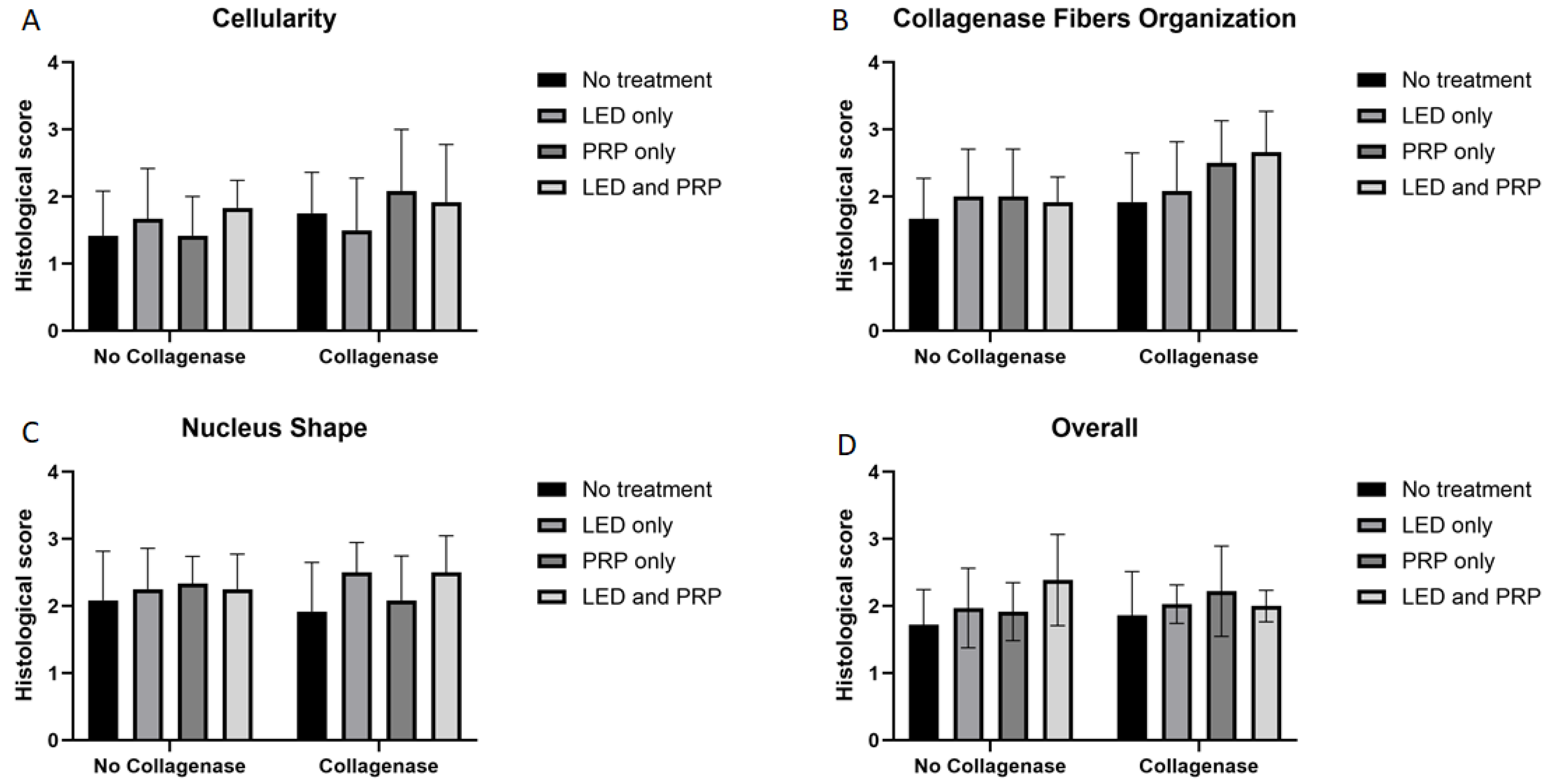
3.2. Histological Findings
3.3. Effect Size η2 and R2 Interpretations
4. Discussion
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Primers 2021, 7, 1. [Google Scholar] [CrossRef]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon basic science: Development, repair, regeneration, and healing. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.P.; Gumucio, J.P.; Awan, T.M.; Mendias, C.L.; Sugg, K.B. Pathogenesis and Management of Tendinopathies in Sports Medicine. Transl. Sports Med. 2018, 1, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.T.; Malmgaard-Clausen, N.M.; Puggaard, R.S.; Svensson, R.B.; Nybing, J.D.; Hansen, P.; Schjerling, P.; Zinglersen, A.H.; Couppé, C.; Boesen, M.; et al. Early development of tendinopathy in humans: Sequence of pathological changes in structure and tissue turnover signaling. FASEB J. 2020, 34, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.B.; Pizzari, T.; Kinsella, R.; Hope, D.; Cook, J.L. Current trends in tendinopathy management. Best Pract. Res. Clin. Rheumatol. 2019, 33, 122–140. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Hanlon, S.; Sprague, A. Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. J. Athl. Train. 2020, 55, 438–447. [Google Scholar] [CrossRef]
- Selestin Raja, I.; Kim, C.; Oh, N.; Park, J.H.; Hong, S.W.; Kang, M.S.; Mao, C.; Han, D.W. Tailoring photobiomodulation to enhance tissue regeneration. Biomaterials 2024, 309, 122623. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Lopes Silva, R.S.D.; Pessoa, D.R.; Mariano, R.R.; Castro, A.B.S.; de Oliveira, R.A.; Ferraresi, C. Systematic Review of Photobiomodulation Therapy (PBMT) on the Experimental Calcaneal Tendon Injury in Rats. Photochem. Photobiol. 2020, 96, 981–997. [Google Scholar] [CrossRef]
- Alzyoud, J.A.M.; Al Najjar, S.A.; Talat, S.; Abu-Irmaileh, B.; Bustanji, Y.; Al-Shudiefat, A.A.S. Effect of light-emitting diodes, platelet-rich plasma, and their combination on the activity of sheep tenocytes. Lasers Med. Sci. 2019, 34, 759–766. [Google Scholar] [CrossRef]
- Xavier, M.; de Souza, R.A.; Pires, V.A.; Santos, A.P.; Aimbire, F.; Silva, J.A., Jr.; Albertini, R.; Villaverde, A.B. Low-level light-emitting diode therapy increases mRNA expressions of IL-10 and type I and III collagens on Achilles tendinitis in rats. Lasers Med. Sci. 2014, 29, 85–90. [Google Scholar] [CrossRef]
- Bastos, J.L.N.; Lizarelli, R.F.Z.; Parizotto, N.A. Comparative study of laser and LED systems of low intensity applied to tendon healing. Laser Phys. 2009, 19, 1925–1931. [Google Scholar] [CrossRef]
- Moura Júnior Mde, J.; Maia Filho, A.L.; Pessoa, D.R.; Alves, M.D.; Justino Jde, S.; Andrade Mdos, S.; Rebêlo, A.M.; de Lima, C.J.; Pinheiro, A.L.; Silveira, L., Jr. Assessing the biochemical changes of tendons of rats in an experimental model of tenotomy under therapeutic ultrasound and LEDs (625 and 945 nm) by near-infrared Raman spectroscopy. Lasers Med. Sci. 2015, 30, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 2008, 33, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Madhi, M.I.; Yausep, O.E.; Khamdan, K.; Trigkilidas, D. The use of PRP in treatment of Achilles Tendinopathy: A systematic review of literature. Study design: Systematic review of literature. Ann. Med. Surg. 2020, 55, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, L.; Altamura, S.A.; Reale, D.; Candrian, C.; Zaffagnini, S.; Filardo, G. Nonsurgical Treatments of Patellar Tendinopathy: Multiple Injections of Platelet-Rich Plasma Are a Suitable Option: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2019, 47, 1001–1018. [Google Scholar] [CrossRef]
- Kearney, R.S.; Ji, C.; Warwick, J.; Parsons, N.; Brown, J.; Harrison, P.; Young, J.; Costa, M.L. ATM Trial Collaborators. Effect of Platelet-Rich Plasma Injection vs Sham Injection on Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy: A Randomized Clinical Trial. JAMA 2021, 326, 137–144. [Google Scholar] [CrossRef]
- Chen, P.C.; Wu, K.T.; Chou, W.Y.; Huang, Y.C.; Wang, L.Y.; Yang, T.H.; Siu, K.K.; Tu, Y.K. Comparative Effectiveness of Different Nonsurgical Treatments for Patellar Tendinopathy: A Systematic Review and Network Meta-analysis. Arthroscopy 2019, 35, 3117–3131.e2. [Google Scholar] [CrossRef]
- Morya, V.K.; Shahid, H.; Lang, J.; Kwak, M.K.; Park, S.-H.; Noh, K.-C. Advancements in Therapeutic Approaches for Degenerative Tendinopathy: Evaluating Efficacy and Challenges. Int. J. Mol. Sci. 2024, 25, 11846. [Google Scholar] [CrossRef]
- Mumford, Z.; Nigam, Y. Maggots in Medicine: A Narrative Review Discussing the Barriers to Maggot Debridement Therapy and Its Utilisation in the Treatment of Chronic Wounds. J. Clin. Med. 2024, 13, 6746. [Google Scholar] [CrossRef]
- Dahlgren, L.A.; van der Meulen, M.C.; Bertram, J.E.; Starrak, G.S.; Nixon, A.J. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J. Orthop. Res. 2002, 20, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.H.; Collier, Z.J.; Fang, M.; Howell, A.; Gillenwater, T.J. The Role of Collagenase Ointment in Acute Burns: A Systematic Review and Meta-Analysis. J. Wound Care 2019, 28 (Suppl. S2), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, P.; Heimbach, D.; Meites, H.; Pickus, E.J.; Erofeev, I.; Pons, P. The Use of Collagenase Ointment in the Treatment of Pressure Ulcers and Other Chronic Wounds. J. Am. Acad. Nurse Pract. 2005, 17, 222–228. [Google Scholar]
- Jordan, A.; Khiyani, N.; Bowers, S.R.; Lukaszczyk, J.J.; stanislaw, P. Maggot debridement therapy: A practical review. Int. J. Acad. Med. 2018, 4, 21–34. [Google Scholar] [CrossRef]
- Nezakati, E.; Hasani, M.H.; Zolfaghari, P.; Rashidan, M.; Sohrabi, M.B. Effects of Lucilia sericata Maggot Therapy in Chronic Wound Treatment: A Randomized Clinical Trial. Chronic Wound Care Manag. Res. 2020, 7, 11–17. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 2017, 24, 101. [Google Scholar] [CrossRef]
- Akamatsu, F.E.; Saleh, S.O.; Teodoro, W.R.; Silva, A.Q.; Martinez, C.A.; Duarte, R.J.; Andrade, M.F.; Jacomo, A.L. Experimental model of Achilles tendon injury in rats. Acta Cir. Bras. 2014, 29, 417–422. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.L.; Calderón-Díez, L.; Herrero-Turrión, J.; Méndez-Sánchez, R.; Arias-Buría, J.L.; Fernández-de-Las-Peñas, C. Changes in gene expression associated with collagen regeneration and remodeling of extracellular matrix after percutaneous electrolysis on collagenase-induced Achilles tendinopathy in an experimental animal model: A pilot study. J. Clin. Med. 2020, 9, 3316. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Cossermelli, W.; Brentani, R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch. Histol. Jpn. 1978, 41, 267–274. [Google Scholar] [CrossRef]
- Rich, L.; Whittaker, P. Collagen and picrosirius red staining: A polarized light assessement of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 2005, 22, 97–104. [Google Scholar]
- Wood, V.T.; Pinfildi, C.E.; Neves, M.A.; Parizoto, N.A.; Hochman, B.; Ferreira, L.M. Collagen changes and realignment induced by low-level laser therapy and low-intensity ultrasound in the calcaneal tendon. Lasers Surg. Med. 2010, 42, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Sharifi, D.; Takhtfooladi, M.A.; Hesaraki, S.; Khansari, M.; Dorbeh, S.S. Evaluation of low-level laser therapy, platelet-rich plasma, and their combination on the healing of Achilles tendon in rabbits. Lasers Med. Sci. 2015, 30, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar]
- Riley, G. Tendinopathy—From basic science to treatment. Nat. Rev. Rheumatol. 2008, 4, 82. [Google Scholar] [CrossRef]
- Everhart, J.S.; Cole, D.; Sojka, J.H.; Higgins, J.D.; Magnussen, R.A.; Schmitt, L.C.; Flanigan, D.C. Treatment Options for Patellar Tendinopathy: A Systematic Review. Arthroscopy 2017, 33, 861–872. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Chaudhry, S.; Lei, I.I.; Varone, A.; Riley, G.P.; Birch, H.L.; Clegg, P.D.; Screen, H.R. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand. J. Med. Sci. Sports 2015, 25, e381–e391. [Google Scholar] [CrossRef]
- Ochiai, Y.; Baba, A.; Hiramatsu, M.; Toyota, N.; Watanabe, T.; Yamashita, K.; Yokota, H.; Iwano, H. Blood biochemistry and hematological changes in rats after administration of a mixture of three anesthetic agents. J. Vet. Med. Sci. 2018, 80, 387–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Helrigle, C.; de Carvalho Pd Casalechi, H.L.; Leal-Junior, E.C.; Fernandes, G.H.; Helrigel, P.A.; Rabelo, R.L.; de Oliveira Aleixo-Junior, I.; Aimbire, F.; Albertini, R. Effects of low-intensity non-coherent light therapy on the inflammatory process in the calcaneal tendon of ovariectomized rats. Lasers Med. Sci. 2016, 31, 33–40. [Google Scholar] [CrossRef]
- Barbosa, D.; de Souza, R.A.; de Carvalho, W.R.; Xavier, M.; de Carvalho, P.K.; Cunha, T.C.; Arisawa, E.Â.; Silveira, L., Jr.; Villaverde, A.B. Low-level laser therapy combined with platelet-rich plasma on the healing calcaneal tendon: A histological study in a rat model. Lasers Med. Sci. 2013, 28, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Fernandes de Jesus, J.; Spadacci-Morena, D.D.; Rabelo, N.D.D.A.; Pinfildi, C.E.; Fukuda, T.Y.; Plapler, H. Photobiomodulation of Matrix Metalloproteinases in Rat Calcaneal Tendons. Photobiomodul. Photomed. Laser Surg. 2019, 37, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.L.; Arnold, G.; Magnenet, V.; Rahouadj, R.; Magdalou, J.; Lopes-Martins, R.Á. Biomechanical and biochemical protective effect of low-level laser therapy for Achilles tendinitis. J. Mech. Behav. Biomed. Mater. 2014, 29, 272–285. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, P.K.; Silveira LJr Barbosa, D.; Munin, E.; Salgado, M.A.; Villaverde, A.B. Analysis of experimental tendinitis in rats treated with laser and platelet-rich plasma therapies by Raman spectroscopy and histometry. Lasers Med. Sci. 2016, 31, 19–26. [Google Scholar] [CrossRef]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Itoi, E.; Minagawa, H.; Miyakoshi, N.; Takahashi, S.; Tuoheti, Y.; Okada, K.; Shimada, Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J. Shoulder Elbow Surg. 2006, 15, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.P.; Cheng, Y.Y.; Lee, H.L.; Hsu, T.Y.; Chang, Y.T.; Shen, Y.A. Enhanced Platelet-Rich Plasma (ePRP) Stimulates Wound Healing through Effects on Metabolic Reprogramming in Fibroblasts. Int. J. Mol. Sci. 2021, 22, 12623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, M.; David, D.R.; de Souza, R.A.; Arrieiro, A.N.; Miranda, H.; Santana, E.T.; Silva, J.A., Jr.; Salgado, M.A.C.; Aimbire, F.; Albertini, R. Anti-inflammatory effects of low-level light emitting diode therapy on achilles tendinitis in rats. Lasers Surg. Med. 2010, 42, 553–558. [Google Scholar] [CrossRef]
- Casalechi, H.L.; Nicolau, R.A.; Casalechi, V.L.; Silveira LJr De Paula, A.M.; Pacheco, M.T. The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med. Sci. 2009, 24, 659–665. [Google Scholar] [CrossRef] [PubMed]
| Group Name (Number) | Collagenase Application | Partial Tenotomy of AT | 4 J/cm2 LED | PRP Treatment |
|---|---|---|---|---|
| Normal Control (G1) | No | No | No | No |
| Model (G2 *) | No | Yes | No | No |
| Collagenase-conditioned (G3 *) | Yes | Yes | No | No |
| LED with a collagenase-conditioned (G4 **) | Yes | Yes | Yes | No |
| PRP with a collagenase-conditioned (G5 ***) | Yes | Yes | No | Yes |
| LED and PRP with a collagenase-conditioned (G6) | Yes | Yes | Yes | Yes |
| LED (G7 **) | No | Yes | Yes | No |
| PRP (G8 ***) | No | Yes | No | Yes |
| LED and PRP (G9) | No | Yes | Yes | Yes |
| Criterion | Four-Point Scoring | |||
|---|---|---|---|---|
| 4 | 3 | 2 | 1 | |
| Collagen fibers | Normal wavy | Slight change | Moderate change | Disorganized |
| Cellularity | Normal | Slight increase | Moderate increase | High increase |
| Nucleus shape | Flattened | Semi-flattened | Semi-rounded | Rounded |
| Tested Variables | Effect | Partial η² | Effect Size Category |
|---|---|---|---|
| Weight Change * | 0.089 | Small | Weak |
| Hemoglobin (HB) ** | 0.106 | Small | Weak |
| White Blood Cells (WBCs) ** | 0.236 | Small | Weak |
| Packed Cell Volume (PCV) ** | 0.077 | Small | Weak |
| Creatinine ** | 0.385 | Medium | Moderate |
| Alanine Transaminase (ALT) ** | 0.510 | Large | Moderate |
| Aspartate Transaminase (AST) ** | 0.431 | Large | Moderate |
| Creatine Phosphokinase (CPK) ** | 0.854 | Large | Strong |
| Collagen Organization (Rank) * | 0.150 | Small | Weak |
| Cellularity (Rank) * | 0.127 | Small | Weak |
| Nucleus Shape (Rank) * | 0.166 | Small | Weak |
| Overall Histology Score (Rank) * | 0.107 | Small | Weak |
| Tested Variables | Effect | Partial η² | Effect Size Category |
|---|---|---|---|
| Weight Change * | Phototherapy | 0.029 | Small |
| Collagenase | 0.014 | Small | |
| Interaction | 0.053 | Small | |
| Hemoglobin (HB) ** | Phototherapy | 0.038 | Small |
| Collagenase | 0.052 | Small | |
| Interaction | 0.029 | Small | |
| White Blood Cells (WBC) ** | Phototherapy | 0.198 | Large |
| Collagenase | 0.005 | Small | |
| Interaction | 0.053 | Small | |
| Packed Cell Volume (PCV) ** | Phototherapy | 0.053 | Small |
| Collagenase | 0.026 | Small | |
| Interaction | 0.004 | Small | |
| Creatinine ** | Phototherapy | 0.160 | Large |
| Collagenase | 0.243 | Large | |
| Interaction | 0.123 | Medium | |
| Alanine Transaminase (ALT) ** | Phototherapy | 0.275 | Large |
| Collagenase | 0.158 | Large | |
| Interaction | 0.184 | Large | |
| Aspartate Transaminase (AST) ** | Phototherapy | 0.135 | Medium |
| Collagenase | 0.378 | Large | |
| Interaction | 0.040 | Small | |
| Creatine Phosphokinase (CPK) ** | Phototherapy | 0.641 | Large |
| Collagenase | 0.304 | Large | |
| Interaction | 0.555 | Large | |
| Collagen Organization (Rank) * | Phototherapy | 0.014 | Small |
| Collagenase | 0.014 | Small | |
| Interaction | 0.129 | Medium | |
| Cellularity (Rank) * | Phototherapy | 0.048 | Small |
| Collagenase | 0.001 | Small | |
| Interaction | 0.065 | Medium | |
| Nucleus Shape (Rank) * | Phototherapy | 0.012 | Small |
| Collagenase | 0.012 | Small | |
| Interaction | 0.126 | Medium | |
| Overall Histology Score (Rank) * | Phototherapy | 0.012 | Small |
| Collagenase | 0.001 | Small | |
| Interaction | 0.086 | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzyoud, J.A.M.; Al-Shudiefat, A.A.-R.S.; Ali, H.A.; Omoush, S.A.; Shuqair, D.A.O. Effects of Collagenase Preconditioning on Partially Incised Rat Tendon Treated with Light-Emitting Diodes and Platelet-Rich Plasma. Biomedicines 2025, 13, 1214. https://doi.org/10.3390/biomedicines13051214
Alzyoud JAM, Al-Shudiefat AA-RS, Ali HA, Omoush SA, Shuqair DAO. Effects of Collagenase Preconditioning on Partially Incised Rat Tendon Treated with Light-Emitting Diodes and Platelet-Rich Plasma. Biomedicines. 2025; 13(5):1214. https://doi.org/10.3390/biomedicines13051214
Chicago/Turabian StyleAlzyoud, Jihad A. M., Abd Al-Rahman Salem Al-Shudiefat, Heba A. Ali, Samya A. Omoush, and Dalal A. O. Shuqair. 2025. "Effects of Collagenase Preconditioning on Partially Incised Rat Tendon Treated with Light-Emitting Diodes and Platelet-Rich Plasma" Biomedicines 13, no. 5: 1214. https://doi.org/10.3390/biomedicines13051214
APA StyleAlzyoud, J. A. M., Al-Shudiefat, A. A.-R. S., Ali, H. A., Omoush, S. A., & Shuqair, D. A. O. (2025). Effects of Collagenase Preconditioning on Partially Incised Rat Tendon Treated with Light-Emitting Diodes and Platelet-Rich Plasma. Biomedicines, 13(5), 1214. https://doi.org/10.3390/biomedicines13051214







