Effect of Enhanced Recovery After Surgery (ERAS) Implementation on Postoperative Atrial Fibrillation in Cardiac Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. ERAS Protocols
2.3. Data Collection
2.4. Objectives and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Primary Outcome
3.3. Preoperative, Intraoperative, and Postoperative Metrics
3.4. Complications After Surgery
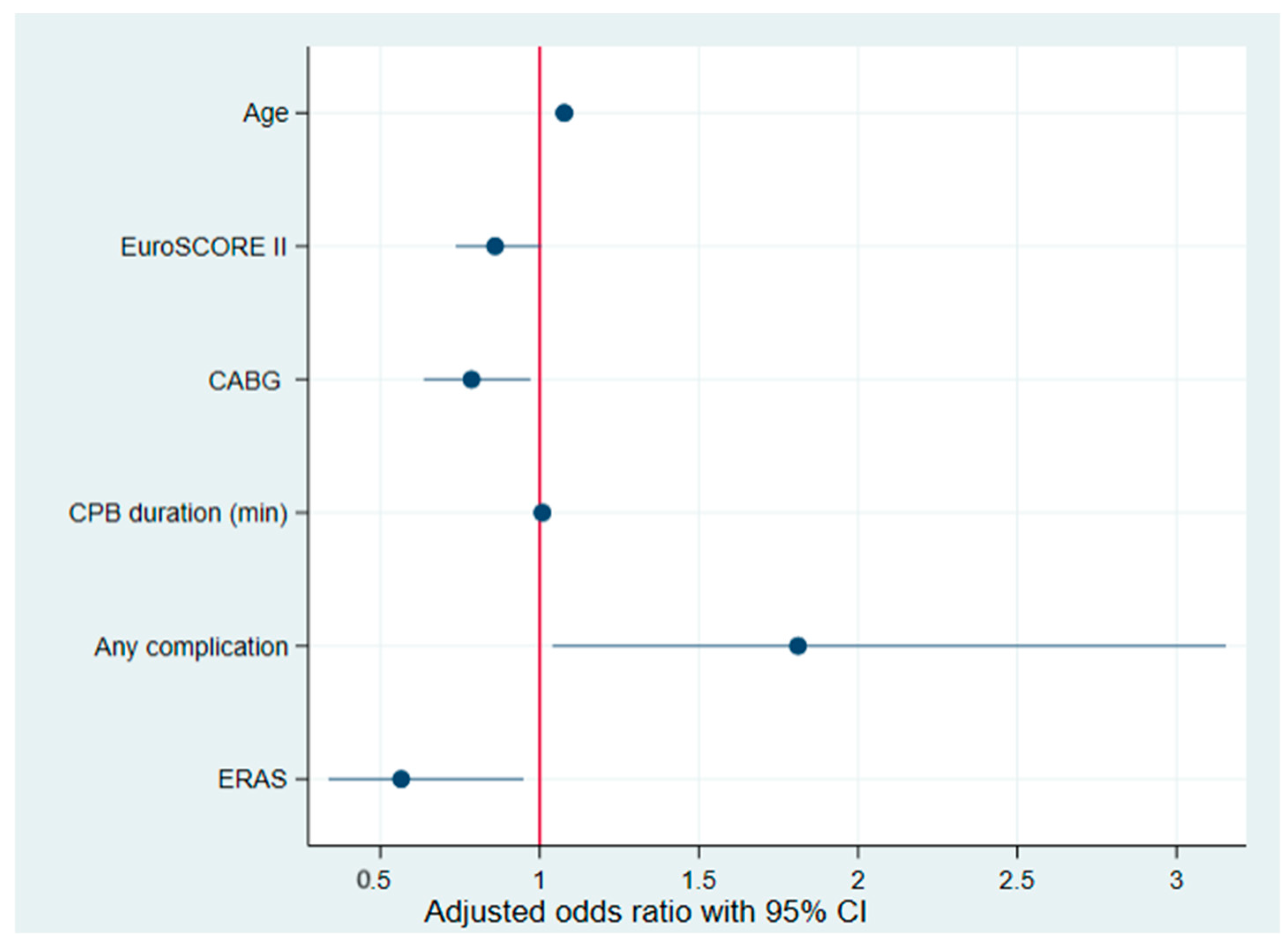
3.5. Predictors of POAF: Univariate and Multivariate Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AATS | American Association for Thoracic Surgery |
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| AF | Atrial fibrillation |
| AKI | Acute kidney injury |
| ARB | Angiotensin II Receptor Blocker |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CPB | Cardiopulmonary bypass |
| EPOα | Erythropoietin alpha |
| ERAS | Enhanced recovery after surgery |
| ESC | European Society of Cardiology |
| EuroSCORE | European System for Cardiac Operative Risk Evaluation |
| HAP | Hospital-acquired pneumonia |
| Hb | Hemoglobin |
| ICU | Intensive care unit |
| LVEF | Left ventricular ejection fraction |
| MME | Morphine milligram equivalent |
| NSAID | Nonsteroidal Anti-Inflammatory Drug |
| OR | Odds ratio |
| PBM | Patient blood management |
| POAF | Postoperative atrial fibrillation |
| POD | Postoperative day |
| PSM | Propensity score matching |
| REDCap | Research Electronic Data Capture |
| RCT | Randomized Clinical Trial |
| STROBE | STrengthening the Reporting of OBservational Studies in Epidemiology |
| ScvO2 | Central venous oxygen saturation |
| TS | transferrin saturation |
| VAS | Visual analog scale |
References
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative atrial fibrillation: From mechanisms to treatment. Eur. Heart J. 2023, 44, 1020–1039. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Yin, X.; Rienstra, M.; Schnabel, R.B.; Walkey, A.J.; Magnani, J.W.; Rahman, F.; McManus, D.D.; Tadros, T.M.; Levy, D.; et al. Long-Term Outcomes of Secondary Atrial Fibrillation in the Community: The Framingham Heart Study. Circulation 2015, 131, 1648–1655. [Google Scholar] [CrossRef]
- Carter-Storch, R.; Dahl, J.S.; Christensen, N.L.; Pecini, R.; Søndergård, E.V.; Øvrehus, K.A.; Møller, J.E. Postoperative atrial fibrillation after aortic valve replacement is a risk factor for long-term atrial fibrillation. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 378–385. [Google Scholar] [CrossRef]
- Frendl, G.; Sodickson, A.C.; Chung, M.K.; Waldo, A.L.; Gersh, B.J.; Tisdale, J.E.; Calkins, H.; Aranki, S.; Kaneko, T.; Cassivi, S.; et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J. Thorac. Cardiovasc. Surg. 2014, 148, e153–e193. [Google Scholar] [CrossRef]
- Gaudino, M.; Sanna, T.; Ballman, K.V.; Robinson, N.B.; Hameed, I.; Audisio, K.; Rahouma, M.; Di Franco, A.; Soletti, G.J.; Lau, C.; et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: An adaptive, single-centre, single-blind, randomised, controlled trial. Lancet 2021, 398, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Landoni, G.; Sparicio, D.; Benussi, S.; Aletti, G.; Pappalardo, F.; Fracasso, G.; Fano, G.; Crescenzi, G. Predictors of atrial fibrillation after off-pump coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2004, 18, 704–708. [Google Scholar] [CrossRef] [PubMed]
- van Boven, W.J.; de Groot, J.R.; Kluin, J. A short cut to prevent postoperative atrial fibrillation. Lancet 2021, 398, 2052–2053. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Cao, D.; Pivato, C.A.; Soletti, G.J.; Chadow, D.; Cancelli, G.; Olaria, R.P.; Gillinov, M.; et al. Pericardial Effusion Provoking Atrial Fibrillation After Cardiac Surgery. J. Am. Coll. Cardiol. 2022, 79, 2529–2539. [Google Scholar] [CrossRef]
- Woldendorp, K.; Farag, J.; Khadra, S.; Black, D.; Robinson, B.; Bannon, P. Postoperative Atrial Fibrillation After Cardiac Surgery: A Meta-Analysis. Ann. Thorac. Surg. 2021, 112, 2084–2093. [Google Scholar] [CrossRef]
- Caldonazo, T.; Kirov, H.; Rahouma, M.; Robinson, N.B.; Demetres, M.; Gaudino, M.; Doenst, T.; Dobrev, D.; Borger, M.A.; Kiehntopf, M.; et al. Atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2023, 165, 94–103.e24. [Google Scholar] [CrossRef]
- Lin, M.H.; Kamel, H.; Singer, D.E.; Wu, Y.L.; Lee, M.; Ovbiagele, B. Perioperative/Postoperative Atrial Fibrillation and Risk of Subsequent Stroke and/or Mortality: A Meta-Analysis. Stroke 2019, 50, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, R.; Sanjanwala, R.; Le, M.L.; Yamashita, M.H.; Arora, R.C. Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 544–554. [Google Scholar] [CrossRef]
- Bowdish, M.E.; Bagiella, E.; Giustino, G.; Atluri, P.; Alexander, J.H.; Thourani, V.H.; Gammie, J.S.; DeRose, J.J.; Taddei-Peters, W.C.; Jeffries, N.O.; et al. Prospective Study of Risk Factors for Postoperative Atrial Fibrillation After Cardiac Surgery. J. Surg. Res. 2024, 294, 262–268. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Lai, C.-H.; Loh, S.-H.; Lin, C.-Y.; Lin, Y.-C.; Lee, C.-Y.; Ke, H.-Y.; Tsai, C.-S. Assessment of the Risk Factors and Outcomes for Postoperative Atrial Fibrillation Patients Undergoing Isolated Coronary Artery Bypass Grafting. Acta Cardiol. Sin. 2015, 31, 436–443. [Google Scholar]
- Yamashita, K.; Hu, N.; Ranjan, R.; Selzman, C.H.; Dosdall, D.J. Clinical Risk Factors for Postoperative Atrial Fibrillation among Patients after Cardiac Surgery. Thorac. Cardiovasc. Surg. 2019, 67, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Schuessler, R.B.; Gaynor, S.L.; Yamada, K.; Fu, A.S.; Boineau, J.P.; Damiano, R.J. Inflammation of Atrium After Cardiac Surgery Is Associated With Inhomogeneity of Atrial Conduction and Atrial Fibrillation. Circulation 2005, 111, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Grieshaber, P. Retained blood syndrome after cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2025, 67 (Suppl. S1), i3–i8. [Google Scholar] [CrossRef] [PubMed]
- Kalman, J.M.; Munawar, M.; Howes, L.G.; Louis, W.J.; Buxton, B.F.; Gutteridge, G.; Tonkin, A.M. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann. Thorac. Surg. 1995, 60, 1709–1715. [Google Scholar] [CrossRef]
- Auer, J.; Weber, T.; Berent, R.; Puschmann, R.; Hartl, P.; Ng, C.-K.; Schwarz, C.; Lehner, E.; Strasser, U.; Lassnig, E.; et al. A comparison between oral antiarrhythmic drugs in the prevention of atrial fibrillation after cardiac surgery: The pilot study of prevention of postoperative atrial fibrillation (SPPAF), a randomized, placebo-controlled trial. Am. Heart J. 2004, 147, 636–643. [Google Scholar] [CrossRef]
- Ozaydin, M.; Icli, A.; Yucel, H.; Akcay, S.; Peker, O.; Erdogan, D.; Varol, E.; Dogan, A.; Okutan, H. Metoprolol vs. carvedilol or carvedilol plus N-acetyl cysteine on post-operative atrial fibrillation: A randomized, double-blind, placebo-controlled study. Eur. Heart J. 2013, 34, 597–604. [Google Scholar] [CrossRef]
- Gorenek, B. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Europace 2017, 19, 190–225. [Google Scholar] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [PubMed]
- Arsenault, K.A.; Yusuf, A.M.; Crystal, E.; Healey, J.S.; Morillo, C.A.; Nair, G.M.; Whitlock, R.P. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst. Rev. 2013, 2013, CD003611. [Google Scholar]
- Soletti, G.J.; Perezgrovas-Olaria, R.; Harik, L.; Rahouma, M.; Dimagli, A.; Alzghari, T.; Demetres, M.; Bratton, B.A.; Yaghmour, M.; Satija, D.; et al. Effect of posterior pericardiotomy in cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 1090102. [Google Scholar] [CrossRef] [PubMed]
- Levy, T.; Fotopoulos, G.; Walker, S.; Rex, S.; Octave, M.; Paul, V.; Amrani, M. Randomized controlled study investigating the effect of biatrial pacing in prevention of atrial fibrillation after coronary artery bypass grafting. Circulation 2000, 102, 1382–1387. [Google Scholar] [CrossRef]
- Gozdek, M.; Pawliszak, W.; Hagner, W.; Zalewski, P.; Kowalewski, J.; Paparella, D.; Carrel, T.; Anisimowicz, L.; Kowalewski, M. Systematic review and meta-analysis of randomized controlled trials assessing safety and efficacy of posterior pericardial drainage in patients undergoing heart surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 865–875.e12. [Google Scholar] [CrossRef]
- Ltaief, Z.; Verdugo-Marchese, M.; Carel, D.; Gunga, Z.; Nowacka, A.; Melly, V.; Addor, V.; Botteau, C.; Hennemann, M.; Lavanchy, L.; et al. Implementation of cardiac enhanced recovery after surgery at Lausanne University Hospital, our roadbook to certification. Interdiscip. Cardiovasc. Thorac. Surg. 2024, 39, ivae118. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ben Ali, W.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef]
- Othenin-Girard, A.; Ltaief, Z.; Verdugo-Marchese, M.; Lavanchy, L.; Vuadens, P.; Nowacka, A.; Gunga, Z.; Melly, V.; Abdurashidova, T.; Botteau, C.; et al. Enhanced Recovery After Surgery (ERAS) Protocols in Cardiac Surgery: Impact on Opioid Consumption. J. Clin. Med. 2025, 14, 1768. [Google Scholar] [CrossRef]
- Ballas, C.; Katsouras, C.S.; Tourmousoglou, C.; Siaravas, K.C.; Tzourtzos, I.; Alexiou, C. A Review on the Etiologies of the Development of Atrial Fibrillation After Cardiac Surgery. Biomolecules 2025, 15, 374. [Google Scholar] [CrossRef]
- Knez, N.; Kopjar, T.; Tokic, T.; Gasparovic, H. Atrial Fibrillation Prediction Model Following Aortic Valve Replacement Surgery. J. Cardiovasc. Dev. Dis. 2025, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Nirgude, A.; Gujjar, P.; Sharma, R. Incidence and risk factors for development of atrial fibrillation after cardiac surgery under cardiopulmonary bypass. Indian. J. Anaesth. 2018, 62, 887. [Google Scholar] [CrossRef] [PubMed]
- Mertes, P.-M.; Kindo, M.; Amour, J.; Baufreton, C.; Camilleri, L.; Caus, T.; Chatel, D.; Cholley, B.; Curtil, A.; Grimaud, J.-P.; et al. Guidelines on enhanced recovery after cardiac surgery under cardiopulmonary bypass or off-pump. Anaesth. Crit. Care Pain Med. 2022, 41, 101059. [Google Scholar] [CrossRef]
- St-Onge, S.; Ben Ali, W.; Bouhout, I.; Bouchard, D.; Lamarche, Y.; Perrault, L.P.; Demers, P. Examining the impact of active clearance of chest drainage catheters on postoperative atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2017, 154, 501–508. [Google Scholar] [CrossRef] [PubMed]
- von Ballmoos, M.C.W.; Hui, D.S.; Mehaffey, J.H.; Malaisrie, S.C.; Vardas, P.N.; Gillinov, A.M.; Sundt, T.M.; Badhwar, V. The Society of Thoracic Surgeons 2023 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann. Thorac. Surg. 2024, 118, 291–310. [Google Scholar] [CrossRef]
- Akintoye, E.; Sellke, F.; Marchioli, R.; Tavazzi, L.; Mozaffarian, D. Factors associated with postoperative atrial fibrillation and other adverse events after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2018, 155, 242–251.e10. [Google Scholar] [CrossRef]
- Bradley, D.; Creswell, L.L.; Hogue, C.W.; Epstein, A.E.; Prystowsky, E.N.; Daoud, E.G. Pharmacologic Prophylaxis. Chest 2005, 128, 39S–47S. [Google Scholar] [CrossRef]
- Fleming, I.O.; Garratt, C.; Guha, R.; Desai, J.; Chaubey, S.; Wang, Y.; Leonard, S.; Kunst, G. Aggregation of Marginal Gains in Cardiac Surgery: Feasibility of a Perioperative Care Bundle for Enhanced Recovery in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 665–670. [Google Scholar] [CrossRef]
- Diz-Ferreira, E.; Díaz-Vidal, P.; Fernández-Vázquez, U.; Gil-Casado, C.; Luna-Rojas, P.; Diz, J.C. Effect of Enhanced Recovery After Surgery (ERAS) Programs on Perioperative Outcomes in Patients Undergoing Cardiac Surgery: A Systematic Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2025, 39, 1325–1334. [Google Scholar] [CrossRef]
- Bruggmann, C.; Astaneh, M.; Lu, H.; Tozzi, P.; Ltaief, Z.; Voirol, P.; Sadeghipour, F. Management of Atrial Fibrillation Following Cardiac Surgery: Observational Study and Development of a Standardized Protocol. Ann. Pharmacother. 2021, 55, 830–838. [Google Scholar] [CrossRef]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, J.; Gan, T.J.; Qin, G.; Wang, L.; Zhu, M.; Zhang, Z.; Pan, Y.; Ye, Z.; Zhang, F.; et al. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: A randomized clinical trial. Eur. J. Cardio-Thorac. Surg. 2018, 54, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Weber, B.; Tung, R.; Choudhry, N.K.; Singh, J.P.; Upadhyay, G.A. Preventing Postoperative Atrial Fibrillation After Noncardiac Surgery: A Meta-analysis. Am. J. Med. 2018, 131, 795–804.e5. [Google Scholar] [CrossRef] [PubMed]
- Muehlschlegel, J.D.; Burrage, P.S.; Ngai, J.Y.; Prutkin, J.M.; Huang, C.-C.; Xu, X.; Chae, S.H.; Bollen, B.A.; Piccini, J.P.; Schwann, N.M.; et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists Practice Advisory for the Management of Perioperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. Anesth. Analg. 2019, 128, 33–42. [Google Scholar] [CrossRef]
- Zhao, B.-C.; Huang, T.-Y.; Deng, Q.-W.; Liu, W.-F.; Liu, J.; Deng, W.-T.; Liu, K.-X.; Li, C. Prophylaxis Against Atrial Fibrillation After General Thoracic Surgery. Chest 2017, 151, 149–159. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [Google Scholar]
- Li, L.; Ai, Q.; Lin, L.; Ge, P.; Yang, C.; Zhang, L. Efficacy and safety of landiolol for prevention of atrial fibrillation after cardiac surgery: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2015, 8, 10265–10273. [Google Scholar]
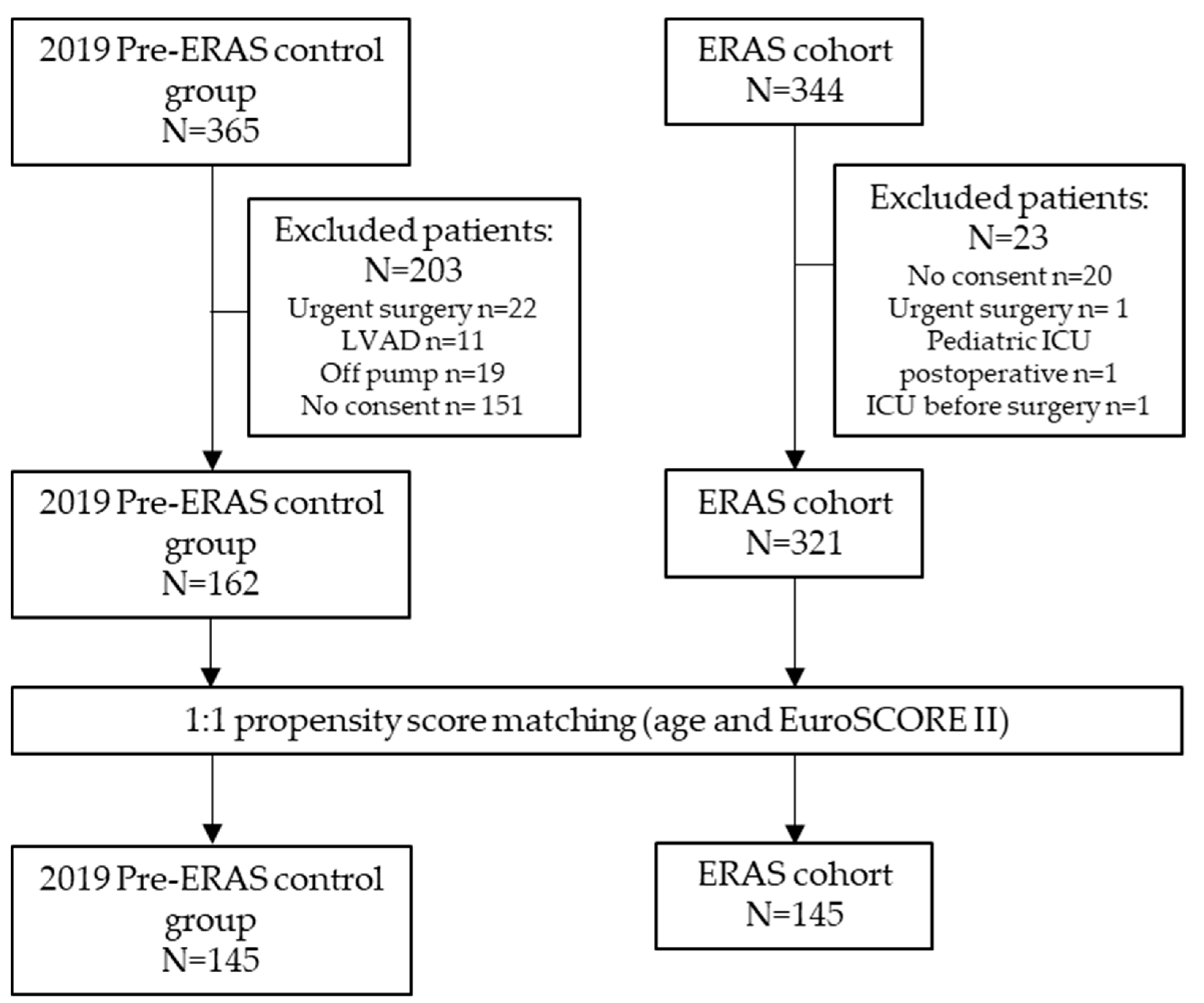
| Characteristics | All Patients | Matched Patients | ||||
|---|---|---|---|---|---|---|
| 2019 Pre-ERAS (n = 162) | ERAS (n = 321) | p-Value | 2019 Pre-ERAS (n = 145) | ERAS (n = 145) | p-Value | |
| Age (years) | 67.0 [59.0; 74.0] | 64.0 [56.0; 71.0] | 0.011 | 66.0 [58.0; 74.0] | 63.0 [54.0; 72.0] | 0.114 |
| Gender (female) | 50 (30.9%) | 78 (24.3%) | 0.151 | 44 (30.3%) | 36 (24.8%) | 0.358 |
| Previous cardiac surgery | 18 (11.1%) | 21 (9%) | 0.636 | 14 (9.66%) | 16 (14.2%) | 0.355 |
| LVEF (%) | 60 [55; 65] | 60 [55; 65] | 0.653 | 60 [55; 65] | 60 [55; 65] | 0.964 |
| Atrial fibrillation | 25 (15%) | 54 (16%) | 0.458 | 21 (14%) | 26 (17%) | 0.529 |
| Preoperative diabetes | 28 (17.3%) | 66 (20%) | 0.461 | 23 (15.9%) | 26 (17.9%) | 0.754 |
| Hypertension | 113 (69%) | 212 (66%) | 0.493 | 100 (68%) | 92 (63%) | 0.238 |
| Dyslipidemia | 78 (48.1%) | 192 (59%) | 0.019 | 68 (46.9%) | 81 (55.9%) | 0.159 |
| Smoker | 40 (24.7%) | 74 (23%) | 0.774 | 36 (24.8%) | 30 (20.7%) | 0.484 |
| COPD BMI | 25 (15.4%) 25.8.0 [22.2; 30.5] | 49 (15.2%) 26.7 [22.7; 29.7] | 0.210 0.009 | 21 (14.4%) 26.1 [23.3; 29.7] | 25 (17.2%) 26.3 [23.3; 30.5] | 0.813 0.153 |
| ASA physical status | 0.191 | 0.050 | ||||
| Class 2 | 14 (8.70%) | 37 (11.6%) | 14 (9.72%) | 12 (8.39%) | ||
| Class 3 | 121 (75.2%) | 214 (67.1%) | 111 (77.1%) | 96 (67.1%) | ||
| Class 4 | 26 (16.1%) | 68 (21.3%) | 19 (13.2%) | 35 (24.5%) | ||
| EuroSCORE II | 1.49 [0.94; 3.12] | 1.12 [0.75; 1.84] | 0.001 | 1.32 [0.91; 2.38] | 1.24 [0.73; 2.25] | 0.269 |
| Surgery type | ||||||
| Isolated CABG | 50 (30.9%) | 97 (30%) | 0.884 | 47 (32.4%) | 31 (21.4%) | 0.034 |
| Valvular surgery | 42 (25.9%) | 133 (41%) | 40 (27.6%) | 65 (44.8%) | ||
| Aortic root surgery | 22 (13.6%) | 38 (11%) | 20 (13.8%) | 20 (13.8%) | ||
| CABG + valvular surgery | 21 (13.0%) | 24 (7%) | 14 (9.66%) | 13 (8.97%) | ||
| Isolated ascending aorta | 12 (7.41%) | 15 (4%) | 10 (6.90%) | 10 (6.90%) | ||
| Other | 15 (9.26%) | 14 (4%) | 14 (9.66%) | 6 (4.14%) | ||
| All Patients | Matched Patients | |||||
|---|---|---|---|---|---|---|
| Pre-ERAS | ERAS | p-Value | Pre-ERAS | ERAS | p-Value | |
| All patients | n = 162 | n = 321 | n = 145 | n = 145 | ||
| POAF AATS 2014 | 64 (39%) | 76 (20%) | 0.001 | 57 (39%) | 34 (23%) | 0.004 |
| No AF history | n = 137 | n = 267 | n = 124 | n = 119 | ||
| POAF ESC 2024 | 51(37%) | 57 (21%) | 0.001 | 45 (36%) | 24(20%) | 0.005 |
| All Patients | Matched Patients | |||||
|---|---|---|---|---|---|---|
| 2019 Pre-ERAS (n = 162) | ERAS (n = 321) | p-Value | 2019 Pre-ERAS (n = 145) | ERAS (n = 145) | p-Value | |
| POAF prophylaxis (%) | 36 (22%) | 215 (66%) | 0.001 | 31(21%) | 101(70%) | 0.001 |
| β-blocker reinitiation (%) | 27 (16%) | 153 (47%) | 0.001 | 23 (15%) | 60 (41%) | 0.028 |
| CPB duration (min) | 94.0 [71.0; 125] | 72.0 [56.0; 91.0] | <0.001 | 90.0 [70.8; 124] | 77.0 [56.8; 94.8] | <0.001 |
| Aortic cross-clamp (min) | 71.0 [49.0; 96.8] | 53.0 [42.0; 70.0] | <0.001 | 68.0 [49.0; 95.0] | 57.0 [43.0; 73.0] | 0.001 |
| Extubation in OR (%) | 72 (44.4%) | 209 (65.3%) | <0.001 | 69 (47.6%) | 92 (63.9%) | 0.008 |
| Extubation time (hours) | 2.15 [0.00; 5.65] | 0.00 [0.00; 3.53] | <0.001 | 1.25 [0.00; 4.50] | 0.00 [0.00; 4.03] | 0.024 |
| Opioid use (MME) | ||||||
| POD 0 | 20.0 [9.25; 38.0] | 14.0 [7.50; 22.6] | <0.001 | 19.0 [8.00; 36.0] | 15.0 [7.50; 23.0] | 0.022 |
| POD 1 | 23.9 [14.9; 46.8] | 16.5 [10.6; 27.8] | <0.001 | 22.3 [13.9; 44.8] | 19.0 [10.8; 29.7] | 0.025 |
| POD 2 | 12.4 [5.00; 19.8] | 3.30 [0.00; 10.0] | <0.001 | 12.4 [5.00; 19.8] | 3.30 [0.00; 9.90] | <0.001 |
| POD 3 | 5.00 [0.00; 12.4] | 0.00 [0.00; 0.00] | <0.001 | 5.00 [0.00; 12.4] | 0.00 [0.00; 0.00] | <0.001 |
| Mobilization for first meal | 4 (2.55%) | 142 (44.4%) | <0.001 | 4 (2.86%) | 71 (49.0%) | <0.001 |
| Mobilization POD 1 (%) | 77 (59.2%) | 224 (83.9%) | <0.001 | 76 (63.9%) | 98 (81.7%) | 0.003 |
| Drainage duration (days) | 3.85 ± 0.40 | 2.41 ± 0.44 | 0.039 | 3.73 ± 0.43 | 2.79 ± 0.25 | 0.060 |
| CVC duration (days) | 5.39 ± 0.45 | 3.86 ± 0.46 | 0.041 | 5.43 ± 0.50 | 4.34 ± 0.28 | 0.061 |
| Bowel recovery (days) | 4.50 [4.00; 5.00] | 3.00 [3.00; 4.00] | <0.001 | 4.50 [4.00; 5.00] | 3.00 [3.00; 4.00] | <0.001 |
| ICU stay (days) | 1.08 [0.94; 2.11] | 1.11 [0.95; 1.99] | 0.626 | 1.08 [0.94; 2.02] | 1.10 [0.94; 1.92] | 0.681 |
| LOS (days) | 12.0 [9.00; 16.0] | 9.00 [7.00; 13.0] | <0.001 | 12.0 [8.00; 15.0] | 9.00 [8.00; 12.0] | <0.001 |
| Variables | All Patients | Matched Patients | ||||
|---|---|---|---|---|---|---|
| 2019 Pre-ERAS (n = 162) | ERAS (n = 321) | p-Value | 2019 Pre-ERAS (n = 145) | ERAS (n = 145) | p-Value | |
| Any complications | 70 (43%) | 78 (24%) | <0.001 | 56 (38%) | 39 (26%) | 0.033 |
| 30-day mortality | 3 (1.85%) | 1 (0.31%) | 0.112 | 1 (0.69%) | 1 (0.69%) | 1.000 |
| Reoperation (%) | 15 (9.26%) | 22 (6.94%) | 0.472 | 12 (8.28%) | 13 (9.09%) | 0.971 |
| ICU readmission (%) | 7 (4.32%) | 13 (4.10%) | 1.000 | 5 (3.45%) | 8 (5.59%) | 0.553 |
| Acute confusional state (%) | 13 (8.02%) | 15 (4.67%) | 0.200 | 11 (7.59%) | 6 (4.14%) | 0.317 |
| Stroke (%) | 4 (2.47%) | 14 (4.36%) | 0.434 | 3 (2.07%) | 7 (4.83%) | 0.334 |
| Postoperative MI (%) | 1 (0.62%) | 1 (0.31%) | 1.000 | 1 (0.69%) | 0 (0.00%) | 1.000 |
| HAP (%) | 21 (13.0%) | 26 (8.10%) | 0.124 | 17 (11.7%) | 8 (5.52%) | 0.094 |
| Other hospital-acquired infections (%) | 12 (7.41%) | 15 (4.67%) | 0.305 | 10 (6.90%) | 7 (4.83%) | 0.617 |
| Pleural effusion (%) | 28 (17.3%) | 18 (5.61%) | <0.001 | 23 (15.9%) | 10 (6.90%) | 0.026 |
| AKI requiring dialysis (%) | 2 (1.23%) | 6 (1.87%) | 0.724 | 2 (1.38%) | 4 (2.76%) | 0.684 |
| Outcome | No POAF (n = 341) | POAF (n = 142) | p-Value |
|---|---|---|---|
| ICU readmission | 10 (2.9%) | 10 (7.2%) | 0.035 |
| Reoperation | 20 (5.9%) | 17 (11.9%) | 0.020 |
| Pleural effusion | 26 (7.5%) | 20 (14.2%) | 0.023 |
| Pericardial effusion | 6 (1.7%) | 10 (7.1%) | 0.003 |
| Acute confusional state | 10 (2.9%) | 18 (12.8%) | 0.000 |
| Hospital stay | 9 [7–12.8] | 12 [9–18] | 0.034 |
| 30-day mortality | 3 (0.8%) | 1 (0.7%) | 0.860 |
| Variables | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.05 (1.03–1.07) | 0.001 | 1.078 (1.0489–1.1070) | 0.001 |
| Gender | 1.10 (0.70–1.71) | 0.666 | - | - |
| BMI | 1.02 (0.98–1.06) | 0.220 | - | - |
| Diabetes | 0.95 (0.76–1.19) | 0.703 | - | - |
| Hypertension | 0.98 (0.92–1.05) | 0.697 | - | - |
| COPD | 0.95 (0.85–1.07) | 0.482 | - | - |
| EuroSCORE II | 1.09 (1.00–1.20) | 0.041 | 0.860 (0.7365–1.0047) | 0.057 |
| LVEF | 0.99 (0.97–1.01) | 0.419 | - | - |
| Isolated CABG | 0.58 (0.37–0.92) | 0.022 | 0.786 (0.6361–0.9714) | 0.026 |
| CPB Duration (min) | 1.00 (1.00–1.00) | 0.026 | 1.008 (1.0021–1.0149) | 0.009 |
| Any Complication | 2.17 (1.43–3.28) | 0.001 | 1.812 (1.0402–3.1549) | 0.036 |
| ERAS | 0.47 (0.31–0.71) | 0.001 | 0.566 (0.3369–0.9493) | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niessen, R.; Rancati, V.; Verdugo-Marchese, M.; Gunga, Z.; Nowacka, A.; Melly, V.; Abellan, C.; Alouazen, K.; Abdurashidova, T.; Botteau, C.; et al. Effect of Enhanced Recovery After Surgery (ERAS) Implementation on Postoperative Atrial Fibrillation in Cardiac Surgery. Biomedicines 2025, 13, 1212. https://doi.org/10.3390/biomedicines13051212
Niessen R, Rancati V, Verdugo-Marchese M, Gunga Z, Nowacka A, Melly V, Abellan C, Alouazen K, Abdurashidova T, Botteau C, et al. Effect of Enhanced Recovery After Surgery (ERAS) Implementation on Postoperative Atrial Fibrillation in Cardiac Surgery. Biomedicines. 2025; 13(5):1212. https://doi.org/10.3390/biomedicines13051212
Chicago/Turabian StyleNiessen, Romain, Valentina Rancati, Mario Verdugo-Marchese, Ziyad Gunga, Anna Nowacka, Valentine Melly, Christophe Abellan, Karima Alouazen, Tamila Abdurashidova, Caroline Botteau, and et al. 2025. "Effect of Enhanced Recovery After Surgery (ERAS) Implementation on Postoperative Atrial Fibrillation in Cardiac Surgery" Biomedicines 13, no. 5: 1212. https://doi.org/10.3390/biomedicines13051212
APA StyleNiessen, R., Rancati, V., Verdugo-Marchese, M., Gunga, Z., Nowacka, A., Melly, V., Abellan, C., Alouazen, K., Abdurashidova, T., Botteau, C., Kirsch, M., & Ltaief, Z. (2025). Effect of Enhanced Recovery After Surgery (ERAS) Implementation on Postoperative Atrial Fibrillation in Cardiac Surgery. Biomedicines, 13(5), 1212. https://doi.org/10.3390/biomedicines13051212






