The Effect of Fungal Nutraceutical Supplementation on Postoperative Complications, Inflammatory Factors and Fecal Microbiota in Patients Undergoing Colorectal Cancer Surgery with Curative Intent: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Enrollment
2.2. Nutraceutical and Placebo Treatment
2.3. Intervention Monitoring
2.4. Assessment of Postoperative Complications
2.5. Microbiota Characterization
2.6. Sample Size Calculation
2.7. Statistical Analysis
- Postoperative Complications: The complication rate was compared between the two groups, stratified by severity and type of complication, using chi-square tests. A subgroup analysis was performed based on the type of surgery (robotic vs. non-robotic).
- Adverse Events (AEs): AEs were documented and compared between groups using chi-square tests.
- Inflammatory Markers: Differences between groups were analyzed using t-tests (for normally distributed variables) or Wilcoxon tests (for non-normally distributed variables).
- Dietary Patterns and Quality of Life: Changes in qualitative variables were assessed using McNemar’s test, while quantitative data were analyzed with paired t-tests.
3. Results
3.1. Patient’s Baseline Characteristics
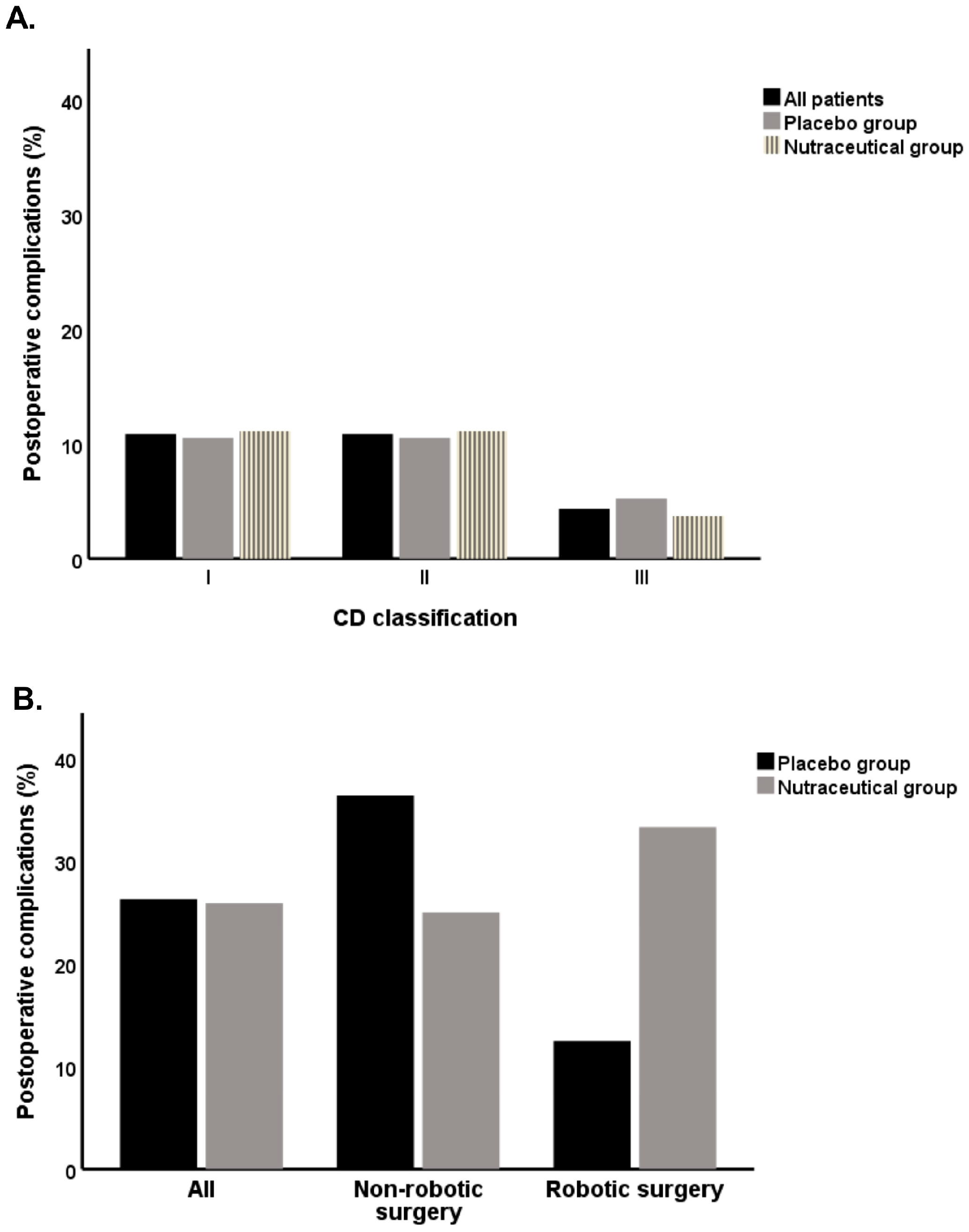
3.2. Association of Nutraceutical Supplementation with Postoperative Complications
3.3. Association of Nutraceutical Supplementation with Inflammatory Biomarkers, Nutritional Status, and Quality of Life
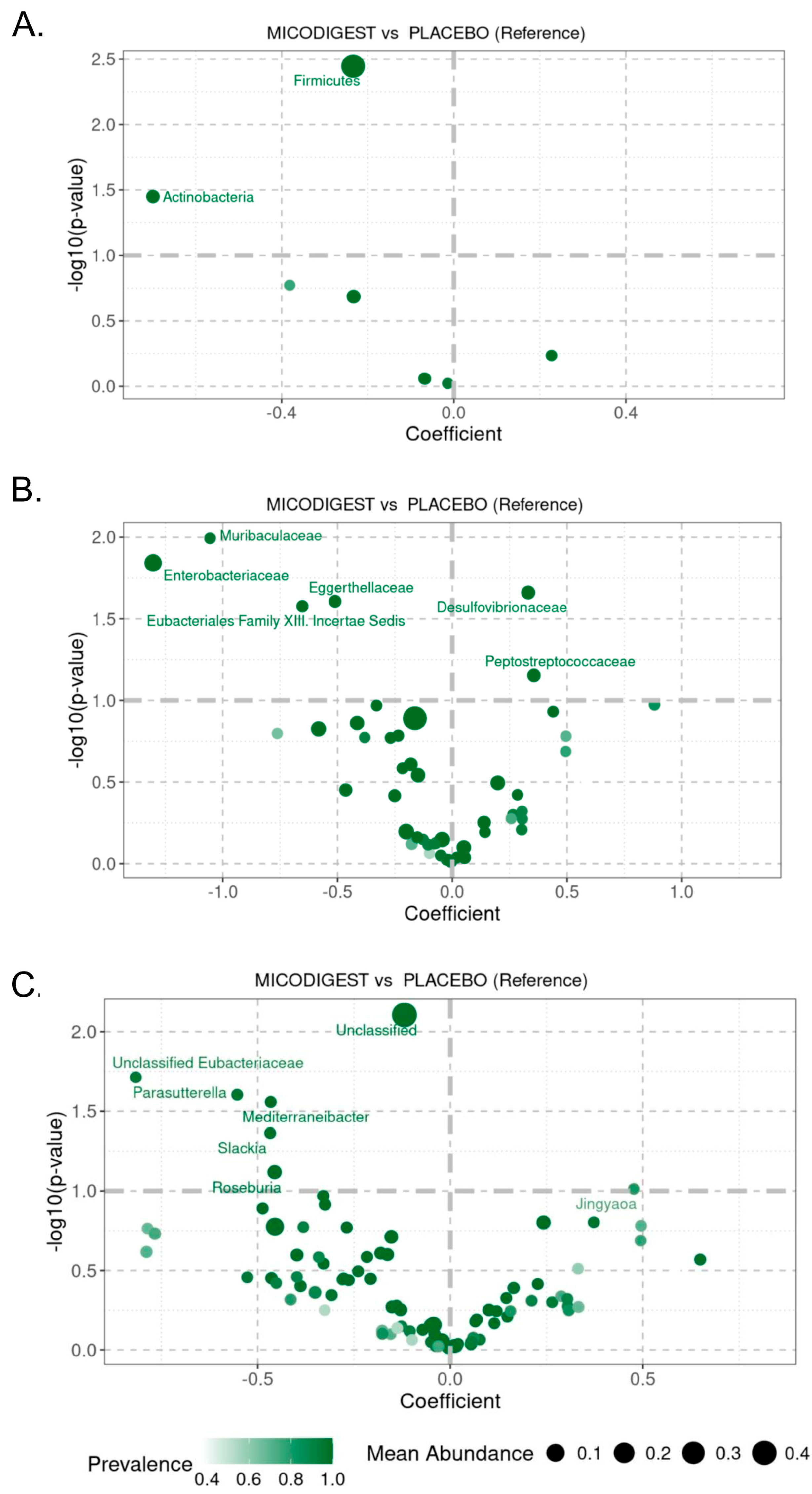
3.4. Effect of Nutraceutical Supplementation on Fecal Microbial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cancer Today. International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today/home (accessed on 23 May 2020).
- Gutierrez-Stampa, M.A.; Aguilar, V.; Sarasqueta, C.; Cubiella, J.; Portillo, I.; Bujanda, L. Impact of the faecal immunochemical test on colorectal cancer survival. BMC Cancer 2020, 20, 616. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.M.; Antón-Ladisla, A.; González, N.; Lázaro, S.; Baré, M.; Fernández de Larrea, N.; Redondo, M.; Briones, E.; Escobar, A.; Sarasqueta, C.; et al. Outcomes of open versus laparoscopic surgery in patients with colon cancer. Eur. J. Surg. Oncol. 2018, 44, 1344–1353. [Google Scholar] [CrossRef]
- Tevis, S.E.; Kennedy, G.D. Postoperative Complications: Looking Forward to a Safer Future. Clin. Colon Rectal Surg. 2016, 29, 246–252. [Google Scholar] [CrossRef]
- Cubiella, J.; González, A.; Almazán, R.; Rodríguez-Camacho, E.; Fontenla Rodiles, J.; Domínguez Ferreiro, C.; Tejido Sandoval, C.; Sánchez Gómez, C.; de Vicente Bielza, N.; Lorenzo, I.P.; et al. pT1 Colorectal Cancer Detected in a Colorectal Cancer Mass Screening Program: Treatment and Factors Associated with Residual and Extraluminal Disease. Cancers 2020, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yan, X.; Cao, Y.; Bao, T.; Li, G.; Gu, S.; Xiong, K.; Xiao, T. Meta-analysis of Glutamine on Immune Function and Post-Operative Complications of Patients with Colorectal Cancer. Front. Nutr. 2021, 8, 765809. [Google Scholar] [CrossRef]
- Bartolini, I.; Risaliti, M.; Ringressi, M.N.; Melli, F.; Nannini, G.; Amedei, A.; Muiesan, P.; Taddei, A. Role of gut microbiota-immunity axis in patients undergoing surgery for colorectal cancer: Focus on short and long-term outcomes. World J. Gastroenterol. 2020, 26, 2498–2513. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Martins dos Santos, V.A.; Ott, S.J.; Moya, A. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut Microbes 2014, 5, 64–70. [Google Scholar] [CrossRef]
- Stavrou, G.; Kotzampassi, K. Gut microbiome, surgical complications and probiotics. Ann. Gastroenterol. 2017, 30, 45–53. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Trone, K.; Rahman, S.; Green, C.H.; Venegas, C.; Martindale, R.; Stroud, A. Synbiotics and Surgery: Can Prebiotics and Probiotics Affect Inflammatory Surgical Outcomes? Curr. Nutr. Rep. 2023, 12, 238–246. [Google Scholar] [CrossRef]
- Adiamah, A.; Skořepa, P.; Weimann, A.; Lobo, D.N. The Impact of Preoperative Immune Modulating Nutrition on Outcomes in Patients Undergoing Surgery for Gastrointestinal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 270, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Adiamah, A.; Kushairi, A.; Varadhan, K.K.; Krznaric, Z.; Kulkarni, A.D.; Neal, K.R.; Lobo, D.N. Perioperative Probiotics or Synbiotics in Adults Undergoing Elective Abdominal Surgery: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2020, 271, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Gano derma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 23, 7489. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Kimatu, B.M.; Zhao, L.; Yang, W.; Pei, F.; Hu, Q. In vivo fermentation of a Pleutorus eryngii polysaccharid and its effects on fecal microbiota composition and immune response. Food Funct. 2017, 8, 1810–1821. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, C.; Yang, J.; Li, J.; Su, J.; Xie, Y.; Lai, G. Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota. Front. Immunol. 2017, 8, 666. [Google Scholar]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of Rich in B-Glucans Edible Mushrooms on Aging Gut Microbiota Chracteristics: An In Vitro Study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The Impact of Mushroom Polysaccharides on Gut Microbiota and Its Beneficial Effects to Host: A Review. Carbohydr. Polym. 2020, 15, 116942. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.P.; Yang, X.X.; Huang, M.; Gao, Y.; Tang, W.; Chan, S.Y.; Dai, X.; Ye, J.; Ho, P.C.; et al. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int. Immunopharmacol. 2006, 6, 499–508. [Google Scholar] [CrossRef]
- Simadibrata, M.; Rachman, A.; Budimutiar, F.; Simadibrata, P.; Abdullah, M.; Haloho, R.M.; Wijaya, A.E.; Bisuk, B.; Maharani, S.; Mustikarani, D.; et al. Combination Treatment in Ulcerative Colitis Using 5-Aminosalysilic Acid (5-ASA) and Polysaccharide Peptide of Indonesian Ganoderma lucidum Mycelium Extract. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 2023, 24, 2–11. [Google Scholar] [CrossRef]
- Jakopovich, I. New dietary supplements from medicinal mushrooms: Dr Myko San--a registration report. Int. J. Med. Mushrooms 2011, 13, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Regueiro, C.; Codesido, L.; García-Nimo, L.; Zarraquiños, S.; Remedios, D.; Rodríguez-Blanco, A.; Sinde, E.; Fernández-de-Ana, C.; Cubiella, J. Effect of the Nutraceutical Micodigest 2.0 on the Complication Rate of Colorectal Cancer Surgery with Curative Intent: Protocol for a Placebo-Controlled Double-blind Randomized Clinical Trial. JMIR Res. Protoc. 2022, 11, 34292. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Ræder, H.; Henriksen, C.; Bøhn, S.K.; O’de Fey Vilbo, A.R.; Henriksen, H.B.; Kværner, A.S.; Rolid, K.; Paur, I.; Smeland, S.; Blomhoff, R. Agreement between PG-SGA category and fat-free mass in colorectal cancer patients. Clin. Nutr. ESPEN 2018, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. The Spanish version of the Short Form 36 Health Survey: A decade of experience and new developments. Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef]
- National-Cancer-Institute. Common Terminology Criteria for Adverse Events (CTCAE), 5th ed.; National-Cancer-Institute: Rockville, MD, USA, 2020. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 17 November 2020).
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- La Reau, A.J.; Strom, N.B.; Filvaroff, E.; Mavrommatis, K.; Ward, T.L.; Knights, D. Shallow shotgun sequencing reduces technical variation in microbiome analysis. Sci. Rep. 2023, 13, 7668. [Google Scholar] [CrossRef]
- Puente-Sánchez, F.; García-García, N.; Tamames, J. SQMtools: Automated processing and visual analysis of ‘omics data with R and anvi’o. BMC Bioinform. 2020, 21, 358. [Google Scholar] [CrossRef]
- Zhou, H.; He, K.; Chen, J.; Zhang, X. LinDA: Linear models for differential abundance analysis of microbiome compositional data. Genome Biol. 2022, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Dan, A.; Swain, R.; Belonce, S.; Jacobs, R.J. Therapeutic Effects of Medicinal Mushrooms on Gastric, Breast, and Colorectal Cancer: A Scoping Review. Cureus 2023, 15, e37574. [Google Scholar] [CrossRef] [PubMed]
- Algehani, R.A.; Abou Khouzam, R.; Hegazy, G.A.; Alamoudi, A.A.; El-Halawany, A.M.; El Dine, R.S.; Ajabnoor, G.A.; Al-Abbasi, F.A.; Baghdadi, M.A.; Elsayed, I.; et al. Colossolactone-G synergizes the anticancer properties of 5-fluorouracil and gemcitabine against colorectal cancer cells. Biomed. Pharmacother. 2021, 140, 111730. [Google Scholar] [CrossRef] [PubMed]
- Macharia, J.M.; Zhang, L.; Mwangi, R.W.; Rozmann, N.; Kaposztas, Z.; Varjas, T.; Sugár, M.; Alfatafta, H.; Pintér, M.; Bence, R.L. Are chemical compounds in medical mushrooms potent against colorectal cancer carcinogenesis and antimicrobial growth? Cancer Cell Int. 2022, 22, 379. [Google Scholar] [CrossRef]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.; Chan, G.C. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst. Rev. 2016, 4, CD007731. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar]
- Lagunas-Rangel, F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020, 92, 1733–1734. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.X.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, I.; Serban, D.; Simion, L.; Motofei, I.; Cristea, B.M.; Dumitrescu, D.; Tudor, C.; Dascalu, A.M.; Serboiu, C.; Tribus, L.C.; et al. Clinical Significance of Blood Cell-Derived Inflammation Markers in Assessing Potential Early and Late Postoperative Complications in Patients with Colorectal Cancer: A Systematic Review. J. Clin. Med. 2025, 14, 2529. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.E.; Viana, P.; Persson, M.; Relvas, J.H.; Danielski, L.G. Perioperative or Postoperative Probiotics Reduce Treatment-Related Complications in Adult Colorectal Cancer Patients Undergoing Surgery: A Systematic Review and Meta-analysis. J. Gastrointest. Cancer 2024, 55, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; He, J.; Li, H.; You, J.; Qin, H. Alterations in intestinal microbiota of colorectal cancer patients receiving radical surgery combined with adjuvant CapeOx therapy. Sci. China Life Sci. 2019, 62, 1178–1193. [Google Scholar] [CrossRef]
- Mizuta, M.; Endo, I.; Yamamoto, S.; Inokawa, H.; Kubo, M.; Udaka, T.; Sogabe, O.; Maeda, H.; Shirakawa, K.; Okazaki, E.; et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci. Microbiota Food Health 2016, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, T.; Wang, Y.; Gao, Y.; Kong, Y.; Liu, Z.; Deng, X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J. Cancer 2019, 10, 568–576. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Ma, Y.; Liu, J.; Cui, Y.; Yuan, Y.; Xiang, C.; Ma, D.; Liu, H. The microbiome types of colorectal tissue are potentially associated with the prognosis of patients with colorectal cancer. Front. Microbiol. 2023, 14, 1100873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trinh, B.B.; Jackson, N.R.; Hauch, A.T.; Hu, T.; Kandil, E. Robotic versus laparoscopic colorectal surgery. JSLS J. Soc. Laparoendosc. Surg. 2014, 8, e2014-00187. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, S.; Huang, Y.; Luo, R.; Liang, W. Comparison of robotic-assisted versus conventional laparoscopic surgery in colorectal cancer resection: A systemic review and meta-analysis of randomized controlled trials. Front. Oncol. 2023, 13, 273378. [Google Scholar] [CrossRef] [PubMed]
- Vilsan, J.; Maddineni, S.A.; Ahsan, N.; Mathew, M.; Chilakuri, N.; Yadav, N.; Munoz, E.J.; Nadeem, M.A.; Abbas, K.; Razzaq, W.; et al. Open, Laparoscopic, and Robotic Approaches to Treat Colorectal Cancer: A Comprehensive Review of Literature. Cureus 2003, 15, e38956. [Google Scholar] [CrossRef]


| Placebo Group (n = 19) | Nutraceutical Group (n = 27) | p 1 | |
|---|---|---|---|
| Age, (years) | 68.0 (63.0–73.0) | 67 (62.3–72.8) | 0.4 |
| Gender, male/female | 11/8 | 12/15 | 0.4 |
| Tumor location, n (%) | 0.4 | ||
| 10 (52.6) | 15 (55.6) | |
| 5 (26.3) | 10 (37.0) | |
| 4 (21.1) | 2 (7.4) | |
| CRC diagnosis, n (%) | 0.6 | ||
| 10 (52.6) | 13 (48.1) | |
| 3 (15.8) | 3 (11.2) | |
| 6 (31.6) | 9 (33.4) | |
| 0 | 2 (7.4) | |
| Duration of symptoms (weeks) | 0 (0–15.0) | 0 (0–14.8) | 0.5 |
| BMI (Kg/m2) | 25.3 (23.6–30.1) | 26.0 (23.5–30.2) | 0.7 |
| Fat mass (%) | 30.8 (24.7–37.7) | 31.8 (24.5–40.3) | 0.4 |
| Muscle mass (%) | 65.7 (59.1–71.1) | 64.6 (56.7–70.1) | 0.5 |
| Hemoglobin (g/dL) | 13.7 (12.5–14.5) | 13.7 (12.6–14.3) | 0.3 |
| White blood cells (103/µL) | 6200 (5200–6970) | 6020 (5180–6740) | 0.7 |
| Lymphocytes (103/µL) | 1590 (1233–1830) | 1441 (1090–1755) | 0.4 |
| Neutrophils (103/µL) | 3680 (3128–4868) | 3690 (2875–4555) | 0.6 |
| Neutrophil/Lymphocyte ratio | 2.63 (2.24–3.08) | 2.49 (2.06–3.47) | 0.9 |
| Creatinine (mg(dL) | 0.9 (0.7–0.9) | 0.8 (0.7–0.9) | 0.4 |
| Albumin (g/dL) | 4.5 (4.3–4.6) | 4.4 (4.3–4.5) | 0.3 |
| Prothrombin time (seg) | 10.8 (10.5–11.4) | 10.7 (9.9–11.1) | 0.1 |
| IL-6 (pg/mL) | 3.6 (2.3–4.8) | 3.0 (2.5–4.7) | 0.6 |
| IL-10 < 1.6 pg/mL, n (%) | 18 (94.7) | 27 (100) | 0.2 |
| TNF-α (pg/mL) | 9.0 (7.6–11.3) | 6.0 (7.0–11.1) | 0.1 |
| Placebo Group (n = 19) | Nutraceutical Group (n = 27) | p 1 | |
|---|---|---|---|
| Duration of treatment (weeks) | 3 (4–3) | 3 (4–2) | 0.9 |
| Adherence to treatment (>80%) | 18 (94.7) | 24 (88.9) | 0.5 |
| Adverse events | 8 (42.1) | 7 (25.9) | 0.2 |
| Adverse events (≥III) | 0 (0) | 1 (14.3) | 0.2 |
| Type of surgery: | |||
| 5 (26.3) | 9 (33.3) | 0.6 |
| 5 (26.3) | 9 (33.2) | 0.6 |
| 0 (0) | 2 (7.4) | 0.2 |
| 4 (2.1) | 1 (3.7) | 0.1 |
| 3 (15.8) | 1 (3.7) | 0.2 |
| 2 (10.5) | 1 (3.7) | 0.4 |
| 0 (0) | 4 (14.8) | 0.1 |
| Surgical approach: | |||
| 9 (47.4) | 20 (74.1) | 0.07 |
| 2 (10.5) | 2 (7.4) | 0.7 |
| 0 (0) | 2 (7.4) | 0.0 |
| 8 (42.1) | 3 (11.1) | 0.02 |
| Postoperative complications | |||
| 2 (10.5) | 3 (11.) | 0.9 |
| 2 (10.5) | 3 (11.1) | 0.9 |
| 1 (5.3) | 1 (3.7) | 0.8 |
| Placebo Group (n = 19) | Nutraceutical Group (n = 27) | p 1 | |
|---|---|---|---|
| BMI (Kg/m2) | 26.2 (23.6–29.7) | 26.0 (23.5–30.1) | 0.8 |
| Fat mass (%) | 30.1 (23.6–37.4) | 30.9 (23.6–38.9) | 0.6 |
| Muscle mass (%) | 66.04 (59.4–72.7) | 65.6 (58.1–72.7) | 0.3 |
| Quality of life: | |||
| 100 (90–100) | 100 (77.5–100) | 0.4 |
| 100 (100–100) | 100(100–100) | 1.0 |
| 100 (66.7–100) | 100 (66.7–100) | 0.8 |
| 70 (55–90) | 70 (56.3–85) | 0.8 |
| 80 (52–96) | 80 (61–95) | 1.0 |
| 100 (75–100) | 100 (87.5–100) | 0.8 |
| 100 (80–100) | 100 (70–100) | 0.5 |
| 60 (45–80) | 65 (46.3–80) | 0.4 |
| Blood parameters: | |||
| 13.3 (12.8–13.95) | 13.3 (12.7–13.9) | 0.5 |
| 6160 (5290–7185) | 5990 (4650–7130) | 0.7 |
| 1435 (1093–1780) | 1730 (1105–2090) | 0.3 |
| 4200 (2978–4840) | 3590 (2285–4415) | 0.1 |
| 2.62 (2.04–3.58) | 2.06 (1.29–2.86) | 0.07 |
| 0.9 (0.8–1.0) | 0.8 (0.8–1.0) | 0.6 |
| 4.3 (4.2–4.5) | 4.2 (4.1–4.4) | 0.1 |
| 11.0 (10.3–11.7) | 9.2 (7.5–10.5) | 0.1 |
| 3.6 (2.4–5.9) | 3.5 (2.7–5.2) | 0.5 |
| 17 (89.5) | 25 (92.6) | 0.7 |
| 9.0 (7.6–12.1) | 6.0 (7.5–10.5) | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regueiro, C.; Diez Martín, A.I.; Pérez, S.; Daviña-Núñez, C.; Zarraquiños, S.; Remedios, D.; Sánchez Gómez, C.A.; Alonso Lorenzo, S.; Fernández Poceiro, R.; de Castro Parga, M.L.; et al. The Effect of Fungal Nutraceutical Supplementation on Postoperative Complications, Inflammatory Factors and Fecal Microbiota in Patients Undergoing Colorectal Cancer Surgery with Curative Intent: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomedicines 2025, 13, 1185. https://doi.org/10.3390/biomedicines13051185
Regueiro C, Diez Martín AI, Pérez S, Daviña-Núñez C, Zarraquiños S, Remedios D, Sánchez Gómez CA, Alonso Lorenzo S, Fernández Poceiro R, de Castro Parga ML, et al. The Effect of Fungal Nutraceutical Supplementation on Postoperative Complications, Inflammatory Factors and Fecal Microbiota in Patients Undergoing Colorectal Cancer Surgery with Curative Intent: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomedicines. 2025; 13(5):1185. https://doi.org/10.3390/biomedicines13051185
Chicago/Turabian StyleRegueiro, Cristina, Astrid Irene Diez Martín, Sonia Pérez, Carlos Daviña-Núñez, Sara Zarraquiños, David Remedios, Cristina Alejandra Sánchez Gómez, Sara Alonso Lorenzo, Romina Fernández Poceiro, María Luisa de Castro Parga, and et al. 2025. "The Effect of Fungal Nutraceutical Supplementation on Postoperative Complications, Inflammatory Factors and Fecal Microbiota in Patients Undergoing Colorectal Cancer Surgery with Curative Intent: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial" Biomedicines 13, no. 5: 1185. https://doi.org/10.3390/biomedicines13051185
APA StyleRegueiro, C., Diez Martín, A. I., Pérez, S., Daviña-Núñez, C., Zarraquiños, S., Remedios, D., Sánchez Gómez, C. A., Alonso Lorenzo, S., Fernández Poceiro, R., de Castro Parga, M. L., Hernández Ramírez, V., Rodríguez-Blanco, A., Sinde, E., Fernández-de-Ana, C., & Cubiella, J. (2025). The Effect of Fungal Nutraceutical Supplementation on Postoperative Complications, Inflammatory Factors and Fecal Microbiota in Patients Undergoing Colorectal Cancer Surgery with Curative Intent: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomedicines, 13(5), 1185. https://doi.org/10.3390/biomedicines13051185







